Abstract
Background
Azathioprine is a commonly prescribed therapy for connective tissue disease-associated interstitial lung disease (CTD-ILD). Combination therapy that included azathioprine was recently shown to increase the risk of death and hospitalization in patients with idiopathic pulmonary fibrosis. Whether azathioprine increases the risk of adverse outcomes in patients with fibrotic CTD-ILD, including those with CTD-associated usual interstitial pneumonia (UIP), remains unknown.
Methods
A retrospective cohort analysis was performed to determine the combined incidence rate of death, transplant and respiratory hospitalization associated with azathioprine exposure. A fibrotic CTD-ILD cohort treated with mycophenolate mofetil served as a comparator group. Incidence rates were compared with an incidence rate ratio (IRR) generated by negative binomial regression. Longitudinal pulmonary function response was then assessed using mixed effects linear regression models.
Results
Fifty-four patients were treated with azathioprine and forty-three with mycophenolate. Medication discontinuation due to non-respiratory side effects occurred in 27% and 5% of the azathioprine and mycophenolate cohorts, respectively. The combined incidence rate of adverse outcomes was 0.013 and 0.015 for azathioprine and mycophenolate, respectively (IRR 1.23; 95% CI 0.49-3.12; p=0.66). Similar incidence rates were observed among those with CTD-UIP (IRR 0.83; 95% CI 0.21-3.31; p=0.79). Both groups demonstrated pulmonary function stability over time, with the azathioprine group demonstrating a marginal improvement.
Conclusions
A significant minority of patients could not tolerate azathioprine due to non-respiratory side effects. Of those who did tolerate azathioprine, a similar incidence of adverse outcomes was observed as those treated with mycophenolate. Both therapies were associated with stability in pulmonary function.
Keywords: rheumatology, usual interstitial pneumonia, mycophenolate mofetil, idiopathic pulmonary fibrosis, interstitial lung disease, connective tissue disease
Introduction
Interstitial lung disease (ILD) is a common manifestation of connective tissue disease (CTD) and may lead to significant morbidity and mortality.(1, 2). The CTDs complicated by ILD include systemic sclerosis, rheumatoid arthritis, polymyositis and dermatomyositis, Sjogren's syndrome, mixed connective tissue disease and systemic lupus erythematosus.(3) CTD-associated ILD is most commonly associated with a pattern of non-specific interstitial pneumonia (NSIP) on high-resolution computed tomography (HRCT) and/or surgical lung biopsy (SLB), followed by usual interstitial pneumonia (UIP).(4, 5) While survival among patients with CTD-associated ILD is generally favorable when compared to patients with idiopathic pulmonary fibrosis (IPF), this survival benefit is less pronounced in the setting of UIP and is likely influenced by CTD etiology.(5-8)
Treatment of CTD-associated ILD generally targets the immune system, which is responsible for the production of autoantibodies that characterize specific CTDs. In addition to corticosteroids, common first-line therapies include azathioprine and mycophenolate mofetil, both of which act to inhibit B and T-lymphocyte proliferation.(9, 10) Data regarding the use of these therapies to treat CTD-associated ILD is sparse and largely confined to case series and a small uncontrolled clinical trial.(11-17) A recent randomized controlled trial conducted in patients with IPF showed that azathioprine, when used in combination with prednisone and N-acetylcysteine, significantly increased the risk of death, hospitalization and IPF exacerbation. It is unknown whether the use of azathioprine in patients with fibrotic CTD-associated ILD, including those with UIP, increases the risk of adverse outcomes in this patient population.
In this investigation we conducted a single-center retrospective longitudinal analysis of patients with fibrotic CTD-associated ILD to determine whether treatment with azathioprine was associated with an increased incidence of adverse outcomes, including death, lung transplantation and respiratory hospitalization. Patients receiving mycophenolate mofetil were used as a control group, as this therapy has been previously shown to be safe and well-tolerated in patients with CTD-associated ILD.(13, 14) We then performed a longitudinal analysis of pulmonary function to determine the change in percent predicted forced vital capacity (FVC) and diffusion capacity of the lung for carbon monoxide (DLCO) associated with azathioprine and mycophenolate mofetil therapy over time.
Methods
Study Design
This investigation was conducted at the University of Chicago and was approved by our Institutional Review Board (IRB protocol #14163-A). The University of Chicago ILD registry was used to identify patients followed from 2006-2015 with a diagnosis of CTD-associated ILD. HRCTs were reviewed by two chest radiologists (JC and SM) to identify patients with fibrotic ILD, defined as the presence of reticulation with traction bronchiectasis, traction bronchiolectasis, or subpleural honeycombing. The electronic medical record was reviewed to identify patients in this cohort treated with azathioprine or mycophenolate mofetil. Other pertinent data extracted from the electronic medical record included demographic information (age, race/ethnicity, gender), tobacco use, medications including systemic corticosteroids and other disease modifying anti-rheumatic drugs (DMARD), including tacrolimus, biologics/tumor necrosis factor-alpha inhibitors, IV immunoglobulin, rituximab, cyclophosphamide, methotrexate, penicillamine, hydroxychloroquine, physical examination findings including clubbing and crackles, laboratory studies including complete blood count and liver function testing (LFT), diagnostic studies (HRCT and SLB) and pulmonary function testing (PFT) including percent predicted FVC, and percent predicted DLCO.
The first period of treatment with either azathioprine or mycophenolate mofetil after establishing care at our institution was used to conduct this analysis. Crossing over from one therapy to another was allowed if it occurred within 4 weeks of therapy initiation and was due to a non-respiratory side effect. One patient was excluded due to receiving both therapies concurrently. Adverse events were defined as death, lung transplantation and respiratory hospitalization. The electronic medical record, social security death index and telephone communication with patients and family members were used to ascertain adverse events. Follow-up time was censored on Dec 1, 2015. Patients with at least 2 PFTs > 90 days apart were included in the longitudinal PFT analysis.
Statistical Analysis
Continuous variables were reported as means with standard deviation (SD) or medians with interquartile range and were compared using a two-tailed student's t-test or Wilcoxon rank sum test, as appropriate. Categorical variables were reported as counts and percentages and compared using the Chi-square test or Fisher's exact test, as appropriate. Adverse outcomes, including death, transplant and respiratory hospitalization were treated as count data with multiple events possible for a given patient. A combined endpoint incidence rate was determined for each treatment group and incidence rate ratio (IRR) determined using negative binomial regression.
Longitudinal analysis of pulmonary function change associated with azathioprine and mycophenolate mofetil therapy was conducted using mixed-effects regression models. Based on exploratory analysis with restricted maximum likelihood modeling, an exchangeable variance-covariance-correlation structure was chosen for FVC modeling while an autoregressive structure was chosen for DLCO modeling. PFTs were grouped into 1-year intervals to allow for time course alignment. Missing observations for DLCO were imputed to the lowest quartile mean of 25% to account for individuals unable to perform this procedure. Longitudinal data are presented graphically using locally weighted scatterplot smoothing. Summary statistics with p<0.05 were considered to be statistically significant. All statistical analyses were performed using Stata (StataCorp. 2013. Release 13. College Station, TX).
Results
Of 1205 patients screened, 209 carried a diagnosis of CTD-ILD, including 182 with evidence of pulmonary fibrosis on HRCT (Figure 1). Of those with fibrotic CTD-associated ILD, 64 were initially treated with azathioprine and 33 with mycophenolate mofetil. Ten patients (16%) initially treated with azathioprine experienced non-respiratory side effects and were subsequently transitioned to mycophenolate mofetil, leaving 54 treated with azathioprine and 43 treated with mycophenolate mofetil for the outcome analysis. Of those, 41 treated with azathioprine and 32 treated with mycophenolate mofetil had multiple PFTs and were included in the longitudinal analysis of pulmonary function.
Figure 1. Consort Diagram.
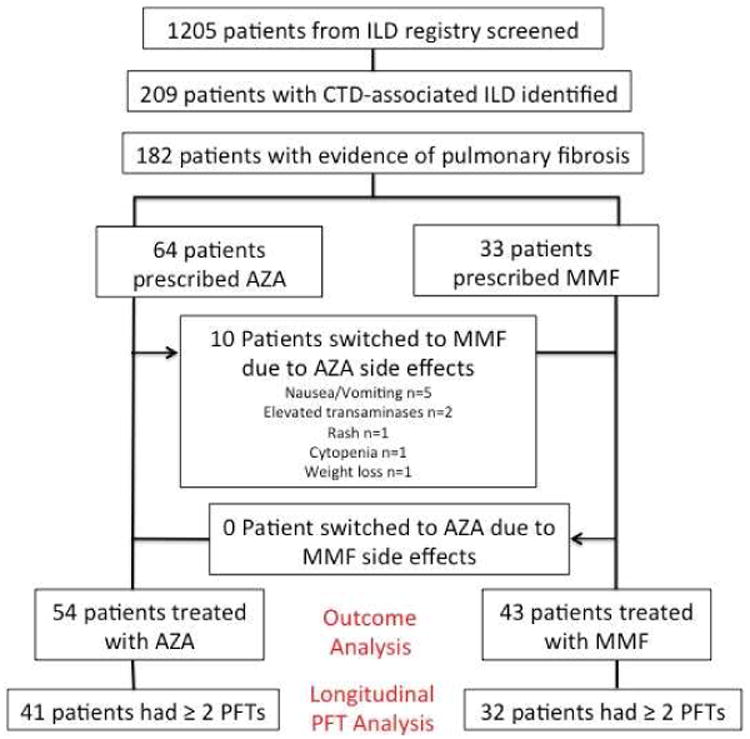
The median daily dosages of azathioprine and mycophenolate mofetil were 125mg and 2000mg, respectively. Among those in the azathioprine group, 33/54 underwent thiopurine methyltransferase (TPMT) enzyme activity testing. Five individuals in this group were found to have an intermediate enzyme activity level, including one who underwent lung transplantation within 6 months of starting therapy and one who discontinued therapy due to nausea. No cases of LFT abnormalities or bone marrow suppression were observed in those with intermediate TPMT activity.
There was no difference between azathioprine and mycophenolate mofetil cohorts (Table 1) with regard to age, gender, concurrent DMARD use, smoking history, crackles, clubbing, HRCT pattern, % fibrosis on HRCT, % ground glass opacity on HRCT or baseline FVC (% predicted). Compared to the myocphenolate mofetil group, the azathioprine group had a lower percentage of whites (33.3% vs. 55.8%), systemic sclerosis (7.4% vs. 27.9%) and UIP by SLB (44.4% vs. 100%). The azathioprine group also received more corticosteroid of ≥20mg (22.2% vs. 4.7%) than the mycophenolate mofetil group and had a lower baseline DLCO (% predicted (47.5% vs. 56.0%). With the exception of systemic sclerosis, the groups were otherwise balanced with regard to CTD etiology. Overall, the most commonly observed pattern by HRCT was NSIP, followed by UIP. The most commonly observed pattern by SLB was UIP, followed by NSIP, but relatively few patients underwent biopsy (n=27). The majority of SLBs (n=18) were performed prior to referral to our institution and the remainder were performed at our institution because ILD etiology remained unclear and CTD diagnosis had not yet been established.
Table 1. Demographic and Clinical Characteristics.
| Characteristic | Azathioprine (n=54)* | Mycophenolate mofetil (n=43)** | p-value |
|---|---|---|---|
| Age, mean (±SD) | 54.1 (12.8) | 52.7 (11.1) | 0.55 |
| Female gender, n (%) | 42 (77.8) | 29 (67.4) | 0.25 |
| Race, n (%) | |||
| White | 18 (33.3) | 24 (55.8) | 0.03 |
| African-American | 27 (50) | 17 (39.5) | 0.3 |
| Hispanic | 6 (11.1) | 2 (4.7) | 0.3 |
| Asian | 3 (5.6) | 0 (0) | 0.25 |
| Diagnosis | |||
| Polymyositis/dermatomyositis | 15 (27.8) | 7 (16.3) | 0.18 |
| Mixed connective tissue disease | 12 (22.2) | 7 (16.3) | 0.61 |
| Rheumatoid arthritis | 15 (27.8) | 8 (18.6) | 0.29 |
| Systemic sclerosis | 4 (7.4) | 12 (27.9) | 0.01 |
| Sjogren's syndrome | 5 (9.3) | 8 (18.6) | 0.18 |
| Systemic lupus erythematosus | 3 (5.6) | 1 (2.3) | 0.43 |
| Concurrent Prednisone Use | |||
| <20mg daily | 32 (59.3) | 28 (65.1) | 0.56 |
| ≥20mg daily | 12 (22.2) | 2 (4.7) | 0.02 |
| Concurrent DMARD Use | |||
| Tacrolimus | 8 (14.8) | 5 (11.6) | 0.78 |
| Biologic/TNF-alpha inhibitor | 4 (7.4) | 2 (4.6) | 0.69 |
| IVIG | 5 (9.3) | 2 (4.6) | 0.46 |
| Hydroxychloroquine | 18 (33.3) | 10 (23.2) | 0.28 |
| Other | 2 (3.7) | 2 (4.6) | 1 |
| Ever smoker, n (%) | 26 (48.2) | 19 (44.2) | 0.7 |
| Crackles | 44 (84.6) | 33 (78.6) | 0.45 |
| Clubbing | 6 (20.7) | 7 (26.9) | 0.75 |
| HRCT pattern, n (%) | |||
| Usual interstitial pneumonia | 16 (29.6) | 14 (32.6) | 0.76 |
| Non-specific interstitial pneumonia | 32 (59.3) | 27 (62.8) | 0.72 |
| Atypical/unclassifiable | 5 (9.3) | 2 (4.7) | 0.46 |
| HRCT % fibrosis, median [IQR] | 15 [10-25] | 20 [10-25] | 0.39 |
| HRCT % ground glass opacity, median [IQR] | 0 [0-10] | 0 [0-5] | 0.19 |
| SLB pattern, n (%) | |||
| Usual interstitial pneumonia | 8 (44.4) | 8 (100) | 0.01 |
| Non-specific interstitial pneumonia | 7 (38.9) | 0 | -- |
| Organizing pneumonia | 3 (16.7) | 0 | -- |
| FVC (% predicted) | 60.9 (18.2) | 60.6 (19.4) | 0.93 |
| DLCO (% predicted) | 47.5 (15.0) | 56.0 (23.9) | 0.05 |
Abbreviations: DMARD=disease modifying anti-rheumatic drug; HRCT = high-resolution computed tomography; SLB = surgical lung biopsy; FVC=forced vital capacity; DLCO=diffusion capacity of the lung for carbon monoxide
Exception for n; crackles n=52; clubbing n=29; SLB pattern n=18; DLCO n=48
Exception for n: crackles n=42; clubbing n=26; SLB pattern n=9; FVC n=42; DLCO n=35
During the follow-up period, 15/54 (28%) patients discontinued azathioprine therapy while 12/43 (28%) patients discontinued mycophenolate therapy (Table 2). Medication discontinuation due to side effects during the follow-up period occurred in 7 (13%) patients treated with azathioprine and 2 (5%) of patients treated with mycophenolate mofetil. Significant side effects observed in the azathioprine group included four cases of elevated transaminases, one case of pancreatitis and one case of recurrent infection. One individual treated with mycophenolate mofetil developed a cytopenia requiring medication discontinuation. Side effects were not observed to occur in any one predominant CTD. Therapy discontinuation due to patient preference occurred in 1 (2%) patient treated with azathioprine and 5 (12%) patients treated with mycophenolate mofefil. One patient in the mycophenolate mofetil group discontinued therapy to attempt pregnancy, but reasons underpinning others decision to stop therapy were not possible to ascertain due to the retrospective nature of this investigation. Therapy discontinuation due to clinical failure, as determined by an attending physician, occurred in approximately 10% of both cohorts.
Table 2. Reasons for azathioprine and mycophenolate mofetil discontinuation during follow-up period.
| Reason | Azathioprine (n=54) | Mycophenolate mofetil (n=43) |
|---|---|---|
| Elevated transaminase | 4 | 0 |
| Pancreatitis | 1 | 0 |
| Nausea/vomiting | 1 | 1 |
| Recurrent infection | 1 | 0 |
| Cytopenia | 0 | 1 |
| Patient Preference | 1 | 5 |
| Disease progression/treatment failure | 6 | 4 |
| Unknown | 1 | 1 |
When comparing adverse outcomes between groups (Table 3), there were 3 deaths, 2 transplants and 17 respiratory hospitalizations over 1445.3 exposure months in the azathioprine group, producing an adverse outcome incidence rate of 0.015. There were 4 deaths, 1 transplant and 11 respiratory hospitalizations over 1236.6 exposure months in the mycophenolate mofetil group, producing an adverse event incidence rate of 0.013. Relative to the mycophenolate mofetil treated group, treatment with azathioprine was associated with a non-significant increase in adverse outcome risk (IRR 1.23 (95% CI 0.49-3.12; p=0.66)). When considering only those with a UIP pattern by HRCT and/or SLB (n=41) (Table 4), azathioprine therapy was associated with a non-significant decrease in adverse outcome risk (IRR 0.83 (95% CI 0.21-3.31; p=0.79).
Table 3. Treatment-associated adverse outcome risk in fibrotic CTD-associated ILD.
| Event | Azathioprine (n=54) | Mycophenolate mofetil (n=43) |
|---|---|---|
|
| ||
| Death, n | 3 | 4 |
| Transplant, n | 2 | 1 |
| Respiratory hospitalization, n | 17 | 11 |
| Total adverse ouctomes, n | 22 | 16 |
|
| ||
| Exposure months | 1445.3 | 1236.6 |
| Adverse outcome incidence rate | 0.015 | 0.013 |
| IRR (95% CI; p-value) | 1.23 (0.49-3.12; p=0.66) | Reference |
Abbreviations: IRR = incidence rate ratio
Table 4. Treatment-associated adverse outcome risk in CTD-associated UIP.
| Event | Azathioprine (n=22) | Mycophenolate (n=19) |
|---|---|---|
|
| ||
| Death, n | 2 | 3 |
| Transplant, n | 0 | 1 |
| Respiratory hospitalization, n | 6 | 3 |
| Total adverse events, n | 8 | 7 |
|
| ||
| Exposure months | 714.5 | 545.3 |
| Adverse Event Incidence Rate | 0.011 | 0.012 |
| IRR (95% CI; p-value) | 0.83 (0.21-3.31;p=0.79) | Reference |
Abbreviations: IRR = incidence rate ratio
Treatment-associated longitudinal change in percent predicted FVC and DLCO is shown in Figure 2. Azathioprine therapy was associated with a significant yearly increase in FVC of 1.53% (95% CI 0.19%-2.87%; p=0.025), while mycophenolate mofetil was associated with a non-significant yearly decline in FVC of 0.56% (95% CI-1.55%-0.43%; p=0.27) (Table 5). Azathioprine was also associated with a significant yearly increase in DLCO of 4.91% (95% CI 1.53%-8.3%; p=0.004), while mycophenolate mofetil was associated with a non-significant yearly decline in DLCO of 2.1% (95% CI -4.62%-0.42%; p=0.1). These findings remained consistent after adjustment for age, gender, race, CTD diagnosis, UIP pattern by HRCT and/or SLB, concurrent DMARD use and concurrent prednisone dose.
Figure 2.
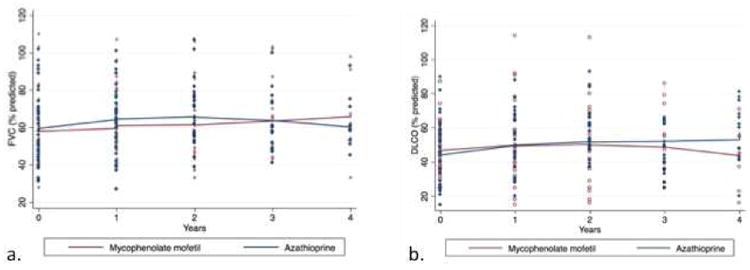
Longitudinal change in percent predicted FVC (a) and DLCO (b) in a cohort of patients with fibrotic CTD- associated ILD treated with azathioprine and mycophenolate mofetil.
Table 5. Yearly change in pulmonary function associated with azathioprine and mycophenolate mofetil therapy.
| Therapy | FVC (% predicted) | DLCO (% predicted) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Unadjusted | Adjusted* | Unadjusted | Adjusted* | |||||||||
| Change | 95% CI | p-value | Change | 95% CI | p-value | Change | 95% CI | p-value | Change | 95% CI | p-value | |
| Azathioprine (n=41) | 1.53% | 0.19% -2.87% | 0.025 | 1.46% | 0.12% -2.79% | 0.033 | 4.91% | 1.53% -8.3% | 0.004 | 4.50% | 1.25% -7.75% | 0.007 |
|
| ||||||||||||
| Mycophenolate mofetil (n=32) | -0.56% | -1.55% -0.43% | 0.27 | -0.52% | -1.51% - 0.47% | 0.3 | -2.10% | -4.62% - 0.42% | 0.1 | -2.00% | -4.41% - 0.41% | 0.1 |
Adjusted for age, gender, race, baseline DLCO (% predicted), CTD diagnosis, UIP pattern by HRCT and/or SLB, concurrent immunosuppressive use and concurrent prednisone dose
Discussion
In this investigation, we demonstrated that treatment with azathioprine was associated with a similar combined incidence of death, transplant and respiratory hospitalization when compared to mycophenolate mofetil in patients with fibrotic CTD-associated ILD, including those with UIP. We also showed that azathioprine was associated with a significant improvement in both FVC and DLCO over four years of follow-up, while mycophenolate mofetil was associated with stability in these metrics. These findings were limited to patients able to tolerate azathioprine, as a large minority discontinued this therapy due to significant side effects.
This study is, to our knowledge, the first to specifically explore the safety of azathioprine in individuals with fibrotic CTD-associated ILD, including CTD-UIP, and suggest that azathioprine may be a reasonable first line agent in patients able to tolerate this therapy. Our findings also provide some degree of reassurance that the treatment of patients with CTD-associated UIP with azathioprine does not increase the risk of death and hospitalization, as was observed in IPF when taken in combination with prednisone and N-acetylcysteine.(18, 19) This discordance supports the paradigm of CTD-associated ILD being driven predominantly by inflammation and IPF from recurrent alveolar injury and aberrant wound healing, despite shared radiographic and histologic features.(20, 21)
Our findings that treatment with azathioprine may improve lung function in patients with CTD-associated ILD are consistent with those of Paone and colleagues,(11) who showed that maintenance therapy with azathioprine after 1 year of cyclophosphamide therapy in 13 patients with systemic sclerosis was associated with a significant improvement in FVC and DLCO. The improvement in FVC associated with azathioprine observed in our cohort was most apparent in the first two years of therapy while the improvement in DLCO persisted throughout the study period. Our findings that mycophenolate mofetil was associated with FVC and DLCO stability, but not improvement, differ from those of Fischer and colleagues,(13) who showed a significant improvement in both FVC and DLCO associated with mycophenolate mofetil in a large CTD cohort. This discordance may be related to the high percentage of patients with UIP, as well as the higher percentage of patients with systemic sclerosis, in our mycophenolate cohort. Concurrent pulmonary hypertension may have also influenced our results with regard to longitudinal change in DLCO, but incomplete data precluded robust analysis of this potential co-morbid condition.
There were several limitations to this investigation. First, because this was a retrospective study, causation could not be assessed, and our findings represent only an association between outcome and immunosuppressant therapy. Next, because the CTDs that comprise CTD-associated ILD are a highly heterogeneous group of disease processes with variable natural histories and response to therapy, our findings may have been biased by the therapeutic responsiveness of individual CTDs. Formal interaction testing did not identify adverse outcomes to be overrepresented in any one CTD etiology (data not shown), but this analysis was limited by the small sample sizes of these subgroups. Another limitation was the concurrent immunosuppressive and systemic corticosteroid use in both groups, which left us unable to test azathioprine and mycophenolate mofetil as monotherapies. Finally, some patients were treated with immunosuppressive therapy prior to referral to our institution. This was most often in the form of corticosteroids, but prior exposure to a non-reported immunosuppressive was possible. This may have biased our results, especially in patients who had already failed one or more therapies prior to referral.
Conclusion
The use of azathioprine to treat patients with fibrotic CTD-associated ILD, including those with UIP, appears to be as safe as treatment with mycophenolate mofetil. However, a significant minority of individuals developed side effects while on azathioprine, leading to discontinuation of this therapy and should be taken into account when developing a treatment plan. While retrospective analyses such as this can provide valuable insight into the response to specific therapies in those with CTD-associated ILD, formal testing of these therapies in a blinded, controlled fashion among well-defined CTD-associated ILD cohorts is desperately needed to establish optimal treatment strategies.
Highlights.
A large minority of patient did not tolerate azathioprine due to side effects
Adverse outcomes were similar between azathioprine and mycophenolate cohorts
Azathioprine was not associated with worse outcomes in those with CTD-UIP
Pulmonary function stability was observed in both treatment groups
Acknowledgments
Funding: This investigation was supported by an NIH T32 training grant (T32-HL007605)
Conflict of Interest Statement: Dr. Oldham has received grants from the American Thoracic Society, Boehringer Ingelheim and the American Lung Association outside the submitted work. He has received speaking honoria from Genentech outside the submitted work.
Drs. Lee, Valenzi, Witt, Adegunsoye, Chung, and Montner, along with Ms. Hsu and Chen, have nothing to disclose. Dr. Vij currently receives a grant from Genentech outside the submitted work. Dr. Noth has received honoraria for advisory boards with Boehringer Ingelheim, InterMune, Anthera within the last 12 months related to IPF. He has also received speaking honoraria from GSK and receives consulting fees for Immuneworks outside the submitted work. He also has study contracts with the NIH, Stromedix, Sanofi, and BI for the conduct of clinical trials in IPF. Dr. Strek has institutional grants from the NIH, Bristol-Myers Sqibb ,Gilead, InterMune, and MedI- mmune for the conduct of clinical trials in IPF. She has received honoraria for serving on a Data Monitoring Committee for Boeh- ringer Ingelheim and an advisory board for InterMune outside the submitted work.
Footnotes
Author contribution: Conception and design: JO, IN, RV, MS. Data collection: JO, CL, EV, LW, SH, LC, AA. Data analysis and interpretation: JO, IN, RV, MS. HRCT interpretation: JC, SM. Drafting the manuscript for important intellectual content: JO, RV, MS. Dr. Oldham is the guarantor of this paper and takes responsibility for the integrity of the work as a whole, from inception to published article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kocheril SV, Appleton BE, Somers EC, Kazerooni EA, Flaherty KR, Martinez FJ, Gross BH, Crofford LJ. Comparison of disease progression and mortality of connective tissue disease-related interstitial lung disease and idiopathic interstitial pneumonia. Arthritis Rheum. 2005;53:549–557. doi: 10.1002/art.21322. [DOI] [PubMed] [Google Scholar]
- 2.Migita K, Arai T, Jiuchi Y, Izumi Y, Iwanaga N, Kawahara C, Suematsu E, Miyamura T, Tsutani H, Kawabe Y, Matsumura R, Mori S, Ohshima S, Yoshizawa S, Suenaga Y, Ogushi F, Kawabata M, Furukawa H, Matsui T, Bito S, Tohma S. Predictors of mortality in patients with interstitial lung disease treated with corticosteroids: Results from a cohort study. Medicine. 2014;93:e175. doi: 10.1097/MD.0000000000000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer A, du Bois R. Interstitial lung disease in connective tissue disorders. Lancet. 2012;380:689–698. doi: 10.1016/S0140-6736(12)61079-4. [DOI] [PubMed] [Google Scholar]
- 4.Tansey D, Wells AU, Colby TV, Ip S, Nikolakoupolou A, du Bois RM, Hansell DM, Nicholson AG. Variations in histological patterns of interstitial pneumonia between connective tissue disorders and their relationship to prognosis. Histopathology. 2004;44:585–596. doi: 10.1111/j.1365-2559.2004.01896.x. [DOI] [PubMed] [Google Scholar]
- 5.Park JH, Kim DS, Park IN, Jang SJ, Kitaichi M, Nicholson AG, Colby TV. Prognosis of fibrotic interstitial pneumonia: Idiopathic versus collagen vascular disease-related subtypes. American journal of respiratory and critical care medicine. 2007;175:705–711. doi: 10.1164/rccm.200607-912OC. [DOI] [PubMed] [Google Scholar]
- 6.Strand MJ, Sprunger D, Cosgrove GP, Fernandez-Perez ER, Frankel SK, Huie TJ, Olson AL, Solomon J, Brown KK, Swigris JJ. Pulmonary function and survival in idiopathic vs secondary usual interstitial pneumonia. Chest. 2014;146:775–785. doi: 10.1378/chest.13-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim EJ, Elicker BM, Maldonado F, Webb WR, Ryu JH, Van Uden JH, Lee JS, King TE, Jr, Collard HR. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. The European respiratory journal. 2010;35:1322–1328. doi: 10.1183/09031936.00092309. [DOI] [PubMed] [Google Scholar]
- 8.Navaratnam V, Ali N, Smith CJ, McKeever T, Fogarty A, Hubbard RB. Does the presence of connective tissue disease modify survival in patients with pulmonary fibrosis? Respiratory medicine. 2011;105:1925–1930. doi: 10.1016/j.rmed.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Maltzman JS, Koretzky GA. Azathioprine: Old drug, new actions. The Journal of clinical investigation. 2003;111:1122–1124. doi: 10.1172/JCI18384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allison AC. Mechanisms of action of mycophenolate mofetil. Lupus. 2005;14(1):s2–8. doi: 10.1191/0961203305lu2109oa. [DOI] [PubMed] [Google Scholar]
- 11.Paone C, Chiarolanza I, Cuomo G, Ruocco L, Vettori S, Menegozzo M, La Montagna G, Valentini G. Twelve-month azathioprine as maintenance therapy in early diffuse systemic sclerosis patients treated for 1-year with low dose cyclophosphamide pulse therapy. Clin Exp Rheumatol. 2007;25:613–616. [PubMed] [Google Scholar]
- 12.Berezne A, Ranque B, Valeyre D, Brauner M, Allanore Y, Launay D, Le Guern V, Kahn JE, Couderc LJ, Constans J, Cohen P, Mahr A, Pagnoux C, Hachulla E, Kahan A, Cabane J, Guillevin L, Mouthon L. Therapeutic strategy combining intravenous cyclophosphamide followed by oral azathioprine to treat worsening interstitial lung disease associated with systemic sclerosis: A retrospective multicenter open-label study. J Rheumatol. 2008;35:1064–1072. [PubMed] [Google Scholar]
- 13.Fischer A, Brown KK, Du Bois RM, Frankel SK, Cosgrove GP, Fernandez-Perez ER, Huie TJ, Krishnamoorthy M, Meehan RT, Olson AL, Solomon JJ, Swigris JJ. Mycophenolate mofetil improves lung function in connective tissue disease-associated interstitial lung disease. J Rheumatol. 2013;40:640–646. doi: 10.3899/jrheum.121043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swigris JJ, Olson AL, Fischer A, Lynch DA, Cosgrove GP, Frankel SK, Meehan RT, Brown KK. Mycophenolate mofetil is safe, well tolerated, and preserves lung function in patients with connective tissue disease-related interstitial lung disease. Chest. 2006;130:30–36. doi: 10.1378/chest.130.1.30. [DOI] [PubMed] [Google Scholar]
- 15.Yu KH, Wu YJ, Kuo CF, See LC, Shen YM, Chang HC, Luo SF, Ho HH, Chen IJ. Survival analysis of patients with dermatomyositis and polymyositis: Analysis of 192 chinese cases. Clin Rheumatol. 2011;30:1595–1601. doi: 10.1007/s10067-011-1840-0. [DOI] [PubMed] [Google Scholar]
- 16.Tzouvelekis A, Galanopoulos N, Bouros E, Kolios G, Zacharis G, Ntolios P, Koulelidis A, Oikonomou A, Bouros D. Effect and safety of mycophenolate mofetil or sodium in systemic sclerosis-associated interstitial lung disease: A meta-analysis. Pulmonary medicine. 2012;2012:143637. doi: 10.1155/2012/143637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deheinzelin D, Capelozzi VL, Kairalla RA, Barbas Filho JV, Saldiva PH, de Carvalho CR. Interstitial lung disease in primary sjogren's syndrome. Clinical-pathological evaluation and response to treatment. American journal of respiratory and critical care medicine. 1996;154:794–799. doi: 10.1164/ajrccm.154.3.8810621. [DOI] [PubMed] [Google Scholar]
- 18.Idiopathic Pulmonary Fibrosis Clinical Research N. Martinez FJ, de Andrade JA, Anstrom KJ, King TE, Jr, Raghu G. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2093–2101. doi: 10.1056/NEJMoa1401739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raghu G, Anstrom KJ, King TE, Jr, Lasky JA, Martinez FJ. Prednisone, azathioprine, and n-acetylcysteine for pulmonary fibrosis. The New England journal of medicine. 2012;366:1968–1977. doi: 10.1056/NEJMoa1113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selman M, King TE, Pardo A American Thoracic S, European Respiratory S, American College of Chest P. Idiopathic pulmonary fibrosis: Prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Annals of internal medicine. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 21.Castelino FV, Varga J. Interstitial lung disease in connective tissue diseases: Evolving concepts of pathogenesis and management. Arthritis research & therapy. 2010;12:213. doi: 10.1186/ar3097. [DOI] [PMC free article] [PubMed] [Google Scholar]


