Abstract
Key points
Obesity induces lymphatic leakiness, decreases initial lymphatic vessel density, impairs collecting vessel pumping and decreases transport of macromolecules.
Obesity results in perilymphatic inducible nitric oxide synthase (iNOS) expression and accumulation of T cells and macrophages.
Deleterious effects of obesity on the lymphatic system correlate with weight gain.
Weight loss restores lymphatic function in obese animals and decreases perilymphatic iNOS and inflammatory cell accumulation.
Abstract
Although clinical and experimental studies have shown that obesity results in lymphatic dysfunction, it remains unknown whether these changes are permanent or reversible with weight loss. In the current study, we used a mouse model of diet‐induced obesity to identify putative cellular mechanisms of obesity‐induced lymphatic dysfunction, determine whether there is a correlation between these deleterious effects and increasing weight gain, and finally examine whether lymphatic dysfunction is reversible with diet‐induced weight loss. We report that obesity is negatively correlated with cutaneous lymphatic collecting vessel pumping rate (r = –0.9812, P < 0.0005) and initial lymphatic vessel density (r = –0.9449, P < 0.005). In addition, we show a significant positive correlation between weight gain and accumulation of perilymphatic inflammatory cells (r = 0.9872, P < 0.0005) and expression of inducible nitric oxide synthase (iNOS; r = 0.9986, P < 0.0001). Weight loss resulting from conversion to a normal chow diet for 8 weeks resulted in more than a 25% decrease in body weight and normalized cutaneous lymphatic collecting vessel pumping rate, lymphatic vessel density, lymphatic leakiness, and lymphatic macromolecule clearance (all P < 0.05). In addition, weight loss markedly decreased perilymphatic inflammation and iNOS expression. Taken together, our findings show that obesity is linearly correlated with lymphatic dysfunction, perilymphatic inflammation and iNOS expression, and that weight loss via dietary modification effectively reverses these deleterious effects.
Keywords: lymphatic vessel function, obesity, weight loss
Key points
Obesity induces lymphatic leakiness, decreases initial lymphatic vessel density, impairs collecting vessel pumping and decreases transport of macromolecules.
Obesity results in perilymphatic inducible nitric oxide synthase (iNOS) expression and accumulation of T cells and macrophages.
Deleterious effects of obesity on the lymphatic system correlate with weight gain.
Weight loss restores lymphatic function in obese animals and decreases perilymphatic iNOS and inflammatory cell accumulation.
Abbreviations
- DC
dendritic cell
- eNOS
endothelial nitric oxide synthase
- HFD
high‐fat diet
- HOMA‐IR
homeostatic model assessment‐insulin resistance
- HPF
high‐power field
- ICG
indocyanine green
- iNOS
inducible nitric oxide synthase
- LYVE‐1
lymphatic vessel hyaluronan receptor 1
- NCD
normal chow diet
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- 99mT
technetium‐99m
- VEGF‐C
vascular endothelial growth factor‐C
Introduction
As the incidence of obesity increases, it has become increasingly evident that this pathological state exerts harmful effects on a variety of organ systems, and complications arising from these effects are a common cause of morbidity and mortality (Flegal et al. 2013). More recent clinical and experimental studies have shown that obesity also has harmful effects on the lymphatic system, resulting in decreased macromolecule clearance, impaired trafficking of antigen presenting cells and abnormal lymph node architecture (Weitman et al. 2013; Blum et al. 2014). This is important because lymphatic dysfunction is thought to amplify the pathology of obesity in other organ systems by modulating inflammatory responses. For example, a recent study suggested that obesity induces vascular endothelial growth factor‐C (VEGF‐C), a growth factor that plays a key role in regulating lymphangiogenesis and lymphatic development, and this effect in turn modulates glucose sensitivity by regulating macrophage infiltration and differentiation (Karaman et al. 2015). Other groups have shown that VEGF‐C deficiency protects against high‐fat diet (HFD)‐induced obesity and metabolic dysfunction (Nurmi et al. 2015) and that lymphatic insufficiency increases atherogenesis (Vuorio et al. 2014). Our laboratory has found that acquired lymphatic defects in obesity markedly increase accumulation of inflammatory cells in dermatitis and, by doing so, heighten the severity of this pathological response (Savetsky et al. 2015). Taken together, these studies suggest that obesity‐related lymphatic injury can act in a feed forward manner to increase the pathological changes of obesity.
While it is clear that obesity results in lymphatic injury, it remains unknown whether these harmful effects are reversible with dietary or pharmacological interventions. This gap in our knowledge is significant because this information can be used to understand the mechanisms that regulate lymphatic dysfunction in obesity, develop therapeutic interventions and identify the optimal time to institute these measures. For example, if late‐stage changes in obesity‐induced lymphatic dysfunction are not reversible, then interventions need to be applied prior to the onset of permanent defects. In addition, based on the known interaction between obesity, lymphatic dysfunction and inflammatory responses, interventions designed to protect the lymphatic system may decrease the pathological effects of obesity.
Numerous studies have examined the benefits of weight loss and its ability to reverse the pathological effects of obesity. For example, weight loss is known to improve glucose intolerance and insulin sensitivity, decrease expression of pro‐inflammatory cytokines in adipose tissue, normalize expression of adipokines and improve cardiac function (Sjostrom et al. 2000, 2004; Poirier et al. 2003; Clement et al. 2004; Moschen et al. 2010). Based on this knowledge, we hypothesized that weight loss in the setting of obesity will have a beneficial effect on the lymphatic system and reverse, at least in part, some of the defects acquired as a result of HFD‐induced obesity in mice. We show that progressive weight gain is directly correlated with impaired lymphatic function characterized by decreased collecting lymphatic pumping frequency and decreased initial lymphatic vessel density. In addition, we report that weight gain has a linear effect on accumulation of perilymphatic inflammatory cells and expression of inducible nitric oxide synthase (iNOS). Weight loss, induced by switching obese mice from a high‐fat to a normal chow diet (NCD), effectively reversed perilymphatic accumulation of inflammatory cells and iNOS expression. These changes correlated with improved collecting vessel pumping frequency, increased lymphatic vessel density and decreased lymphatic leakiness, resulting in significantly increased lymphatic clearance of macromolecules and improved migration of dendritic cells (DCs) to regional lymph nodes. Taken together, our findings suggest that increasing weight gain is directly correlated with lymphatic dysfunction and that weight loss effectively, and rapidly, improves lymphatic function in mice.
Methods
Ethics approval and animals
All experimental protocols were approved by the Institutional Animal Care and Use Committee at Memorial Sloan Kettering Cancer Center. Our institution functions under the Animal Welfare Act (AWA) and Health Research Extension Act of 1985. Male (6–8 weeks old) C57BL/6J mice (Taconic Biosciences, Hudson, NY, USA) were kept in a light‐ and temperature‐controlled environment and fed either a HFD consisting of 60% kcal from fat (Purina TestDiet 58Y1, W.F. Fisher and Son, Somerville, NJ, USA) ad libitum for 1 week (25 g), 3 weeks (30 g), 6 weeks (35 g), 8 weeks (40 g), 10 weeks (45 g) and 12–13 weeks (50 g) or a NCD (13% kcal from fat; Purina PicoLab Rodent Diet 20, Purina Company, Wilkes‐Barre, PA, USA) for 12 weeks. In experiments where anaesthesia was necessary, isofluorane was used under our MSKCC guidelines, and mice were monitored for depth of anaesthesia by pinching the tail and monitoring the respiratory rate every 15 min. As per the Guidelines on Euthanasia by the American Veterinary Medical Association, mice were killed using carbon dioxide asphyxiation. The researchers confirm that this work meets the standards of the animal ethics checklist and understand the ethics principles under which this journal operates.
Lymphatic function was assessed using indocyanine green (ICG) lymphangiography for every 5 g increase in weight as outlined below. In other experiments, in order to induce weight loss in obese mice, a subset of animals fed a HFD for 10–12 weeks to obtain a weight of > 40 g, a threshold we determined to signify successful induction of obesity. Any mice that did not meet the > 40 g threshold were omitted from the study. A subgroup of mice > 40 g were switched to a NCD for an additional 8 weeks to induce weight loss. Subsequently, lymphatic function was determined in lean animals maintained on a NCD throughout the course of the study, in obese animals maintained on a HFD, or in weight loss animals that were initially kept on a HFD before being switched to a NCD. Animal weights were measured weekly and prior to be killed using a digital scale (Sartorius, Bradford, MA, USA).
Analysis of lymphatic function
Lymphatic function was assessed using a variety of techniques as previously described (Weiler et al. 2012; Robinson et al. 2013; Nelson et al. 2014). Collecting lymphatic pumping and analysis of packet frequency was performed using near‐infrared imaging with ICG. Briefly, 15 μl (0.15 mg ml−1) of ICG (a compound bound by albumin and taken up selectively by lymphatic vessels) was injected intradermally in the dorsal web space of the right distal hindlimb and imaged 20 min later while the mouse was maintained under anaesthesia. Time‐lapse images of hindlimb collecting lymphatics were taken every 8 s for 30 min using an EVOS EMCCD camera (Life Technologies, Carlsbad, CA, USA), LED light source (CoolLED, Andover, UK) and a Zeiss V12 Stereolumar microscope (Caliper Life Sciences, Hopington, MA, USA). The images were analysed using region‐of‐interest software (FIJI software; NIH, Bethesda, MA, USA) to measure the transport of packets within the dominant lymphatic collecting vessels (a measure of collecting lymphatic pumping) by measuring ICG signal intensity at a region of interest in a collecting vessel and subtracting the background. Lymphatic packet frequency was quantified by counting the total number of ICG signal peaks in collecting vessels over time and was expressed as pumps per minute. All experiments were performed with a minimum of four animals per group.
Lymphangiography was also performed as previously described using Evans blue dye (Sigma‐Aldrich, St Louis, MO, USA) to identify leaky lymphatic vessels and analyse lymphatic vessel architecture (Jang et al. 2013). Briefly, 2 μl of Evans blue dye (1%, w/v) was injected into the apex of the ear and visualized using photographs 1 min later. In addition, to further analyse functional lymphatic vessel uptake and leakiness of lymphatic vessels, we injected tomato lectin (1 mg ml–1; Sigma‐Aldrich) into the apex of the ear followed by the animals being killed 10 min later as previously described (Nishimura et al. 2009). Whole mount staining was then performed to co‐localize tomato lectin and capillary/collecting lymphatics as previously described (Nitschke et al. 2012). Briefly, ear tissues were fixed with 4% paraformaldehyde, and stained with podoplanin (Abcam, San Francisco, CA, USA) and α‐SMA (Sigma‐Aldrich). Z‐stack confocal images were obtained using a Leica SP5‐U confocal microscope (Leica Microsystems Inc., Buffalo Grove, IL, USA). Images were then acquired using Zeiss Zen 2010 software (Carl Zeiss, Jena, Germany) and analysed using IMARIS version 7.2.3 software (Bitplane, Zurich, Switzerland). All experiments were performed in a minimum of four animals per group.
Lymphoscintigraphy was performed as previously described to measure clearance of macromolecules by the lymphatic system (Weitman et al. 2013). Briefly, 20 μl of technetium‐99m (99mTc) was injected in the plantar side of the left distal hindlimb and popliteal/inguinal lymph node uptake was assessed for 90 min using an X‐SPECT camera (Gamma Medica, Northridge, CA, USA). Region‐of‐interest analysis was used to assess peak nodal uptake of the draining popliteal and inguinal lymph nodes using ASIPro software (CTI Molecular Imaging, Knoxville, TN, USA). Experiments were performed using a minimum of four animals per group.
Trafficking of peripherally injected DCs was performed using a modification of our previously published methods (Weitman et al. 2013). Briefly, CD45.1+ cells were harvested from spleens of B6.SJL‐PtprcaPepcb/BoyJ mice (Jackson Laboratories, Bar Harbor, ME, USA) using CD11c+ magnetic microbead positive selection (Miltenyi Biotech, Gladbach, Germany). Isolated DCs were purified and resuspended, and 1.5 × 106 cells were injected into the plantar side of the left distal hindlimb. Left popliteal lymph nodes were harvested 18 h after injection and analysed using flow cytometry after digestion with a solution of Dispase II, Collagenase D and DNase I (Roche Diagnostics, Mannheim, Germany) to prepare single‐cell suspensions. Cells were stained using fluorescein isothiocyanate‐conjugated anti‐CD45.1, phycoerythrin‐conjugated anti‐CD11c and allophycocyanin‐conjugated MHC‐II antibodies and endogenous Fc receptor binding block was performed using anti‐CD16/anti‐CD32 antibodies (all from EBioscience, San Diego, CA, USA). Quantification of DCs (CD45.1+CD11c+MHC‐IIhigh) that had trafficked into the lymph nodes was performed using an LSRII flow cytometer (BD Biosciences, San Jose, CA, USA) and FlowJo software (Tree Star, Ashland, OR, USA). Cytometer settings were compensated for and optimized using C57BL/6J splenocytes obtained at the time of harvest. Each experiment was repeated in four animals per group.
Histology
Histological sections were fixed for 4 h using 4% paraformaldehyde (PFA; Affymetrix, Cleveland, OH, USA) and embedded in paraffin. Immunohistochemical staining was performed as previously reported (Avraham et al. 2010). Briefly, tissues were rehydrated, followed by antigen unmasking using boiling sodium citrate (Sigma‐Aldrich). Non‐specific binding was blocked using 2% BSA/10% secondary‐antibody animal serum solution. Tissues were then incubated overnight at 4°C in primary antibody, washed in PBS, then incubated in immunofluorescent secondary antibodies. All secondary antibodies used were obtained from Life Technologies (Grand Island, NY, USA). Primary antibodies used were: anti‐lymphatic vessel hyaluronan receptor 1 (LYVE‐1), anti‐CD45, anti‐CD4, anti‐CD11b (R&D Systems; Minneapolis, MN, USA), anti‐CD3 (Dako, Carpinteria, CA, USA), and anti‐iNOS (Abcam). Slides were counter‐stained with 42,6‐diamidino‐2‐phenylindole (DAPI), dehydrated and mounted using Mowiol 40–88 mounting solution (Sigma‐Aldrich). Stained sections were scanned using a Mirax slide scanner (Zeiss, Munich, Germany). Positively stained perilymphatic inflammatory cells were identified in 20× or 40× high powered fields and quantified by two blinded reviewers in a minimum of three randomly selected sections with four animals per group. Inflammatory cells were considered to be perilymphatic if they were located within a 50 μm radius of a lymphatic vessel.
For whole mount imaging, ear tissues were fixed using 4% paraformaldehyde followed by blocking with 12% BSA for prevent non‐specific binding of antibodies. Tissues were incubated with primary antibodies for anti‐podoplanin (Abcam), anti‐CDllb (R&D Systems), anti‐iNOS (BD Biosciences) and DAPI. Fluorescently conjugated secondary antibodies (Life Technologies) were used to visualize primary antibodies. Z‐stacked confocal images were taken as previously described.
Serum analysis
Blood was collected in serum separator tubes (BD Microcontainer, Franklin Lakes, NJ, USA) from the mice using cardiac puncture following terminal general anaesthesia. The blood was spun at 16,060 g for 10 min following which the serum was collected for metabolic analysis, as well as protein analysis. Fasting serum was sent to the Comparative Pathology Lab at MSKCC for a lipid panel [total cholesterol, triglycerides, high‐ (HDL) and low‐density lipoproteins (LDL)], glucose and insulin. Serum cytokine analysis (n = 5 animals per group) was performed according to the manufacturer's protocol for transforming growth factor‐β1 (TGF‐β1) and interferon‐γ (INF‐γ) using enzyme‐linked immunosorbent assay (ELISA; eBioscience, San Diego, CA, USA). These ELISAs were run in duplicate.
Statistical analysis
The GraphPad Prism software was used to analyse and graphically present all data. All data were analysed for Gaussian distribution using the D'Agostino–Pearson omnibus normality test. Normally distributed data were analysed using one‐way ANOVA with post hoc Tukey's multiple comparison test to compare multiple groups. Non‐normally distributed data were analysed using Kruskal–Wallis one‐way ANOVA test with post hoc Dunn's multiple comparison test for comparing multiple groups. Correlations were performed using the Pearson coefficient, and in all experiments P < 0.05 was considered significant. All data are presented as mean plus or minus standard deviation unless otherwise noted. Scattered dot plots represent each individual observation in a particular experiment.
Results
Weight gain is negatively correlated with collecting lymphatic pumping frequency
Male C57BL/6J mice progressively gained weight on a HFD and weight gain was positively correlated with subcutaneous adipose tissue deposition (n = 5, r = 0.9507, P < 0.005; Fig. 1 A, B). Consistent with our previous reports demonstrating decreased initial lymphatic vessel density in obesity (Weitman et al. 2013), we found that weight gain negatively correlated with the number of LYVE‐1+ vessels in the hindlimb skin (n = 21–27, r = –0.9449, P < 0.005; Fig. 1 A, C). In addition, earlier studies have shown that obesity is associated with decreased pumping frequency of collecting lymphatics (Blum et al. 2014; Savetsky et al. 2015). Consistent with these previous findings, we noted that progressive weight gain had a strong negative correlation with packet frequency (a correlate of pumping) in the dominant collecting lymphatic vessels (n = 7–10, r = –0.9812, P < 0.0005; Fig. 1 D, E). Interestingly, similar to our findings with lymphatic vessel density, we found that statistically significant decreases in packet frequency were observed only when animals reached a weight of 35 g, a 40% increase in weight gain from baseline (25 g; Fig. 1 D, E). These changes became even more exaggerated with increasing body weight, suggesting that a threshold of 35 g is necessary to begin to see measurable differences in packet frequency and becomes more obvious with increasing weight gain. As a result, for all subsequent experiments we used mice that weighed greater 40 g or more in the obese groups.
Figure 1. Weight gain is negatively correlated with collecting lymphatic pumping frequency .
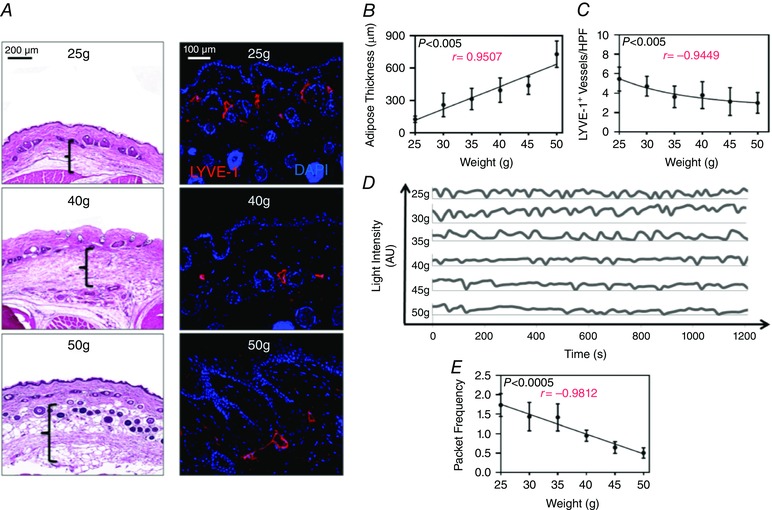
A, left panel: representative photomicrographs of H&E‐stained hindlimb skin from 25, 40 and 50 g mice. Brackets represent adipose thickness. Note increasing adipose thickness with increasing weight. Right panel: representative photomicrograph of hindlimb skin immunofluorescence staining for lymphatic vessels (LYVE‐1+; red) and nuclei (DAPI; blue) for 25, 40 and 50 g mice. Note decreasing lymphatic vessels with increasing weight. B, quantification of subcutaneous adipose thickness per 5 g weight gain starting at 25 g (n = 5 animals per group; Pearson's correlation: r = 0.9507, P = 0.0036). C, quantification of subcutaneous lymphatic vessel density (LYVE+ vessels per 40× high power field) in hindlimb skin per 5 g weight gain starting at 25 g [n = 5 animals per group with 4–6 high‐power fields (HPF) per animal; Pearson's correlation: r = –0.9449, P = 0.0045]. D, representative line graphs demonstrating hindlimb collecting lymphatic vessel packet frequency (correlates with pumping) as seen by changes in light intensity (arbitrary units) over time (seconds) for each weight group. E, quantification of hindlimb collecting lymphatic vessel packet frequency (pumping) for each weight group (n = 7–10 animals per group; Pearson's correlation: r = –0.9812, P = 0.0005).
Weight gain is positively correlated with perilymphatic inflammation
Previous studies have shown that the negative effects of obesity on the vascular system are strongly correlated with perivascular inflammation (Greenstein et al. 2009; Ketonen et al. 2010; Iantorno et al. 2014). For example, it is well known that perivascular inflammatory responses in obesity increase endothelial dysfunction, mediate atherosclerosis and modulate vascular responsiveness (Berg and Scherer 2005; Mauricio et al. 2013; Prieto et al. 2014; Van de Voorde et al. 2014). Consistent with these reports, we found that increasing weight gain had a positive correlation with perilymphatic accumulation of inflammatory cells (cells within a radius of 50 μm) as assessed with co‐localization of CD45 (a pan‐leukocyte marker) and LYVE‐1 (a lymphatic vessel marker; n = 20, r = 0.9872, P < 0.0005; Fig. 2 A, B). Because inflammatory cells are a major source of iNOS and nitric oxide (NO) is a known regulator of collecting lymphatic pumping (Scallan et al. 2015), we next analysed perilymphatic expression of iNOS as a function of increasing weight gain. This analysis, similar to our findings with perilymphatic inflammation, demonstrated a marked accumulation of iNOS+ cells clustering around lymphatic vessels in obese animals and a strong positive correlation with weight gain (n = 20, r = 0.9986, P < 0.0001; Fig. 2 C, D).
Figure 2. Weight gain is positively correlated with perilymphatic inflammation .
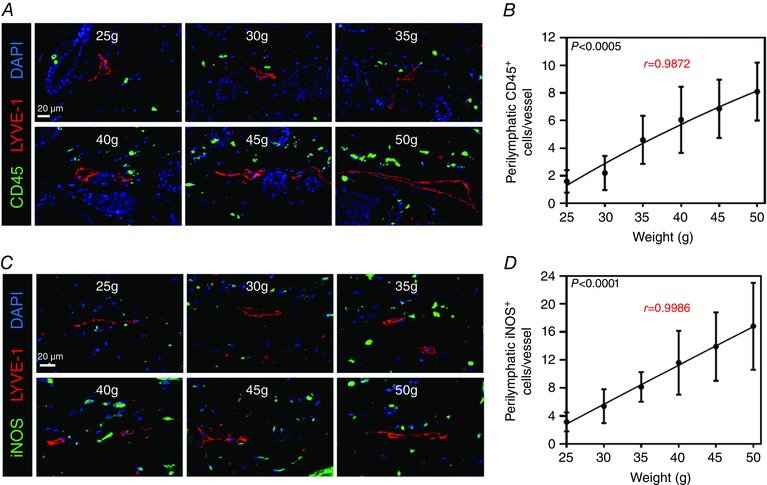
A, representative photomicrographs of hindlimb skin immunofluorescence staining localizing leukocytes (CD45+; green) with lymphatic vessels (LYVE‐1+; red) and nuclei (DAPI; blue). Note the increase in perilymphatic leukocytes as weight increases. B, quantification of perilymphatic CD45+ cells (cells located within 50 μm of lymphatic vessels) for each weight point (n = 5 animals per group with four HPFs per animal; Pearson's correlation: r = 0.9872, P = 0.0002). C, representative photomicrographs of hindlimb skin immunofluorescence staining localizing iNOS (iNOS+; green) with lymphatic vessels (LYVE‐1+; red) and nuclei (DAPI; blue). Note the increase in perilymphatic iNOS+ cells as weight increases. D, quantification of perilymphatic iNOS+ cells (cells located within 50 μm of lymphatic vessels) for each weight point (n = 5 animals per group with four HPFs per animal; Pearson's correlation: r = 0.9986, P < 0.0001).
Calorie restriction results in rapid weight loss and decreased adipose tissue deposition
We next sought to determine whether diet‐induced weight loss could reverse the pathological effects of obesity on the lymphatic system. To accomplish this goal, male C57BL/6J mice were fed a HFD ad libitum until they reached a minimum weight of 40 g. At this point, a group of obese animals was randomly chosen and switched to a NCD for an additional 8 weeks (hereafter ‘weight loss mice’) while the remaining mice were maintained on a HFD for the duration of the experiment (hereafter ‘obese mice’). Age matched control animals were maintained on a NCD for the entire experiment (hereafter ‘lean mice’).
As expected, animals maintained on a HFD initially experienced rapid weight gain and then plateaued to an average weight of 45.1 ± 4.6 g at the end of the experiment (n = 8; Fig. 3 A). Weight loss animals also reached a peak weight of 45 g after HFD but progressively lost weight after placement on a NCD for 8 weeks, reaching a final weight of 33.4 ± 1.4 g at the conclusion of the study (Fig. 3 A). Lean animals also gained a small amount of weight during the course of the experiment, reaching a final weight of 30.5 ± 1.8 g (Fig. 3 A). The weight gain in obese animals correlated with a nearly 4‐fold increase in subcutaneous adipose tissue deposition in the ear skin as compared to lean animals (n = 8, P < 0.0005; Fig. 3 B, C). After switching to a NCD, the weight loss group demonstrated a decrease in adipose tissue deposition, as compared to obese mice (more than 2‐fold decrease) but this was not significant; however, these animals still had more adipose deposition compared to lean controls (Fig. 3 B, C). Mice maintained on HFD had markedly increased total cholesterol, triglycerides, HDL, LDL and serum insulin (n = 4–5, Supplementary Material Fig. S1A, B). Serum glucose levels were also increased in obese mice but did not quite reach statistical significance. In addition, we calculated the homeostatic model assessment‐insulin resistance (HOMA‐IR) for each group and found that this was markedly increased in obese mice, as compared to lean controls (n = 4–5, Fig. S1C). Not surprisingly, these metabolic parameters were greatly improved after weight loss with a return to baseline levels of total cholesterol, triglycerides and HDL. There was also an improvement in serum glucose, insulin and HOMA‐IR following weight loss (Fig. S1A–C).
Figure 3. Calorie restriction results in rapid weight loss and decreased adipose tissue deposition .
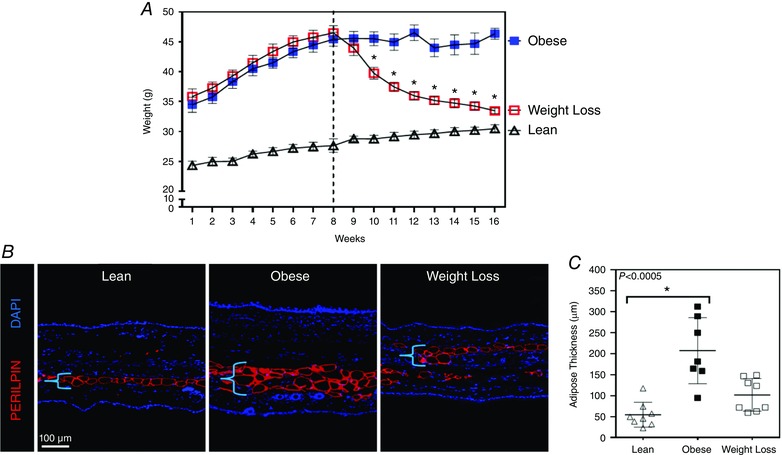
A, weight changes over time for lean mice (fed a NCD throughout the course of the experiment: open triangle), obese mice (fed a HFD throughout the course of the experiment: blue solid square) and weight loss mice (fed a HFD for 12 weeks then placed on a NCD for 8 weeks to induce weight loss: red open square; n = 8 animals per group). Dotted line represents the point at which half of the obese mice were randomized to the weight loss group. B, representative photomicrographs of ear tissue immunofluorescence staining for adipocytes (perilipin+; red) and nuclei (DAPI; blue) for each group. Bracket represents adipose tissue thickness. C, quantification of adipose thickness for each group (Kruskal–Wallis test one‐way ANOVA: n = 8 ears per group, lean vs. obese: P = 0.0003, obese vs. weight loss: P = 0.1139).
Weight loss decreases perilymphatic accumulation of inflammatory cells
Consistent with our findings correlating weight gain and perilymphatic inflammation, we found that obese mice had significant accumulation of CD45+ cells within a 50 μm radius of LYVE‐1+ lymphatics (data not shown). To determine more specifically the makeup of the inflammatory response, we used immunofluorescence to examine T cells (CD3+) and macrophages (F4/80+) in these regions and found that the accumulation of both inflammatory cell types was significantly increased in obese mice, as compared to lean mice (4.4‐fold increase in T cells and 1.9‐fold increase in macrophages; n = 16, P < 0.001 and P < 0.05, respectively; Fig. 4 A left panel, B and Fig. S2). Weight loss effectively reversed this inflammatory response, resulting in normalization of the number of perilymphatic T cells and macrophages to lean control levels (n = 16, P < 0.005 and P < 0.0001, respectively; Fig. 4 A left panel, B and Fig. S2).
Figure 4. Weight loss decreases perilymphatic accumulation of inflammatory cells .
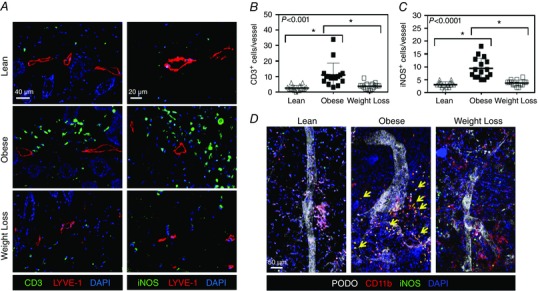
A, left panel: representative photomicrographs of hindlimb skin immunofluorescence staining localizing T cells (CD3+; green), lymphatic vessels (LYVE‐1+; red) and nuclei (DAPI; blue) for lean, obese and weight loss mice. Right panel: representative photomicrographs of hindlimb skin immunofluorescence staining for localizing iNOS (green), lymphatic vessels (LYVE‐1+; red) and nuclei (DAPI; blue) for each group. B, quantification of perilymphatic T cells for lean (open triangle), obese (closed square) and weight loss mice (open square; Kruskal–Wallis test one‐way ANOVA: n = 5 animals per group with 3–4 HFPs per animal, lean vs. obese: P < 0.0001, obese vs. weight loss: P = 0.0010). C, quantification of perilymphatic iNOS+ cells for each group (Kruskal–Wallis test one‐way ANOVA: n = 5 animals per group with 3–4 HFPs per animal, lean vs. obese: P < 0.0001, obese vs. weight loss: P = 0.0001). D, representative ear whole mount imaging of lymphatic vessels (podoplanin; white), macrophages (CD11b+; red), iNOS (green) and nuclei (DAPI; blue). Note the perilymphatic accumulation of CD11b+iNOS+ cells (arrows) in the obese group, which is attenuated in weight loss.
Analysis of perilymphatic iNOS expression revealed a similar distribution to perilymphatic inflammation. As expected, obese mice had a significant increase (3.07‐fold) in the number of perilymphatic iNOS+ cells, as compared to lean controls (n = 14, P < 0.0001; Fig. 4 A right panel, C). This effect was reversed with weight loss, resulting in a significant decrease (2.54‐fold) in perilymphatic iNOS+ cells as compared to obese mice maintained on a HFD throughout the course of the experiment (P = 0.0001; Fig. 4 A right panel, C). Recent studies have indicated that a major source of iNOS in the setting of inflammation and obesity are macrophages (Liao et al. 2011; Torrisi et al. 2016). To further examine the cellular source of iNOS in obese mice, we localized macrophages (CD11b+), iNOS and lymphatic vessels (podoplanin+) in whole mount ear specimens. This analysis demonstrated a perilymphatic distribution of inflammatory cells and co‐localization of iNOS+ and CD11b+ in a large number of cells (Fig. 4 D).
Weight loss restores lymphatic function
We next analysed lymphatic vessel density in obese and weight loss mice. This analysis, consistent with our initial observations, demonstrated that obese mice had a significant decrease (1.88‐fold) in the number of cutaneous LYVE‐1+ lymphatic vessels, as compared to lean mice (n = 16, P = 0.0001; Fig. 5 A, B). Obese animals also had an increase in the average area of their lymphatic vessels, suggesting that the lymphatic vessels in these animals are dilatated, as compared to controls (a finding that has previously been shown to be consistent with lymphatic dysfunction; n = 11–16, P < 0.01; Fig. 5 C) (Tabibiazar et al. 2006). More importantly, we found that weight loss reversed the negative effects of obesity on lymphatic vessel density, restoring the number of cutaneous lymphatics and lymphatic vessel area to levels that were statistically indistinguishable from lean controls (Fig. 5 B, C). TGF‐β1 and INF‐γ are immunomodulatory cytokines that also have potent anti‐lymphangiogenic effects (Clavin et al. 2008; Kataru et al. 2011). Consistent with our observation of decreased lymphatic vessel density and generalized inflammation in obese mice, we noted significantly increased serum levels of these anti‐lymphangiogenic cytokines in obese mice when compared to lean controls (n = 5–10, P < 0.01 for both; Fig. 5 D). These levels normalized after weight loss.
Figure 5. Weight loss results in increased capillary lymphatic vessel density .
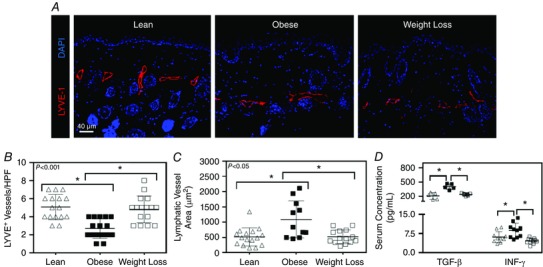
A, representative photomicrographs of hindlimb skin immunofluorescence staining for lymphatic vessels (LYVE‐1+; red) and nuclei (DAPI; blue) for lean, obese and weight loss mice. B, quantification of lymphatic vessel density (LYVE+ vessels per 40× HPF) for lean (open triangle), obese (solid square) and weight loss mice (open square) (Kruskal–Wallis test one‐way ANOVA: n = 5 animals per group with 3–4 HPFs per animal, lean vs. obese: P = 0.0001, obese vs. weight loss: P = 0.0009). C, quantification of lymphatic vessel area (largest area of a LYVE+ vessel per quadrant) for each group (Kruskal–Wallis test one‐way ANOVA: n = 5 animals per group with 2–4 HPFs per animal, lean vs. obese: P = 0.0169, obese vs. weight loss: P = 0.0380). D, ELISA for transforming growth factor‐β1 (TGF‐β1) and interferon‐γ (INF‐γ) from serum for each experimental group (Kruskal–Wallis test one‐way ANOVA: n = 5 animals per group, lean vs. obese: P = 0.0175, obese vs. weight loss: P = 0.0328; one‐way ANOVA: n = 5 animals per group in duplicate, lean vs. obese: P = 0.0297, obese vs. weight loss: P = 0.0006).
Previous studies from our lab and others have suggested obesity increases leakiness of lymphatic vessels (Angeli et al. 2004; Lim et al. 2009; Savetsky et al. 2015). Consistent with these reports, we found that obese mice had leaky initial and collecting lymphatics as assessed by Evans blue lymphangiography (n = 5; Fig. 6 A). In obese mice, the Evans blue dye, which is ordinarily bound by albumin after injection and picked up by lymphatic vessels, was noted to extravasate proximal to the injection site and was eventually picked up by sparsely located lymphatics. In contrast, lean mice had discretely stained lymphatics with little extravasation of dye proximally. Similarly, weight loss animals demonstrated improved uptake of Evans blue dye by the lymphatics with a pattern that was essentially indistinguishable from lean mice (Fig. 6 A).
Figure 6. Weight loss decreases leakiness of lymphatic vessels .
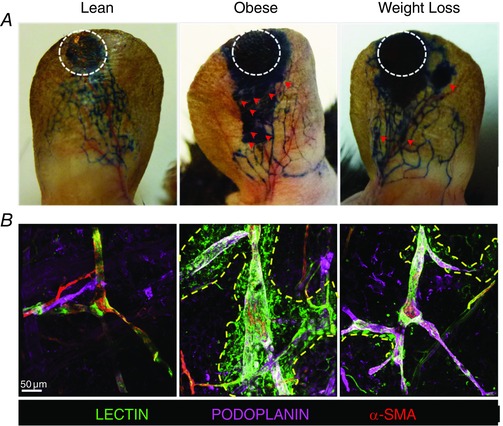
A, representative photographs of microlymphangiography using Evans blue dye for lean, obese and weight loss mice. White dotted circles represent the injection site of Evans blue; red arrows represent areas of lymphatic leakage (n = 5 ears per group). B, representative photomicrographs of whole mount staining of the ear following injection of tomato lectin into the apex of the ear and localizing lymphatic vessels (podoplanin+; purple) and alpha smooth muscle actin (α‐SMA+; red). Note the extensive extravasation of tomato lectin (yellow dotted line) in the obese mice and the significant reduction following weight loss (n = 5 ears per group).
Our findings with Evans blue dye were confirmed with tomato lectin injection, a compound that is preferentially taken up by lymphatic vessels and used to identify functional lymphatics (Nitschke et al. 2012). Indeed, injection of this material in the ears of lean mice led to it being found within the lumen of collecting lymphatics (podoplanin+ vessels with smooth muscle cell coverage) with the presence of very little extra‐lymphatic dye (n = 5; Fig. 6 B). In contrast, obese mice had marked leakiness of lectin outside of the collecting lymphatic vessels with accumulation in the extracellular matrix. Similar to our findings with Evans blue, we found that lectin uptake by the collecting lymphatics was improved with more normal appearing uptake after weight loss although a small amount of dye could still be observed outside the vessels (Fig. 6 B).
As expected, analysis of collecting lymphatic vessel package frequency demonstrated a marked inhibition (more than 2‐fold decrease) of collecting lymphatic pumping capacity in obese mice, as compared to age‐matched lean controls (n = 5, P < 0.01; Fig. 7 A, B). Weight loss effectively reversed this pathological response and returned lymphatic pumping frequency back to lean levels (Fig. 7 A, B). Similarly, we found that deficits in transport of DCs to regional lymph nodes noted in obese mice were reversed with weight loss (n = 6–8, P < 0.0005; Fig. 7 C). In this analysis, obese mice had a 15‐fold decrease in the percentage of peripherally injected DCs that had migrated to the draining popliteal lymph nodes, as compared to lean mice. In contrast, the percentage of migrating DCs in weight loss animals was essentially the same as those observed in lean controls (Fig. 7 C).
Figure 7. Weight loss restores lymphatic function .
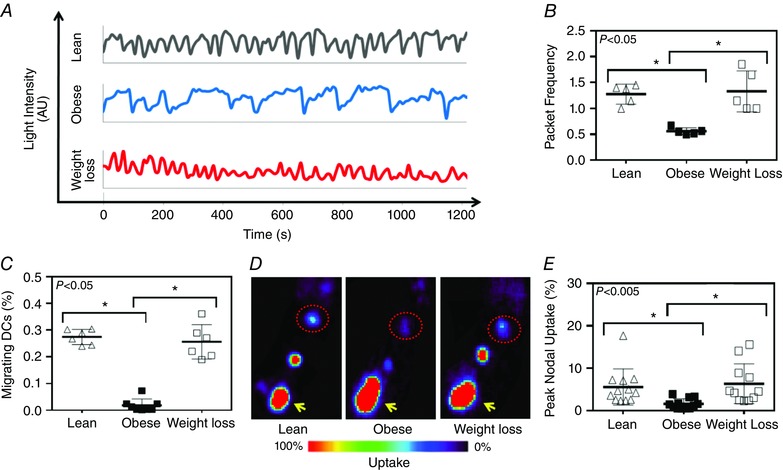
A, representative line graphs demonstrating hindlimb collecting lymphatic vessel packet frequency (correlates with pumping) as seen by changes in light intensity (arbitrary units) over time (seconds) for lean (black), obese (blue) and weight loss mice (red). B, quantification of hindlimb collecting lymphatic vessel packet frequency (pumping) for each group (Kruskal–Wallis test one‐way ANOVA: n = 5 animals per group, lean vs. obese: P = 0.0233, obese vs. weight loss: P = 0.0233). C, quantification of DC migration to popliteal lymph node following injection of DCs into the mouse's hindpaw (Kruskal–Wallis test one‐way ANOVA: n = 6–8, lean vs. obese P = 0.0021; obese vs. weight loss: P = 0.0124). D, representative lymph node heat map images following 99mTc injections into the hindpaw. Yellow arrow represents the injection site and red dotted circle represents inguinal lymph nodes. E, quantification of peak nodal uptake of 99mTc to regional lymph nodes (popliteal and inguinal) following injection into the hindpaw following 1 h of transit (Kruskal–Wallis test one‐way ANOVA: n = 12, lean vs. obese P = 0.0023; obese vs. weight loss: P = 0.0019).
Consistent with our observations in lymphatic pumping and DC trafficking, we noted a marked decrease in clearance of macromolecules in obese animals as assessed with 99mTc lymphatic clearance following injection into the hindpaw (n = 12; P < 0.0005; Fig. 7 D, E). Obese animals had a more than 3‐fold decrease in peripherally injected 99mTc uptake by the draining popliteal and inguinal lymph nodes as compared to controls. This deficit improved significantly in weight loss animals with macromolecule clearance being restored by to normal (lean) levels (Fig. 7 D, E).
Discussion
In this study, we have shown that obesity has a nearly linear negative correlation with both initial lymphatic vessel density and collecting lymphatic packet frequency. These changes occurred in association with accumulation of perilymphatic inflammatory and iNOS+ cells. We have shown that lymphatic defects in obesity are most reproducibly measured in mice that weigh more than 40 g. Furthermore, we have shown that diet‐induced weight loss can effectively reverse the pathological effects of obesity on the lymphatic system by decreasing perilymphatic inflammation and iNOS expression, restoring initial lymphatic vessel density, decreasing capillary and collecting lymphatic leakiness, reversing deficits in collecting lymphatic pumping and dendritic cell trafficking to regional lymph nodes, and resolving obesity‐mediated defects in macromolecule clearance.
These findings are important and add to the previous literature because they show that there is a threshold effect from weight gain, beyond which lymphatic deficits become measurable. Our hypothesis that there is a weight threshold necessary to observe obesity‐related pathological changes in the lymphatic system is supported by previous clinical studies in obese patients reporting that primary lower extremity lymphoedema can spontaneously develop in massively obese patients with a body mass index of 59 or higher (Greene et al. 2012). In addition, our data help to explain disparate findings in the literature on the effects of obesity on the lymphatic system since most previous studies have not utilized a standardized minimum weight as a baseline for comparison. In fact, some authors have described experiments on ‘obese’ animals fed a HFD for relatively short periods of time (as little as 2 weeks) (Katagiri et al. 2007). Thus, it is possible that some reports demonstrating relatively minor or statistically insignificant changes in various aspects of lymphatic function utilized mice that were either not obese enough (i.e. less than 40 g) or the test of lymphatic function used to analyse outcomes was not sensitive enough to elucidate modest changes.
An interesting observation in the current study was the correlation between increasing weight and accumulation of inflammatory cells within a 50 μm radius of cutaneous lymphatic vessels. These findings add to previous reports on white adipose tissue inflammation by suggesting that obesity‐induced inflammatory responses are not randomly distributed. In addition, our findings suggest that perilymphatic inflammation in obesity may play an important role in obesity‐induced lymphatic dysfunction and that weight loss may provide an effective means of reversing this phenomenon. This hypothesis is supported by numerous previous studies demonstrating that perivascular inflammation, acting via a variety of paracrine and autocrine mechanisms (i.e. altered production of adipokines, changes in NO gradients, production of reactive oxygen species and expression of inflammatory cytokines), is a key regulator of endothelial dysfunction and atherosclerosis in obesity (Greenstein et al. 2009; Ketonen et al. 2010). In addition, other studies have shown that insulin resistance is associated with vascular inflammation (Zhang et al. 2003; Zhao et al. 2015). More specifically, insulin resistance in the vasculature appears to precede peripheral tissue insulin resistance, suggesting that the former is more susceptible to injury or metabolic changes (Kim et al. 2008; Zhao et al. 2015). Consistent with this, in the current study, we found that there was a significant increase in insulin and HOMA‐IR score in the obese mice and that these changes normalized after weight loss, thus raising the possibility that improved insulin sensitivity in lymphatic vessels may also be protective. This continues to be an area of interest and future studies will be required to investigate these findings. In addition, other changes in obesity such as alterations in adipokine expression, cytokine production and endocrine changes may also play a role in the regulation of lymphatic function in obesity. However, these changes require further study.
NO is a gas that has potent effects on the lymphatic system and is produced by three different isoforms of nitric oxide synthase: eNOS (endothelial nitric oxide synthase), nNOS (neuronal) and iNOS (inducible) (Alderton et al. 2001). Under basal conditions, eNOS expression by lymphatic endothelial cells is regulated by intracellular calcium levels and vessel shear stress, resulting in gradients of NO that, in turn, regulate lymphatic dilatation and contraction (Ferguson & DeFilippi, 1994; Leak et al. 1995; Gashev et al. 2002; Liao et al. 2011). In contrast, iNOS is produced primarily by inflammatory cells (and to a lesser extent smooth muscle cells), and its expression is not regulated by intracellular calcium levels (Aktan 2004). Previous studies have shown that high levels of NO produced by iNOS in inflammatory cells disrupt endogenous gradients of eNOS‐derived NO and, as a result, impair collecting lymphatic contraction and function (Liao et al. 2011). In addition, obesity has been recently shown to increase the number of perilymphatic iNOS‐expressing cells (Hespe et al. 2016). Lymphatic dysfunction in ageing mesenteric lymphatics, as well as in response to lipopolysaccharide inflammation, is thought to be mediated at least in part by production of iNOS by macrophages and mast cells (Chatterjee and Gashev 2012; Chakraborty et al. 2015). Consistent with these findings, we found that the expression of iNOS was markedly increased in obesity and mirrored the perilymphatic distribution of inflammatory cells we had observed. More specifically, we have found that the majority of these iNOS‐expressing inflammatory cells also expressed the surface receptor CD11b, suggesting these cells are macrophages.
Consistent with these reports, we found that weight gain was almost linearly correlated with perilymphatic inflammation and iNOS expression and that these changes, in turn, were correlated with decreased collecting lymphatic pumping frequency. Weight loss effectively decreased this inflammatory response and the expression of perilymphatic iNOS, as well as normalized collecting lymphatic pumping frequency, suggesting that this is an important mechanism regulating lymphatic dysfunction in obesity. This hypothesis is supported by other studies in our lab demonstrating that iNOS inhibitors increase lymphatic pumping frequency and lymphatic function (Torrisi et al. 2016). In addition, it is possible that NO has deleterious effects on the lymphatics independent of changes in contractility. For example, previous studies have shown that lymphatic endothelial cells (LECs) are highly sensitive to reactive oxygen species; excess NO can be converted to peroxynitrite, a powerful oxidant, which can potentially damage lymphatic vessels (Thangaswamy et al. 2012; Kasuya et al. 2014).
The findings of our study are clinically relevant because numerous studies have shown that obesity is a risk factor for the development of lymphoedema (Werner et al. 1991; Helyer et al. 2010). In addition, Greene and colleagues have observed that patients who are morbidly obese can spontaneously develop extremity lymphoedema (Greene et al. 2012; Greene & Maclellan, 2013). More importantly, several randomized control studies have shown that weight loss in patients with lymphoedema decreases their symptoms and that this strategy is an effective means of reducing limb volume in patients with established lymphoedema (Shaw et al. 2007). Thus, while it is likely that improvements in lymphoedema symptoms after weight loss are in part due to decreased adipose deposition, our findings suggest that these changes may also be related to improved lymphatic function in general.
In conclusion, we have demonstrated that weight gain has a nearly linear negative correlation with lymphatic function and that obesity results in decreased lymphatic vessel density, decreased collecting vessel pumping frequency and leaky lymphatics. In addition, we have shown that weight gain and obesity result in perilymphatic accumulation of inflammatory cells (T cells and macrophages) and increased levels of iNOS. Weight loss effectively and rapidly reverses these pathological changes and restores lymphatic function to normal levels. Future studies are warranted to further elucidate the role of iNOS in modulating lymphatic function. Whether weight loss‐induced changes in adipokine levels affect lymphatic function should also be determined.
Additional information
Competing interests
The authors have no conflicts of interest or disclosures. This work has not been previously presented and is not in consideration for publication at any other journals.
Author contributions
M.D.N., A.J.D., R.P.K. and B.J.M. conceived and designed the work. M.D.N., G.E.H., G.G.N., I.L.S., J.S.T., J.C.G., and R.P.K. conducted acquisition and analysis of data; M.D.N., G.E.H., G.G.N., I.L.S., J.S.T., J.C.G. and R.P.K. interpreted the data; M.D.N., G.E.H., G.G.N., I.L.S., J.S.T., J.C.G., A.J.D., R.P.K. and B.J.M. drafted the paper or revised it critically for important intellectual content.
Funding
NIH R01 HL111130‐01 awarded to B.J.M.; NIH T32 CA009685‐21A1 grant to G.G.N.; Plastic Surgery Foundation Pilot Research Grant awarded to J.C.G.; Plastic Surgery Foundation Pilot Research Grant awarded to G.G.N.; Sharp Foundation Fund philanthropic gift to B.J.M.; Breast Cancer Research Foundation awarded to A.J.D.; MSKCC Core Grant P30 CA008748.
Translational perspective
Obesity has significant deleterious effects on the lymphatic system. This is important because acquired defects in the lymphatic system can modulate the pathology of obesity in other organ systems by impairing clearance and regulating differentiation of inflammatory cells. In this study we show that weight gain has a nearly linear correlation with decreasing lymphatic function characterized by decreased lymphatic vessel density and decreasing lymphatic vessel pumping frequency. In addition, we show that obesity results in perilymphatic accumulation of inflammatory cells and increased expression of iNOS. More importantly, we show that weight loss effectively reverses these pathological effects and markedly improves lymphatic function. These findings are translationally relevant to patients with lymphoedema since obesity is a major risk factor for disease development and because weight loss has been shown to improve the symptoms of lymphoedema. Thus, it is possible that obese patients have impaired lymphatic function at baseline and are more susceptible to developing lymphoedema after injury. Moreover, patients with lymphoedema may show improvements in symptomatology after weight loss due to improvements in lymphatic function. More broadly, our study suggests that the harmful effects of obesity on the lymphatic system are reversible. Therefore, devising pharmacological or behavioural interventions that protect the lymphatic system in obese individuals may be an effective means of decreasing the pathology of obesity in other organ systems.
Supporting information
Disclaimer: Supporting information has been peer‐reviewed but not copyedited.
Figure S1. Weight loss restores metabolic parameters.
Figure S2. Weight loss decreases perilymphatic macrophage infiltration.
Video S1. Lymphatic collecting vessel pumping in lean control mice.
Video S2. Lymphatic collecting vessel pumping in obese mice.
Video S3. Lymphatic collecting vessel pumping in weight loss mice.
Acknowledgements
We are grateful to Mesruh Turkekul, Navid Paknejad, Sho Fujisawa and Yevgeniy Romin of the Molecular Cytology Core at MSKCC for assistance with histology and tissue imaging (Core Grant (P30 CA008748).
Linked articles This article is highlighted by a Perspective by Barton et al. To read this Perspective, visit http://dx.doi.org/10.1113/JP273253.
This is an Editor's Choice article from the 1 December 2016 issue.
References
- Aktan F (2004). iNOS‐mediated nitric oxide production and its regulation. Life Sci 75, 639–653. [DOI] [PubMed] [Google Scholar]
- Alderton WK, Cooper CE & Knowles RG (2001). Nitric oxide synthases: structure, function and inhibition. Biochem J 357, 593–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli V, Llodra J, Rong JX, Satoh K, Ishii S, Shimizu T, Fisher EA & Randolph GJ (2004). Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity 21, 561–574. [DOI] [PubMed] [Google Scholar]
- Avraham T, Daluvoy S, Zampell J, Yan A, Haviv YS, Rockson SG & Mehrara BJ (2010). Blockade of transforming growth factor‐β1 accelerates lymphatic regeneration during wound repair. Am J Pathol 177, 3202–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AH & Scherer PE (2005). Adipose tissue, inflammation, and cardiovascular disease. Circ Res 96, 939–949. [DOI] [PubMed] [Google Scholar]
- Blum KS, Karaman S, Proulx ST, Ochsenbein AM, Luciani P, Leroux JC, Wolfrum C & Detmar M (2014). Chronic high‐fat diet impairs collecting lymphatic vessel function in mice. PLoS One 9, e94713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S, Zawieja SD, Wang W, Lee Y, Wang YJ, von der Weid PY, Zawieja DC & Muthuchamy M (2015). Lipopolysaccharide modulates neutrophil recruitment and macrophage polarization on lymphatic vessels and impairs lymphatic function in rat mesentery. Am J Physiol Heart Circ Physiol 309, H2042–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee V & Gashev AA (2012). Aging‐associated shifts in functional status of mast cells located by adult and aged mesenteric lymphatic vessels. Am J Physiol Heart Circ Physiol 303, H693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavin NW, Avraham T, Fernandez J, Daluvoy SV, Soares MA, Chaudhry A & Mehrara BJ (2008). TGF‐β1 is a negative regulator of lymphatic regeneration during wound repair. Am J Physiol Heart Circ Physiol 295, H2113–2127. [DOI] [PubMed] [Google Scholar]
- Clement K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, Sicard A, Rome S, Benis A, Zucker JD, Vidal H, Laville M, Barsh GS, Basdevant A, Stich V, Cancello R & Langin D (2004). Weight loss regulates inflammation‐related genes in white adipose tissue of obese subjects. FASEB J 18, 1657–1669. [DOI] [PubMed] [Google Scholar]
- Ferguson MK & DeFilippi VJ (1994). Nitric oxide and endothelium‐dependent relaxation in tracheobronchial lymph vessels. Microvasc Res 47, 308–317. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Kit BK, Orpana H & Graubard BI (2013). Association of all‐cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta‐analysis. JAMA 309, 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gashev AA, Davis MJ & Zawieja DC (2002). Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol 540, 1023–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene AK, Grant FD & Slavin SA (2012). Lower‐extremity lymphedema and elevated body‐mass index. N Engl J Med 366, 2136–2137. [DOI] [PubMed] [Google Scholar]
- Greene AK & Maclellan RA (2013). Obesity‐induced upper extremity lymphedema. Plast Reconstr Surg Glob Open 1, e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA & Heagerty AM (2009). Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 119, 1661–1670. [DOI] [PubMed] [Google Scholar]
- Helyer LK, Varnic M, Le LW, Leong W & McCready D (2010). Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients. Breast J 16, 48–54. [DOI] [PubMed] [Google Scholar]
- Hespe GE, Kataru RP, Savetsky IL, Garcia Nores GD, Torrisi JS, Nitti MD, Gardenier JC, Zhou J, Yu JZ, Jones LW & Mehrara BJ (2016). Exercise training improves obesity‐related lymphatic dysfunction. J Physiol 594, 4267–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iantorno M, Campia U, Di Daniele N, Nistico S, Forleo GB, Cardillo C & Tesauro M (2014). Obesity, inflammation and endothelial dysfunction. J Biol Regul Homeost Agents 28, 169–176. [PubMed] [Google Scholar]
- Jang JY, Koh YJ, Lee SH, Lee J, Kim KH, Kim D, Koh GY & Yoo OJ (2013). Conditional ablation of LYVE‐1+ cells unveils defensive roles of lymphatic vessels in intestine and lymph nodes. Blood 122, 2151–2161. [DOI] [PubMed] [Google Scholar]
- Karaman S, Hollmen M, Robciuc MR, Alitalo A, Nurmi H, Morf B, Buschle D, Alkan HF, Ochsenbein AM, Alitalo K, Wolfrum C & Detmar M (2015). Blockade of VEGF‐C and VEGF‐D modulates adipose tissue inflammation and improves metabolic parameters under high‐fat diet. Mol Metab 4, 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuya A, Sakabe J & Tokura Y (2014). Potential application of in vivo imaging of impaired lymphatic duct to evaluate the severity of pressure ulcer in mouse model. Sci Rep 4, 4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri K, Arakawa S, Kurahashi R & Hatano Y (2007). Impaired contact hypersensitivity in diet‐induced obese mice. J Dermatol Sci 46, 117–126. [DOI] [PubMed] [Google Scholar]
- Kataru RP, Kim H, Jang C, Choi DK, Koh BI, Kim M, Gollamudi S, Kim YK, Lee SH & Koh GY (2011). T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity 34, 96–107. [DOI] [PubMed] [Google Scholar]
- Ketonen J, Shi J, Martonen E & Mervaala E (2010). Periadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet‐induced obese C57Bl/6 mice. Circ J 74, 1479–1487. [DOI] [PubMed] [Google Scholar]
- Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A & Schwartz MW (2008). Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol 28, 1982–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leak LV, Cadet JL, Griffin CP & Richardson K (1995). Nitric oxide production by lymphatic endothelial cells in vitro. Biochem Biophys Res Commun 217, 96–105. [DOI] [PubMed] [Google Scholar]
- Liao S, Cheng G, Conner DA, Huang Y, Kucherlapati RS, Munn LL, Ruddle NH, Jain RK, Fukumura D & Padera TP (2011). Impaired lymphatic contraction associated with immunosuppression. Proc Natl Acad Sci USA 108, 18784–18789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HY, Rutkowski JM, Helft J, Reddy ST, Swartz MA, Randolph GJ & Angeli V (2009). Hypercholesterolemic mice exhibit lymphatic vessel dysfunction and degeneration. Am J Pathol 175, 1328–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauricio MD, Aldasoro M, Ortega J & Vila JM (2013). Endothelial dysfunction in morbid obesity. Curr Pharm Des 19, 5718–5729. [DOI] [PubMed] [Google Scholar]
- Moschen AR, Molnar C, Geiger S, Graziadei I, Ebenbichler CF, Weiss H, Kaser S, Kaser A & Tilg H (2010). Anti‐inflammatory effects of excessive weight loss: potent suppression of adipose interleukin 6 and tumour necrosis factor alpha expression. Gut 59, 1259–1264. [DOI] [PubMed] [Google Scholar]
- Nelson TS, Akin RE, Weiler MJ, Kassis T, Kornuta JA & Dixon JB (2014). Minimally invasive method for determining the effective lymphatic pumping pressure in rats using near‐infrared imaging. Am J Physiol Regul Integr Comp Physiol 306, R281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T & Nagai R (2009). CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15, 914–920. [DOI] [PubMed] [Google Scholar]
- Nitschke M, Aebischer D, Abadier M, Haener S, Lucic M, Vigl B, Luche H, Fehling HJ, Biehlmaier O, Lyck R & Halin C (2012). Differential requirement for ROCK in dendritic cell migration within lymphatic capillaries in steady‐state and inflammation. Blood 120, 2249–2258. [DOI] [PubMed] [Google Scholar]
- Nurmi H, Saharinen P, Zarkada G, Zheng W, Robciuc MR & Alitalo K (2015). VEGF‐C is required for intestinal lymphatic vessel maintenance and lipid absorption. EMBO Mol Med 7, 1418–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier P, Hernandez TL, Weil KM, Shepard TJ & Eckel RH (2003). Impact of diet‐induced weight loss on the cardiac autonomic nervous system in severe obesity. Obes Res 11, 1040–1047. [DOI] [PubMed] [Google Scholar]
- Prieto D, Contreras C & Sanchez A (2014). Endothelial dysfunction, obesity and insulin resistance. Curr Vasc Pharmacol 12, 412–426. [DOI] [PubMed] [Google Scholar]
- Robinson HA, Kwon S, Hall MA, Rasmussen JC, Aldrich MB & Sevick‐Muraca EM (2013). Non‐invasive optical imaging of the lymphatic vasculature of a mouse. J Vis Exp 73, e4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savetsky IL, Albano NJ, Cuzzone DA, Gardenier JC, Torrisi JS, Garcia Nores GD, Nitti MD, Hespe GE, Nelson TS, Kataru RP, Dixon JB & Mehrara BJ (2015). Lymphatic function regulates contact hypersensitivity dermatitis in obesity. J Invest Dermatol 135, 2742–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan JP, Hill MA & Davis MJ (2015). Lymphatic vascular integrity is disrupted in type 2 diabetes due to impaired nitric oxide signalling. Cardiovasc Res 107, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw C, Mortimer P & Judd PA (2007). A randomized controlled trial of weight reduction as a treatment for breast cancer‐related lymphedema. Cancer 110, 1868–1874. [DOI] [PubMed] [Google Scholar]
- Sjostrom CD, Peltonen M, Wedel H & Sjostrom L (2000). Differentiated long‐term effects of intentional weight loss on diabetes and hypertension. Hypertension 36, 20–25. [DOI] [PubMed] [Google Scholar]
- Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjostrom CD, Sullivan M, Wedel H & Swedish Obese Subjects Study Scientific Group (2004). Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 351, 2683–2693. [DOI] [PubMed] [Google Scholar]
- Tabibiazar R, Cheung L, Han J, Swanson J, Beilhack A, An A, Dadras SS, Rockson N, Joshi S, Wagner R & Rockson SG (2006). Inflammatory manifestations of experimental lymphatic insufficiency. PLoS Med 3, e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangaswamy S, Bridenbaugh EA & Gashev AA (2012). Evidence of increased oxidative stress in aged mesenteric lymphatic vessels. Lymphat Res Biol 10, 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi JS, Hespe GE, Cuzzone DA, Savetsky IL, Nitti MD, Gardenier JC, Garcia Nores GD, Jowhar D, Kataru RP & Mehrara BJ (2016). Inhibition of inflammation and iNOS improves lymphatic function in obesity. Sci Rep 6, 19817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Voorde J., Boydens C, Pauwels B & Decaluwe K (2014). Perivascular adipose tissue, inflammation and vascular dysfunction in obesity. Curr Vasc Pharmacol 12, 403–411. [DOI] [PubMed] [Google Scholar]
- Vuorio T, Nurmi H, Moulton K, Kurkipuro J, Robciuc MR, Ohman M, Heinonen SE, Samaranayake H, Heikura T, Alitalo K & Yla‐Herttuala S (2014). Lymphatic vessel insufficiency in hypercholesterolemic mice alters lipoprotein levels and promotes atherogenesis. Arterioscler Thromb Vasc Biol 34, 1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler M, Kassis T & Dixon JB (2012). Sensitivity analysis of near‐infrared functional lymphatic imaging. J Biomed Opt 17, 066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitman ES, Aschen SZ, Farias‐Eisner G, Albano N, Cuzzone DA, Ghanta S, Zampell JC, Thorek D & Mehrara BJ (2013). Obesity impairs lymphatic fluid transport and dendritic cell migration to lymph nodes. PLoS One 8, e70703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner RS, McCormick B, Petrek J, Cox L, Cirrincione C, Gray JR & Yahalom J (1991). Arm edema in conservatively managed breast cancer: obesity is a major predictive factor. Radiology 180, 177–184. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zalewski A, Liu Y, Mazurek T, Cowan S, Martin JL, Hofmann SM, Vlassara H & Shi Y (2003). Diabetes‐induced oxidative stress and low‐grade inflammation in porcine coronary arteries. Circulation 108, 472–478. [DOI] [PubMed] [Google Scholar]
- Zhao L, Fu Z, Wu J, Aylor KW, Barrett EJ, Cao W & Liu Z (2015). Inflammation‐induced microvascular insulin resistance is an early event in diet‐induced obesity. Clin Sci (Lond) 129, 1025–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclaimer: Supporting information has been peer‐reviewed but not copyedited.
Figure S1. Weight loss restores metabolic parameters.
Figure S2. Weight loss decreases perilymphatic macrophage infiltration.
Video S1. Lymphatic collecting vessel pumping in lean control mice.
Video S2. Lymphatic collecting vessel pumping in obese mice.
Video S3. Lymphatic collecting vessel pumping in weight loss mice.


