Abstract
Key points
Reducing excessive oxidative stress, through chronic exercise or antioxidants, can decrease the negative effects induced by excessive amounts of oxidative stress. Transient increases in oxidative stress produced during acute exercise facilitate beneficial vascular training adaptations, but the effects of non‐specific antioxidants on exercise training‐induced vascular adaptations remain elusive.
Circulating angiogenic cells (CACs) are an exercise‐inducible subset of white blood cells that maintain vascular integrity.
We investigated whether mitochondria‐specific antioxidant (MitoQ) supplementation would affect the response to 3 weeks of endurance exercise training in CACs, muscle mitochondrial capacity and maximal oxygen uptake in young healthy men.
We show that endurance exercise training increases multiple CAC types, an adaptation that is not altered by MitoQ supplementation. Additionally, MitoQ does not affect skeletal muscle or whole‐body aerobic adaptations to exercise training.
These results indicate that MitoQ supplementation neither enhances nor attenuates endurance training adaptations in young healthy men.
Abstract
Antioxidants have been shown to improve endothelial function and cardiovascular outcomes. However, the effects of antioxidants on exercise training‐induced vascular adaptations remain elusive. General acting antioxidants combined with exercise have not impacted circulating angiogenic cells (CACs). We investigated whether mitochondria‐specific antioxidant (MitoQ) supplementation would affect the response to 3 weeks of endurance exercise training on CD3+, CD3+/CD31+, CD14+/CD31+, CD31+, CD34+/VEGFR2+ and CD62E+ peripheral blood mononuclear cells (PBMCs), muscle mitochondrial capacity, and maximal oxygen uptake () in healthy men aged 22.1 ± 0.7 years, with a body mass index of 26.9 ± 0.9 kg m–2, and 24.8 ± 1.3% body fat. Analysis of main effects revealed that training induced 33, 105 and 285% increases in CD14+/CD31+, CD62E+ and CD34+/VEGFR2+ CACs, respectively, and reduced CD3+/CD31− PBMCs by 14%. There was no effect of MitoQ on CAC levels. Also independent of MitoQ supplementation, exercise training significantly increased quadriceps muscle mitochondrial capacity by 24% and by roughly 7%. In conclusion, endurance exercise training induced increases in multiple CAC types, and this adaptation is not modified by MitoQ supplementation. Furthermore, we demonstrate that a mitochondrial‐targeted antioxidant does not influence skeletal muscle or whole‐body aerobic adaptations to exercise training.
Keywords: circulating angiogenic cells, endurance training, mitochondria, mitoq, near infrared spectroscopy
Key points
Reducing excessive oxidative stress, through chronic exercise or antioxidants, can decrease the negative effects induced by excessive amounts of oxidative stress. Transient increases in oxidative stress produced during acute exercise facilitate beneficial vascular training adaptations, but the effects of non‐specific antioxidants on exercise training‐induced vascular adaptations remain elusive.
Circulating angiogenic cells (CACs) are an exercise‐inducible subset of white blood cells that maintain vascular integrity.
We investigated whether mitochondria‐specific antioxidant (MitoQ) supplementation would affect the response to 3 weeks of endurance exercise training in CACs, muscle mitochondrial capacity and maximal oxygen uptake in young healthy men.
We show that endurance exercise training increases multiple CAC types, an adaptation that is not altered by MitoQ supplementation. Additionally, MitoQ does not affect skeletal muscle or whole‐body aerobic adaptations to exercise training.
These results indicate that MitoQ supplementation neither enhances nor attenuates endurance training adaptations in young healthy men.
Abbreviations
- BMI
body mass index
- CAC
circulating angiogenic cell
- CD
cluster differentiation
- CD62E
E‐selectin
- FFM
fat‐free mass
- FITC
fluorescein isothiocyanate
- MDA
malondialdehyde
- MitoQ
mitochondria‐targeted ubiquinone antioxidant
- NIRS
near infrared spectroscopy
- PBMC
peripheral blood mononuclear cell
- PE
phycoerythrin
- ROS
reactive oxygen species
- VEGFR2
vascular endothelial growth factor receptor 2
maximal oxygen consumption
Introduction
Reactive oxygen species (ROS) are important signalling molecules with roles in both health and disease. At physiological concentrations, ROS play a regulatory role in vascular homeostasis, beta oxidation, glucose uptake and myokine production (Quintero et al. 2006; Zhang & Gutterman, 2007; Silveira et al. 2008; Scheele et al. 2009; Finkel, 2012; Merry & McConell, 2012; Freed & Gutterman, 2013). Importantly, transient increases in ROS produced during exercise facilitate beneficial vascular and skeletal muscle adaptations to exercise training (Lauer et al. 2005; Ji et al. 2006; Ushio‐Fukai, 2006; Powers et al. 2011; Nikolaidis et al. 2012). Minimizing excessive oxidative stress, via chronic exercise or exogenous antioxidants, can attenuate the deleterious effects induced by excessive amounts of ROS (Dudgeon et al. 1998; Kojda & Hambrecht, 2005; Jablonski et al. 2007; Zhang & Gutterman, 2007; Widlansky & Gutterman, 2011).
The effects of antioxidant supplementation on training‐induced changes in vascular function are unclear (Polytarchou & Papadimitriou, 2004; Lauer et al. 2005; Polytarchou & Papadimitriou, 2005; Ristow et al. 2009; Wray et al. 2009; Theodorou et al. 2011), but literature suggests non‐specific antioxidants do not alter training‐induced changes in circulating angiogenic cells (CACs) (Balestrieri et al. 2008; Fiorito et al. 2008 a,b). CACs are an exercise‐inducible subset of peripheral blood mononuclear cells (PBMCs) with endothelial‐specific antigens and angiogenic characteristics thought to facilitate endothelial repair by homing to sites of damage and integrating into the endothelial monolayer or releasing paracrine factors (Asahara et al. 1997; Kim et al. 2011; Lansford et al. 2016). Importantly, the maintenance of vascular integrity is thought to be contingent on the number and function of CACs (Koutroumpi et al. 2012). Exercise training enhances CACs and their regenerative capacity (Koutroumpi et al. 2012; Landers‐Ramos et al. 2015), but the effect of mitochondria‐specific antioxidant supplementation on training‐induced changes in CACs is unknown.
The mitochondria‐targeted antioxidant MitoQ is engineered to accumulate to the matrix surface of the inner mitochondrial membrane and exert beneficial effects through oxidation of ubiquinol to ubiquinone and subsequent re‐reduction, thereby reducing electron backup and ROS formation (Smith & Murphy, 2010). Moreover, the ubiquinone moiety appears to have some beneficial effects on the vascular endothelium. For example, evidence from animal and in vitro studies has demonstrated that MitoQ administration mitigates intracellular PBMC oxidative stress, prevents endothelial apoptosis and re‐establishes endothelial function in disease states (Dhanasekaran et al. 2004; Graham et al. 2009; Marthandan et al. 2011; Gioscia‐Ryan et al. 2014). However, the efficacy of MitoQ supplementation is uncertain (Doughan & Dikalov, 2007; Gane et al. 2010; Snow et al. 2010). The majority of studies indicate that non‐specific exogenous antioxidant supplementation does not alter or can even blunt exercise training adaptations in muscle (Ristow et al. 2009; Wray et al. 2009; Nikolaidis et al. 2012). Interestingly, however, our group recently reported that a resveratrol/piperine supplement in combination with endurance training enhanced skeletal muscle oxidative capacity compared to training alone in the human forearm (Polley et al. 2016). Collectively, the mixed evidence on whether antioxidant supplements alter training adaptations indicates that further studies are warranted.
Therefore, the present study investigated the effect of endurance exercise training with and without MitoQ supplementation on CACs, skeletal muscle oxidative capacity and maximal oxygen uptake. Consistent with previously observed effects of non‐specific antioxidant supplementation during aerobic training (Balestrieri et al. 2008; Fiorito et al. 2008 a; Ristow et al. 2009; Wray et al. 2009; Theodorou et al. 2011) and MitoQ's putative mechanism, it was hypothesized that MitoQ would either attenuate or have no effect on training‐induced adaptations in CACs, muscle oxidative capacity or maximal oxygen uptake.
Methods
Ethical approval
The University of Georgia Institutional Review Board approved all study procedures, which conformed to Declaration of Helsinki standards, and subjects provided written informed consent prior to participation.
Screening
Twenty 18–40‐year‐old men free of cardiovascular or metabolic diseases, participating in less than 45 min of vigorous activity 3 days per week, or less than 60 min of moderate activity 4 days per week for the previous 6 months were recruited. As previously described, vigorous activity was defined as activities requiring greater than or equal to six metabolic equivalents (e.g. running), and moderate activity was defined as activities requiring between three and six metabolic equivalents (e.g. brisk walking) (Pescatello et al. 2014). Subjects were excluded based on the following: any known food allergies, body mass index (BMI) < 20 or > 35 kg m–2, currently smoking or previous use within 2 years, currently taking more than three pharmacological agents including prescription, non‐prescription, cardiovascular and/or metabolic drugs or oral antioxidants ≥ 300 mg day–1 within 120 days of enrollment. A 7‐day washout period was required for subjects currently taking oral antioxidants at doses between 25 and 300 mg day–1 and any multivitamin or mineral supplements.
Testing protocol
Baseline and post‐training visits consisted of a blood draw and assessments of body composition, skeletal muscle mitochondrial capacity and maximal oxygen consumption (). Subjects arrived in the laboratory between 06.00 and 09.00 h for their baseline visit in a fasted state (> 12 h) and refrained from exercise, alcohol consumption and caffeine ingestion for the preceding 24 h. Blood samples were obtained using standard venipuncture techniques. Dual‐energy X‐ray absorptiometry was used to assess body composition (iDXA, GE Healthcare, Pittsburg, PA, USA). Skeletal muscle mitochondrial oxidative capacity was measured as previously described (Ryan et al. 2012, 2014). Briefly, near infrared spectroscopy (NIRS) was used to measure metabolic recovery kinetics in the left vastus lateralis muscle following contractions induced by neuromuscular electrical stimulation. The recovery kinetics of muscle oxygen consumption were fitted to a mono‐exponential curve where the rate constant (min−1) derived from the curve is directly related to the muscles’ maximal oxidative capacity (Ryan et al. 2014). Whole‐body was assessed via a progressive cycle ergometer protocol. To elicit within 6–12 min, the first stage commenced at a workload between 150 and 200 W and increased by 25 W every 2 min until subjects could no longer maintain a pedal cadence of 60 r.p.m. Expired gas (Parvo Medics TrueOne 2400, Parvo Medics, Salt Lake City, UT, USA) and heart rate (Polar, Polar Electro Inc., Lake Success, NY, USA) were collected throughout the graded exercise test. was considered maximal if a plateau was achieved (< 250 ml min–1 increase in with increased work). If a plateau was not apparent, the following criteria verified maximal effort: respiratory exchange ratio > 1.15 and peak heart rate within 10 beats min–1 of age‐predicted maximum. In a randomized and double blind fashion, subjects were assigned to consume a placebo or MitoQ pill every morning, with breakfast, throughout study enrollment. Further, subjects were instructed to maintain normal exercise routines and abstain from alcohol. Placebo pills contained rice flour (Arrowhead Mills, The Hain Celestial Group, Inc., Lake Success, NY, USA). MitoQ (Antipodean Pharmaceuticals Inc., San Francisco, CA, USA; obtained from Amazon.com, Inc., Seattle, WA, USA) capsules were prescribed in accordance with the manufacturer's recommended dose of 10 mg daily. Baseline measurements were repeated 24–48 h after the last training session. For post‐testing measurements, subjects ingested their assigned pill with water before blood sampling, but otherwise remained in a fasted state and did not consume caffeine for the previous 24 h.
Exercise training
Subjects completed three exercise sessions during the first week of training, with each session consisting of 45 min of stationary cycling at an intensity of 50–60% . During the second and third weeks, training load increased to 60 min sessions at 60–70% on 5 days per week. Research assistants supervised all exercise‐training sessions and monitored intensity by heart rate and ratings of perceived exertion.
Lipid peroxidation assay
EDTA‐collected plasma samples were prepared by centrifugation and stored at –80°C until examined in duplicate for concentrations of malondialdehyde (MDA) using the TBARS Assay Kit (Cayman Chemical Company, Ann Arbor, MI, USA), according to the manufacturer's instructions. Absorbance values read at 530 nm yielded a standard curve (R 2 = 0.9956) to determine the amount of MDA in each sample.
CAC preparation and data acquisition
PBMCs were isolated from 10 ml of EDTA‐collected blood samples (Vacutainer K2EDTA, Becton Dickinson, Franklin Lakes, NJ, USA). After centrifugation on a density gradient (Ficoll, GE Healthcare, Pittsburg, PA, USA), cells were washed with PBS three times and characterized with the following fluorescein isothiocyanate (FITC) and phycoerythrin (PE) conjugated cluster differentiation (CD) antibody combinations: FITC‐CD3/PE‐CD31, FITC‐CD14/PE‐CD31, FITC‐CD34/PE‐VEGFR2 (vascular endothelial growth factor receptor 2), and PE‐CD62E (E‐selectin) (all BD Biosciences, Becton Dickinson, Franklin Lakes, NJ, USA). After fluorochrome staining, PBMCs were incubated in the dark and fixed. Fluorescence was acquired from 50 000 positive events via flow cytometry (CyAn ADP, Beckman Coulter, Hialeah, FL, USA). Data were gated from unstained and single‐stained controls in respective populations and analysed in FlowJo v.10.0.08 (FlowJo, LLC, Ashland, OR, USA), as previously described (Shill et al. 2016).
Statistics
Statistical analysis was performed in SPSS, Version 23.0 (IBM Corp., Armonk, NY, USA). Data were analysed using two‐factor repeated measures ANOVA and Fisher's Least Significant Difference tests for simple effects. Data are presented as mean ± SEM. Statistical significance was accepted at P < 0.05. Approaching statistical significance was defined as P ≤ 0.10.
Results
Subject characteristics are presented in Table 1. MitoQ did not produce any adverse side effects among study participants, and no adverse events occurred during exercise training. Exercise training adherence was 99% (258/260 sessions completed during the study). Additionally, placebo/MitoQ pill compliance was 100%, as monitored through daily questionnaires. No interactions between training and MitoQ were observed (P > 0.05); therefore, main effects of training are reported.
Table 1.
Subject characteristics
| Combined (n = 20) | Placebo (n = 10) | MitoQ (n = 10) | ||||
|---|---|---|---|---|---|---|
| Baseline | After training | Baseline | After training | Baseline | After training | |
| Age (years) | 22.1 ± 0.7 | – | 20.8 ± 0.7 | – | 23.4 ± 0.9 | – |
| Height (m) | 1.75 ± 0.01 | – | 1.74 ± 0.02 | – | 1.76 ± 0.02 | – |
| Mass (kg) | 81.9 ± 2.8 | 81.7 ± 2.7 | 82.3 ± 4.9 | 81.9 ± 4.8 | 81.6 ± 3.0 | 81.6 ± 3.0 |
| BMI (kg m–2) | 26.9 ± 0.9 | 26.8 ± 0.9 | 27.4 ± 1.7 | 27.3 ± 1.7 | 26.3 ± 0.8 | 26.3 ± 0.7 |
| Body fat (%) | 24.8 ± 1.3 | 23.9 ± 1.3* | 24.8 ± 2.1 | 23.9 ± 2.0 | 24.1 ± 1.7 | 24.0 ± 1.7 |
| Fat mass (kg) | 20.1 ± 1.6 | 19.3 ± 1.5* | 20.5 ± 2.7 | 19.5 ± 2.6 | 19.7 ± 1.7 | 19.0 ± 1.6 |
| FFM (kg) | 62.4 ± 1.7 | 62.8 ± 1.7 | 62.4 ± 2.5 | 62.8 ± 2.5 | 62.5 ± 2.4 | 62.8 ± 2.4 |
| Lean mass (kg) | 59.3 ± 1.6 | 59.6 ± 1.6 | 59.3 ± 2.4 | 59.7 ± 2.3 | 59.3 ± 2.3 | 59.6 ± 2.3 |
| MDA (μM) | 9.4 ± 0.6 | 8.4 ± 0.6 | 9.6 ± 0.6 | 9.1 ± 0.9 | 9.2 ± 1.1 | 7.7 ± 0.8 |
Values are mean ± SEM. BMI, body mass index; FFM, fat‐free mass = mass – fat mass; lean mass = mass – fat mass – bone; MDA, malondialdehyde.
*Statistically significant effect of training (P < 0.05).
Training did not elicit significant changes in body mass, BMI, fat‐free mass (FFM), lean mass or MDA concentrations (Table 1) (P > 0.05). Body fat percentage and fat mass were reduced by 4% after training (Table 1) (P < 0.05). Training induced 7% increases in absolute (Fig. 1 A) and relative (Fig. 1 B) (P < 0.05). relative to FFM increased by 6% (Fig. 1 C) (P < 0.05). Additionally, training increased quadriceps muscle mitochondrial capacity by 24% (Fig. 1 D) (P < 0.05).
Figure 1. Oxidative capacity before and after endurance training .

Absolute (A), relative (B), fat‐free mass (C) and skeletal muscle oxidative capacity (D) before and after 3 weeks of endurance training in placebo (white) and MitoQ (grey) groups. FFM, fat‐free mass (kg); skeletal muscle oxidative capacity data available on 19 subjects (Placebo = 10, MitoQ = 9). *Statistically significant effect of training (P < 0.05).
Training induced 105 and 285% increases in CD62E+ (Fig. 2 A) and CD34+/VEGR2+ (Fig. 2 B) PBMCs, respectively (P < 0.05). A 33% increase in CD14+/CD31+ cells approached statistical significance after training (Fig. 3 A) (P = 0.056). Training did not alter CD14+/CD31− or CD14−/CD31+ PBMCs (Fig. 3 B, C) (P > 0.05). Exercise training reduced CD3+/CD31− PBMCs by 14% (Fig. 4 A) (P < 0.05). Training did not alter CD3+/CD31+, CD3−/CD31+ or CD3+ PBMCs (Fig. 4 B–D) (P > 0.05).
Figure 2. CD62E+ and CD34+/VEGFR2+ CACs before and after endurance training .
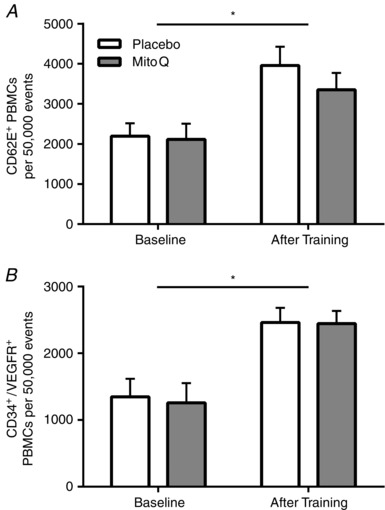
CD62E+ (A) and CD34+/VEGFR2+ (B) PBMCs before and after 3 weeks of endurance training in placebo (white) and MitoQ (grey) groups. *Statistically significant effect of training (P < 0.05).
Figure 3. CD14+ PBMCs before and after endurance training .
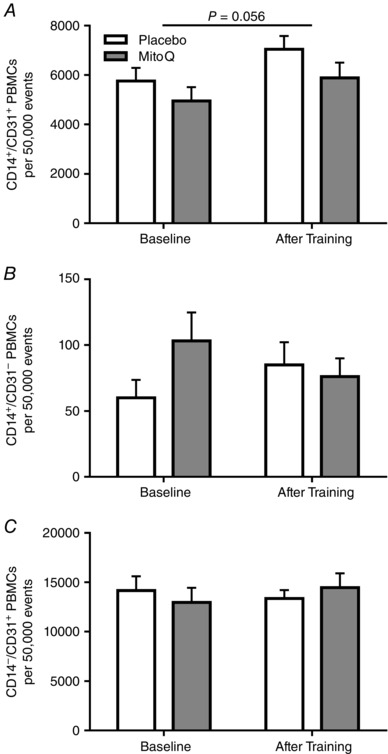
CD14+/CD31+ (A), CD14+/CD31− (B) and CD14−/CD31+ (C) PBMCs before and after 3 weeks of endurance training in placebo (white) and MitoQ (grey) groups. Brackets with P‐values indicate data approaching statistical significance (P ≤ 0.10).
Figure 4. CD3+ PBMCs before and after endurance training .
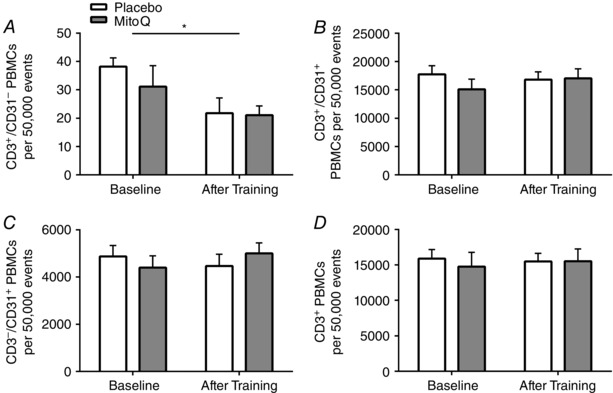
CD3+/CD31− (A), CD3+/CD31+ (B), CD3−/CD31+ (C) and CD3+ (D) PBMCs before and after 3 weeks of endurance training in placebo (white) and MitoQ (grey) groups. *Statistically significant effect of trainng (P < 0.05).
Discussion
The present investigation demonstrates that supplementation of commercially available MitoQ at the manufacturer's recommended dose did not influence training‐induced changes in CAC subpopulations or aerobic training adaptations in young healthy men. Additionally, our observations of training‐induced increases in CD62E+, CD34+/VEGR2+ and CD14+/CD31+ cells, but a lack of change in CD3+ or CD3+/CD31+ cells, suggest that training‐induced adaptations in CACs are heterogeneous among cell types. Finally, we demonstrate that MitoQ does not influence training‐induced changes in skeletal muscle or whole‐body aerobic capacity.
Vascular‐derived mitochondrial ROS are cell‐signalling molecules that facilitate CAC recruitment and function, contributing to the maintenance of vascular integrity (Schroder et al. 2009; Ushio‐Fukai & Urao, 2009; Fleissner & Thum, 2011; Widlansky & Gutterman, 2011; Urao & Ushio‐Fukai, 2013). While exercise‐induced ROS have been shown to modulate CAC mobilization and angiogenesis, the present study demonstrates MitoQ does not influence the training‐induced CAC enhancement (Polytarchou & Papadimitriou, 2004; Ushio‐Fukai, 2006; Urao et al. 2008; Fiorito et al. 2008 b; Ushio‐Fukai & Urao, 2009; Suvorava et al. 2010). Consistent with these results, previous studies have found CACs were not altered by antioxidant supplementation during exercise training (Balestrieri et al. 2008; Fiorito et al. 2008 a,b). However, these studies used vitamins C and E, polyphenols and l‐arginine, which do not specifically target mitochondrial ROS. Taken together, our current results and the available evidence support the notion that antioxidant supplements, mitochondrial‐targeted or otherwise, do not alter training‐induced effects on CACs.
Although mitochondria in contracting muscles have generally been considered the source of ROS during exercise (Kanter, 1994; Urso & Clarkson, 2003), accumulating evidence suggests mitochondrial bioenergetics are not responsible for producing excessive ROS, indicating an origin other than skeletal muscle mitochondria, such as NADPH‐ or xanthine oxidases (Herrero & Barja, 1997; Di Meo & Venditti, 2001; St‐Pierre et al. 2002; Kozlov et al. 2005; Jackson et al. 2007; Powers & Jackson, 2008). Furthermore, CACs’ predominant source of ROS is derived from sources other than mitochondria, for example NADPH‐oxidases (Ushio‐Fukai & Urao, 2009). Thus, non‐mitochondrial ROS production during exercise could prevent MitoQ from being an effective modulator of exercise‐induced ROS, which may potentially explain our results. Moreover, although MitoQ did not influence training‐induced changes in CACs, a main effect of training was noted in several CAC subpopulations. Our study is the first to report that short‐term, aerobic exercise training affects multiple CACs including CD62E+, CD34+/VEGFR2+ and CD14+/CD31+ PBMCs, with a reduction in CD3+/CD31− PBMCs. As our study included only young healthy males, future studies are warranted to determine whether training enhances numbers of these cells in other populations (e.g. patients with chronic diseases) or alters their angiogenic function.
CD62E, an endothelial adhesion molecule, is critical to cell adhesion, angiogenesis and CAC recruitment to ischaemic tissue (Koch et al. 1995; Mazo et al. 1998; Oh et al. 2007). The present study documents, for the first time, an increase in CD62E+ CACs after an endurance exercise training intervention. We recently reported that CD62E+ PBMCs are an acute exercise‐inducible cell type with angiogenic potential (Lansford et al. 2016). The cumulative effect of transient increases in CD62E+ cells after each training session is a plausible explanation for our finding of enhanced basal CD62E+ PBMCs after training. Although post‐training blood samples were obtained 24–48 h after the final exercise session, it is nevertheless possible we captured a residual effect of the final acute exercise bout rather than training effects per se. Future studies are necessary to determine the time course of acute exercise‐induced increases in CD62E+ PBMCs.
CD34+ cell subpopulations, largely studied for their role in regenerative medicine, facilitate angiogenesis and rescue ischaemic tissue probably through paracrine signalling (Asahara et al. 1997, 1999; Weissman, 2000; Kim et al. 2011; Landers‐Ramos et al. 2015). We demonstrate increased CD34+/VEGFR2+ PBMCs after exercise training, consistent with previous investigations (Koutroumpi et al. 2012). Inherent antioxidative phenotypes in CD34+ cells potentially explain why MitoQ did not impact changes in CD34+/VEGFR2+ PBMCs with aerobic training (Dernbach et al. 2004).
Monocytes, identified by the CD14 surface antigen, migrate to sites of vascular injury and contribute to vascular homeostasis (Awad et al. 2006). Although we recently showed a decrease in CD14+/CD31+ CACs after acute exercise (Lansford et al. 2016), the present study found an increase in CD14+/CD31+ PBMCs after 3 weeks of aerobic training. Not all investigations have reported an influence of exercise training on CD14+ cells (Stewart et al. 2005; Czepluch et al. 2011). Enhanced CD14+/CD31+ CACs after endurance exercise training may be indicative of an anti‐inflammatory state and active endothelial repair (Harraz et al. 2001; Timmerman et al. 2008), but future studies are needed to address the influence of angiogenic monocytes on inflammation.
The influence of exercise training on CD3+ cells appears to be mediated by training status, duration and intensity (Shore et al. 1999; Lancaster et al. 2004; Wang et al. 2011). The present study observed no changes in CD3+ cells, but a reduction in CD3+/CD31− PBMCs after exercise training. Although the precise mechanism underlying this effect of training remains unclear, it is possible that the training‐induced decrease of CD3+/CD31− PBMCs was linked to a reduction in fat mass and associated systemic inflammation (Rajala & Scherer, 2003).
Our data indicate MitoQ supplementation neither enhances nor attenuates endurance training adaptations in skeletal muscle oxidative capacity and in young healthy men. The aerobic stimulus produced a 24% increase in oxidative capacity, concurring with previous training studies in different muscles (Polley et al. 2016), and 6–7% increases in . The effects of antioxidant supplementation on exercise‐induced adaptations could be attributable to training intensity and duration, the dose of antioxidant, and the differential impact of exercise on systemic‐tissue ROS production (Nikolaidis et al. 2012; Durand & Gutterman, 2014). The effect of antioxidant supplementation may also be influenced by the relationship between endogenous antioxidant scavenging capacity and the source of ROS responsible for exercise‐induced training adaptations. Assuming MitoQ's aforementioned biochemical formula and molecular function were operative in the present study, the observed increases in and mitochondrial capacity may have been driven by non‐mitochondrial ROS production, supporting previous studies investigating the role of non‐mitochondrial muscle ROS contributing to exercise adaptations (Herrero & Barja, 1997; Di Meo & Venditti, 2001; St‐Pierre et al. 2002; Kozlov et al. 2005; Jackson et al. 2007). It is also possible that the relatively lower dose of MitoQ administered in the present study, 10 mg day–1 as recommended by the manufacturer vs. 20–80 mg day–1 in previous clinical trials (Gane et al. 2010; Snow et al. 2010), was not sufficient to suppress the exercise‐induced ROS in our study subjects. Importantly, neither exercise training nor MitoQ supplementation impacted plasma MDA concentrations, an indicator of oxidative stress, although we did not assay mitochondrial‐derived ROS production in CACs or muscle biopsy samples. Future examinations incorporating these measures would be informative.
Our study has limitations that warrant mention. First, our study involved only young, healthy males. Accordingly, our findings on the influence of MitoQ on training‐induced adaptations should not be extrapolated to other populations, e.g. women, older adults and/or patients with chronic diseases. Additionally, we did not measure levels of MitoQ in blood or muscle tissue. Such measurements would have provided insight into whether the 10 mg dose, as recommended by the manufacturer and therefore used in the present study, could induce biological effects. Future studies should address these limitations by examining other study populations and incorporating muscle biopsies and/or CAC oxidative stress measurements into their experimental approach.
In conclusion, the present study indicates that CAC subpopulations and aerobic training adaptations are not affected by supplementing commercially available MitoQ, at the manufacturer's recommended dose, during exercise training in young healthy men. We provide the first evidence of a heterogeneous CAC adaptation to endurance exercise training not limited to cells of progenitor origin. Specifically, we demonstrate an increase in CD62E+, CD34+/VEGFR2+ and CD14+/CD31+ CACs and a reduction in CD3+/CD31− PBMCs after training. Moreover, our study is the first to use NIRS to non‐invasively detect a training‐induced increase in the oxidative capacity of the human vastus lateralis. We extend previous findings of antioxidant effects on training adaptations by demonstrating that mitochondria‐targeted antioxidant administration neither enhances nor attenuates short‐term aerobic training adaptations in muscle oxidative capacity or whole‐body .
Additional information
Competing interests
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions
N.T.J. conception and design of research; D.D.S., W.M.S., T.B.W. and K.A.L. performed experiments; D.D.S. and W.M.S. analysed data; D.D.S., W.M.S., T.B.W., K.K.M. and N.T.J. interpreted results; D.D.S. prepared figures; D.D.S., W.M.S. and T.B.W. drafted manuscript; D.D.S., W.M.S., T.B.W., K.A.L., K.K.M. and N.T.J. edited and revised manuscript; D.D.S., W.M.S., T.B.W., K.A.L., K.K.M. and N.T.J. approved final version of manuscript.
Funding
This study was supported by funding from the Office of the Vice President for Research and the College of Education at the University of Georgia (to N.T.J.).
Acknowledgements
We thank Julie Nelson and Meagan Marshburn for their technical assistance and the subjects for their participation.
References
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M & Isner JM (1999). Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85, 221–228. [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G & Isner JM (1997). Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967. [DOI] [PubMed] [Google Scholar]
- Awad O, Dedkov EI, Jiao C, Bloomer S, Tomanek RJ & Schatteman GC (2006). Differential healing activities of CD34+ and CD14+ endothelial cell progenitors. Arterioscler Thromb Vasc Biol 26, 758–764. [DOI] [PubMed] [Google Scholar]
- Balestrieri ML, Fiorito C, Crimi E, Felice F, Schiano C, Milone L, Casamassimi A, Giovane A, Grimaldi V, del Giudice V, Minucci PB, Mancini FP, Servillo L, D'Armiento FP, Farzati B & Napoli C (2008). Effect of red wine antioxidants and minor polyphenolic constituents on endothelial progenitor cells after physical training in mice. Int J Cardiol 126, 295–297. [DOI] [PubMed] [Google Scholar]
- Czepluch FS, Barres R, Caidahl K, Olieslagers S, Krook A, Rickenlund A, Zierath JR & Waltenberger J (2011). Strenuous physical exercise adversely affects monocyte chemotaxis. Thromb Haemost 105, 122–130. [DOI] [PubMed] [Google Scholar]
- Dernbach E, Urbich C, Brandes RP, Hofmann WK, Zeiher AM & Dimmeler S (2004). Antioxidative stress‐associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood 104, 3591–3597. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran A, Kotamraju S, Kalivendi SV, Matsunaga T, Shang T, Keszler A, Joseph J & Kalyanaraman B (2004). Supplementation of endothelial cells with mitochondria‐targeted antioxidants inhibit peroxide‐induced mitochondrial iron uptake, oxidative damage, and apoptosis. J Biol Chem 279, 37575–37587. [DOI] [PubMed] [Google Scholar]
- Di Meo S & Venditti P (2001). Mitochondria in exercise‐induced oxidative stress. Biol Signals Recept 10, 125–140. [DOI] [PubMed] [Google Scholar]
- Doughan AK & Dikalov SI (2007). Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxid Redox Signal 9, 1825–1836. [DOI] [PubMed] [Google Scholar]
- Dudgeon S, Benson DP, MacKenzie A, Paisley‐Zyszkiewicz K & Martin W (1998). Recovery by ascorbate of impaired nitric oxide‐dependent relaxation resulting from oxidant stress in rat aorta. Br J Pharmacol 125, 782–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand MJ & Gutterman DD (2014). Exercise and vascular function: how much is too much? Can J Physiol Pharmacol 92, 551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T (2012). Signal transduction by mitochondrial oxidants. J Biol Chem 287, 4434–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorito C, Balestrieri ML, Crimi E, Giovane A, Grimaldi V, Minucci PB, Servillo L, D'Armiento FP, Farzati B & Napoli C (2008. a). Effect of l‐arginine on circulating endothelial progenitor cells and VEGF after moderate physical training in mice. Int J Cardiol 126, 421–423. [DOI] [PubMed] [Google Scholar]
- Fiorito C, Rienzo M, Crimi E, Rossiello R, Balestrieri ML, Casamassimi A, Muto F, Grimaldi V, Giovane A, Farzati B, Mancini FP & Napoli C (2008. b). Antioxidants increase number of progenitor endothelial cells through multiple gene expression pathways. Free Radic Res 42, 754–762. [DOI] [PubMed] [Google Scholar]
- Fleissner F & Thum T (2011). Critical role of the nitric oxide/reactive oxygen species balance in endothelial progenitor dysfunction. Antioxid Redox Signal 15, 933–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed JK & Gutterman DD (2013). Mitochondrial reactive oxygen species and vascular function: less is more. Arterioscler Thromb Vasc Biol 33, 673–675. [DOI] [PubMed] [Google Scholar]
- Gane EJ, Weilert F, Orr DW, Keogh GF, Gibson M, Lockhart MM, Frampton CM, Taylor KM, Smith RA & Murphy MP (2010). The mitochondria‐targeted anti‐oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int 30, 1019–1026. [DOI] [PubMed] [Google Scholar]
- Gioscia‐Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP & Seals DR (2014). Mitochondria‐targeted antioxidant (MitoQ) ameliorates age‐related arterial endothelial dysfunction in mice. J Physiol 592, 2549–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cocheme HM, Murphy MP & Dominiczak AF (2009). Mitochondria‐targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension 54, 322–328. [DOI] [PubMed] [Google Scholar]
- Harraz M, Jiao C, Hanlon HD, Hartley RS & Schatteman GC (2001). CD34– blood‐derived human endothelial cell progenitors. Stem Cells 19, 304–312. [DOI] [PubMed] [Google Scholar]
- Herrero A & Barja G (1997). ADP‐regulation of mitochondrial free radical production is different with complex I‐ or complex II‐linked substrates: implications for the exercise paradox and brain hypermetabolism. J Bioenerg Biomembr 29, 241–249. [DOI] [PubMed] [Google Scholar]
- Jablonski KL, Seals DR, Eskurza I, Monahan KD & Donato AJ (2007). High‐dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. J Appl Physiol (1985) 103, 1715–1721. [DOI] [PubMed] [Google Scholar]
- Jackson MJ, Pye D & Palomero J (2007). The production of reactive oxygen and nitrogen species by skeletal muscle. J Appl Physiol (1985) 102, 1664–1670. [DOI] [PubMed] [Google Scholar]
- Ji LL, Gomez‐Cabrera MC & Vina J (2006). Exercise and hormesis: activation of cellular antioxidant signaling pathway. Ann NY Acad Sci 1067, 425–435. [DOI] [PubMed] [Google Scholar]
- Kanter MM (1994). Free radicals, exercise, and antioxidant supplementation. Int J Sport Nutr 4, 205–220. [DOI] [PubMed] [Google Scholar]
- Kim SW, Kim H & Yoon YS (2011). Advances in bone marrow‐derived cell therapy: CD31‐expressing cells as next generation cardiovascular cell therapy. Regen Med 6, 335–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch AE, Halloran MM, Haskell CJ, Shah MR & Polverini PJ (1995). Angiogenesis mediated by soluble forms of E‐selectin and vascular cell adhesion molecule‐1. Nature 376, 517–519. [DOI] [PubMed] [Google Scholar]
- Kojda G & Hambrecht R (2005). Molecular mechanisms of vascular adaptations to exercise. Physical activity as an effective antioxidant therapy? Cardiovasc Res 67, 187–197. [DOI] [PubMed] [Google Scholar]
- Koutroumpi M, Dimopoulos S, Psarra K, Kyprianou T & Nanas S (2012). Circulating endothelial and progenitor cells: evidence from acute and long‐term exercise effects. World J Cardiol 4, 312–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov AV, Szalay L, Umar F, Kropik K, Staniek K, Niedermuller H, Bahrami S & Nohl H (2005). Skeletal muscles, heart, and lung are the main sources of oxygen radicals in old rats. Biochim Biophys Acta 1740, 382–389. [DOI] [PubMed] [Google Scholar]
- Lancaster GI, Halson SL, Khan Q, Drysdale P, Wallace F, Jeukendrup AE, Drayson MT & Gleeson M (2004). Effects of acute exhaustive exercise and chronic exercise training on type 1 and type 2 T lymphocytes. Exerc Immunol Rev 10, 91–106. [PubMed] [Google Scholar]
- Landers‐Ramos RQ, Sapp RM, Jenkins NT, Murphy AE, Cancre L, Chin ER, Spangenburg EE & Hagberg JM (2015). Chronic endurance exercise affects paracrine action of CD31+ and CD34+ cells on endothelial tube formation. Am J Physiol Heart Circ Physiol 309, H407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansford KA, Shill DD, Dicks AB, Marshburn MP, Southern WM & Jenkins NT (2016). Effect of acute exercise on circulating angiogenic cell and microparticle populations. Exp Physiol 101, 155–167. [DOI] [PubMed] [Google Scholar]
- Lauer N, Suvorava T, Ruther U, Jacob R, Meyer W, Harrison DG & Kojda G (2005). Critical involvement of hydrogen peroxide in exercise‐induced up‐regulation of endothelial NO synthase. Cardiovasc Res 65, 254–262. [DOI] [PubMed] [Google Scholar]
- Marthandan S, Murphy MP, Billett E & Barnett Y (2011). An investigation of the effects of MitoQ on human peripheral mononuclear cells. Free Radic Res 45, 351–358. [DOI] [PubMed] [Google Scholar]
- Mazo IB, Gutierrez‐Ramos JC, Frenette PS, Hynes RO, Wagner DD & von Andrian UH (1998). Hematopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. J Exp Med 188, 465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merry TL & McConell GK (2012). Do reactive oxygen species regulate skeletal muscle glucose uptake during contraction? Exerc Sport Sci Rev 40, 102–105. [DOI] [PubMed] [Google Scholar]
- Nikolaidis MG, Kyparos A, Spanou C, Paschalis V, Theodorou AA & Vrabas IS (2012). Redox biology of exercise: an integrative and comparative consideration of some overlooked issues. J Exp Biol 215, 1615–1625. [DOI] [PubMed] [Google Scholar]
- Oh IY, Yoon CH, Hur J, Kim JH, Kim TY, Lee CS, Park KW, Chae IH, Oh BH, Park YB & Kim HS (2007). Involvement of E‐selectin in recruitment of endothelial progenitor cells and angiogenesis in ischemic muscle. Blood 110, 3891–3899. [DOI] [PubMed] [Google Scholar]
- Pescatello LS, Arena R, Riebe D & Thompson PD (2014). ACSM's Guidelines for Exercise Testing and Prescription 9th Ed Lippincott Williams & Wilkins, Philadelphia, PA. [DOI] [PubMed] [Google Scholar]
- Polley KR, Jenkins N, O'Connor P & McCully K (2016). Influence of exercise training with resveratrol supplementation on skeletal muscle mitochondrial capacity. Appl Physiol Nutr Metab 41, 26–32. [DOI] [PubMed] [Google Scholar]
- Polytarchou C & Papadimitriou E (2004). Antioxidants inhibit angiogenesis in vivo through down‐regulation of nitric oxide synthase expression and activity. Free Radic Res 38, 501–508. [DOI] [PubMed] [Google Scholar]
- Polytarchou C & Papadimitriou E (2005). Antioxidants inhibit human endothelial cell functions through down‐regulation of endothelial nitric oxide synthase activity. Eur J Pharmacol 510, 31–38. [DOI] [PubMed] [Google Scholar]
- Powers SK & Jackson MJ (2008). Exercise‐induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88, 1243–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Talbert EE & Adhihetty PJ (2011). Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J Physiol 589, 2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero M, Colombo SL, Godfrey A & Moncada S (2006). Mitochondria as signaling organelles in the vascular endothelium. Proc Natl Acad Sci USA 103, 5379–5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala MW & Scherer PE (2003). Minireview: The adipocyte – at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology 144, 3765–3773. [DOI] [PubMed] [Google Scholar]
- Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR & Bluher M (2009). Antioxidants prevent health‐promoting effects of physical exercise in humans. Proc Natl Acad Sci USA 106, 8665–8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan TE, Brophy P, Lin CT, Hickner RC & Neufer PD (2014). Assessment of in vivo skeletal muscle mitochondrial respiratory capacity in humans by near‐infrared spectroscopy: a comparison with in situ measurements. J Physiol 592, 3231–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan TE, Erickson ML, Brizendine JT, Young HJ & McCully KK (2012). Noninvasive evaluation of skeletal muscle mitochondrial capacity with near‐infrared spectroscopy: correcting for blood volume changes. J Appl Physiol (1985) 113, 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele C, Nielsen S & Pedersen BK (2009). ROS and myokines promote muscle adaptation to exercise. Trends Endocrinol Metab 20, 95–99. [DOI] [PubMed] [Google Scholar]
- Schroder K, Kohnen A, Aicher A, Liehn EA, Buchse T, Stein S, Weber C, Dimmeler S & Brandes RP (2009). NADPH oxidase Nox2 is required for hypoxia‐induced mobilization of endothelial progenitor cells. Circ Res 105, 537–544. [DOI] [PubMed] [Google Scholar]
- Shill DD, Marshburn MP, Hempel HK, Lansford KA & Jenkins NT (2016). Heterogeneous circulating angiogenic cell responses to acute maximal exercise. Med Sci Sports Exerc, in press. [DOI] [PubMed] [Google Scholar]
- Shore S, Shinkai S, Rhind S & Shephard RJ (1999). Immune responses to training: how critical is training volume? J Sports Med Phys Fitness 39, 1–11. [PubMed] [Google Scholar]
- Silveira LR, Fiamoncini J, Hirabara SM, Procopio J, Cambiaghi TD, Pinheiro CH, Lopes LR & Curi R (2008). Updating the effects of fatty acids on skeletal muscle. J Cell Physiol 217, 1–12. [DOI] [PubMed] [Google Scholar]
- Smith RA & Murphy MP (2010). Animal and human studies with the mitochondria‐targeted antioxidant MitoQ. Ann NY Acad Sci 1201, 96–103. [DOI] [PubMed] [Google Scholar]
- Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O'Sullivan JD, Fung V, Smith RA, Murphy MP, Taylor KM & Protect Study G (2010). A double‐blind, placebo‐controlled study to assess the mitochondria‐targeted antioxidant MitoQ as a disease‐modifying therapy in Parkinson's disease. Mov Disord 25, 1670–1674. [DOI] [PubMed] [Google Scholar]
- St‐Pierre J, Buckingham JA, Roebuck SJ & Brand MD (2002). Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277, 44784–44790. [DOI] [PubMed] [Google Scholar]
- Stewart LK, Flynn MG, Campbell WW, Craig BA, Robinson JP, McFarlin BK, Timmerman KL, Coen PM, Felker J & Talbert E (2005). Influence of exercise training and age on CD14+ cell‐surface expression of toll‐like receptor 2 and 4. Brain Behav Immun 19, 389–397. [DOI] [PubMed] [Google Scholar]
- Suvorava T, Kumpf S, Rauch BH, Dao VT, Adams V & Kojda G (2010). Hydrogen peroxide inhibits exercise‐induced increase of circulating stem cells with endothelial progenitor capacity. Free Radic Res 44, 199–207. [DOI] [PubMed] [Google Scholar]
- Theodorou AA, Nikolaidis MG, Paschalis V, Koutsias S, Panayiotou G, Fatouros IG, Koutedakis Y & Jamurtas AZ (2011). No effect of antioxidant supplementation on muscle performance and blood redox status adaptations to eccentric training. Am J Clin Nutr 93, 1373–1383. [DOI] [PubMed] [Google Scholar]
- Timmerman KL, Flynn MG, Coen PM, Markofski MM & Pence BD (2008). Exercise training‐induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti‐inflammatory influence of exercise? J Leukoc Biol 84, 1271–1278. [DOI] [PubMed] [Google Scholar]
- Urao N, Inomata H, Razvi M, Kim HW, Wary K, McKinney R, Fukai T & Ushio‐Fukai M (2008). Role of nox2‐based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circ Res 103, 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urao N & Ushio‐Fukai M (2013). Redox regulation of stem/progenitor cells and bone marrow niche. Free Radic Biol Med 54, 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urso ML & Clarkson PM (2003). Oxidative stress, exercise, and antioxidant supplementation. Toxicology 189, 41–54. [DOI] [PubMed] [Google Scholar]
- Ushio‐Fukai M (2006). Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc Res 71, 226–235. [DOI] [PubMed] [Google Scholar]
- Ushio‐Fukai M & Urao N (2009). Novel role of NADPH oxidase in angiogenesis and stem/progenitor cell function. Antioxid Redox Signal 11, 2517–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JS, Chen WL & Weng TP (2011). Hypoxic exercise training reduces senescent T‐lymphocyte subsets in blood. Brain Behav Immun 25, 270–278. [DOI] [PubMed] [Google Scholar]
- Weissman IL (2000). Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science 287, 1442–1446. [DOI] [PubMed] [Google Scholar]
- Widlansky ME & Gutterman DD (2011). Regulation of endothelial function by mitochondrial reactive oxygen species. Antioxid Redox Signal 15, 1517–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray DW, Uberoi A, Lawrenson L, Bailey DM & Richardson RS (2009). Oral antioxidants and cardiovascular health in the exercise‐trained and untrained elderly: a radically different outcome. Clin Sci (Lond) 116, 433–441. [DOI] [PubMed] [Google Scholar]
- Zhang DX & Gutterman DD (2007). Mitochondrial reactive oxygen species‐mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol 292, H2023–2031. [DOI] [PubMed] [Google Scholar]


