Synopsis
There is significant inter-patient variability in clopidogrel effectiveness, which is due in part to cytochrome P450 (CYP) 2C19 genotype. Approximately 30% of individuals carry CYP2C19 loss-of-function (LOF) alleles, which have been consistently shown to reduce clopidogrel effectiveness after an acute coronary syndrome and percutaneous coronary intervention (PCI). Guidelines recommend consideration of prasugrel or ticagrelor in these patients. A clinical trial examining outcomes with CYP2C19-genotype guided antiplatelet therapy in ongoing. In the meantime, based on the evidence available to date, several institutions have started clinically implementing CYP2C19 genotyping to assist with antiplatelet selection after PCI.
Keywords: clopidogrel, prasugrel, ticagrelor, genotype, CYP2C19, pharmacogenomics
Introduction
Clopidogrel is commonly prescribed in combination with aspirin for the prevention of ischemic events in patients with an ACS, whether managed medically or with percutaneous coronary intervention (PCI).1, 2 However, there is substantial interpatient response variability with clopidogrel. Contributions to this variability have been well studied and include both clinical factors and genotype.3-5 Prasugrel and ticagrelor are alternative agents shown to be superior to clopidogrel in clinical trials, but are associated with increased bleeding risk.6, 7 This review describes genetic contributions to variable response to platelet P2Y12 inhibitors, guidelines for selecting antiplatelet therapy based on genotype, and examples of clinical implementation of genotype-guided antiplatelet therapy after PCI.
Pharmacology of ADP Antagonists / P2Y12 Receptor Inhibitors
Clopidogrel and prasugrel are thienopyridines that irreversibly bind to platelet P2Y12 receptors thereby inhibiting ADP-mediated platelet activation and aggregation. Both are administered as prodrugs that require hepatic bioactivation to their active moieties.8 As shown in Figure 1, a number of cytochrome P450 (CYP) enzymes are involved in the two-step biotransformation of clopidogrel to its active compound (R1309641).8, 9 Notably, only 15% of ingested clopidogrel is converted into the active compound, and the remainder is inactivated by carboxyl esterases.8, 10
Figure 1. Depiction of clopidogrel metabolic pathway.
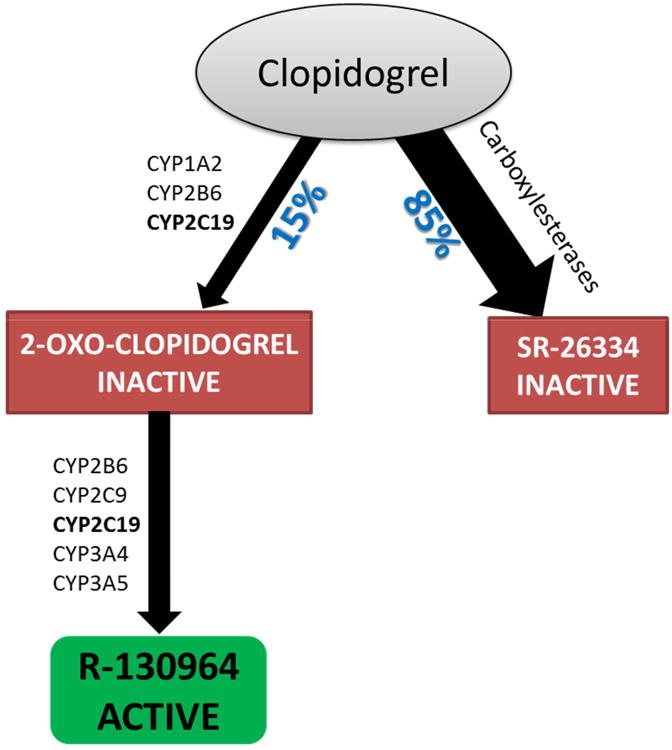
A lack of uniformity in platelet inhibition after clopidogrel treatment has been observed, and patients with high pre-treatment platelet reactivity tend to respond poorly to the drug, with an increased risk for major adverse cardiovascular events (MACE), including stent thrombosis.11-14 Clopidogrel response variability has been attributed to factors such as age, body mass index, co-medications, diabetes, renal failure, cardiac failure and most importantly loss-of-function (LOF) polymorphisms in the CYP2C19 gene.3-5
Prasugrel also undergoes hepatic bioactivation mediated by multiple enzymes, as shown in Figure 2.8 It has a more rapid onset of action and exhibits greater platelet inhibition than clopidogrel, with much less variability in response.15 However, prasugrel is associated with an increased risk of major bleeding compared to clopidogrel, which has led to reduced dose (5mg per day) recommendations for patients weighing 60 kg or less and those 75 years of age or older, who have excess formation of active metabolites.15, 16 Like clopidogrel, high on treatment platelet reactivity has been reported with prasugrel, which confers a higher risk of MACE after PCI.17 Prasugrel is contraindicated in patients with pathological bleeding or a history of transient ischemic attack or stroke.
Figure 2.
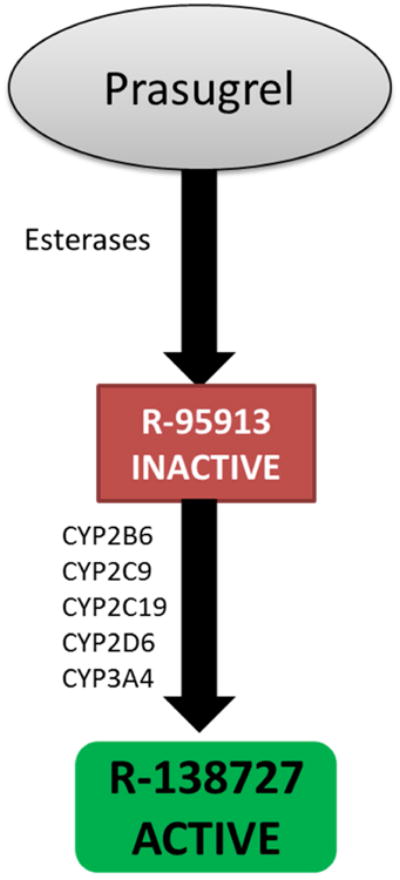
Depiction of prasugrel metabolic pathway
Ticagrelor is a non-thienopyridine that reversibly binds the P2Y12 receptor and does not require bioactivation. It has a quicker onset and offset of antiplatelet activity compared to clopidogrel and this results in greater efficacy in terms of reduction in the risk for MACE in ACS patients.7, 18 While risk for major bleeding is similar between ticagrelor and clopidogrel, ticagrelor is associated with an increased risk of non-coronary artery bypass graft related bleeding. Other adverse events with ticagrelor include dyspnea, bradyarrhythmia and minor spikes in serum creatinine and serum uric acid levels.7, 19, 20 Ticagrelor is contraindicated in patients a history of intracranial hemorrhage, active bleeding, moderate to severe hepatic impairment and any hypersensitivity reactions to ticagrelor.20
Genetic Determinants of Clopidogrel Response
CYP2C19 Genotype
Polymorphisms in the CYP2C19 gene (specifically loss-of-function variants) have been consistently implicated in clopidogrel response heterogeneity in both candidate gene and genome-wide association studies (GWAS).21-26 CYP2C19 or cytochrome P450, family 2, subfamily C, polypeptide 19, is located on chromosome 10 among a cluster of CYP genes including CYP2C18, CYP2C9, and CYP2C8.27 Like many other cytochrome P450 genes, CYP2C19 possesses genetic polymorphisms that lead to variable hepatic expression, which in turn alters the function of the resultant protein (i.e. CYP2C19 enzyme).
Over 30 CYP2C19 alleles have been identified.(http://www.cypalleles.ki.se/). The CYP2C19*1 allele represents a fully functional or normal activity allele. The most common loss-of-function (LOF) allele is the *2 allele (c.681 G>A; rs4244285), which occurs secondary to an aberrant splice site in exon 5 leading to a truncated protein.27 The less common CYP2C19*3 (c.636G>A; rs4986893) variation results in loss of enzyme activity secondary to premature termination of the amino acid sequence. CYP2C19*4 through *8 are LOF alleles observed in less than 1% of the general population.27, 28 Approximately 60% to 70% of East Asians carry a LOF allele, while the rate is lower (about 30%) in an ethnically and racially diverse population.28, 29 The CYP2C19*17 allele (c. -806C>T) occurs in the gene promoter region and results in enhanced enzymatic activity and is thus called a gain-of-function allele. While several groups have observed greater clopidogrel-induced platelet inhibition and high bleeding risk in patients with a *17 allele compared to noncarriers, data are inconsistent.22, 30-32
As shown in Table 1, CYP2C19 genotype confers 5 phenotypes:
Normal (or extensive) metabolizers (NMs)
Poor metabolizers (PMs)
Intermediate metabolizers (IMs)
Rapid metabolizers (RMs)
Ultra-rapid metabolizers (UMs)
Table 1. CYP2C19 phenotypes derived from CYP2C19 genotype.
| Genotype | Phenotype |
|---|---|
| *1/*1 | Normal metabolizer (NM) |
| *2/*2, *2/*3, or other combination of two loss-of-function alleles | Poor metabolizer (PM) |
| *1/*2, *1/*3, *2/*17† or other genotypes with a single loss-of-function allele | Intermediate metabolizer (IM) |
| *1/*17 | Rapid metabolizer (RM) |
| *17/*17 | Ultra-rapid metabolizer (UM) |
The IM phenotype assignment for genotypes with one loss-of-function and one gain-of-function allele (e.g. *2/*17) is based on evidence of increased platelet aggregation among clopidogrel treated patients with this genotype compared to the *1/*1 genotype, indicating that that the *17 allele is unable to completely compensate for reduced activity with the *2 allele.29 However, the data are not completely consistent, and thus the IM phenotype assignment is considered provisional.
The prevalence of CYP2C19 phenotypes by race is shown in Table 2. Consistent with having a higher frequency of LOF alleles, Asians have the highest prevalence of the PM and IM phenotypes.
Table 2. Prevalence of CYP2C19 phenotypes by race.
| Race | Phenotype | ||
|---|---|---|---|
| PMs | IMs | RMs or UMs | |
| White | 2% | 25% | 40% |
| Black | 4% | 30% | 45% |
| Asian | 14% | 50% | <5% |
PMs, Poor metabolizers; IMs, intermediate metabolizers; RMs, rapid metabolizers; UMs, ultra-rapid metabolizers
CYP2C19 genotype and clopidogrel pharmacokinetics and pharmacodynamics
Carriers of a CYP2C19 LOF allele (i.e. PMs and IMs) have diminished capacity to bioactive clopidogrel. A relative reduction of 32% in plasma concentrations of the active metabolite was reported in LOF carriers following clopidogrel exposure.33 Consistent with this, LOF genotype is associated with high on-treatment platelet reactivity (HTPR) after PCI, which is an independent risk factor for MACE.34 Therefore, it would follow that clopidogrel-treated LOF allele carriers may be at greater risk for MACE after PCI compared to non-carriers.
CYP2C19 genotype and clinical outcomes with clopidogrel
In an early study of nearly 800 PCI patients, a 3-fold increase in the incidence of death and myocardial infarction at one year was observed in CYP2C19*2 allele carriers compared to the non-carriers.21 This association was replicated in a number of subsequent studies.22-25 In a meta-analysis including 9 studies and over 9600 patients (54% with ACS and 91% with PCI), carriers of a LOF allele had an increased risk for MACE compared to noncarriers (hazard ratio, 1.57; 95% confidence interval, 1.13-2.16).35 The risk for stent thrombosis was even more marked, with a hazard ratio of 2.67 (95% CI, 1.69-4.22) in IMs and 3.97 (95% CI, 1.75-9.02) in PMs compared to non-LOF allele carriers.
The association between CYP2C19 genotype and adverse outcomes has not been demonstrated with all indications of clopidogrel. Specifically, among lower risk patients, such as those receiving clopidogrel for stable coronary disease, atrial fibrillation, or with ACS managed medically, no difference in clinical outcomes has been reported by genotype.32, 36 Similarly, a meta-analysis including studies of lower risk patients found only a modest association between genotype and clinical outcomes, which was abrogated when smaller studies were excluded.37
A more recent meta-analysis specifically aimed to assess outcomes among patients with and without PCI.38 In non-PCI patients, no increased risk of cardiovascular events was apparent (relative risk 0.99, 95% CI 0.84-1.17). However, among those who underwent PCI, there were significantly more events among LOF allele carriers compared to noncarriers (relative risk 1.20, 95% CI 1.10-1.31). Overall, the data strongly and consistently support reduced clopidogrel effectiveness in carriers of a CYP2C19 LOF allele after ACS and PCI, but not in those at lower risk for adverse cardiovascular events.
Other genes associated with clopidogrel response
Other genes involved in the metabolic and pharmacodynamics pathways of clopidogrel have also been examined for their association with clopidogrel effectiveness. These include the ABCB1, CES1, CYP2B6, CYP2C9, CYP3A4, PON1, and P2Y12 genes.22, 39-43 However, none of these have been consistently shown to contribute to clopidogrel response heterogeneity. In a GWAS conducted in healthy Amish volunteers given clopidogrel, only a cluster of polymorphisms on chromosome 10q24 in linkage disequilibrium with CYP2C19*2 reached genome wide significance for its association with platelet aggregation, suggesting that no other gene has major contributions to clopidogrel response.23
Therapeutic approaches based on CYP2C19 genotype
Clopidogrel dose escalation
Clopidogrel dose escalation as a means of compensating for reduced clopidogrel activation in the presence of a CYP2C19 LOF allele has been the subject of several studies. In healthy volunteers, a clopidogrel maintenance dose of 150 mg in IMs and 300 mg in PMs resulted in similar inhibition of ADP-induced platelet aggregation as a 75 mg dose in NMs.44 However, among patients with coronary heart disease, a 300 mg dose failed to reduce platelet reactivity in PMs to a level achieved with a 75 mg dose in NMs.45 In IMs, a dose of 225 mg in non-diabetics and 300 mg in diabetics resulted in desired on-treatment platelet reactivity. Taken together, these data suggest that while adequate antiplatelet activity may be attained with tripling or quadrupling the dose in IMs, such an approach may not be effective in PMs. Use of alternative agents whose effects are not influenced by CYP2C19 genotype is a more viable option in IMs and PMs.
Alternative antiplatelet therapy
Unlike clopidogrel, CYP2C19 genotype does not affect prasugrel pharmacokinetics or pharmacodynamics despite being involved in prasugrel bioactivation.22 This is likely because CYP2C19 has a minor role in the bioactivation of prasugrel compared to other enzymes involved.46 Since ticagrelor does not require bioactivation, CYP450 enzymes do not influence the amount of drug initially entering the body.
Genetic sub-studies of large randomized controlled trials that compared the efficacy of clopidogrel to prasugrel or ticagrelor have been conducted.47, 48 In carriers of a CYP2C19 LOF allele, both prasugrel and ticagrelor were shown to significantly reduce ischemic events compared to clopidogrel. However, prasugrel and clopidogrel were similarly effective in patients without a LOF allele.48 Ticagrelor tended to remain superior to clopidogrel in the absence of the LOF genotype (p=0.06).47 The test for interaction between genotype and treatment group was not significant, leading the authors to conclude that ticagrelor is superior in reducing ischemic events compared to clopidogrel regardless of genotype.
Guidelines for CYP2C19 genotyping with clopidogrel
In March, 2010, the FDA approved a revision to the clopidogrel labeling to add a boxed warning stating that:49
The efficacy of clopidogrel is reduced in PMs with ACS or undergoing PCI;
Tests are available to determine genotype for clinical purposes; and
Alternative treatment strategies should be considered for PMs.
This followed two previous revisions to the label, the first of which added initial information about reduced clopidogrel response in PMs, and the second of which advised avoidance of clopidogrel in patients with decreased CYP2C19 enzyme activity secondary to LOF genotype or concomitant use of CYP2C19 inhibitors.
Following the clopidogrel label revision, the American College of Cardiology Foundation and the American Heart Association issued guidance on the use of genetic testing to guide antiplatelet selection after PCI.49 They state that the evidence base is insufficient to recommend routine genetic testing, citing a lack of data that routine testing improves outcomes. However, genetic testing may be considered before starting clopidogrel in patients at moderate to high risk for poor outcomes, such as those undergoing high-risk PCI procedures. For patients found to be PMs, then alternative therapy is recommended in the absence of contraindications.
The Clinical Pharmacogenetics Implementation Consortium (CPIC) also provides guidelines for clopidogrel use based on CYP2C19 genotype.29 The guidelines do not provide recommendations on whether or not to order a genetic test, but rather on how to interpret genetic test results and use them to optimize drug therapy. The clopidogrel guidelines were the second of 17 CPIC gene-drug pair guidelines available as of mid-2016, indicating the high level of evidence supporting CYP2C19-guided clopidogrel use relevant to genotype-guided therapy for other drugs. The recommendations are outlined in Figure 3, with prasugrel or ticagrelor recommended after ACS and PCI in patients with a loss-of-function allele.29 The recommendations are graded as strong for PMs, meaning that the desirable effects clearly outweigh the undesirable effects, and moderate for IMs, meaning that there is close or uncertain balance between desirable and undesirable effects.
Figure 3.
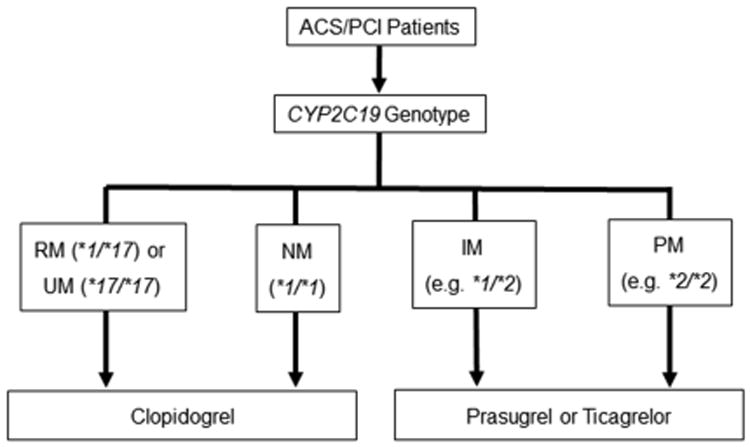
Recommendations by the Clinical Pharmacogenetics Implementation Consortium (CPIC) for CYP2C19-guided antiplatelet therapy; ACS, acute coronary syndrome; IM, intermediate metabolizer; PCI, percutaneous coronary intervention; PM, poor metabolizer; RM, rapid metabolizer; UM, ultra-rapid metabolizer
Clinical implementation of CYP2C19 genotyping
Examples of clinical implementation
A number of institutions have established a process for providing CYP2C19 genotyping to help direct antiplatelet prescribing for patients undergoing PCI. Approaches vary from preemptive genetic testing in advance of patients needing dual antiplatelet therapy to reactive testing at the time of PCI when dual antiplatelet therapy is deemed necessary. For example, Vanderbilt University and University of Maryland implemented CYP2C19 testing for patients scheduled to undergo left heart catheterization so that results would be available in the event that the patient proceeded to PCI.50, 51 At the University of Florida, the approach is more reactive, with the genotype test order defaulted on the post-PCI order set so that patients are automatically genotyped unless the physician chooses to unselect the order.52
Patient selection for genotyping also varies by site, with some sites broadly genotyping all patients undergoing left heart catheterization or PCI, such as described above. Other sites focus on high risk patients. This is the approach at the University of North Carolina where testing is recommended in PCI patients with high-risk anatomic findings.53 Rather than being a defaulted order, genetic testing is actively ordered after angiography-guided risk stratification by the interventional cardiologist.
In the U.S., genotyping must be performed in Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory for results to be entered into the electronic health record and used for clinical purposes. Recommendations for alternative antiplatelet therapy for patients with LOF genotype may be provided to physicians via electronic decision support tools or through other forms of communication (e.g. telephone call, secure email). 50-52 For example, at the University of Florida, an alert will pop up in the EHR in response to an order for clopidogrel for a patient with a loss-of-function genotype, warning the clinician of potential reduced clopidogrel effectiveness and advising consideration of prasugrel or ticagrelor. Dosing information and contraindications for alternative agents are included in the alert to assist with prescribing decisions, and the physician may place the order for alternative therapy directly from the alert. Ultimately, the choice of antiplatelet therapy is left to the discretion of the physician. Recent preliminary data suggest that patients with a LOF allele who are switched to alternative therapy have a significant reduction in risk for major adverse cardiovascular events compared to loss-of-function allele carriers continued on clopidogrel.54
Barriers to clinical implementation
Implementation of CYP2C19 testing requires overcoming a number of barriers. These include establishing a process for genetic testing and the need to obtain genotype results prior to or soon after PCI. Ideally, a point-of-care platform would be available in the cardiac catheterization laboratory to rapidly provide genotype results. While such a platform is available, placing it in the cardiac catheterization laboratory is inconsistent with quality standard in the U.S., which requires that testing be done in a CLIA-licensed laboratory. Thus, a process must be established to transport samples efficiently to a CLIA-licensed laboratory for testing and efficient return of genotype results.
Another major barrier is the lack of data from large randomized clinical trials showing improved outcomes with genotype-guided clopidogrel use. Obviously, many sites feel that the available data linking CYP2C19 genotype to poor outcomes with clopidogrel are sufficient to support clinical implementation. However, others argue that implementation is premature in the absence of prospective clinical trial data. While a clinical trial is currently underway, it is not expected to be completed until 2019.(clinical trials.gov identifier: NCT01742117) In the meantime, it is anticipated that clinical outcome data will emerge from sites implementing genetic testing, which may influence the landscape of clopidogrel pharmacogenetics.
The need for clinician preparedness to utilize genetic testing results is an additional barrier. Equipping clinicians with tools to translate genetic results into prescribing decisions at the time of care may be addressed through electronic decision support, as described above at the University of Florida, consultation with pharmacogenetic experts, or other means. Recognizing the knowledge and awareness barrier for pharmacogenetic testing, the National Institutes of Health and other stakeholders have also taken steps to better educate the clinician workforce on genomic medicine.(Genetics/Genomics Competency Center http://g-2-c-2.org//)
Pharmacogenetics of prasugrel and ticagrelor
Several investigators have examined associations between CYP450 genotypes and prasugrel response. One study reported overrepresentation of the CYP2C9*2 variant among individuals with a lower level of platelet inhibition with prasugrel,55 whereas others have found no significant effect of CYP2C9, CYP2C19, CYP2B6, CYP3A4, or CYP1A2 genotypes on either prasugrel metabolite concentrations or antiplatelet effects.22, 41
A GWAS was conducted to identify associations with ticagrelor pharmacokinetics and clinical response.56 A variant in the CYP3A4 gene was found to be associated with ticagrelor concentrations. Two additional polymorphisms, one in the SLCO1B1 gene, encoding for the organic anion transporter polypeptide, and another in UGT2B7, encoding for UDP-glucuronosyltransferase 2B7, were associated with concentrations of the major active metabolite of ticagrelor. However, effects were modest, and none of the polymorphisms were associated with reductions in ischemic events or risk for bleeding or dyspnea with ticagrelor.
Summary and future projections for antiplatelet pharmacogenomics
Data clearly and consistently demonstrate reduced clopidogrel effectiveness in preventing ischemic events in patients with a CYP2C19 LOF allele. Given the high prevalence of the LOF genotype, a substantial portion of the population is at risk for inadequate anti-platelet response to clopidogrel. The data have accumulated to the extent that an increasing number of institutions are beginning to offer CYP2C19 genotyping for patients undergoing PCI to assist with antiplatelet selection. Randomized controlled trial data, considered the gold standard evidence needed to broadly influence practice patterns, are forthcoming on the efficacy of genotype-guided antiplatelet therapy. In the meantime, efforts to implement CYP2C19 testing into practice to predict clopidogrel response will help to establish procedures for overcoming implementation barriers and may also provide useful “real world” data on outcomes with pharmacogenetics testing to complement clinical trial findings.
Key Points.
There is significant inter-patient variability in clopidogrel effectiveness in patients with an acute coronary syndrome and percutaneous coronary intervention (PCI).
Clopidogrel is a prodrug that requires bioactivation, and cytochrome P450 (CYP) 2C19 is involved in both steps of the bioactivation process.
Data consistently demonstrate reduced clopidgrel effectiveness after PCI in patients with the CYPC19 loss-of-function genotype.
Neither prasugrel nor ticagrelor are affected by CYP2C19 genotype, and guidelines recommend consideration of one of these agents for PCI patients with the CYP2C19 loss-of-function genotype.
A number of institutions have implemented CYP2C19 genotyping for patients undergoing PCI to assist with antiplatelet selection.
Acknowledgments
Funding Sources: Work by LHC is supported by NIH/NHGRI (U01 HG 007269). AOO is supported in part by NIH/NHGRI (U01HG006380). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosure statement: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Larisa H. Cavallari, Email: lcavallari@cop.ufl.edu, Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics, University of Florida, Gainesville, FL; 1333 Center Drive, PO Box 100486, Gainesville, FL 32610; Fax: (352) 273-6485; Tel: (352) 273-8245.
Aniwaa Owusu Obeng, Email: aniwaa.owusu-obeng@mssm.edu, The Charles Bronfman Institute for Personalized Medicine and Division of General Internal Medicine, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, and the Department of Pharmacy, The Mount Sinai Hospital, New York, NY; Tel: (212) 241-7371.
References
- 1.Sabatine MS, Cannon CP, Gibson CM, Lopez-Sendon JL, Montalescot G, Theroux P, Lewis BS, Murphy SA, McCabe CH, Braunwald E Clopidogrel as Adjunctive Reperfusion Therapy -Thrombolysis in Myocardial Infarction I. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA. 2005;294:1224–1232. doi: 10.1001/jama.294.10.1224. [DOI] [PubMed] [Google Scholar]
- 2.Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, Malmberg K, Rupprecht H, Zhao F, Chrolavicius S, Copland I, Fox KA Clopidogrel in Unstable angina to prevent Recurrent Events trial I. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358:527–533. doi: 10.1016/s0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]
- 3.Khalil BM, Shahin MH, Solayman M, Langaee T, Schaalan MF, Gong Y, Hammad LN, Al-Mesallamy HO, Hamdy NM, El-Hammady WA, Johnson JA. Genetic and Nongenetic Factors Affecting Clopidogrel Response in the Egyptian Population. Clin Transl Sci. 2016;9:23–28. doi: 10.1111/cts.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuisset T, Quilici J, Grosdidier C, Fourcade L, Gaborit B, Pankert M, Molines L, Morange PE, Bonnet JL, Alessi MC. Comparison of platelet reactivity and clopidogrel response in patients </= 75 Years Versus > 75 years undergoing percutaneous coronary intervention for non-ST-segment elevation acute coronary syndrome. Am J Cardiol. 2011;108:1411–1416. doi: 10.1016/j.amjcard.2011.06.060. [DOI] [PubMed] [Google Scholar]
- 5.Hochholzer W, Trenk D, Fromm MF, Valina CM, Stratz C, Bestehorn HP, Buttner HJ, Neumann FJ. Impact of cytochrome P450 2C19 loss-of-function polymorphism and of major demographic characteristics on residual platelet function after loading and maintenance treatment with clopidogrel in patients undergoing elective coronary stent placement. J Am Coll Cardiol. 2010;55:2427–2434. doi: 10.1016/j.jacc.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 6.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM Investigators T-T. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 7.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Investigators P, Freij A, Thorsen M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 8.Laine M, Paganelli F, Bonello L. P2Y12-ADP receptor antagonists: Days of future and past. World J Cardiol. 2016;8:327–332. doi: 10.4330/wjc.v8.i5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, Ikeda T, Kurihara A. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. 2010;38:92–99. doi: 10.1124/dmd.109.029132. [DOI] [PubMed] [Google Scholar]
- 10.Karazniewicz-Lada M, Danielak D, Burchardt P, Kruszyna L, Komosa A, Lesiak M, Glowka F. Clinical pharmacokinetics of clopidogrel and its metabolites in patients with cardiovascular diseases. Clin Pharmacokinet. 2014;53:155–164. doi: 10.1007/s40262-013-0105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurbel PA, Bliden KP, Hiatt BL, O'Connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107:2908–2913. doi: 10.1161/01.CIR.0000072771.11429.83. [DOI] [PubMed] [Google Scholar]
- 12.Jaremo P, Lindahl TL, Fransson SG, Richter A. Individual variations of platelet inhibition after loading doses of clopidogrel. J Intern Med. 2002;252:233–238. doi: 10.1046/j.1365-2796.2002.01027.x. [DOI] [PubMed] [Google Scholar]
- 13.Stone GW, Witzenbichler B, Weisz G, Rinaldi MJ, Neumann FJ, Metzger DC, Henry TD, Cox DA, Duffy PL, Mazzaferri E, Gurbel PA, Xu K, Parise H, Kirtane AJ, Brodie BR, Mehran R, Stuckey TD Investigators AD. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet. 2013;382:614–623. doi: 10.1016/S0140-6736(13)61170-8. [DOI] [PubMed] [Google Scholar]
- 14.Angiolillo DJ, Bernardo E, Sabate M, Jimenez-Quevedo P, Costa MA, Palazuelos J, Hernandez-Antolin R, Moreno R, Escaned J, Alfonso F, Banuelos C, Guzman LA, Bass TA, Macaya C, Fernandez-Ortiz A. Impact of platelet reactivity on cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol. 2007;50:1541–1547. doi: 10.1016/j.jacc.2007.05.049. [DOI] [PubMed] [Google Scholar]
- 15.Wiviott SD, Trenk D, Frelinger AL, O'Donoghue M, Neumann FJ, Michelson AD, Angiolillo DJ, Hod H, Montalescot G, Miller DL, Jakubowski JA, Cairns R, Murphy SA, McCabe CH, Antman EM, Braunwald E Investigators P-T. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation. 2007;116:2923–2932. doi: 10.1161/CIRCULATIONAHA.107.740324. [DOI] [PubMed] [Google Scholar]
- 16.Roe MT, Goodman SG, Ohman EM, Stevens SR, Hochman JS, Gottlieb S, Martinez F, Dalby AJ, Boden WE, White HD, Prabhakaran D, Winters KJ, Aylward PE, Bassand JP, McGuire DK, Ardissino D, Fox KA, Armstrong PW. Elderly patients with acute coronary syndromes managed without revascularization: insights into the safety of long-term dual antiplatelet therapy with reduced-dose prasugrel versus standard-dose clopidogrel. Circulation. 2013;128:823–833. doi: 10.1161/CIRCULATIONAHA.113.002303. [DOI] [PubMed] [Google Scholar]
- 17.Bonello L, Pansieri M, Mancini J, Bonello R, Maillard L, Barnay P, Rossi P, Ait-Mokhtar O, Jouve B, Collet F, Peyre JP, Wittenberg O, de Labriolle A, Camilleri E, Cheneau E, Cabassome E, Dignat-George F, Camoin-Jau L, Paganelli F. High on-treatment platelet reactivity after prasugrel loading dose and cardiovascular events after percutaneous coronary intervention in acute coronary syndromes. J Am Coll Cardiol. 2011;58:467–473. doi: 10.1016/j.jacc.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Gurbel PA, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, Teng R, Antonino MJ, Patil SB, Karunakaran A, Kereiakes DJ, Parris C, Purdy D, Wilson V, Ledley GS, Storey RF. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120:2577–2585. doi: 10.1161/CIRCULATIONAHA.109.912550. [DOI] [PubMed] [Google Scholar]
- 19.Yousuf O, Bhatt DL. The evolution of antiplatelet therapy in cardiovascular disease. Nat Rev Cardiol. 2011;8:547–559. doi: 10.1038/nrcardio.2011.96. [DOI] [PubMed] [Google Scholar]
- 20.Htun WW, Steinhubl SR. Ticagrelor: the first novel reversible P2Y(12) inhibitor. Expert Opin Pharmacother. 2013;14:237–245. doi: 10.1517/14656566.2013.757303. [DOI] [PubMed] [Google Scholar]
- 21.Trenk D, Hochholzer W, Fromm MF, Chialda LE, Pahl A, Valina CM, Stratz C, Schmiebusch P, Bestehorn HP, Buttner HJ, Neumann FJ. Cytochrome P450 2C19 681G>A polymorphism and high on-clopidogrel platelet reactivity associated with adverse 1-year clinical outcome of elective percutaneous coronary intervention with drug-eluting or bare-metal stents. J Am Coll Cardiol. 2008;51:1925–1934. doi: 10.1016/j.jacc.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 22.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias WL, Braunwald E, Sabatine MS. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation. 2009;119:2553–2560. doi: 10.1161/CIRCULATIONAHA.109.851949. [DOI] [PubMed] [Google Scholar]
- 23.Shuldiner AR, O'Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, Damcott CM, Pakyz R, Tantry US, Gibson Q, Pollin TI, Post W, Parsa A, Mitchell BD, Faraday N, Herzog W, Gurbel PA. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–857. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sibbing D, Stegherr J, Latz W, Koch W, Mehilli J, Dorrler K, Morath T, Schomig A, Kastrati A, von Beckerath N. Cytochrome P450 2C19 loss-of-function polymorphism and stent thrombosis following percutaneous coronary intervention. Eur Heart J. 2009;30:916–922. doi: 10.1093/eurheartj/ehp041. [DOI] [PubMed] [Google Scholar]
- 25.Collet JP, Hulot JS, Pena A, Villard E, Esteve JB, Silvain J, Payot L, Brugier D, Cayla G, Beygui F, Bensimon G, Funck-Brentano C, Montalescot G. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009;373:309–317. doi: 10.1016/S0140-6736(08)61845-0. [DOI] [PubMed] [Google Scholar]
- 26.Hulot JS, Bura A, Villard E, Azizi M, Remones V, Goyenvalle C, Aiach M, Lechat P, Gaussem P. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2006;108:2244–2247. doi: 10.1182/blood-2006-04-013052. [DOI] [PubMed] [Google Scholar]
- 27.Scott SA, Sangkuhl K, Shuldiner AR, Hulot JS, Thorn CF, Altman RB, Klein TE. PharmGKB summary: very important pharmacogene information for cytochrome P450, family 2, subfamily C, polypeptide 19. Pharmacogenet Genomics. 2012;22:159–165. doi: 10.1097/FPC.0b013e32834d4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeong YH, Bliden KP, Park Y, Tantry US, Gurbel PA. Pharmacogenetic guidance for antiplatelet treatment. Lancet. 2012;380:725. doi: 10.1016/S0140-6736(12)61398-1. author reply 725-726. [DOI] [PubMed] [Google Scholar]
- 29.Scott SA, Sangkuhl K, Stein CM, Hulot JS, Mega JL, Roden DM, Klein TE, Sabatine MS, Johnson JA, Shuldiner AR Clinical Pharmacogenetics Implementation C. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317–323. doi: 10.1038/clpt.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sibbing D, Gebhard D, Koch W, Braun S, Stegherr J, Morath T, Von Beckerath N, Mehilli J, Schomig A, Schuster T, Kastrati A. Isolated and interactive impact of common CYP2C19 genetic variants on the antiplatelet effect of chronic clopidogrel therapy. J Thromb Haemost. 2010;8:1685–1693. doi: 10.1111/j.1538-7836.2010.03921.x. [DOI] [PubMed] [Google Scholar]
- 31.Tiroch KA, Sibbing D, Koch W, Roosen-Runge T, Mehilli J, Schomig A, Kastrati A. Protective effect of the CYP2C19 *17 polymorphism with increased activation of clopidogrel on cardiovascular events. Am Heart J. 2010;160:506–512. doi: 10.1016/j.ahj.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 32.Pare G, Mehta SR, Yusuf S, Anand SS, Connolly SJ, Hirsh J, Simonsen K, Bhatt DL, Fox KA, Eikelboom JW. Effects of CYP2C19 genotype on outcomes of clopidogrel treatment. N Engl J Med. 2010;363:1704–1714. doi: 10.1056/NEJMoa1008410. [DOI] [PubMed] [Google Scholar]
- 33.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias W, Braunwald E, Sabatine MS. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 34.Palmerini T, Calabro P, Piscione F, De Servi S, Cattaneo M, Maffeo D, Toso A, Bartorelli A, Palmieri C, De Carlo M, Capodanno D, Barozzi C, Tomasi L, Della Riva D, Mariani A, Taglieri N, Reggiani LB, Bianchi R, De Rosa R, Mariani M, Podda G, Genereux P, Stone GW, Angiolillo DJ. Impact of gene polymorphisms, platelet reactivity, and the SYNTAX score on 1-year clinical outcomes in patients with non-ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention: the GEPRESS study. JACC Cardiovasc Interv. 2014;7:1117–1127. doi: 10.1016/j.jcin.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 35.Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, Cannon CP, Danchin N, Giusti B, Gurbel P, Horne BD, Hulot JS, Kastrati A, Montalescot G, Neumann FJ, Shen L, Sibbing D, Steg PG, Trenk D, Wiviott SD, Sabatine MS. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821–1830. doi: 10.1001/jama.2010.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doll JA, Neely ML, Roe MT, Armstrong PW, White HD, Prabhakaran D, Winters KJ, Duvvuru S, Sundseth SS, Jakubowski JA, Gurbel PA, Bhatt DL, Ohman EM, Fox KA Investigators TA. Impact of CYP2C19 Metabolizer Status on Patients With ACS Treated With Prasugrel Versus Clopidogrel. J Am Coll Cardiol. 2016;67:936–947. doi: 10.1016/j.jacc.2015.12.036. [DOI] [PubMed] [Google Scholar]
- 37.Holmes MV, Perel P, Shah T, Hingorani AD, Casas JP. CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: a systematic review and meta-analysis. JAMA. 2011;306:2704–2714. doi: 10.1001/jama.2011.1880. [DOI] [PubMed] [Google Scholar]
- 38.Sorich MJ, Rowland A, McKinnon RA, Wiese MD. CYP2C19 genotype has a greater effect on adverse cardiovascular outcomes following percutaneous coronary intervention and in Asian populations treated with clopidogrel: a meta-analysis. Circ Cardiovasc Genet. 2014;7:895–902. doi: 10.1161/CIRCGENETICS.114.000669. [DOI] [PubMed] [Google Scholar]
- 39.Mega JL, Close SL, Wiviott SD, Shen L, Walker JR, Simon T, Antman EM, Braunwald E, Sabatine MS. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet. 2010;376:1312–1319. doi: 10.1016/S0140-6736(10)61273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis JP, Horenstein RB, Ryan K, O'Connell JR, Gibson Q, Mitchell BD, Tanner K, Chai S, Bliden KP, Tantry US, Peer CJ, Figg WD, Spencer SD, Pacanowski MA, Gurbel PA, Shuldiner AR. The functional G143E variant of carboxylesterase 1 is associated with increased clopidogrel active metabolite levels and greater clopidogrel response. Pharmacogenet Genomics. 2013;23:1–8. doi: 10.1097/FPC.0b013e32835aa8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brandt JT, Close SL, Iturria SJ, Payne CD, Farid NA, Ernest CS, 2nd, Lachno DR, Salazar D, Winters KJ. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost. 2007;5:2429–2436. doi: 10.1111/j.1538-7836.2007.02775.x. [DOI] [PubMed] [Google Scholar]
- 42.Harmsze A, van Werkum JW, Bouman HJ, Ruven HJ, Breet NJ, Ten Berg JM, Hackeng CM, Tjoeng MM, Klungel OH, de Boer A, Deneer VH. Besides CYP2C19*2, the variant allele CYP2C9*3 is associated with higher on-clopidogrel platelet reactivity in patients on dual antiplatelet therapy undergoing elective coronary stent implantation. Pharmacogenet Genomics. 2010;20:18–25. doi: 10.1097/FPC.0b013e328333dafe. [DOI] [PubMed] [Google Scholar]
- 43.Malek LA, Kisiel B, Spiewak M, Grabowski M, Filipiak KJ, Kostrzewa G, Huczek Z, Ploski R, Opolski G. Coexisting polymorphisms of P2Y12 and CYP2C19 genes as a risk factor for persistent platelet activation with clopidogrel. Circ J. 2008;72:1165–1169. doi: 10.1253/circj.72.1165. [DOI] [PubMed] [Google Scholar]
- 44.Horenstein RB, Madabushi R, Zineh I, Yerges-Armstrong LM, Peer CJ, Schuck RN, Figg WD, Shuldiner AR, Pacanowski MA. Effectiveness of clopidogrel dose escalation to normalize active metabolite exposure and antiplatelet effects in CYP2C19 poor metabolizers. J Clin Pharmacol. 2014;54:865–873. doi: 10.1002/jcph.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mega JL, Hochholzer W, Frelinger AL, 3rd, Kluk MJ, Angiolillo DJ, Kereiakes DJ, Isserman S, Rogers WJ, Ruff CT, Contant C, Pencina MJ, Scirica BM, Longtine JA, Michelson AD, Sabatine MS. Dosing clopidogrel based on CYP2C19 genotype and the effect on platelet reactivity in patients with stable cardiovascular disease. JAMA. 2011;306:2221–2228. doi: 10.1001/jama.2011.1703. [DOI] [PubMed] [Google Scholar]
- 46.Rehmel JL, Eckstein JA, Farid NA, Heim JB, Kasper SC, Kurihara A, Wrighton SA, Ring BJ. Interactions of two major metabolites of prasugrel, a thienopyridine antiplatelet agent, with the cytochromes P450. Drug Metab Dispos. 2006;34:600–607. doi: 10.1124/dmd.105.007989. [DOI] [PubMed] [Google Scholar]
- 47.Wallentin L, James S, Storey RF, Armstrong M, Barratt BJ, Horrow J, Husted S, Katus H, Steg PG, Shah SH, Becker RC investigators P. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376:1320–1328. doi: 10.1016/S0140-6736(10)61274-3. [DOI] [PubMed] [Google Scholar]
- 48.Sorich MJ, Vitry A, Ward MB, Horowitz JD, McKinnon RA. Prasugrel vs. clopidogrel for cytochrome P450 2C19-genotyped subgroups: integration of the TRITON-TIMI 38 trial data. J Thromb Haemost. 2010;8:1678–1684. doi: 10.1111/j.1538-7836.2010.03923.x. [DOI] [PubMed] [Google Scholar]
- 49.Society for Cardiovascular A, Interventions, Society of Thoracic S, Writing Committee M. Holmes DR, Jr, Dehmer GJ, Kaul S, Leifer D, O'Gara PT, Stein CM. ACCF/AHA Clopidogrel clinical alert: approaches to the FDA “boxed warning”: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the American Heart Association. Circulation. 2010;122:537–557. doi: 10.1161/CIR.0b013e3181ee08ed. [DOI] [PubMed] [Google Scholar]
- 50.Peterson JF, Field JR, Unertl K, Schildcrout JS, Johnson DC, Shi Y, Danciu I, Cleator JH, Pulley JM, McPherson JA, Denny JC, Laposata M, Roden DM, Johnson KB. Physician response to implementation of genotype-tailored antiplatelet therapy. Clin Pharmacol Ther. 2015 doi: 10.1002/cpt.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shuldiner AR, Palmer K, Pakyz RE, Alestock TD, Maloney KA, O'Neill C, Bhatty S, Schub J, Overby CL, Horenstein RB, Pollin TI, Kelemen MD, Beitelshees AL, Robinson SW, Blitzer MG, McArdle PF, Brown L, Jeng LJ, Zhao RY, Ambulos N, Vesely MR. Implementation of pharmacogenetics: the University of Maryland Personalized Anti-platelet Pharmacogenetics Program. Am J Med Genet C Semin Med Genet. 2014;166C:76–84. doi: 10.1002/ajmg.c.31396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weitzel KW, Elsey AR, Langaee TY, Burkley B, Nessl DR, Obeng AO, Staley BJ, Dong HJ, Allan RW, Liu JF, Cooper-Dehoff RM, Anderson RD, Conlon M, Clare-Salzler MJ, Nelson DR, Johnson JA. Clinical pharmacogenetics implementation: approaches, successes, and challenges. Am J Med Genet C Semin Med Genet. 2014;166C:56–67. doi: 10.1002/ajmg.c.31390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee JA, Lee CR, Reed BN, Plitt DC, Polasek MJ, Howell LA, Cicci JD, Tasca KE, Weck KE, Rossi JS, Stouffer GA. Implementation and evaluation of a CYP2C19 genotype-guided antiplatelet therapy algorithm in high-risk coronary artery disease patients. Pharmacogenomics. 2015;16:303–313. doi: 10.2217/pgs.14.180. [DOI] [PubMed] [Google Scholar]
- 54.Cavallari LH, Magvanjav O, Anderson RD, Gong Y, Owusu-Obeng A, Kong B, Vo T, Ashton JN, Staley BJ, Elsey AR, Allan RW, Starostik P, Cooper-DeHoff RM, Weitzel KW, Clare-Salzler MJ, Nelson DR, Johnson JA. Clinical implementation of CYP2C19 genotype guided antiplatelet therapy reduces cardiovascular events after PCI. Circulation. 2015;132:A11802. [Google Scholar]
- 55.Franken CC, Kaiser AF, Kruger JC, Overbeck K, Mugge A, Neubauer H. Cytochrome P450 2B6 and 2C9 genotype polymorphism--a possible cause of prasugrel low responsiveness. Thromb Haemost. 2013;110:131–140. doi: 10.1160/TH13-01-0021. [DOI] [PubMed] [Google Scholar]
- 56.Varenhorst C, Eriksson N, Johansson A, Barratt BJ, Hagstrom E, Akerblom A, Syvanen AC, Becker RC, James SK, Katus HA, Husted S, Steg PG, Siegbahn A, Voora D, Teng R, Storey RF, Wallentin L Investigators P. Effect of genetic variations on ticagrelor plasma levels and clinical outcomes. Eur Heart J. 2015;36:1901–1912. doi: 10.1093/eurheartj/ehv116. [DOI] [PubMed] [Google Scholar]


