Abstract
Bone morphogenetic proteins (BMPs) are secreted signals that regulate apical ectodermal ridge (AER) functions and interdigital programmed cell death (PCD) of developing limb. However the identities of the intracellular mediators of these signals are unknown. To investigate the role of Smad proteins in BMP-regulated AER functions in limb development, we inactivated Smad1 and Smad5 selectively in AER and ventral ectoderm of developing limb, using Smad1 or/and Smad5 floxed alleles and an En1Cre/+ knock-in allele. Single inactivation of either Smad1 or Smad5 did not result in limb abnormalities. However, the Smad1/Smad5 double mutants exhibited syndactyly due to a reduction in interdigital PCD and an increase in interdigital cell proliferation. Cell tracing experiments in the Smad1/Smad5 double mutants showed that ventral ectoderm became thicker and the descendents of ventral En1Cre/+ expressing ectodermal cells were located at dorsal interdigital regions. At the molecular level, Fgf8 expression was prolonged in the interdigital ectoderm of embryonic day (E) 13 Smad1/Smad5 double mutants, suggesting that the ectopic Fgf8 expression may serve as a survival signal for interdigital epithelial and mesenchymal cells. Our result suggests that Smad1 and Smad5 are required and function redundantly as intracellular mediators for BMP signaling in the AER and ventral ectoderm. Smad1/Smad5 signaling in the AER and ventral ectoderm regulates interdigital tissue regression of developing limb. Our mutants with defects in interdigital PCD could also serve as a valuable model for investigation of PCD regulation machinery.
Keywords: Receptor Smads, Programmed cell death, Limb development, AER, Conditional inactivation
Introduction
The developing limb has easily identified discrete pattern and structure formation and is conveniently accessible for experimental manipulation. It has therefore long been an excellent model for investigating how different signals interact to control different cellular activities during pattern formation (Towers and Tickle, 2009). The mouse limbs develop from the embryonic limb buds, coming out from either side of body wall at the appropriate positions along the body axis. Early limb bud is made up of a mass of undifferentiated mesenchymal cells covered with surface ectoderm. The growth and patterning of the limb bud along the proximal–distal, anterior–posterior and dorsal–ventral axes are coordinated by reciprocal interactions between specialized regions known as apical ectodermal ridge (AER), zone of polarizing activity and the non-ridge ectoderm of the limb bud respectively (Capdevila and Izpisua Belmonte, 2001; Martin, 1990). AER is a stratified columnar epithelial structure located at the apex of the forelimb and hindlimb bud starting from embryonic day (E) 10.5 and 11.5 respectively. It is a source of secreted factors required for limb outgrowth (Dudley et al., 2002). Alternatively, this structure is also involved in regulating programmed cell death (PCD) in the developing limb (Maatouk et al., 2009; Pajni-Underwood et al., 2007). PCD is an important genetically controlled process that regulates development and homeostasis inmulticellular organisms (Fadeel and Orrenius, 2005; Jacobson et al., 1997). PCD is mainly observed in the interdigital undifferentiated mesenchymal cells and the AER (Fernandez-Teran et al., 2006; Zuzarte-Luis and Hurle, 2005). This process has to be controlled precisely so that the developing limb is sculpted into a particular shape and structure that is particular to an organism (Zuzarte-Luis and Hurle, 2005). Interdigital region of developing limb is of particular interest because the fate of interdigital mesenchymal cells to undergo cell death or not or chondrogenesis is subjected to precise genetic control and appropriate signaling between the ectoderm and underlying mesenchyme (Bandyopadhyay et al., 2006; Hernández-Martínez and Covarrubias, 2011; Hurle and Ganan, 1986). Dying cells located in the mesenchyme between the forming digits of the developing limbs are referred as interdigital PCD that is responsible in restricting interdigital tissue growth and promoting tissue regression in order to separate digits (Hernández-Martínez and Covarrubias, 2011; Macias et al., 1997; Montero et al., 2001). Defects in patterning and interdigital PCD in the developing limb are correlated with various types of congenital limb malformations (Al-Qattan et al., 2009; Goodman, 2002).
Interdigital PCD in developing limb is tightly controlled under the effects of different signaling factors like fibroblast growth factors (FGFs) (Delgado et al., 2008; Montero et al., 2001; Pajni-Underwood et al., 2007; Salas-Vidal et al., 2001), BMPs (Ganan et al., 1996; Guha et al., 2002; Macias et al., 1997; Pajni-Underwood et al., 2007; Salas-Vidal et al., 2001; Zou and Niswander, 1996), transforming growth factor-beta (TGF-β) (Ganan et al., 1996), Msx-2 (Marazzi et al., 1997; Salas-Vidal et al., 2001), Wnts (Grotewold and Ruther, 2002), Shh (Buscher et al., 1997) and retinoic acid (Ali-Khan and Hales, 2006; Galdones et al., 2006). BMP signaling in the AER has been shown to regulate interdigital PCD but the identity of the intracellular mediator remains unclear (Maatouk et al., 2009; Pajni-Underwood et al., 2007; Zuzarte-Luis and Hurle, 2005). One of the pathways involves receptor-regulated Smad proteins (Zuzarte-Luis and Hurle, 2005; Zuzarte-Luis et al., 2004). Binding of BMP ligand to membrane-bound serine/threonine kinase receptors results in receptor phosphorylation and subsequent activation of receptor-regulated Smad proteins (R-Smads) Smads1/5/8 by phosphorylation. Activated Smad1, 5 or 8 form complexes with common partner Smad (CoSmad), Smad4 and translocate into the nucleus where they regulate the transcription of target genes (Itoh et al., 2000).
In this study, we investigated the identity of the Smad proteins involved in BMP-regulated AER functions during limb development. Knockout of either Smad1 or Smad5 results in early embryonic lethality, hindering the studies of Smad function in limb formation (Chang et al., 1999; Lechleider et al., 2001). Therefore, Cre/loxP approach was used to inactivate Smad1 and/or Smad5 in the developing limb ectoderm in a tissue-specific manner by the use of Smad1 and/or Smad5 conditional null (floxed) alleles (Huang et al., 2002; Umans et al., 2003) and an Engrailed1-Cre-recombinase knock-in allele (En1Cre/+) (Kimmel et al., 2000). En1Cre/+ first expresses in the ventral ectoderm and the ventral part of the developing AER of forelimb bud at E9.5 (Kwan et al., 2004). After E11.5, En1Cre/+ expressing lineage is present throughout the dorsal–ventral extent of the AER (Kimmel et al., 2000). Our data showed that conditional inactivation of either Smad1 or Smad5 in the limb AER and ventral ectoderm does not result in limb abnormalities. However, double inactivation of both Smad1/Smad5 resulted in syndactyly due to a reduction in interdigital PCD and an increase in interdigital cell proliferation. Our data suggest that Smad1 and Smad5 act as the intracellular mediator downstream of the BMP receptor Ia (BmprIa) to transduce BMPs signal in the AER. The two proteins are required and function redundantly to regulate AER functions. The Smad1/Smad5 signaling in AER indirectly regulates interdigital PCD and cell proliferation in developing limb. Furthermore, we have investigated on the possible molecular mechanism of the Smad1/Smad5 signaling in regulating the interdigital tissue regression.
Materials and methods
Mouse strain generation and genotyping
The generation and genotyping of conditional (floxed) alleles of Smad1 (Smad1f) and Smad5 (Smad5f) have been described previously (Huang et al., 2002; Pangas et al., 2008; Umans et al., 2003). The En1Cre knock-in allele has been described previously (Kimmel et al., 2000; Kwan et al., 2004). To generate the AER and ventral ectoderm conditional Smad1/Smad5 knockout mutant, we crossed En1Cre/+ mice with Smad1f/f; Smad5f/f mice. Their En1Cre/+; Smad1f/+; Smad5f/+ female offspring were then crossed with Smad1f/f; Smad5f/f male to produce En1Cre/+; Smad1f/+; Smad5f/f males. To generate the En1Cre/+; Smad1f/f; Smad5f/f mutant embryos for analysis, Smad1f/f; Smad5f/f females were then crossed with En1Cre/+; Smad1f/+; Smad5f/f males. Their littermates, En1Cre/+; Smad1f/+; Smad5f/f, which developed normally, were used as controls. Since the En1Cre is a knock-in allele, we used En1Cre/+; Smad1f/+; Smad5f/f mice as the controls to eliminate the possible effect of the En1 heterozygous null genotype on the phenotypic analysis of the double Smad1/Smad5 conditional mutants. All mouse strains were on a mixed genetic background. All animal procedures were conducted with the approval of the Animal Experimentation Ethics Committee of The Chinese University of Hong Kong.
Immunofluorescence, cell death and cell proliferation analysis, section and whole mount RNA in situ hybridization
Embryos or embryonic limbs were isolated, fixed in 4% paraformaldehyde (PFA) at 4 °C overnight, paraffin-embedded and sectioned using standard techniques. Antibodies against Smad1 (Invitrogen) at a dilution of 4 µg/ml, Smad5 at a dilution of 1:50 (Santa Cruz), phospho-Smad1/5/8 at a dilution of 1:100 (Cell Signaling Technology) and CD44 at a dilution of 1:50 (Cell Signaling Technology) were used for immunofluorescence as described previously (Andl et al., 2004; Flanders et al., 2001; Maatouk et al., 2009). To detect cell death, the TUNEL assay (Roche) and anti-cleaved caspase3 antibody at a 1:150 dilution (Cell Signaling Technology) were performed according to the manufacturers' protocol. BrdU labeling (Roche) was used to detect cell proliferation level. For BrdU labeling, pregnant females at 13.5 dpc were injected with BrdU (1 mg per 10 g body weight) and sacrificed after 2 h. Embryonic limbs were isolated, fixed in 4% PFA at 4 °C overnight, paraffin-embedded and sectioned. BrdU incorporation was detected by antibody against BrdU (Chemicon) at dilution of 1:500. The number of cleaved caspase3 positive and BrdU positive cells were counted from a fixed area circle within the interdigital regions between digit2/digit3, digit3/digit4 and digit4/digit5 under the ectoderm from 7 µm sections from each embryo. Percentage of BrdU positive cells were calculated by dividing number of BrdU positive cells by the number of Hoechst-stained nuclei in fixed area circle within the interdigital area. Section in situ hybridization was performed according to the protocol previously described (He et al., 2001; Kwan et al., 2004). Whole mount RNA in situ hybridization was performed according to protocol previously described (Wilkinson, 1992). Probes used to detect Bmp2, Bmp4, Bmp7, En1, Fgf8, Gremlin, Lmx1b, Msx1, Msx2 and Shh have been described previously (Maatouk et al., 2009; Pajni-Underwood et al., 2007). At least three embryos for each genotype were examined in the aforementioned experiments. Embryos from the same litter were used for comparisons between the mutant and control embryos.
Cell tracing and skeletal preparations
To trace the limb ectodermal cells and their descendents whose Smad1/Smad5 were inactivated by En1Cre/+, we stained for the Cre dependent β-gal activity of the R26R reporter allele in En1Cre/+; Smad1f/f; Smad5f/f; Rosa26R26R/+ embryos as previously described (Soriano, 1999). Stained embryos were postfixed in 4% PFA at 4 °C overnight, paraffin-embedded and stained with eosin for histological analysis of the limbs. Skeletal staining using Alcian blue and Alizarin red was performed as previously described (Ovchinnikov, 2009).
Results
En1Cre/+ inactivation of Smad1 and Smad5 conditional alleles in the AER and limb ventral ectoderm
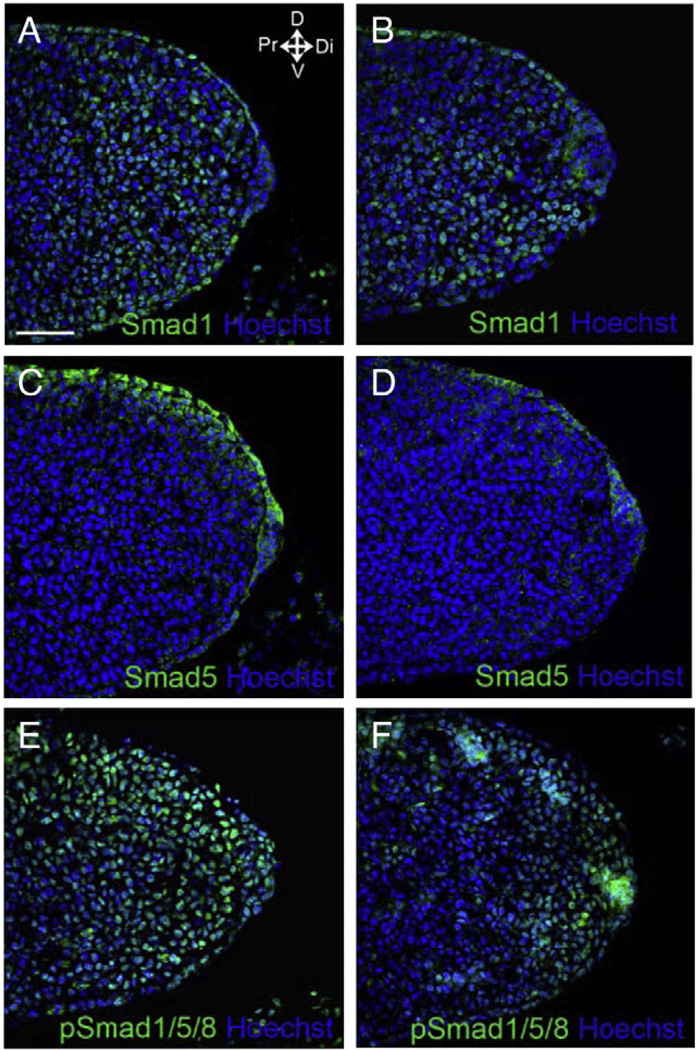
To investigate the functions of Smad1 and/or Smad5 signaling in limb ectoderm and AER, Smad1 and/or Smad5 were inactivated in the AER and ventral ectoderm, using Smad1 and/or Smad5 conditional null alleles and an En1Cre/+ knock-in allele. Previous reports have shown that En1Cre/+ knock-in allele is active in the ventral ectoderm and the ventral part of the developing AER in the forelimb buds as early as E9.5 (Kimmel et al., 2000; Kwan et al., 2004). At later stages, from E11.5, En1Cre/+ expressing cells are also found throughout the dorsal–ventral extent of the AER (Kimmel et al., 2000). To determine whether the inactivation of Smad genes by En1Cre/+ knock-in allele followed this pattern; we performed immunofluorescence on the forelimb buds of E10.5 Smad1/Smad5 double conditional knockout mutant (hereafter referred as Smad1/5 mutant) embryo using anti-Smad1 and anti-Smad5 antibodies. Smad1 and Smad5 were absent in the ventral ectoderm and ventral AER of the Smad1/5 mutants (Fig. 1A–D), suggesting En1Cre/+ knock-in allele successfully recombined Smad1 and Smad5 conditional alleles in the limb ventral ectoderm of the mutant. Smad1 and Smad5 were present in the dorsal ectoderm. When using antibody against pSmad1/5/8 for performing immunofluorescence, the signal was detected in the AER and throughout ectoderm of both the control and Smad1/5 mutant suggesting that the expression of another BMPR-Smad, Smad8, was still present in the limb AER and ectoderm of the Smad1/5 mutants (Fig. 1E, F).
Fig. 1.
En1-Cre inactivated Smad1 and Smad5 conditional null alleles in ventral ectoderm and ventral AER of forelimb buds. (A–D) Immunofluorescence detecting Smad1 (A, B) and Smad5 (C, D) in E10.5 control (A, C) (Smad1f/+; Smad5f/f) and Smad1/5 mutant (B, D) (En1Cre/+; Smad1f/f; Smad5f/f) forelimb buds respectively. Smad1 and Smad5 were detected in the dorsal ectoderm but not in the ventral ectoderm and ventral half of AER of Smad1/5 mutant. (E, F) Immunofluorescence detecting pSmad1/5/8 in the forelimb buds of E10.5 control (E) (En1Cre/+; Smad1f/+; Smad5f/f) and Smad1/5 mutant (F) (En1Cre/+; Smad1f/f; Smad5f/f). pSmad1/5/8 signal was detected throughout AER and ectoderm in both control and Smad1/5 mutant. The histological samples are sectioned saggitally along the dorsal–ventral axis of the limb buds. Arrows indicate the major axes: D, dorsal; V, ventral; Di, distal; Pr, proximal. Scale bar: 50 µm.
Inactivation of both Smad1/Smad5 signaling in the limb AER and ventral ectoderm results in interdigital tissue regression defects
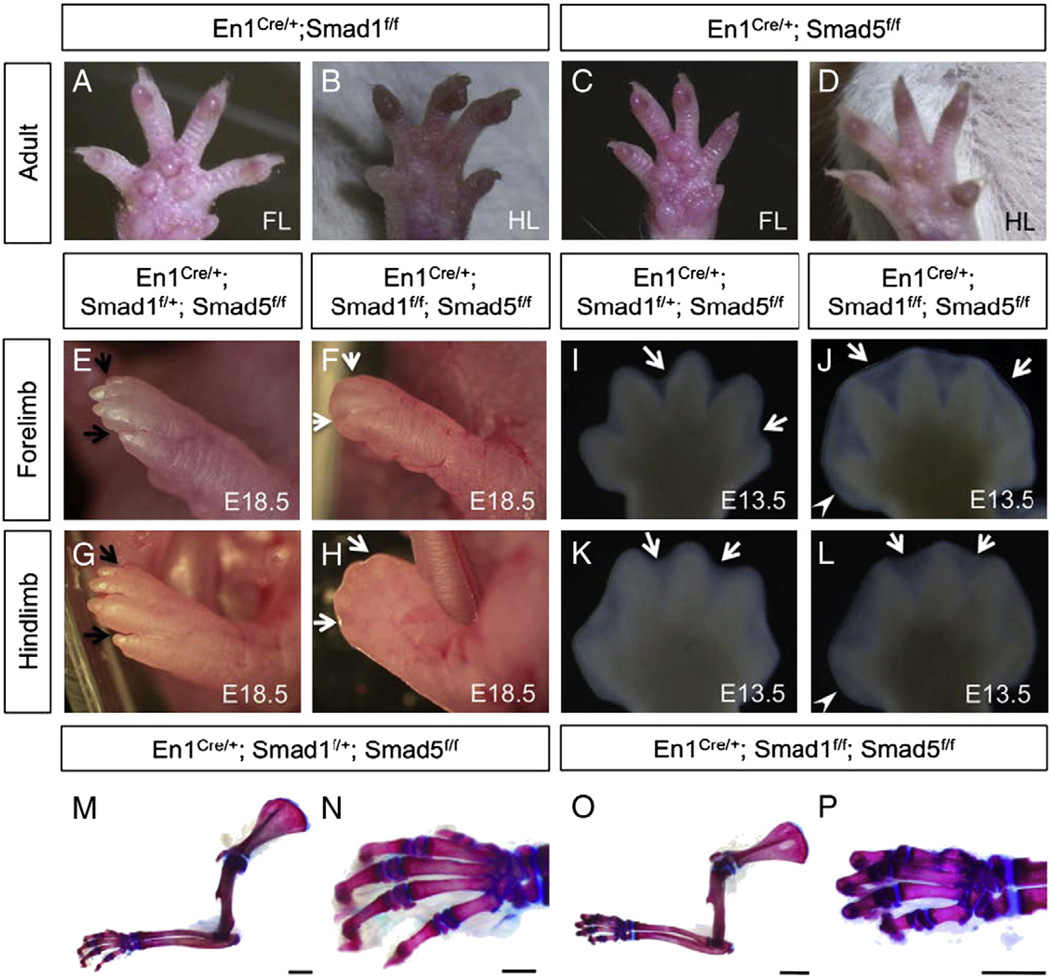
Ectodermal BMP receptor BmprIa and the ligands Bmp2/4 are important for regulating interdigital PCD (Maatouk et al., 2009; Pajni-Underwood et al., 2007). However, it remains unclear whether canonical BMP signaling through R-Smads are involved and what are their roles in regulating the interdigital PCD. To investigate which of the ectodermal R-Smads signaling is essential for the interdigital PCD, single Smad1 or Smad5 and double conditional knockout mutants were examined. Single inactivation of either Smad1 (En1Cre/+; Smad1f/f) or Smad5 (En1Cre/+; Smad5f/f) does not result in limb development abnormalities (Fig. 2A–D). Embryos harboring only one functional allele of either Smad1 allele (En1Cre/+; Smad1f/+; Smad5f/f) (Fig. 2E, G) or Smad5 allele (En1Cre/+; Smad1f/f; Smad5f/+) (data not shown) also had normal limbs. Thus, inactivation of three alleles of the Smad1/5 genes in the AER and ventral ectoderm did not result in a visible limb abnormality. Embryos of En1Cre/+; Smad1f/+; Smad5f/f genotype were used as control throughout the study. Interestingly, combined deletion of both Smad1 and Smad5 in limb AER and ventral ectoderm resulted in limb abnormalities similar to embryos lacking BmprIa or BMP2/BMP4 in the AER (Maatouk et al., 2009; Pajni-Underwood et al., 2007). Both the forelimbs and hindlimbs of our Smad1/5 mutants were severely affected at E18.5. Interdigital tissue was retained and syndactyly was observed in the mutant limbs (Fig. 2F, H). The defect of interdigital tissue regression could be observed at E13.5 when forming digits started to separate. (Fig. 2I–L). In controls, the forming digits started to separate at this stage and individual digits could be observed in both forelimbs and hindlimbs (Fig. 2I, K). However, the mutant limb autopods at E13.5 remained as a plate-like structure and the interdigital tissue was maintained between the forming digits of both forelimbs and hindlimbs (Fig. 2J, L). The autopods of Smad1/5 mutant were expanded slightly along the anterior to posterior axis. This demonstrates that Smad1 and Smad5 in the ectoderm and AER function redundantly to regulate the interdigital mesenchymal tissue regression.
Fig. 2.
Inactivation of Smad1 and Smad5 in the AER and ventral ectoderm by En1Cre/+ knock-in allele resulted in syndactyly and retention of interdigital tissue. Inactivation of either Smad1 (A, B) or Smad5 (C, D) in the AER and ventral ectoderm did not result in visible phenotypes in both forelimbs (FL) and hindlimbs (HL) of the individuals at adult stage. (E–H) Fore- and hindlimb from the control (E, G) and Smad1/5 mutant fetuses (F, H) at E18.5. Black arrows indicate separated digits in the control, while white arrows indicate the retained interdigital tissue in the mutants. (I–L) The defect in interdigital tissue regression was observed in both fore- and hindlimbs of the Smad1/5 mutants starting from E13.5. Syndactyly (webbing) is observed in the double mutants (J, L). White arrows indicate interdigital tissue. White arrowheads indicate that the posterior end of the autopod of Smad1/5 mutant is broadened at E13.5. (M–P) Skeletal preparations of the forelimbs from the control (M) and Smad1/5 mutants (O) at postnatal day 10. Higher magnification view of the autopod of the controls (N) and the mutants (P). Inactivation of Smad1 and Smad5 in the AER and ventral ectoderm did not result in skeletal defects in the autopod and along proximal–distal axis. A–D are the ventral views of the limb with anterior to the right. E–L are dorsal views of the limb plates with anterior to the right. Scale bars: M, O=5 mm; N, P=500 µm.
Alcian blue/alizarin red skeletal staining revealed no major differences of limb skeletal elements along the proximal to distal axis between the control and the Smad1/5 mutant limbs at P10 (Fig. 2M–P). Skeletal elements along the proximal to distal axis were formed properly in Smad1/5 mutants. Inactivation of both Smad1 and Smad5 in the AER and ventral ectoderm did not result in defects in proximal–distal patterning of limb. Interestingly, our Smad1/5 mutants exhibited skeletal alteration in the anterior to posterior axis. Postaxial polydactyly was observed at E18.5 Smad 1/5 mutants (four out of four Smad1/5 mutants analyzed) (Suppl. Fig. 1F). The ectopic rudiment attaches to the base of the distal phalange of the digitus minimus.
Smad1/Smad5 signaling in the limb AER and ventral ectoderm is required for regulating interdigital cell death and cell proliferation
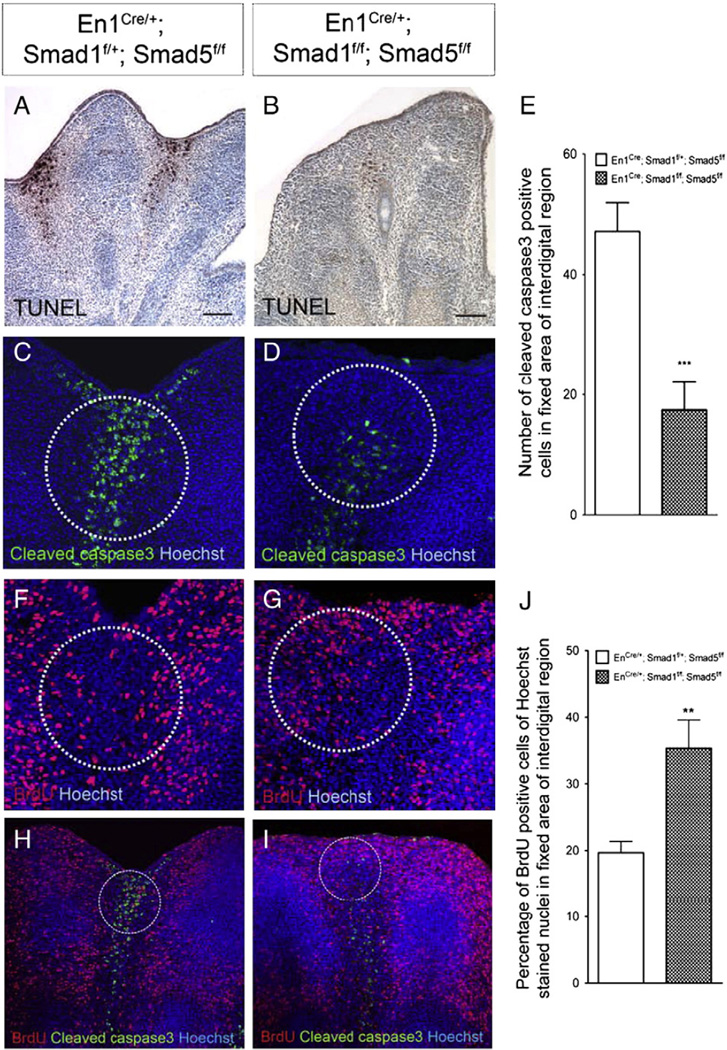
To examine whether the syndactylyl phenotype in the Smad1/5 mutants is caused by a decrease in apoptosis in the interdigital regions, the TUNEL assay was employed to mark the apoptotic cells in the E13.5 limb. Prominent apoptosis was observed in the interdigital regions of the control embryos (Fig. 3A). However, there was marked decrease in apoptosis in the interdigital regions of the Smad1/5 mutants (Fig. 3B). This suggests that a reduction in interdigital cell death leads to the retention of interdigital tissue. Dying cells may activate caspase3 to execute interdigital apoptosis. To investigate whether there was reduction in caspase3 activation, immunofluorescence was employed to detect activated cleaved caspase3 in limbs from the Smad1/5 mutants at E13.5. Interdigital mesenchyme of mutant limbs showed a drastic decrease in cleaved caspase3 positive cells compared with controls (p<0.005) (Fig. 3C–E), suggesting that activation of caspase3 in interdigital mesenchyme may be compromised after both Smad1 and Smad5 are inactivated in the limb ectoderm. These results demonstrate that Smad1/5 signaling in the AER and ventral ectoderm is required to regulate activation of caspase3 in interdigital mesenchyme and hence interdigital PCD.
Fig. 3.
Smad1/Smad5 signaling in the AER and ventral ectoderm is required for regulating cell death and cell proliferation in interdigital regions. (A, B) TUNEL assay and (C, D) immunofluorescence detecting cleaved caspase3 to measure cell death in E13.5 forelimbs. Inactivation of Smad1 and Smad5 resulted in a decrease in cell death. (E) Quantification of the number of anti-cleaved caspase3 positive cells demonstrated that there was a significantly decrease in the number of cells undergoing cell death in the Smad1/5 mutant limbs (***p<0.005). (F, G) Smad1/5 signaling in the AER and ventral ectoderm is required for regulating cell proliferation in the underlying mesenchyme. BrdU labeling assay showed that inactivation of Smad1 and Smad5 resulted in an increase in cell proliferation in distal interdigital mesenchymal regions. (H, I) Cleaved caspase3 and BrdU co-immunostaining indicated that distal interdigital areas (white circle) of Smad1/5 mutant are abundant in BrdU positive cells. (J) Quantification of the percentage of BrdU positive cells with Hoechst stained nuclei demonstrated that there was a significant increase in the percentage of proliferating cells in the distal interdigital areas of the Smad1/5 mutant limbs (**p<0.005). Scale bars: A–B=100 µm.
Apart from the reduction in apoptosis, it is possible that ectopic cell proliferation in the interdigital mesenchyme can also contribute to the syndactyly in the Smad1/5 mutants. Thus, BrdU labeling was employed to measure cell proliferation in the limbs of the mutant embryos at E13.5 (Fig. 3F–J). There was an increase in percentage of BrdU positive cells in the distal interdigital mesenchymal regions of the mutant limbs when comparing to the controls. This result demonstrates that Smad1/5 signaling in the AER and ventral ectoderm also regulates cell proliferation in the interdigital mesenchyme.
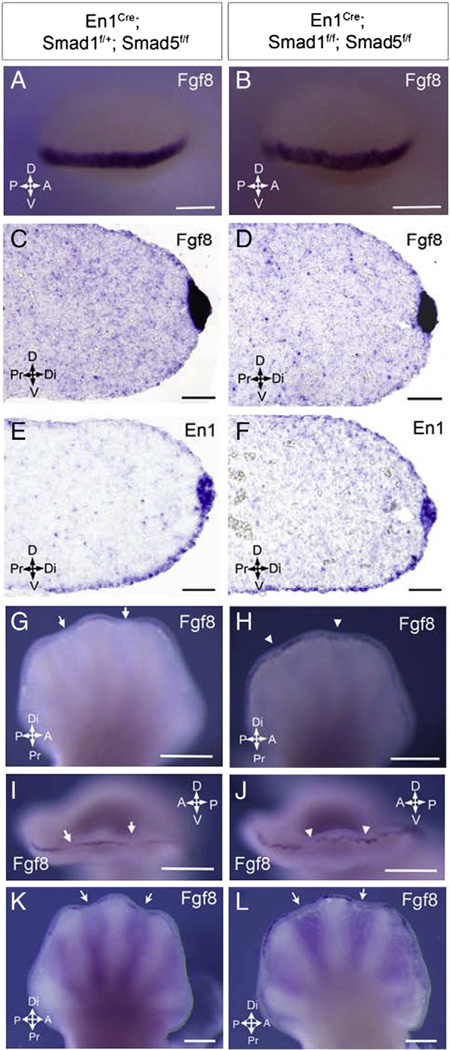
Fgf8 is persisted at the interdigital distal ectoderm and serves as survival signal for interdigital mesenchyme upon inactivation of the Smad1/Smad5 signaling in AER and ventral ectoderm
AER is an important signaling center in the developing limb bud which secretes various factors regulating limb development (Dudley et al., 2002; Pajni-Underwood et al., 2007). To analyze the roles of Smad1/5 signaling in regulating AER structure and function, the expression of Fgf8 and En1, markers for AER, was examined in the mutant limbs. At E10.5, similar pattern of Fgf8 expression was observed at the AER of the Smad1/5 mutant and the control (Fig. 4A–B). At E11.5, similar pattern and level of Fgf8 and En1 expressions within the AER were observed in the Smad1/5 mutant and control limb buds as shown by the section in-situ hybridization on sections along the dorsal–ventral axis (Fig. 4C–F). This result showed that En1 expression was maintained in the absence of Smad1/5 signaling in the AER and ventral ectoderm while the morphology of the AER was not altered in the Smad1/5 mutant at E11.5. At E13, Fgf8 expression was expressed in the AER overlying the developing digits but ceased over the interdigital region when interdigital cell death was initiated in the controls (Fig. 4G, I). However, we found that Fgf8 expression was maintained at the AER over the interdigital region in the E13 Smad1/5 mutant limbs, consistent with the previous findings in the BmprIa mutants (Pajni-Underwood et al., 2007). The expression of Fgf8 was punctate throughout the mutant AER (Fig. 4H, J). The Fgf8 expression in AER over the interdigital region ceased by E13.5 in the Smad1/5 mutant (Fig. 4L). This suggests that FGF signaling in the Smad1/5 mutant limbs may be elevated. The ectopic AER-specific Fgf8 expression over the interdigital region may serve as survival signal for interdigital cells in Smad1/5 the mutants (Macias et al., 1996; Martin, 1998; Pajni-Underwood et al., 2007; Weatherbee et al., 2006).
Fig. 4.
AER and ventral ectoderm Smad1/Smad5 signaling is required to repress expression of Fgf8 in the interdigital distal ectoderm. (A, B) Whole mount in situ hybridization of E10.5 mutant forelimb buds showed no expansion of Fgf8 expression along dorsal–ventral or anterior–posterior axis. (C–F) Section in situ hybridization of E11.5 mutant forelimb buds showed that the expression of AER marker Fgf8 (C, D) and En1 (E, F) was restricted in AER and ventral ectoderm along the dorsal–ventral axis that was comparable with the control. The morphology of AER in the Smad1/5 mutant did not show any abnormality. (G–J) Ectopic Fgf8 expression was observed in the forelimb interdigital ectoderm of the Smad1/5 mutants at E13 (white arrowhead) and the Fgf8 expression was punctuate throughout the distal ectoderm of the mutants. (K, L) Fgf8 expression faded in AER over interdigit of Smad1/5 mutants at E13.5 indicated by white arrows. Arrows indicate the major axes: A, anterior; P, posterior; D, dorsal; V, ventral; Di, distal; Pr, proximal. Scale bars: A–B=500 µm; C–F=50 µm; G–L=1 mm.
Mesenchymal BMP signals are not altered in the developing autopod of the Smad1/5 mutants
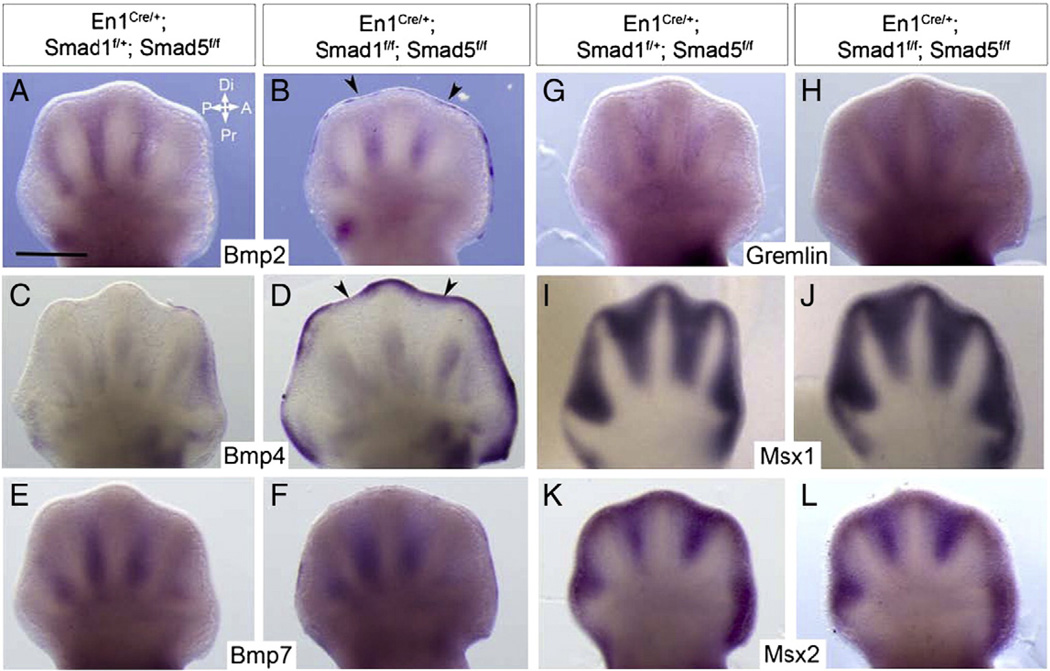
BMP signals acting on the interdigital mesenchyme are suggested to be the triggering factors for interdigital PCD. Bmp2, Bmp4 and Bmp7 are expressed in the interdigital regions at E12.5 and suggested to be the candidate ligands in these regions (Lyons et al., 1990, 1995; Zou and Niswander, 1996). To examine if the decrease in interdigital PCD in our Smad1/5 mutants was related to a decrease in mesenchymal BMP signals, expression of the BMP signaling components was examined in E12.5 autopod. The expression of the three BMP ligands, Bmp2, Bmp4 and Bmp7, in the interdigital regions was similar between the Smad1/5 mutants and the controls at E12.5 (Fig. 5A–F). Interestingly, there were enhanced expressions of Bmp2 (Fig. 5B) and Bmp4 (Fig. 5D and Supp. Fig. 1B) in the AER of the Smad1/5 mutants. Gremlin is known to be antagonist of BMP signaling (Khokha et al., 2003; Zuniga et al., 1999). No significant difference in interdigital Gremlin expression was found between the Smad1/5 mutant and control (Fig. 5G, H). Msx1 and Msx2 are downstream targets of BMP signaling in the interdigital regions (Guha et al., 2002; Marazzi et al., 1997; Zou and Niswander, 1996). No significant decrease in the expression of Msx1 and Msx2 was detected in the Smad1/5 mutant interdigital mesenchyme. Taken together, these results indicate that the mesenchymal BMP signaling does not alter in our Smad1/5 mutants and the defect of interdigital PCD in the mutants is not caused by the decrease in mesenchymal BMPs signaling levels. Interestingly, the ectopic Bmp2 and Bmp4 expression in the distal ectoderm in the Smad1/5 mutants suggests that there may be an auto-regulatory loop of BMP signaling and BMP signals in the limb AER (Pajni-Underwood et al., 2007).
Fig. 5.
Syndactyly phenotype of the Smad1/5 mutants is not caused by changes in mesenchymal BMP signaling of the developing autopod. Whole mount in situ hybridization of E12.5 controls (A, C, E, G, I and K) and mutants (B, D, F, H, J and L) forelimb buds using the probes as indicated. All are dorsal views. Arrowheads indicated ectopic expression of the genes indicated in the distal ectoderm. Arrows indicate the major axes: A, anterior; P, posterior; Di, distal; Pr, proximal. Scale bars = 500 µm.
Smad1/Smad5 inactivation in the limb ventral ectoderm resulted in ventral ectoderm thickening and ectopic En1-expressing cells and their descendents in the dorsal interdigital ectoderm
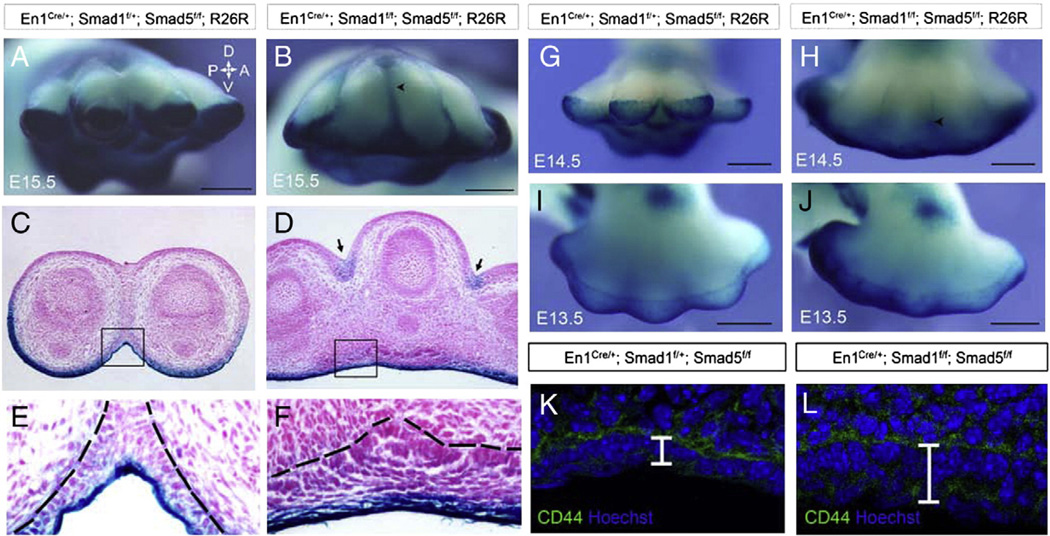
The fate of mesenchymal cells of the developing limb bud has been shown to be regulated by signaling from the ectoderm (Hurle and Ganan, 1986; Maatouk et al., 2009; Pajni-Underwood et al., 2007). Interdigital mesenchyme showed a reduction in apoptosis and increase in cell proliferation after Smad1/5 inactivation in the AER and ventral ectoderm. However, the fate of the ventral ectodermal cells after Smad1/Smad5 inactivation remains unclear. To investigate the possible changes in the ventral ectoderm after Smad1/5 inactivation, a R26R lacZ reporter system was employed to trace the ventral ectodermal cells. The morphology of the ventral ectoderm was examined in E15.5 limbs. Transverse sections of the control limbs showed that the ventral ectoderm was a thin structure consisting of 1–2 layers of cells (Figs. 6E and 7E). However, the ventral ectoderm of the Smad1/5 mutant became thicker and consisted of 8–10 layers of cells (Figs. 6F and 7F). To determine if this change occurs in the earlier stage limb buds, we examined an ectodermal marker CD44 at E13.5. CD44 immunostaining showed that the ectoderm of E13.5 control limb was thin and made up of 1–2 layers of cells (Fig. 6K). However, the ectoderm of the mutant was thicker, which was made up of multiple layers of cells (Fig. 6L). These results suggest that Smad1/5 signaling regulates the number of cell layers formation at the ventral ectoderm. When Smad1 and Smad5 were inactivated, the ventral limb ectoderm became thickened with multiple layers of cells. Very interestingly, some ventral En1-expressing cells and their descendents were located at the interdigital webbing of the developing limb of the Smad1/5 mutants at E14.5 (Fig. 6G, H) and E15.5 (Fig. 6A, B) while the digits of the controls were separated. The border of interdigital blue streaks extend more proximally at E15.5 compared to E14.5 (Fig. 6B, H). Transverse sections of the E15.5mutant limbs showed that some En1-expressing cells and their descendents were located ectopically at the dorsal interdigital ectoderm (Fig. 6D). The boundary of ventral ectoderm was shown to displace dorsally slightly in E13.5 mutant limb buds (Fig. 6I, J).
Fig. 6.
Smad1/5 signaling in the ventral AER and ectoderm is required for regulating the cell fate of ventral ectodermal cells. The Cre/loxP system was employed to map the ventral ectodermal cells and their descendenst after Smad1/5 inactivation in ventral AER and ectoderm. (A–B, G–J) Whole-mount X-gal staining of forelimb plates at E15.5 (A, B), E14.5 (G, H) and E13.5 (I, J) to identify En1-expressing cells and their descendents after Smad1/Smad5 inactivation. The border of the interdigital blue streak (arrowheads) extended more proximally at E15.5 (B) compared to E14.5 (H). (C–F) Transverse sections of the X-gal stained E15.5 forelimb plates showing β-galactosidase activity. (D, F) Ventral ectoderm was thickened in the Smad1/5 mutant. Blue lacZ staining was observed in the ventral ectoderm and interdigital ectoderm in the dorsal regions of the Smad1/5 mutant (arrows). (E, F) Higher magnification views of the areas indicated by the squares in (C and D). Ventral ectoderm layers of the Smad1/5 mutants became thicker and consisted of multiple layers of cells at E15.5 (compare E and F). (K–L) Immunostaining using ectodermal marker CD44 showed that ectoderm thickening was observed in mutant forelimb plates at E13.5. Dotted lines (E and F) indicated the basement membrane of the epithelium. Arrows indicate the major axes: A, anterior; P, posterior; D, dorsal; V, ventral. Scale bars: A–B, G–J=500 µm.
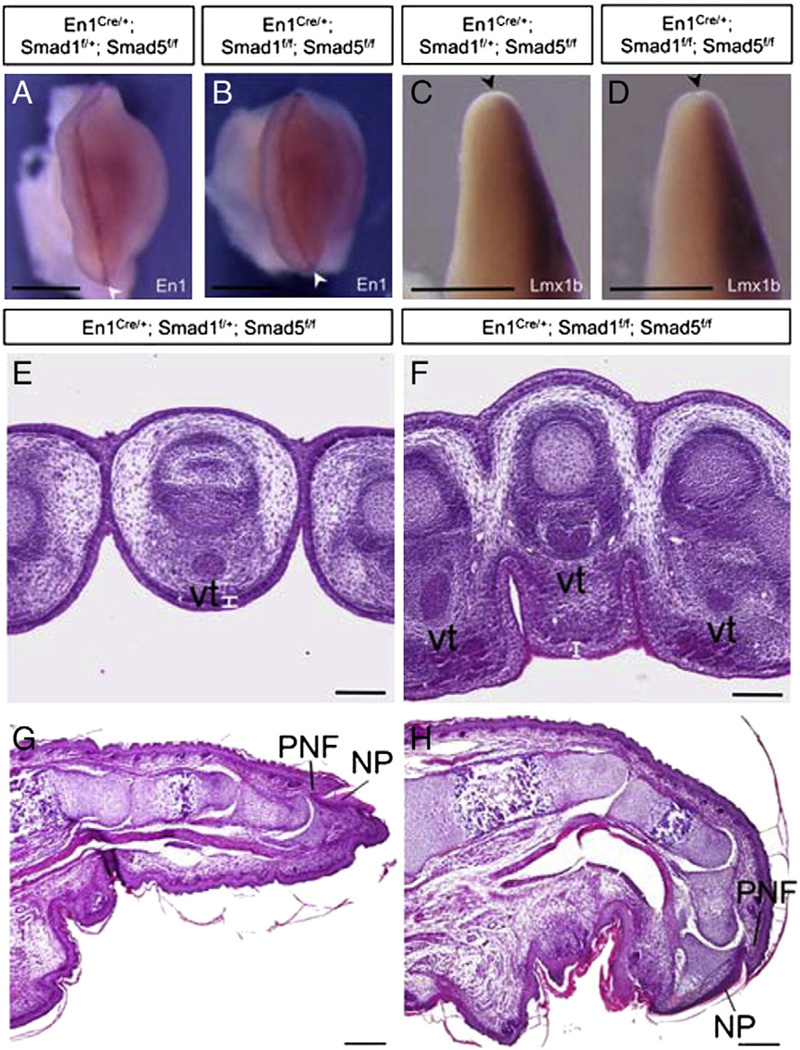
Fig. 7.
Smad1 and Smad5 signaling in the AER and ventral ectoderm are not required for dorsal–ventral patterning of the limb. (A, B) En1 expression in the AER (white arrowheads) and the ventral ectoderm of the forelimb buds from control (A) and Smad1/5 mutant (B) at E11.5. (C, D) Lmx1b, which is a dorsal limb mesoderm marker, showed restricted expression in the dorsal mesoderm of the mutant forelimb bud at E11.5 (D), similar to the control (C). Black arrowheads mark the location of AER. For panel A–D, dorsal side of limb buds towards the right. (E, F) Transverse sections through the E15.5 forelimb hand-plates are shown. The tendons, which are a ventral mesodermal structure, were formed and located properly at the ventral side of the mutant limbs. Aberrant thickening of ventral epidermal structure is observed in the Smad1/5 mutant limb (white bars). (G, H) Sagittal section Smad1/5 mutant limbs at E18.5 does not show ectopic nail plate on the ventral side. NP, nail plate; PNF, Proximal nail fold; Vt, ventral tendon. Scale bars: A–D=1 mm; E–F=100 µm; G–H=200 µm.
Inactivation of both Smad1 and Smad5 in ventral limb ectoderm and AER does not cause defects in dorsal–ventral patterning
Previous report shows that BMP signaling in the preAER/AER is required for dorsal–ventral patterning. Inactivation of BmprIa in limb ectoderm by Brn4-Cre would result in double dorsal structures in the limb and absence of En1 expression (Ahn et al., 2001). To investigate if the inactivation of ectodermal Smad1 and Smad5 would affect dorsal–ventral patterning, the expressions of En1 and Lmx1b were examined. En1 was expressed normally in the AER and ventral ectoderm of E11.5 forelimb buds of the Smad1/5 mutants. The expression pattern of En1 in the mutants was comparable to the control limbs (Figs. 4E, F and 7A, B). Lmx1b is expressed at the dorsal mesenchyme which promotes dorsal structure differentiation. Lmx1b was expressed normally at the dorsal mesenchyme of the Smad1/5 mutant limb buds at E11.5. No ectopic Lmx1b expression was observed at the ventral mesenchyme of mutant limbs (Fig. 7C, D). Histological sections across the limb dorsal–ventral axis at E15.5 revealed that ventral mesenchyme structure such as tendon were formed and located properly in the ventral positions of the Smad1/5 mutant limbs, which is in contrast with loss of ventral mesenchyme structure in the conditional BmprIa mutant (Ahn et al., 2001). Also, no ectopic dorsal ectodermal structure was observed at the ventral side of our Smad1/5 mutant limbs (Fig. 7G, H), contrasting with the conditional BmprIa mutant.
Smad1/Smad5 inactivation in the limb AER and ventral ectoderm resulted in postaxial polydactyly and expanded Shh expression in the posterior limb mesenchyme
Shh is expressed at the posterior mesenchyme of the developing limb bud to regulate anterior–posterior axis formation (Chang et al., 1994; Riddle et al., 1993). Anterior expansion of Shh signaling within the limb mesenchyme is suggested to be one of the mechanisms underlying the polydactyly (Selever et al., 2004). Alcian blue/alizarin red skeletal staining revealed that our Smad1/5 mutants exhibited postaxial polydactyly (Suppl. Fig. 1F). Shh expression was expanded in the posterior mesenchyme of the Smad1/5 mutant forelimb buds when compared with the control at E10.5 (Suppl. Fig. 1C, D). Thus, the lost of ventral ectodermal Smad1/5 signaling may lead to the expanded Shh expression that induce the postaxial polydactyly.
Discussion
Previous reports have shown that BMP signaling in the AER plays an essential role in regulating limb patterning and interdigital PCD (Ahn et al., 2001; Guha et al., 2002; Maatouk et al., 2009; Pajni-Underwood et al., 2007). However, these studies do not address the intracellular mechanism downstream of the receptor BmprIa mediating the BMP signals in the AER. In addition, complete inactivation of Smad1 or Smad5 or heterozygous inactivation of both Smad1 and Smad5 would lead to early embryonic lethality (Chang et al., 1999; Lechleider et al., 2001). Thus, the functions and the interactions of ectodermal R-Smads in AER and ventral ectoderm were investigated by conditional inactivation approach in this study. First, we discovered that Smad1 and Smad5 are employed as intracellular mediators of BMP signaling in the limb AER and ventral ectoderm. Second, the AER and ectoderm Smad1 and Smad5 function redundantly to regulate interdigital PCD and cell proliferation of developing limb indirectly. Third, the interaction between the limb ectoderm and mesenchyme is required to regulate the aforementioned processes. Smad1/Smad5 signaling regulates AER Fgf expression to control cell death and survival of the interdigital mesenchyme of limb. Finally, apart from the interdigital mesechyme, the development of ectoderm is also regulated by ectodermal Smad1/Smad5 signaling.
Functional redundancy between Smad1 and Smad5 in BMP-regulated AER and ventral ectoderm functions
The syndactyly phenotype of our mutant embryos in which both Smad1 and Smad5 have been inactivated is similar to the mice lacking BmprIa or Bmp2/Bmp4 in the ectoderm of developing limb bud (Maatouk et al., 2009; Pajni-Underwood et al., 2007). This suggests that R-Smad1 and R-Smad5 act as the intracellular mediator downstream of BmprIa to transduce BMP signals in the AER and ventral ectoderm to regulate interdigital PCD and digit separation. Since single inactivation of either Smad1 or Smad5 does not result in limb abnormality, we suggest that there is functional redundancy between the two R-Smads in the AER and ventral ectoderm. Smad1 and Smad5 can compensate the functions of one another to regulate digit separation and even one allele of either Smad1 or Smad5 is sufficient to transduce BMP signals in the AER for regulating interdigital PCD. In addition, this study provides further evidence that functional redundancy between Smad1 and Smad5 is not restricted to cancer development (Pangas et al., 2008) and bone development (Retting et al., 2009) but also in the limb AER function and PCD. However, the expression of Smad8 alone, another member of BMP receptor-regulated Smad, cannot compensate for the function of Smad1 and Smad5 in AER and ventral ectoderm. This may be explained by the early divergence of Smad1/5 and Smad8 during vertebrate evolution (Arnold et al., 2006).
The syndactyly defect of the Smad1/5 mutant is caused by reduction of interdigital PCD as suggested by TUNEL assay. The interdigital mesenchymal cells fail to undergo normal PCD and are maintained in the interdigital regions of the mutant to form the webbing. This result suggests that the ectodermal Smad1/Smad5 signaling in the AER indirectly regulate interdigital mesenchymal cell death. On the other hand, controversies remain regarding whether the dying cells activate caspase3 to execute interdigital apoptosis (Zuzarte-Luis and Hurle, 2005). Caspase3 activation is compromised in our Smad1/5 mutant as detected by anti-cleaved caspase3 antibody. This suggests that caspase3 activation is involved in interdigital PCD and Smad1/Smad5 signaling in the ectoderm regulates activation of caspase3 in interdigital mesenchyme and hence interdigital apoptosis. Interdigital mesenchyme of our Smad1/5 mutants also undergoes ectopic cell proliferation that contributes to the formation of the webbing. Since the AER and ventral ectodermal inactivation of both Smad1 and Smad5 leads to the decrease in cell death and ectopic cell proliferation in mesenchyme, this suggests that appropriate interactions between the ectoderm and the mesenchyme are required to regulate cell death and cell proliferation in the mesenchyme.
Molecular mechanisms of limb AER and ventral ectodermal Smad1/Smad5 signaling to control cell death and survival in the interdigital regions
Previous studies suggested that removal of BMP signaling components in the AER would result in failure in AER maturation and expansion of Fgf8 expression. The prolonged expression of Fgf8 at the AER would maintain interdigital cell survival in the Bmpr1a (Pajni-Underwood et al., 2007) and Bmp2/4 (Maatouk et al., 2009) conditional mutants. The BMPs expressed in AER control Fgf activity of the AER to regulate interdigital cell death. In our Smad1/5 mutants, Fgf8 was ectopically expressed in the AER over the interdigits and was punctate throughout the AER at E13, which is consistent with the previous findings. We propose that Smad1 and Smad5 are employed as intracellular mediator to transduce BMPs signal acting on the AER and ventral ectoderm to repress Fgf expression in the AER over interdigits. Interdigital PCD is triggered by the reduction in Fgf signaling in AER over interdigital regions and this leads to separation of digits (Macias et al., 1996; Martin, 1998). Smad1 and Smad5 function redundantly to repress the Fgf8 expression in AER over interdigital region. Simultaneous inactivation of both Smad1 and Smad5 in the AER and ventral ectoderm would result in prolonged Fgf8 expression. This prolonged expression of Fgf8 in the AER over interdigital region serves as survival signal to the interdigital mesenchyme, leading to decrease in cell death and increase in cell proliferation in this tissue (Fig. 8). In addition, our data showing the unaltered expression of mesenchymal BMP signaling components in our Smad1/5 mutants further support the idea that the BMP signaling within the AER and ectoderm does not regulate the mesenchymal BMP signaling of developing limbs (Pajni-Underwood et al., 2007). The Smad1/5 signaling in the AER and ventral ectoderm only indirectly regulates interdigital PCD through regulating the ectodermal FGF expression. On the other hand, previous report showed that knockout of BMP ligands in the mesenchyme resulted in expanded Fgf8 expression in AER of E11.5 limb bud (Bandyopadhyay et al., 2006). The question of whether mesenchymal BMP signaling can regulate BMP signaling within AER and ectoderm awaits to be elucidated.
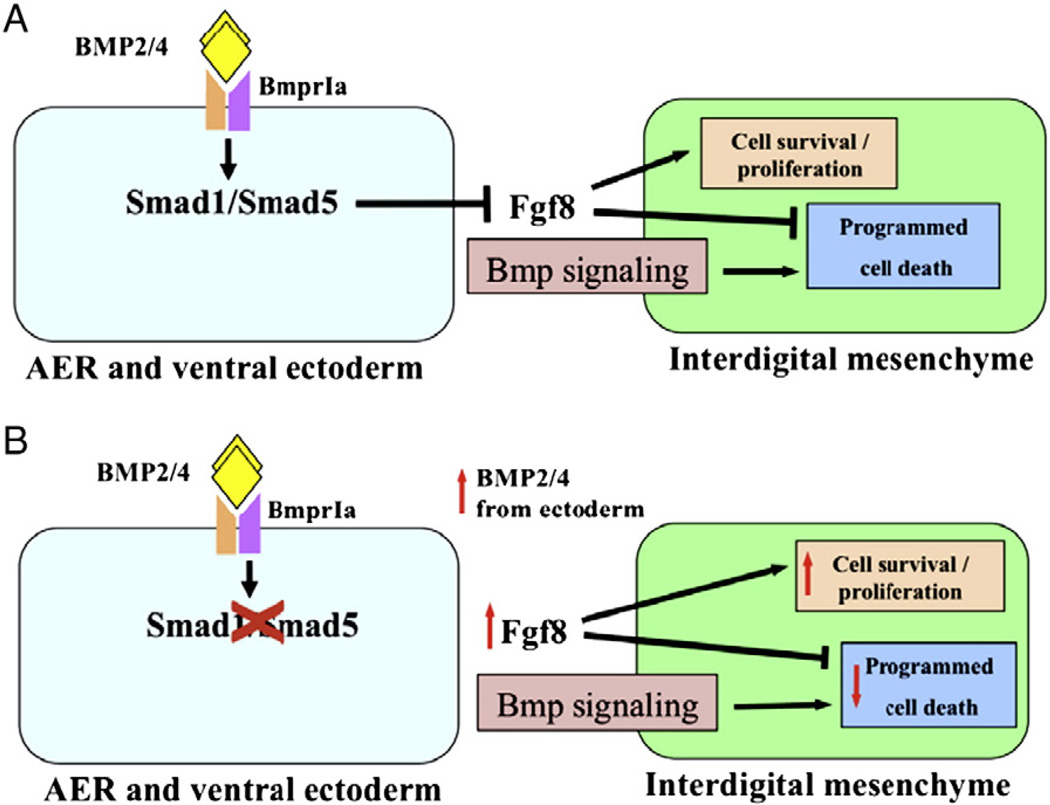
Fig. 8.
Our proposed model for the roles and interactions of Smad1/Smad5 in AER and ventral ectoderm of developing limbs. (A) Smad1 and Smad5 functions redundantly as the intracellular mediator of the BMP signaling downstream of BmprIa in the AER and ventral ectoderm (Maatouk et al., 2009; Pajni-Underwood et al., 2007). Fgf8 expression at the AER serves as cell survival signal to the interdigital mesenchyme (arrow). Smad1/5 signaling at the AER and ventral ectoderm inhibits Fgf8 expression. The termination of Fgf8 expression initiates programmed cell death in the intedigital mesenchyme. BMP signaling within the mesenchyme was shown to regulate interdigital PCD (Bandyopadhyay et al., 2006). (B) Inactivation of Smad1/Smad5 in the AER and ventral ectoderm would result in persisted Fgf8 expression. Fgf8 is expressed ectopically in the AER over interdigital region that leads to inhibition of PCD and ectopic cell survival and proliferation in the interdigital mesenchyme. In addition, there is an auto-regulatory loop of BMP signaling and BMP signals in the limb AER. In the absence of Smad1/5 signaling, BMP signals in the AER is elevated.
Interestingly, the expression of Fgf8 in our E10.5 Smad1/5 mutants did not expanded as previously reported for the Bmpr1a and Bmp2/4 conditional mutants, suggesting that the AER of our Smad1/5 mutants instead undergoes maturation properly. The difference in Fgf8 expression of our Smad1/5 mutants at E10.5 could possibly be due to the different Cre mouse line used in our study. At E10.5, the En1-Cre knockin allele is only active in the ventral half of the developing AER and the ventral ectoderm (Kwan et al., 2004) while the Msx2-Cre employed in previous reports is active in the entire AER of the early limb bud and the ectoderm (Barrow et al., 2003; Maatouk et al., 2009; Pajni-Underwood et al., 2007). Our results suggest that BMP signaling in the dorsal and ventral AER at E10.5 may be responsible for differential functions during limb development. Dorsal ectodermal BMP signaling may be required for the maturation of the AER and restricting the expression of Fgf8 at this earlier stage of limb bud development. Our experiments give insights into the possible differential functions of different parts of limb ectoderm in AER development.
The role of Smad1/Smad5 signaling in limb ectoderm structure formation
The epidermis is an important organ throughout the body that provides protection against dehydration, injury and infection. During development, the epidermis is a well-organized structure which consists of 2–3 layers of cells at E15.5. The inner layer is the stratum germinativum and contains proliferating cells that divide continuously to replace the outer layer. The outer layer gives rise to periderm which is a temporary covering of the skin and will shed continuously. The balance between proliferation of the stratum geminativum and the shedding of the outer layer is critical for the maintenance of the structure of the epidermis and its protection function (Simpson et al., 2011). When both Smad1 and Smad5 were inactivated in the ventral ectoderm of the developing limb, the ectoderm lost the well-organized structure of 2–3 layers of cells. The ectoderm of the Smad1/Smad5 mutants became a thick structure densely packed with multiple layers of cells. These multiple layers of cells are descendents of the Smad1/Smad5-inactivated ventral ectodermal cells. Furthermore, the ventral En1-expressing cells and their descendents were found ectopically at the dorsal interdigital ectoderm. Our data suggests that Smad1/Smad5 signaling in the ventral ectoderm is important in regulating its own structural development and hence the functions of the epidermis. We propose that Smad1/Smad5 signaling may also function redundantly in the ventral ectoderm to limit ectodermal cells in the ventral position. When both Smad1 and Smad5 are inactivated in the ectodermal cells at the distal end of the developing limb, these ectodermal cells may undergo ectopic proliferation and migrate to the dorsal position as suggested by our cell tracing experiment and the thickening of ventral ectoderm in the Smad1/5 mutants.
The role of ventral ectodermal Smad1/5 signaling in dorsal–ventral patterning
Previous report showed that preAER/AER BMP signaling is required for dorsal–ventral patterning. Inactivation of the receptor BmprIa in limb ectoderm by Brn4-Cre would result in double dorsal defects including the absence of ventral mesenchymal structures such as flexor digitorum profundus tendon and sesamoid process and the loss of En1 expression (Ahn et al., 2001). Moreover, knockout of BMP ligands Bmp2/Bmp4 in the AER would result in ectopic nail plate on the ventral side (Maatouk et al., 2009). In contrast, expression of En1 and Lmx1b in the developing limb buds of our Smad1/5 mutants was comparable to the controls. No visible dorsal–ventral defects were observed in the Smad1/5 mutants. Ventral mesenchyme structures are formed and located properly in the Smad1/5 mutant limbs, which is in contrast with loss of ventral mesenchyme structure in the conditional BmprIa mutant (Ahn et al., 2001). No ectopic dorsal ectodermal structure was observed at the ventral side of the mutant limb, contrasting with the conditional Bmp2/4 mutant (Maatouk et al., 2009). The lack of defects in the dorsal–ventral patterning of our Smad1/5 mutant could possibly due to the different Cre mouse line used in our study. The Brn4-Cre and Msx2-Cre show their activities in the entire ectoderm during limb bud formation while the En1-Cre is only active in the AER and ventral ectoderm. The signaling through the Smad1/Smad5 in the dorsal ectodermal region of our Smad1/5 mutant limb may be sufficient to maintain the En1 expression and the patterning of dorsal–ventral axis. It is also possible that the AER-expressed BMP ligands may function through alternative pathways, other than R-Smad, upstream of En1 to regulate dorsal–ventral patterning.
Supplementary Material
Acknowledgments
The authors thank Ms. Sze-Nee Lim for technical assistance, Dr Yasuhide Furuta (University of Texas MD Anderson Cancer Center, USA) for providing the probes for Bmp2, Bmp4 and Bmp7, and Dr James Li (University of Connecticut Health Center, USA) for providing the probes for En1 and Lmx1b. This research was supported by Hong Kong Research Grant Council General Research Fund (CUHK 466708) and Focused Investments Scheme — Scheme B of The Chinese University of Hong Kong (BL08650).
Footnotes
Supplementary materials related to this article can be found online at doi:10.1016/j.ydbio.2011.12.037.
References
- Ahn K, Mishina Y, Hanks MC, Behringer RR, Crenshaw EB., III BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal–ventral patterning of the limb. Development. 2001;128:4449–4461. doi: 10.1242/dev.128.22.4449. [DOI] [PubMed] [Google Scholar]
- Ali-Khan SE, Hales BF. Retinoid receptor antagonists alter the pattern of apoptosis in organogenesis stage mouse limbs. Toxicol. Sci. 2006;90:208–220. doi: 10.1093/toxsci/kfj060. [DOI] [PubMed] [Google Scholar]
- Al-Qattan MM, Yang Y, Kozin SH. Embryology of the upper limb. J. Hand Surg. Am. 2009;34:1340–1350. doi: 10.1016/j.jhsa.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, Croft NJ, Cebra-Thomas JA, Metzger D, Chambon P, Lyons KM, Mishina Y, Seykora JT, Crenshaw EB, III, Millar SE. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- Arnold SJ, Maretto S, Islam A, Bikoff EK, Robertson EJ. Dose-dependent Smad1, Smad5 and Smad8 signaling in the early mouse embryo. Dev. Biol. 2006;296:104–118. doi: 10.1016/j.ydbio.2006.04.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, Tabin CJ. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2006;2:e216. doi: 10.1371/journal.pgen.0020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow JR, Thomas KR, Boussadia-Zahui O, Moore R, Kemler R, Capecchi MR, McMahon AP. Ectodermal Wnt3/beta-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge. Genes Dev. 2003;17:394–409. doi: 10.1101/gad.1044903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscher D, Bosse B, Heymer J, Ruther U. Evidence for genetic control of Sonic hedgehog by Gli3 in mouse limb development. Mech. Dev. 1997;62:175–182. doi: 10.1016/s0925-4773(97)00656-4. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Izpisua Belmonte JC. Patterning mechanisms controlling vertebrate limb development. Annu. Rev. Cell Dev. Biol. 2001;17:87–132. doi: 10.1146/annurev.cellbio.17.1.87. [DOI] [PubMed] [Google Scholar]
- Chang DT, Lopez A, von Kessler DP, Chiang C, Simandl BK, Zhao R, Seldin MF, Fallon JF, Beachy PA. Products, genetic linkage and limb patterning activity of a murine hedgehog gene. Development. 1994;120:3339–3353. doi: 10.1242/dev.120.11.3339. [DOI] [PubMed] [Google Scholar]
- Chang H, Huylebroeck D, Verschueren K, Guo Q, Matzuk MM, Zwijsen A. Smad5 knockout mice die at mid-gestation due to multiple embryonic and extra-embryonic defects. Development. 1999;126:1631–1642. doi: 10.1242/dev.126.8.1631. [DOI] [PubMed] [Google Scholar]
- Delgado I, Dominguez-Frutos E, Schimmang T, Ros MA. The incomplete inactivation of Fgf8 in the limb ectoderm affects the morphogenesis of the anterior autopod through BMP-mediated cell death. Dev. Dyn. 2008;237:649–658. doi: 10.1002/dvdy.21452. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Ros MA, Tabin CJ. A re-examination of proximodistal patterning during vertebrate limb development. Nature. 2002;418:539–544. doi: 10.1038/nature00945. [DOI] [PubMed] [Google Scholar]
- Fadeel B, Orrenius S. Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J. Intern. Med. 2005;258:479–517. doi: 10.1111/j.1365-2796.2005.01570.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Teran MA, Hinchliffe JR, Ros MA. Birth and death of cells in limb development: a mapping study. Dev. Dyn. 2006;235:2521–2537. doi: 10.1002/dvdy.20916. [DOI] [PubMed] [Google Scholar]
- Flanders KC, Kim ES, Roberts AB. Immunohistochemical expression of Smads 1–6 in the 15-day gestation mouse embryo: signaling by BMPs and TGF-βs. Dev. Dyn. 2001;220:141–154. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1096>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Galdones E, Lohnes D, Hales BF. Role of retinoic acid receptors alpha1 and gamma in the response of murine limbs to retinol in vitro. Birth Defects Res. A. Clin. Mol. Teratol. 2006;76:39–45. doi: 10.1002/bdra.20219. [DOI] [PubMed] [Google Scholar]
- Ganan Y, Macias D, Duterque-Coquillaud M, Ros MA, Hurle JM. Role of TGF beta s and BMPs as signals controlling the position of the digits and the areas of interdigital cell death in the developing chick limb autopod. Development. 1996;122:2349–2357. doi: 10.1242/dev.122.8.2349. [DOI] [PubMed] [Google Scholar]
- Goodman FR. Limb malformations and the human HOX genes. Am. J. Med. Genet. 2002;112:256–265. doi: 10.1002/ajmg.10776. [DOI] [PubMed] [Google Scholar]
- Grotewold L, Ruther U. Bmp, Fgf and Wnt signalling in programmed cell death and chondrogenesis during vertebrate limb development: the role of Dickkopf-1. Int. J. Dev. Biol. 2002;46:943–947. [PubMed] [Google Scholar]
- Guha U, Gomes WA, Kobayashi T, Pestell RG, Kessler JA. In vivo evidence that BMP signaling is necessary for apoptosis in the mouse limb. Dev. Biol. 2002;249:108–120. doi: 10.1006/dbio.2002.0752. [DOI] [PubMed] [Google Scholar]
- He W, Cao T, Smith DA, Myers TE, Wang XJ. Smads mediate signaling of the TGFbeta superfamily in normal keratinocytes but are lost during skin chemical carcinogenesis. Oncogene. 2001;20:471–483. doi: 10.1038/sj.onc.1204117. [DOI] [PubMed] [Google Scholar]
- Hernández-Martínez R, Covarrubias L. Interdigital cell death function and regulation: new insights on an old programmed cell death model. Dev. Growth Differ. 2011;53:245–258. doi: 10.1111/j.1440-169X.2010.01246.x. [DOI] [PubMed] [Google Scholar]
- Huang S, Tang B, Usoskin D, Lechleider RJ, Jamin SP, Li C, Anzano MA, Ebendal T, Deng C, Roberts AB. Conditional knockout of the Smad1 gene. Genesis. 2002;32:76–79. doi: 10.1002/gene.10059. [DOI] [PubMed] [Google Scholar]
- Hurle JM, Ganan Y. Interdigital tissue chondrogenesis induced by surgical removal of the ectoderm in the embryonic chick leg bud. J. Embryol. Exp. Morphol. 1986;94:231–244. [PubMed] [Google Scholar]
- Itoh S, Itoh F, Goumans MJ, ten Dijke P. Signaling of transforming growth factor-beta family members through Smad proteins. Eur. J. Biochem. 2000;267:6954–6967. doi: 10.1046/j.1432-1327.2000.01828.x. [DOI] [PubMed] [Google Scholar]
- Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- Khokha MK, Hsu D, Brunet LJ, Dionne MS, Harland RM. Gremlin is the BMP antagonist required for maintenance of Shh and Fgf signals during limb patterning. Nat. Genet. 2003;34:303–307. doi: 10.1038/ng1178. [DOI] [PubMed] [Google Scholar]
- Kimmel RA, Turnbull DH, Blanquet V, Wurst W, Loomis CA, Joyner AL. Two lineage boundaries coordinate vertebrate apical ectodermal ridge formation. Genes Dev. 2000;14:1377–1389. [PMC free article] [PubMed] [Google Scholar]
- Kwan KM, Li AG, Wang XJ, Wurst W, Behringer RR. Essential roles of BMPR-IA signaling in differentiation and growth of hair follicles and in skin tumorigenesis. Genesis. 2004;39:10–25. doi: 10.1002/gene.20021. [DOI] [PubMed] [Google Scholar]
- Lechleider RJ, Ryan JL, Garrett L, Eng C, Deng C, Wynshaw-Boris A, Roberts AB. Targeted mutagenesis of Smad1 reveals an essential role in chorioallantoic fusion. Dev. Biol. 2001;240:157–167. doi: 10.1006/dbio.2001.0469. [DOI] [PubMed] [Google Scholar]
- Lyons KM, Pelton RW, Hogan BL. Organogenesis and pattern formation in the mouse: RNA distribution patterns suggest a role for bone morphogenetic protein-2A (BMP-2A) Development. 1990;109:833–844. doi: 10.1242/dev.109.4.833. [DOI] [PubMed] [Google Scholar]
- Lyons KM, Hogan BL, Robertson EJ. Colocalization of BMP 7 and BMP 2 RNAs suggests that these factors cooperatively mediate tissue interactions during murine development. Mech. Dev. 1995;50:71–83. doi: 10.1016/0925-4773(94)00326-i. [DOI] [PubMed] [Google Scholar]
- Maatouk DM, Choi KS, Bouldin CM, Harfe BD. In the limb AER Bmp2 and Bmp4 are required for dorsal–ventral patterning and interdigital cell death but not limb outgrowth. Dev. Biol. 2009;327:516–523. doi: 10.1016/j.ydbio.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Macias D, Gañan Y, Ros MA, Hurle JM. In vivo inhibition of programmed cell death by local administration of FGF-2 and FGF-4 in the interdigital areas of the embryonic chick leg bud. Anat. Embryol. (Berl) 1996;193:533–541. doi: 10.1007/BF00187925. [DOI] [PubMed] [Google Scholar]
- Macias D, Gañan Y, Sampath TK, Piedra ME, Ros MA, Hurle JM. Role of BMP-2 and OP-1 (BMP-7) in programmed cell death and skeletogenesis during chick limb development. Development. 1997;124:1109–1117. doi: 10.1242/dev.124.6.1109. [DOI] [PubMed] [Google Scholar]
- Marazzi G, Wang Y, Sassoon D. Msx2 is a transcriptional regulator in the BMP4-mediated programmed cell death pathway. Dev. Biol. 1997;186:127–138. doi: 10.1006/dbio.1997.8576. [DOI] [PubMed] [Google Scholar]
- Martin P. Tissue patterning in the developing mouse limb. Int. J. Dev. Biol. 1990;34:323–336. [PubMed] [Google Scholar]
- Martin GR. The roles of FGFs in the early development of vertebrate limbs. Genes Dev. 1998;12:1571–1586. doi: 10.1101/gad.12.11.1571. [DOI] [PubMed] [Google Scholar]
- Montero JA, Ganan Y, Macias D, Rodriguez-Leon J, Sanz-Ezquerro JJ, Merino R, Chimal-Monroy J, Nieto MA, Hurle JM. Role of FGFs in the control of programmed cell death during limb development. Development. 2001;128:2075–2084. doi: 10.1242/dev.128.11.2075. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov D. Alcian blue/alizarin red staining of cartilage and bone in mouse. Cold Spring Harb. Protoc. 2009;4(3):prot5170. doi: 10.1101/pdb.prot5170. [DOI] [PubMed] [Google Scholar]
- Pajni-Underwood S, Wilson CP, Elder C, Mishina Y, Lewandoski M. BMP signals control limb bud interdigital programmed cell death by regulating FGF signaling. Development. 2007;134:2359–2368. doi: 10.1242/dev.001677. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Li X, Umans L, Zwijsen A, Huylebroeck D, Gutierrez C, Wang D, Martin JF, Jamin SP, Behringer RR, Robertson EJ, Matzuk MM. Conditional deletion of Smad1 and Smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol. Cell. Biol. 2008;28:248–257. doi: 10.1128/MCB.01404-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retting KN, Song B, Yoon BS, Lyons KM. BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development. 2009;136:1093–1104. doi: 10.1242/dev.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- Salas-Vidal E, Valencia C, Covarrubias L. Differential tissue growth and patterns of cell death in mouse limb autopod morphogenesis. Dev. Dyn. 2001;220:295–306. doi: 10.1002/dvdy.1108. [DOI] [PubMed] [Google Scholar]
- Selever J, Liu W, Lu MF, Behringer RR, Martin JF. Bmp4 in limb bud mesoderm regulates digit pattern by controlling AER development. Dev. Biol. 2004;276:268–279. doi: 10.1016/j.ydbio.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Simpson CL, Patel DM, Green KJ. Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis. Nat. Rev. Mol. Cell Biol. 2011;129:65–80. doi: 10.1038/nrm3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Towers M, Tickle C. Generation of pattern and form in the developing limb. Int. J. Dev. Biol. 2009;53:805–812. doi: 10.1387/ijdb.072499mt. [DOI] [PubMed] [Google Scholar]
- Umans L, Vermeire L, Francis A, Chang H, Huylebroeck D, Zwijsen A. Generation of a floxed allele of Smad5 for cre-mediated conditional knockout in the mouse. Genesis. 2003;37:5–11. doi: 10.1002/gene.10219. [DOI] [PubMed] [Google Scholar]
- Weatherbee SD, Behringer RR, Rasweiler JJ, IV, Niswander LA. Interdigital webbing retention in bat wings illustrates genetic changes underlying amniote limb diversification. Proc. Natl. Acad. Sci. U. S. A. 2006;103:15103–15107. doi: 10.1073/pnas.0604934103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DG. Whole mount in situ hybridization to vertebrate embryos. In: Wilkinson DG, editor. In Situ Hybridization: a Practical Approach. Oxford: IRL Press; 1992. pp. 75–83. [Google Scholar]
- Zou H, Niswander L. Requirement for BMP signaling in interdigital apoptosis and scale formation. Science. 1996;272:738–741. doi: 10.1126/science.272.5262.738. [DOI] [PubMed] [Google Scholar]
- Zuniga A, Haramis AP, McMahon AP, Zeller R. Signal relay by BMP antagonism controls the SHH/FGF4 feedback loop in vertebrate limb buds. Nature. 1999;401:598–602. doi: 10.1038/44157. [DOI] [PubMed] [Google Scholar]
- Zuzarte-Luis V, Hurle JM. Programmed cell death in the embryonic vertebrate limb. Semin. Cell Dev. Biol. 2005;16:261–269. doi: 10.1016/j.semcdb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Zuzarte-Luis V, Montero JA, Rodriguez-Leon J, Merino R, Rodriguez-Rey JC, Hurle JM. A new role for BMP5 during limb development acting through the synergic activation of Smad and MAPK pathways. Dev. Biol. 2004;272:39–52. doi: 10.1016/j.ydbio.2004.04.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.