Summary
Francisella tularensis causes a lethal human disease known as tularemia. As an intracellular pathogen, Francisella survives and replicates in phagocytic cells, such as macrophages. However, to establish an intracellular niche, Francisella must overcome the oxidative stress posed by the reactive oxygen species (ROS) produced by the infected macrophages. OxyR and SoxR/S are two well-characterized transcriptional regulators of oxidative stress responses in several bacterial pathogens. Only the OxyR homolog is present in F. tularensis, while the SoxR homologs are absent. The functional role of OxyR has not been established in F. tularensis. We demonstrate that OxyR regulates oxidative stress responses and provides resistance against ROS, thereby contributing to the survival of the F. tularensis subsp. holarctica live vaccine strain (LVS) in macrophages and epithelial cells and contributing to virulence in mice. Proteomic analysis reveals the differential production of 128 proteins in the oxyR gene deletion mutant, indicating its global regulatory role in the oxidative stress response of F. tularensis. Moreover, OxyR regulates the transcription of the primary antioxidant enzyme genes by binding directly to their putative promoter regions. This study demonstrates that OxyR is an important virulence factor and transcriptional regulator of the oxidative stress response of the F. tularensis LVS.
Graphical Abstract
Introduction
Francisella tularensis is a gram-negative, facultative intracellular bacterium that causes a zoonotic disease known as tularemia. Francisella is classified into four subspecies: F. tularensis subspecies tularensis, F. tularensis subspecies holarctica, F. tularensis subspecies mediasiatica, and F. tularensis subspecies novicida. Of these four subspecies, F. tularensis subspecies tularensis (type A) and subspecies holarctica (type B) are associated with human disease. F. tularensis subspecies tularensis is highly virulent in humans; as low as 10 colony forming units (CFUs) can cause fatal disease (Pechous et al., 2009). Due to its extremely high virulence and ability to cause extensive mortality, Francisella has been used in bioweapon programs in the past (Dennis et al., 2001). The Centers for Disease Control has classified Francisella as a Tier 1 Category A Select Agent due to its potential to be used as a bioterror agent. The live vaccine strain (LVS) is derived from F. tularensis subspecies holarctica. The LVS has attenuated virulence in humans but retains its virulence in mice and is widely used as a model to study tularemia pathogenesis (Fortier et al., 1991).
As an intracellular pathogen, F. tularensis survives and replicates inside neutrophils, dendritic cells, and macrophages (Sjostedt, 2006; Santic et al., 2006; Allen, 2006; Chase et al., 2009). To establish an intracellular niche, Francisella has to overcome reactive oxygen and nitrogen species (ROS/RNS) generated following respiratory burst in these phagocytic cells. The ROS include highly microbicidal superoxide radicals (O2−), hydrogen peroxide (H2O2) and highly reactive hydroxyl radicals (HO−) produced from H2O2 via the Fenton reaction (Storz and Imlay, 1999). As a primary defense mechanism against ROS generated by the host’s phagocytic cells, Francisella produces antioxidant enzymes such as Fe-containing superoxide dismutase (SodB) and Cu-Zn containing superoxide dismutase (SodC) to dismutate O2− into H2O2 as well as a catalase (KatG) and alkyl-hydro-peroxide reductase (AhpC) to convert H2O2 into water and oxygen. SodB and SodC contribute to the oxidative stress resistance and virulence of the F. tularensis LVS (Bakshi et al., 2006; Melillo et al., 2009; Melillo et al., 2010; Lindgren et al., 2007; Binesse et al., 2015); however, their role against resistance to oxidative stress and the virulence of F. tularensis SchuS4 remains unknown. The catalase activity in F. tularensis strains does not correlate with their virulence (Vi-Dor and Yaniv, 1952); accordingly, KatG has been shown to be required for the virulence of the F. tularensis LVS, but not for the SchuS4 strain (Lindgren et al., 2007). In addition to these primary antioxidant enzymes, MoxR-like ATPase (Dieppedale et al., 2011) and proteins with sequence similarities to the ohr gene product of Xanthomonas campestris have also been shown to resist oxidative stress in F. tularensis (Meireles et al., 2014; Llewellyn et al., 2011). The antioxidant enzymes KatG, SodC, and AhpC of F. tularensis are induced in response to oxidative stress (Wehrly et al., 2009) and are secreted into the cytosol of the infected macrophages (Lee et al., 2006). Our previous study has demonstrated that a fusion protein of the multidrug efflux pump EmrA1 confers oxidative stress resistance by affecting the secretion of KatG and SodB (Ma et al., 2014). The expression of these primary antioxidant enzymes starts immediately upon phagocytosis and remains significantly upregulated during the phagosomal and cytosolic phases of growth (Wehrly et al., 2009), indicating that their expression is highly regulated in these distinct intracellular locations of F. tularensis.
OxyR, SoxR, PerR and RNA polymerase of stationary phase (RpoS) serve as primary regulators of oxidative stress in bacteria (Chiang and Schellhorn, 2012). Additionally, the regulators NorR and IscR are also induced in response to oxidative stress to mitigate RNS and the formation of iron-sulfur (Fe-S) clusters (Gardner et al., 2003). OxyR belongs to the LysR family of transcriptional regulators, consisting of an N-terminal DNA-binding domain with a winged helix-turn-helix motif and a C-terminal regulatory domain (Schell, 1993a). OxyR in E. coli regulates the expression of genes required for protection against H2O2 toxicity, heat stress, oxidant-mediated cell damage, and phagocyte-mediated killing (Staudinger et al., 2002). The activation of OxyR occurs via the oxidation of two conserved cysteine residues (Cys199 and Cys208) in response to H2O2-induced oxidative stress (Lee et al., 2004). In contrast, SoxR protects against O2− radicals and is induced in response to O2−-generating compounds, NO or high levels of H2O2 (Nunoshiba et al., 1992). Sequence analysis of the F. tularensis genome reveals the presence of OxyR and RpoS, while the SoxR/SoxS regulons are absent (Larsson et al., 2005).
The mechanisms of regulation of oxidative stress responses in F. tularensis are not known. This study investigated the role of OxyR of the F. tularensis LVS in tularemia pathogenesis and the regulation of oxidative stress responses. The results from this study demonstrate that OxyR of F. tularensis regulates oxidative stress responses to promote resistance against ROS, thereby contributing to its intracellular survival and promoting its virulence in mice. Proteomic analysis reveals that OxyR modulates the level of 128 proteins, indicating a broader regulatory role of OxyR in overcoming oxidative stress. Moreover, OxyR regulates the transcription of the primary antioxidant enzyme genes ahpC and katG by binding directly to their putative promoter regions. These results indicate that OxyR is an important regulator of the oxidative stress response and renders F. tularensis a pathoadaptive advantage to establish an intracellular niche. An understanding of these unique pathogenic mechanisms is essential for the development of effective therapeutics and prophylactics against this important biothreat agent.
Results
Genomic organization and generation of an oxyR gene deletion mutant of the F. tularensis LVS
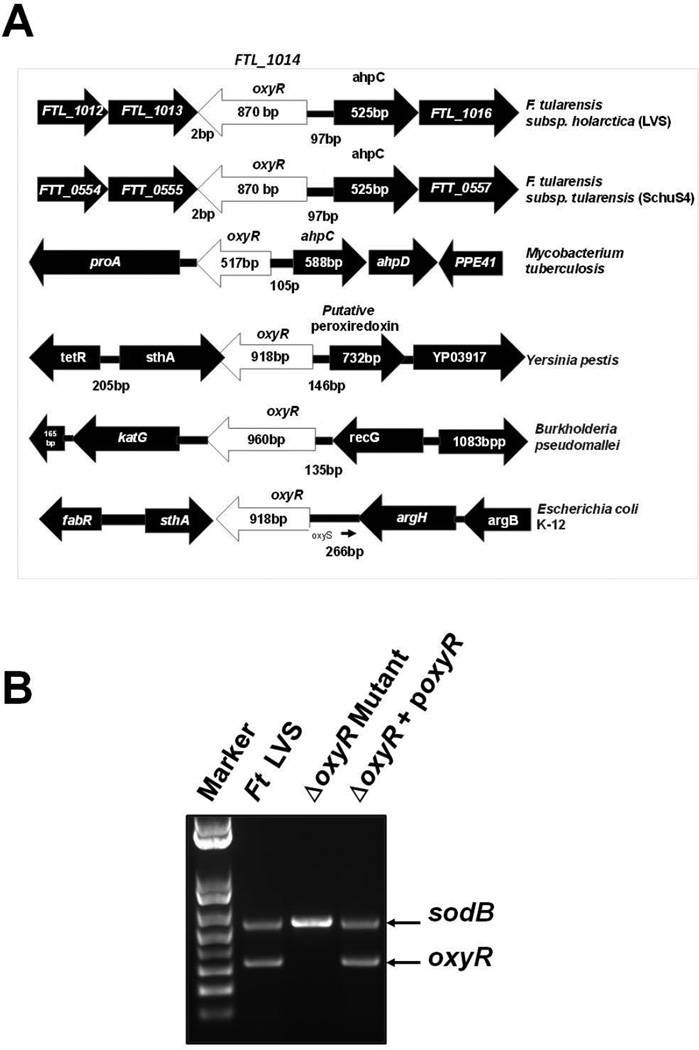
The 289-amino-acid, 34.6 kDa OxyR protein in the F. tularensis LVS is encoded by the FTL_1014 (oxyR) gene. The oxyR gene in F. tularensis is located upstream of and transcribed divergently from the gene encoding AhpC. A similar genomic organization is also present in the virulent F. tularensis SchuS4 strain and in Mycobacterium tuberculosis (Fig. 1A). However, the oxyR gene in Yersinia pestis, B. pseudomallei, and E. coli K-12 is bigger in size and has neighboring genes other than ahpC (Fig. 1A). The sequence analysis of the oxyR gene reveals a conserved N-terminal (1–60 amino acid) helix-turn-helix domain, which serves as a sequence-specific DNA binding domain, found in the LysR family of transcriptional regulators. The C-terminal domain is a Type 2 periplasmic binding superfamily domain and is required for the uptake of many substrates. The OxyR protein of F. tularensis has two redox-sensitive cysteine residues (Cys199 and Cys208) that are highly conserved and are required for its oxidant-dependent activation. Sequence alignment analyses show that OxyR of the F. tularensis LVS exhibits 99% identity with the OxyR of F. tularensis SchuS4, 36% identity with E. coli and Yersinia pestis, and 34% identity with that of B. pseudomallei (Fig. S1).
Figure 1. Genomic organization and generation of the oxyR gene deletion mutant of the F. tularensis LVS.
(A) The genomic organization of the oxyR gene of the F. tularensis LVS and its comparison with the oxyR gene from the indicated bacterial pathogens. (B) Multiplex colony PCR using oxyR gene-specific primers and sodB gene primers as internal controls. Amplification of the sodB gene confirmed the presence of the DNA template in the reaction, whereas the absence of the oxyR gene product in the ΔoxyR mutant confirmed the deletion of the oxyR gene.
To investigate the role of OxyR in the pathogenesis of tularemia and the transcriptional regulation of the genes involved in the oxidative stress response, we generated an in-frame oxyR gene deletion mutant (ΔoxyR mutant) and a transcomplemented strain (ΔoxyR + poxyR) of the F. tularensis LVS. The oxyR gene deletion and transcomplementation was confirmed by PCR using oxyR gene-specific primers (Fig. 1B). sodB gene primers were used as internal controls. The in-frame gene deletion of the oxyR gene in the ΔoxyR mutant was also confirmed by DNA sequencing (data not shown).
OxyR of F. tularensis is not essential for cell viability but is required for growth under acidic conditions
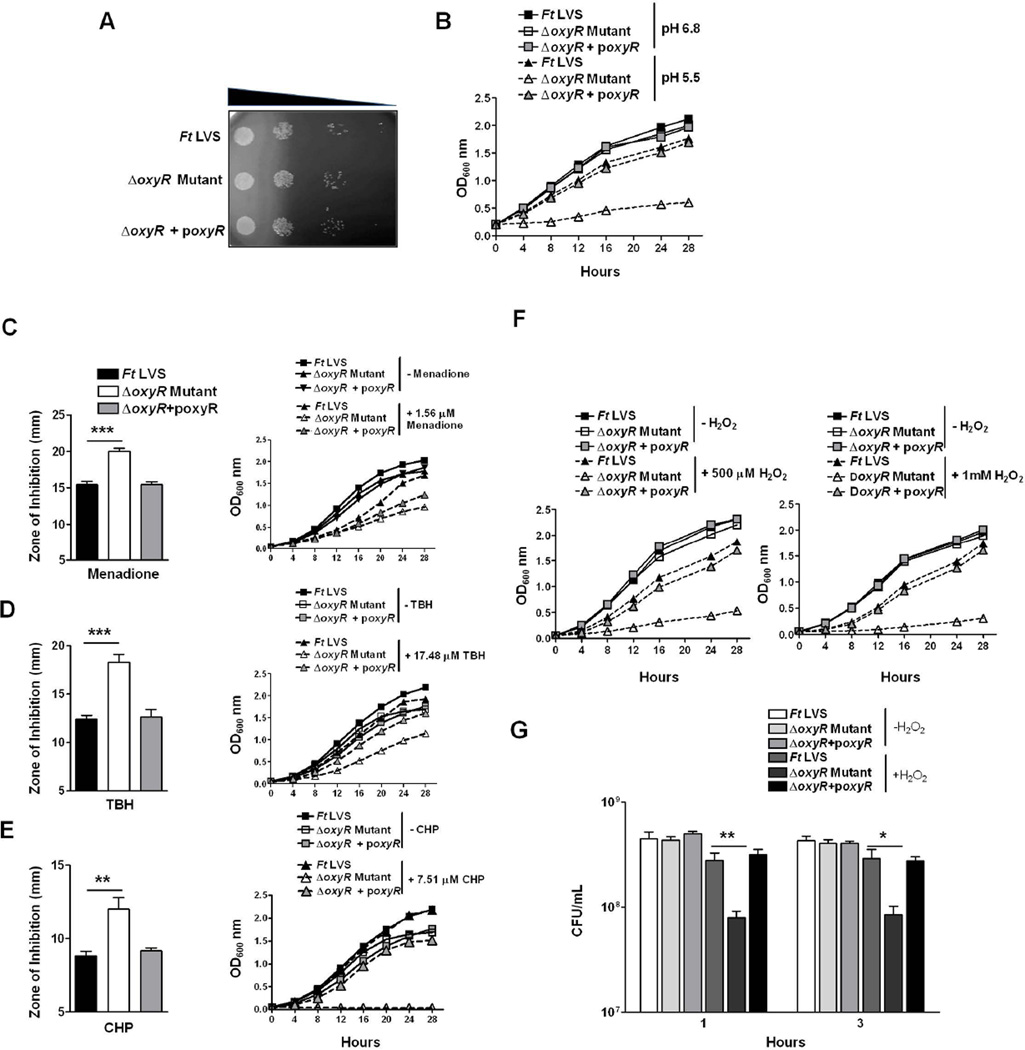
Loss of the oxyR gene in several bacterial pathogens is associated with a viability-deficient phenotype known as “plating defect” (Jiang et al., 2014; Shi et al., 2015; Gonzalez-Flecha and Demple, 1997). We investigated whether the ΔoxyR mutant of the F. tularensis LVS exhibits a similar phenotype. Cultures of the wild-type F. tularensis LVS, the ΔoxyR mutant and the transcomplemented strain grown to mid-log phase were collected, centrifuged, diluted 10-fold and plated on chocolate agar plates to determine the differences in bacterial viability. No differences were observed in the number of colonies recovered from these bacterial strains, indicating that the loss of the oxyR gene is not associated with decreased viability (Fig. 2A). We also determined whether the loss of oxyR results in enhanced sensitivity towards stressors such as SDS, sodium deoxycholate, Triton X-100 or ethidium bromide by performing disc diffusion assays. Identical zones of inhibition were obtained for all the three bacterial strains against these stressors, indicating that OxyR does not contribute to bacterial membrane integrity (Fig. S2). We further investigated whether the ΔoxyR mutant exhibits growth defects when grown aerobically in liquid culture. No differences in growth were observed between the F. tularensis LVS, the ΔoxyR mutant or the transcomplemented strain grown aerobically in Mueller Hinton broth (MHB) at a pH of 6.8. However, when these bacterial strains were grown in MHB adjusted to a pH of 5.5, the growth of the ΔoxyR mutant was severely impaired. On the other hand, the growth of the F. tularensis LVS or the transcomplemented strain remained unaltered (Fig. 2B). Collectively, these results demonstrate that OxyR is not required for the viability or structural integrity of F. tularensis; however, it does play a role in the survival of Francisella at an acidic pH.
Figure 2. OxyR of F. tularensis is not essential for cell viability but is required for growth under acidic conditions and resistance against superoxide radicals, hydrogen peroxide, and organic peroxides.
(A) A cell viability assay was performed by growing the indicated bacterial strains aerobically. The cultures were diluted 10-fold and spotted on MH-chocolate agar plates. (B) The F. tularensis LVS, the ΔoxyR mutant and the ΔoxyR + poxyR transcomplemented strains were grown aerobically in MHB at pH 6.8 and pH 5.5, and OD600 readings were recorded at 4 hr intervals. (C–E) The susceptibility of the ΔoxyR mutant to the superoxide-generating compounds menadione (C), organic peroxide tert-Butyl hydroperoxide (TBH; D) and cumene hydroperoxide (CHP; E) was tested by disk diffusion assay as described in Experimental Procedures. The plates were incubated for 48–72 hrs, and the zones of inhibition around the discs were measured (left panels). Growth curves of the F. tularensis (Ft) LVS, the ΔoxyR mutant and the transcomplemented strain in the presence of the indicated concentrations of menadione (C), TBH (D) and CHP (E), right panels. (F) Growth curves in the presence of 500 µM and 1 mM concentrations of hydrogen peroxide (H2O2). (G) Bacterial killing assay in the presence of 750 µM H2O2. The indicated bacterial strains were exposed to H2O2 for 1 and 3 hrs, diluted 10-fold and plated on MH-chocolate agar plates to recover viable bacteria. The results are expressed as CFU/mL. The data are representative of at least 4–5 independent experiments and represented as the mean ± SD. The data were analyzed by ANOVA, and the P values were recorded. *P<0.05; **P<0.01; ***P<0.001.
OxyR protects F. tularensis from the growth-inhibitory effects of superoxide radicals, hydrogen peroxide and organic peroxides
We next determined the role of OxyR in providing resistance against oxidants using disc diffusion assays, growth curves and bacterial killing assays. In disc diffusion assays, the ΔoxyR mutant showed enhanced sensitivity towards the superoxide-generating compound menadione and the organic peroxides TBH and CHP, as indicated by significantly larger zones of inhibition (20.0±1.3 mm, 18.2±2.2 mm, and 12.0±1.8 mm for menadione, TBH and CHP, respectively) than those observed for the wild-type F. tularensis LVS (15.4±1.4, 12.4±0.9, and 8.8±0.7 mm for menadione, TBH and CHP, respectively) or the transcomplemented strain (15.4±1.23, 12.6±1.86, and 9.1±0.4 mm for menadione, TBH and CHP, respectively). Similarly, when growth curves were generated in the presence of these oxidants, the growth of the ΔoxyR mutant was found to be severely impaired (Fig. 2C, D and E). The ΔoxyR mutant showed extreme sensitivity towards H2O2 compared to the wild-type F. tularensis LVS or the transcomplemented strain and failed to grow in the presence of 500 µM or 1 mM concentrations of H2O2 (Fig. 2F). The sensitivity of the ΔoxyR mutant towards H2O2 was also tested using a bacterial killing assay. Significantly fewer viable ΔoxyR mutant bacteria were recovered at 1 and 3 hrs post-exposure to H2O2 compared to those observed for the wild-type F. tularensis LVS or the transcomplemented strain (Fig. 2G). Collectively, these results demonstrate that OxyR of F. tularensis mediates resistance against oxidative stress generated by superoxide radicals, organic peroxides and H2O2.
OxyR of F. tularensis is required for intracellular survival and virulence in mice
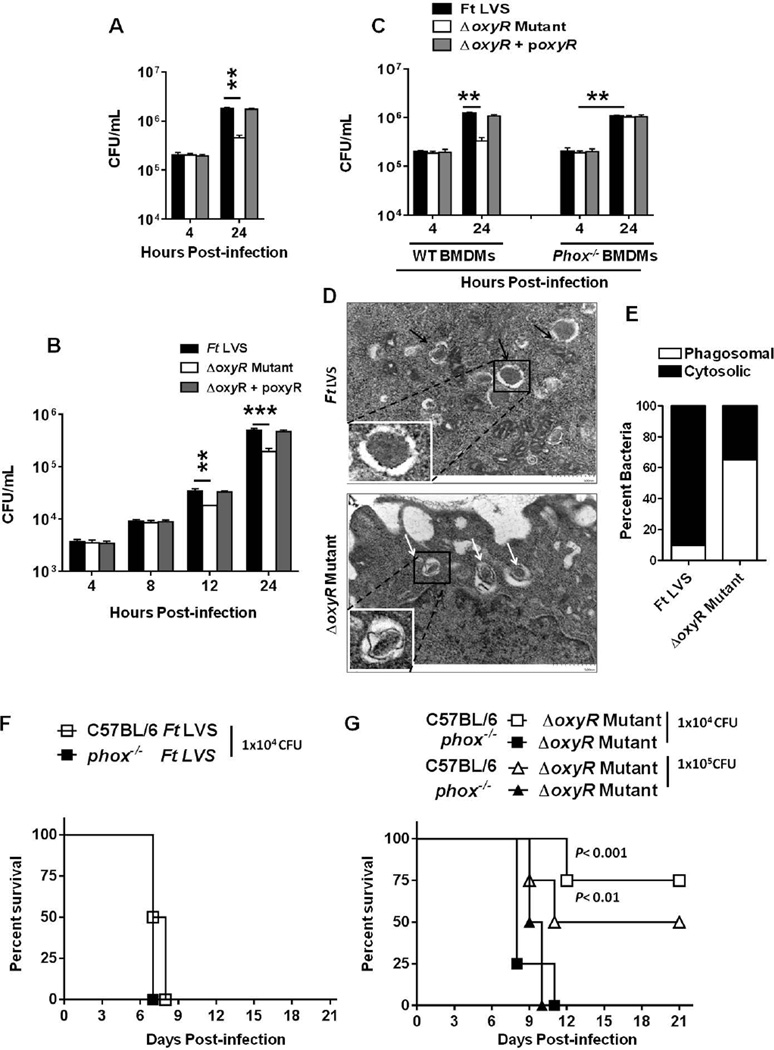
The role of OxyR of F. tularensis in intramacrophage survival was determined by performing gentamicin protection assays in the RAW macrophage cell line and in primary bone marrow derived macrophages (BMDMs) derived from wild-type or gp91phox−/− C57BL/6 mice. Equal numbers of the F. tularensis LVS or the ΔoxyR mutant bacteria were recovered from the infected RAW macrophages after 4 hrs of infection indicating not only an equivalent uptake but also an equivalent bacterial survival in early phagosomes. However, ten-fold fewer ΔoxyR mutant bacteria compared to the wild-type F. tularensis LVS or the transcomplemented strain were recovered from the infected macrophages 24 hrs post-infection (Fig. 3A). We further investigated whether the ΔoxyR mutant is sensitive to physiological levels of ROS in the host cell cytosol by performing a gentamicin protection assay in the A549 lung epithelial cell line. Similar to RAW macrophages, significantly fewer ΔoxyR mutant bacteria were recovered at 12 and 24 hrs post-infection from epithelial cells than those from the wild-type F. tularensis LVS or the transcomplemented strain infected cells (Fig. 3B). Similar results were observed in BMDMs derived from wild-type C57BL/6 mice, indicating that the ΔoxyR mutant is deficient for intracellular survival. However, the growth of the ΔoxyR mutant was restored to the wild-type F. tularensis LVS levels in BMDMs derived from gp91phox−/− mice (Fig. 3C). We further examined the localization of the ΔoxyR mutant in macrophages using transmission electron microscopy (TEM) at 1 and 6 hrs post-infection. The majority of the wild-type F. tularensis LVS and the ΔoxyR mutant bacteria (>90%) were localized in the phagosomes 1 hr post-infection (not shown). However, at 6 hrs post-infection, only 10% wild-type F. tularensis LVS bacteria remained localized to the phagosomes; while 90% bacteria had escaped the phagosomes and were present in the macrophage cytosol. In contrast, 65% of the ΔoxyR mutant bacteria were still trapped inside the phagosomes and only 35% bacteria were present in the macrophage cytosol (Fig. 3D, E). Collectively, these results demonstrate that the ΔoxyR mutant has attenuated intracellular growth. Furthermore, these results also indicate that the ΔoxyR mutant is sensitive to NADPH oxidase-dependent ROS, which may reduce its fitness to escape phagosomes and therefore its ability to replicate in the cytosol.
Figure 3. OxyR of F. tularensis is required for intracellular survival and virulence in mice.
(A) The RAW macrophage cell line, (B) A549 Type II alveolar epithelial cells and (C) primary BMDMs derived from wild-type C57BL/6 or gp91phox−/− mice (n=4 biological replicates) were infected with the F. tularensis (Ft) LVS, the ΔoxyR mutant or the transcomplemented strain (ΔoxyR + poxyR) at an MOI of 100. The macrophages were lysed after 4 and 24 hrs (A and C); while the epithelial cells were lysed at 4, 8, 12 and 24 hrs post-infection (B), diluted 10-fold and plated on MH-chocolate agar plates for the enumeration of bacterial numbers. The data are representative of three independent experiments conducted with identical results (A and C) or cumulative results from two independent experiments (B). The data are expressed as CFU/mL and analyzed by ANOVA with a Tukey-Kramer post-test, and comparisons are shown with Ft LVS. **P<0.01; ***P<0.001. (D) TEM of RAW macrophages infected with the F. tularensis LVS or the ΔoxyR mutant at an MOI of 100, imaged 6 hrs post-infection. Black arrows indicate extra-phagosomal bacteria, while the white arrows indicate phagosomal bacteria. (E) Quantitation of phagosomal and cytosolic bacteria was performed by counting at least 100 bacteria in randomly selected sections of the infected macrophages on a Hitachi HT 7700 TE Microscope (61,000× magnification). The data shown are representative of two independent experiments conducted with similar results. (F) Wild-type or gp91phox−/− mice on a C57BL/6 background (n=6 mice/ group) were infected intranasally with 1×104 CFUs of the F. tularensis LVS. (G) Wild-type or gp91phox−/− mice on a C57BL/6 background (n=6 mice/ group) were infected intranasally with 1×104 or 1×105 CFUs of the ΔoxyR mutant. The mice were observed for morbidity and mortality over a period of 21 days. The results are represented as Kaplan-Meier survival curves, and the statistical significance was determined by log-rank test.
The role of OxyR in virulence was determined by infecting wild-type or gp91phox−/− C57BL/6 mice intranasally with 1×104 (equivalent to 1×LD100) F. tularensis LVS or 1×104 or 1×105 CFUs of the ΔoxyR mutant. The infected mice were observed for survival for a period of 21 days. One hundred percent of the wild-type and gp91phox−/− C57BL/6 mice infected with the F. tularensis LVS succumbed to infection by days 6 and 8 post-infection, respectively (Fig. 3F). In contrast, 80 and 60% of the wild-type C57BL/6 mice inoculated with 1×104 or 1×105 CFUs of the ΔoxyR mutant, respectively, survived the infection. However, 100% of the gp91phox−/− mice inoculated with similar doses of the ΔoxyR mutant succumbed to infection by days 10–11 post-infection (Fig. 3G). Collectively, these results demonstrate that OxyR of F. tularensis plays an important role in intracellular survival as well as in F. tularensis virulence in mice and that OxyR contributes to virulence by overcoming the oxidative stress generated by NADPH oxidase.
OxyR is a global regulator of the oxidative stress response in F. tularensis
Our preceding results demonstrated that the ΔoxyR mutant is highly sensitive to oxidants, deficient for intramacrophage growth, and attenuated for virulence in mice. We next investigated the mechanisms through which OxyR regulates the oxidative stress response of F. tularensis. We took a broad proteomic approach by performing iTRAQ analysis to profile differentially induced proteins in the ΔoxyR mutant compared to those observed in the wild-type F. tularensis LVS. The data analyses were performed using the Paragon algorithm against the database within ProteinPilot V4.5 software. For a protein to be designated as being differentially induced, it must have been quantified in at least three spectra (allowing generation of a P-value), had a fold change of more than 1.3 or less than 0.7 from two independent experiments performed with two biological replicates each, and had a P-value of <0.05. A log2 ratio of the fold change in protein levels (ΔoxyR mutant/F. tularensis LVS) of +0.11 or higher indicated an increase, while a ratio less than −0.15 indicated a decrease in protein levels.
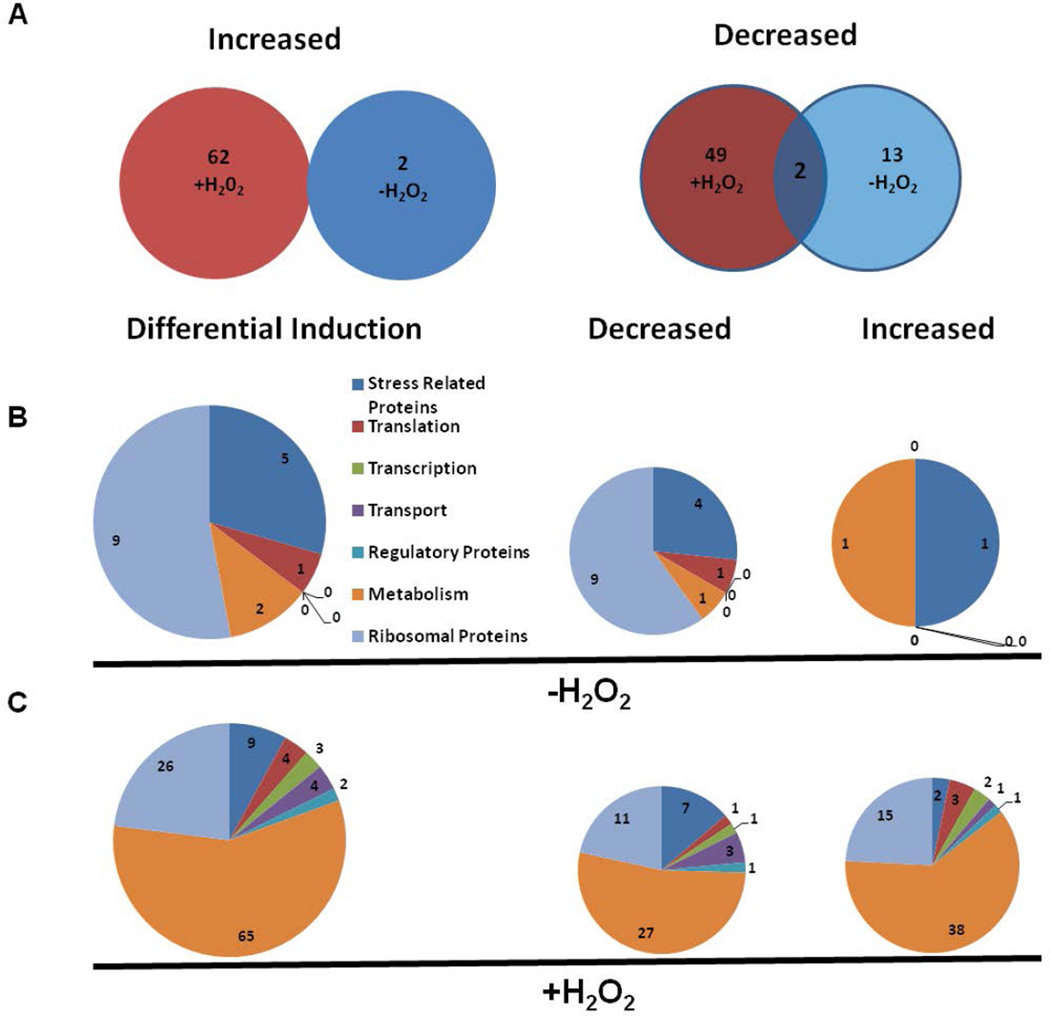
A total of 128 out of 252 proteins identified in the iTRAQ analysis were differentially induced in the ΔoxyR mutant (Table S1). These included proteins involved in oxidative/general stress resistance, translation, transcription, transport, metabolism, regulatory, and ribosomal functions. Of these, 2 proteins were elevated and 15 were reduced in the ΔoxyR mutant not exposed to H2O2. Exposure of the ΔoxyR mutant to H2O2 resulted in increased levels of 62 proteins, while 51 proteins were decreased. Two proteins, AhpC and SodB, remained suppressed in the ΔoxyR mutant irrespective of its exposure to H2O2 (Fig. 4A). The 15 proteins that were suppressed in the ΔoxyR mutant not exposed to H2O2 included 4 proteins involved in stress resistance, 1 in translation, 1 in general metabolism, and 9 ribosomal proteins (Fig. 4B). The 51 proteins that were suppressed in the ΔoxyR mutant upon exposure to H2O2 included 27 proteins involved in metabolism, 11 ribosomal proteins, 7 stress resistance proteins, 3 transport proteins, and 1 protein each involved in transcription, translation and gene regulation (Fig. 4C). The two proteins that were increased in the ΔoxyR mutant not exposed to H2O2 were ClpB and glucosamine fructose-6-phosphate aminotransferase, while a total of 62 proteins were increased when the ΔoxyR mutant was exposed to H2O2. A majority of these proteins were those involved in metabolism and transport function and ribosomal proteins (Fig. 4B, C). Collectively, these results indicate that the loss of OxyR results in the differential induction of several proteins in the ΔoxyR mutant of F. tularensis.
Figure 4. Differential induction of proteins in the ΔoxyR mutant of F. tularensis with or without exposure to H2O2.
(A) Total number of differentially induced proteins in the ΔoxyR mutant compared to the wild-type F. tularensis LVS. (B, C) Profile of differentially induced proteins involved in various cellular functions in the ΔoxyR mutant in the presence or absence of H2O2-induced oxidative stress.
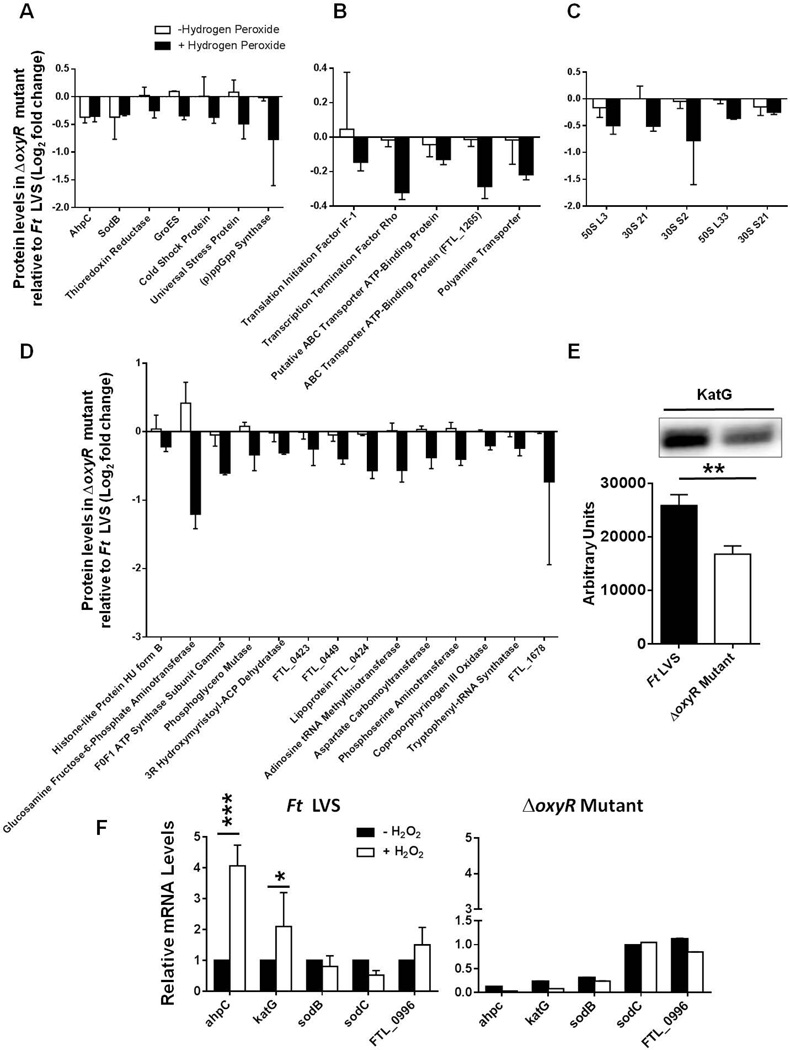
We categorized proteins that were less abundant in the ΔoxyR mutant when exposed to H2O2 into four categories: Category I included proteins required for oxidant and general stress resistance, such as AhpC (FTL_1015), SodB (FTL_1791), thioredoxin reductase (FTL_1571), the chaperonin protein GroES (FTL_1715), cold shock (FTL_0457) and universal stress (FTL_0166) proteins, and alarmone (p)ppGpp synthase (FTL_1413) (Fig. 5A). Category II included proteins involved in transcription, translation and transport functions. The predominantly less abundant proteins in this category included the translation initiation factor IF-1 (FTL_1236), the transcription termination factor rho (FTL_0610), ABC transporter (FTL_1870), ABC binding protein (FTL_1065), and polyamine transporter protein (FTL_0679) (Fig. 5B). The ribosomal proteins that were prominently suppressed in the ΔoxyR mutant were categorized as Category III proteins (Fig. 5C). Category IV included proteins involved in metabolism and cellular function, as well as hypothetical proteins. The prominently suppressed proteins were glucosamine fructose-6-phosphate aminotransferase (FTL_0454), ATP synthase subunit gamma (FTL_1800), adenosine tRNA methylthiotransferase (FTL_0886), and the hypothetical protein FTL_1678 (Fig. 5D). However, our iTRAQ results did not reveal the differential induction of KatG in the ΔoxyR mutant compared to the wild-type F. tularensis LVS. In several bacterial pathogens, it has been reported that OxyR regulates the expression of katG (Ieva et al., 2008;Jagielski et al., 2015;Kim et al., 2015;Kim and Holmes, 2012). To determine the effect of OxyR on the induction of KatG, we performed western blot analysis to determine the levels of KatG in the wild-type F. tularensis LVS and the ΔoxyR mutant. Significantly lower levels of KatG protein were observed in the ΔoxyR mutant compared to the F. tularensis LVS (Fig. 5E). Collectively, these results indicate that OxyR in F. tularensis serves as a positive regulator of the oxidative stress response.
Figure 5. Suppressed proteins in the ΔoxyR mutant of F. tularensis with or without exposure to H2O2.
The most prominently decreased proteins in the ΔoxyR mutant compared to the wild-type F. tularensis LVS are shown from the top 128 differentially induced proteins in the ΔoxyR mutants. The proteins are grouped according to their functional categories. (A) oxidant and general stress resistance (Category I); (B) transcription, translation, and transport function (Category II); (C) ribosomal proteins (Category III); (D) metabolism and hypothetical proteins (Category IV). The data shown are cumulative of two independent iTRAQ experiments, each conducted with duplicate samples. The data are represented as the ratio of the log2-fold change in protein levels between the wild-type F. tularensis LVS and the ΔoxyR mutant. (E) Western blot analysis for the determination of KatG expression in the wild-type F. tularensis LVS and the ΔoxyR mutant. The bottom panel shows quantitation of the bands (n=3 blots). The statistical analysis was performed by Student’s t test. **P<0.01. (F) Transcriptional analysis of the indicated antioxidant enzyme genes in the F. tularensis (Ft) LVS and the ΔoxyR mutant in the presence or absence of H2O2. The data are representative of three independent experiments with similar results and are presented as relative mRNA levels. The data were analyzed by ANOVA, and the P values were recorded. *P<0.05; ***P<0.001.
We next investigated whether decreased levels of the aforementioned proteins in the ΔoxyR mutant are due to differences originating at the level of transcription. We focused our attention on the genes encoding the primary antioxidant enzymes ahpC, sodB and katG of F. tularensis. An additional gene encoding ahpC (FTL_0996) has also been found in F. tularensis. Although the protein levels of FTL_0996 and another primary antioxidant enzyme, SodC, remained unaltered in the iTRAQ analysis, we also included these two primary antioxidant genes as controls. We conducted transcriptional analysis of the ahpC, sodB, katG, sodC, and FTL_0996 genes by qRT-PCR in the wild-type F. tularensis LVS and the ΔoxyR mutant with or without exposure to H2O2. The transcript levels of ahpC and katG were nearly 4- and 2-fold higher, respectively, when the F. tularensis LVS was exposed to H2O2; however, the transcript levels of sodB, sodC and FTL_0996 remained unaltered. In the ΔoxyR mutant, irrespective of its treatment with H2O2, the expression of these genes either remained downregulated (ahpC, katG and sodB) or remained unchanged (sodC, FTL_0996) (Fig. 5F). These results demonstrate that OxyR positively regulates the oxidative stress response, and the regulation occurs at the level of transcription.
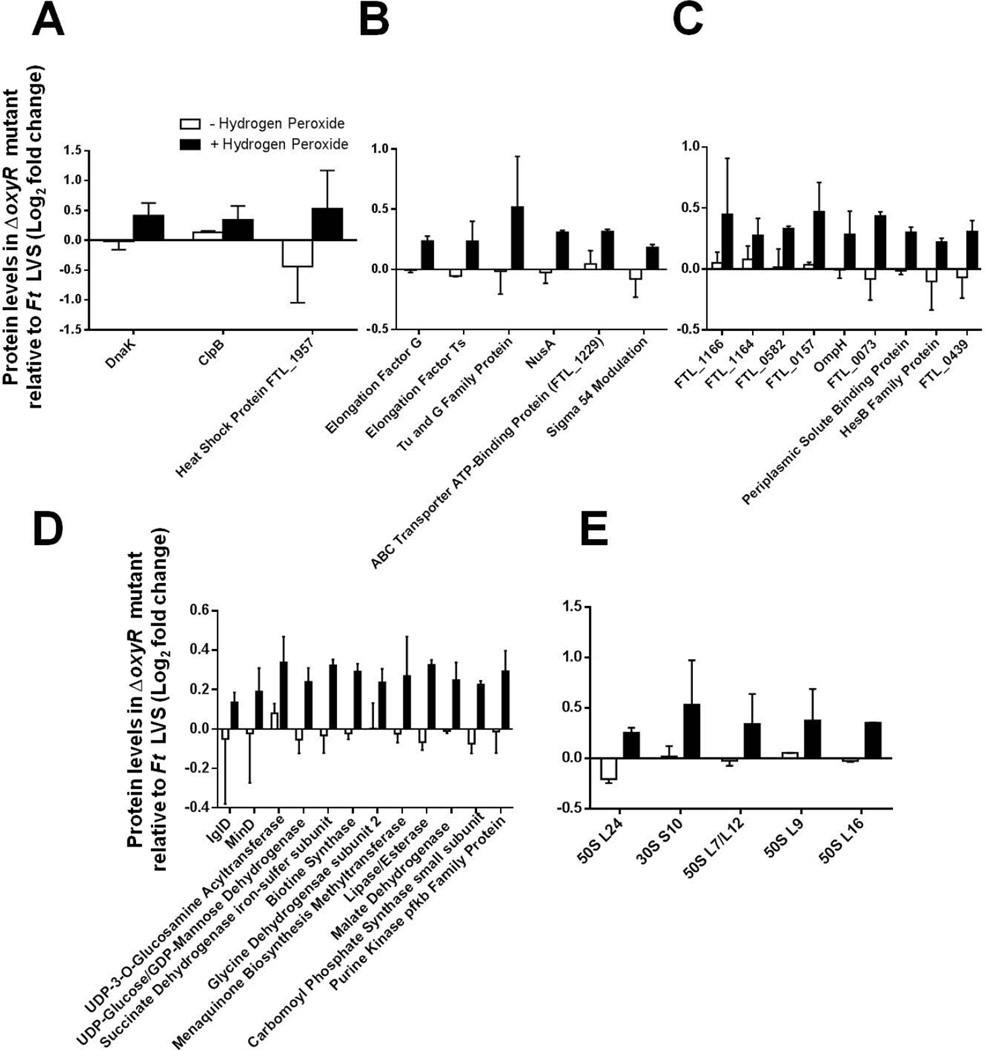
Several proteins were found to be increased in the ΔoxyR mutant upon exposure to H2O2 and were also grouped into four categories. The category I general stress response proteins included DnaK (FTL_1191), ClpB (FTL_0094), and heat shock protein (FTL_1957) (Fig. 6A). The category II proteins involved in translation, transcription, and transport function included: elongation factors G (FTL_0234), Ts (FTL_0225), and TU and G family protein (FTL_0768), the transcription factor NusA (FTL_1810), the ABC transporter ATP binding protein (FTL_1229) and Sigma 54 modulation protein (FTL_1179) (Fig. 6B). A number of hypothetical proteins, ribosomal proteins, and proteins involved in metabolism were also produced at higher levels in the ΔoxyR mutant when exposed to H2O2 (Fig. 6C, D and E). Transcriptional analysis by qRT-PCR confirmed the upregulated expression of a representative group of the abundant proteins (data not shown). Collectively, these results demonstrate that OxyR serves as a global regulator of the oxidative stress response in F. tularensis and regulates the oxidative stress response proteins involved in a multitude of functions, both positively and negatively.
Figure 6. Elevated proteins in the ΔoxyR mutant upon exposure to H2O2.
The most prominently increased proteins in the ΔoxyR mutant compared to the wild-type F. tularensis LVS are shown from the top 62 elevated proteins in the ΔoxyR mutants exposed to H2O2. The proteins are grouped according to their functional categories. (A) oxidant and general stress resistance (Category I); (B) transcription, translation, and transport functions (Category II); (C) hypothetical proteins (Category III); (D) metabolism (Category IV); (E) ribosomal proteins (Category V). The data shown are cumulative of two independent iTRAQ experiments, each conducted with duplicate samples. The data are represented as the ratio of log2-fold changes in the protein levels between the wild-type F. tularensis LVS and the ΔoxyR mutant.
OxyR regulates the transcription of primary antioxidant enzymes by physically interacting with their putative promoter regions
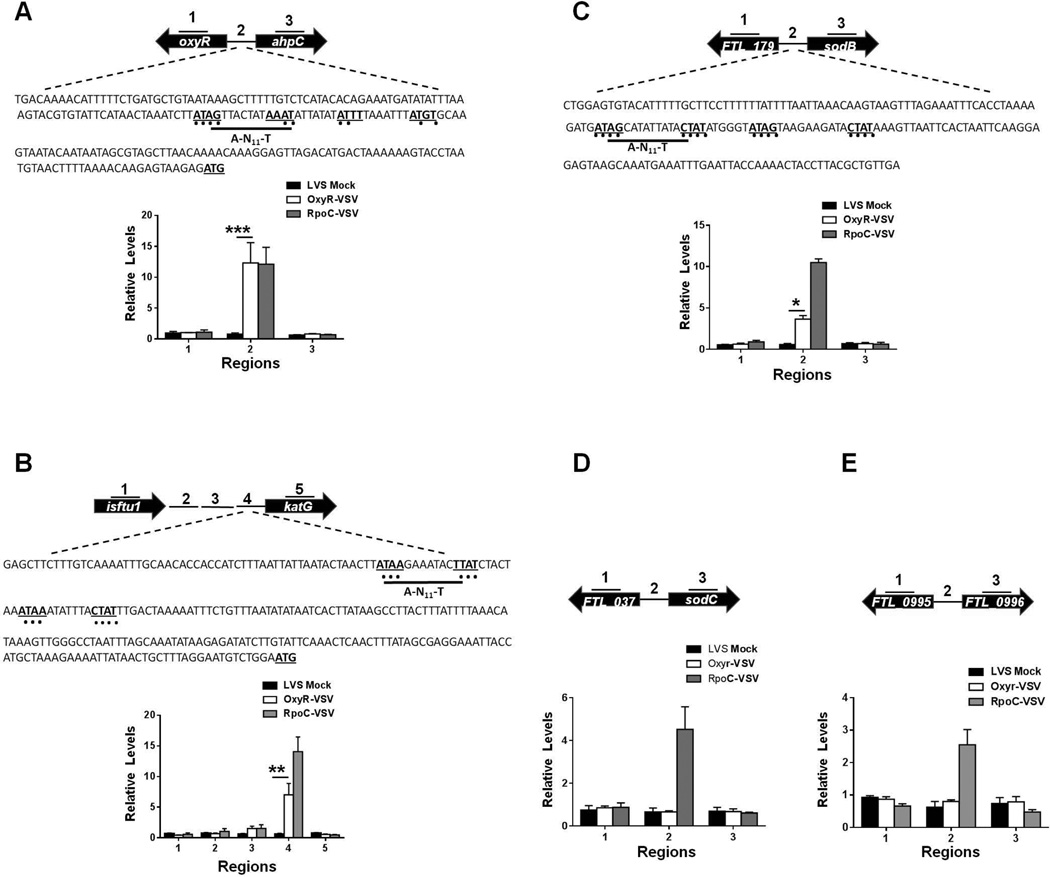
To investigate the mechanism of the transcriptional regulation of the primary antioxidant enzyme genes ahpC, katG and sodB by OxyR, we generated an F. tularensis LVS strain expressing an epitope-tagged version of OxyR by fusing the vesicular stomatitis virus glycoprotein (VSV-G) epitope to its C-terminus (OxyR-VSV-G). This allowed the expression of OxyR-VSV-G from the native oxyR locus. The oxyR-VSV-G strain was similar to the wild-type F. tularensis LVS with respect to its growth attributes and sensitivity to oxidants (data not shown). The oxyR-VSV-G strain was used to detect the in vivo binding of OxyR-VSV-G to the promoters of the ahpC, katG and sodB genes by performing ChIP followed by qRT-PCR analysis. We observed nearly 15-fold enrichment of Region 2, the stretch of DNA immediately upstream of the ahpC gene. We did not observe enrichment of either Region 1 (the region within the oxyR gene) or Region 3 (the region within the ahpC gene). These results indicate that OxyR binds specifically to the upstream promoter region of the ahpC gene. The Region 2 DNA sequence upstream of the ahpC gene also contains a putative OxyR-binding domain, ATAG-N7-AAAT-N7-ATGT, and additional conserved A-N11-T residues that show significant homology to the conserved OxyR-binding domain of E. coli, ATAG-N7-CTAT-N7-ATAG-N7-CTAT (Schell, 1993b;Wei et al., 2012) (Fig. 7A). Similarly, nearly 10-fold enrichment of Region 4, immediately upstream of the katG gene, which also contains conserved OxyR-binding motifs, was observed (Fig. 7B). The region upstream of the sodB gene also contains a conserved OxyR binding domain; however, the binding of OxyR to the sodB promoter region (Region 2) did not appear to be as strong as that observed for either the ahpC or the katG genes, and only 5-fold-enrichment of this region was observed by ChIP analysis (Fig. 7C). No enrichment of the regions upstream of either the sodC or FTL_0996 genes was observed, indicating that OxyR does not bind to the promoter regions of these genes (Fig. 7D and E). The promoter regions of these genes also lacked a putative OxyR-binding domain. Collectively, these results demonstrate that OxyR regulates the expression of the ahpC, katG and sodB genes by binding to the upstream promoter regions of these genes.
Figure 7. OxyR regulates the transcription of genes encoding primary antioxidant enzymes of F. tularensis by physically interacting with their putative promoter regions.
Chromatin immunoprecipitation (ChIP) was performed using anti-VSV-G-agarose beads, as described in the Experimental Procedures section. The fragments of intergenic regions covering the putative promoter sequences and the coding regions of the up- and downstream genes (indicated by numbers) in ChIP and input samples of the indicated bacterial strains were analyzed for enrichment by qRT-PCR. The values were normalized to the input and a fopA coding region as internal controls. The qRT-PCR results of ChIP from Ft oxyR-VSVG along with Ft LVS (mock) and rpoC-VSVG (positive control) are shown (n=3 biological replicates). (A) Illustrated ahpC locus; (B) katG locus (C) sodB locus; (D) sodC locus and (E) FTL_0996 locus. The data shown are representative of three independent experiments with identical results. In A, B and C, the DNA sequences of the upstream regions of the ahpC, katG and sodB genes, respectively, with conserved OxyR binding motifs (bold letters with underlined dots) are shown. The data were analyzed by ANOVA, and the P values were recorded. *P<0.05; **P<0.01; ***P<0.001.
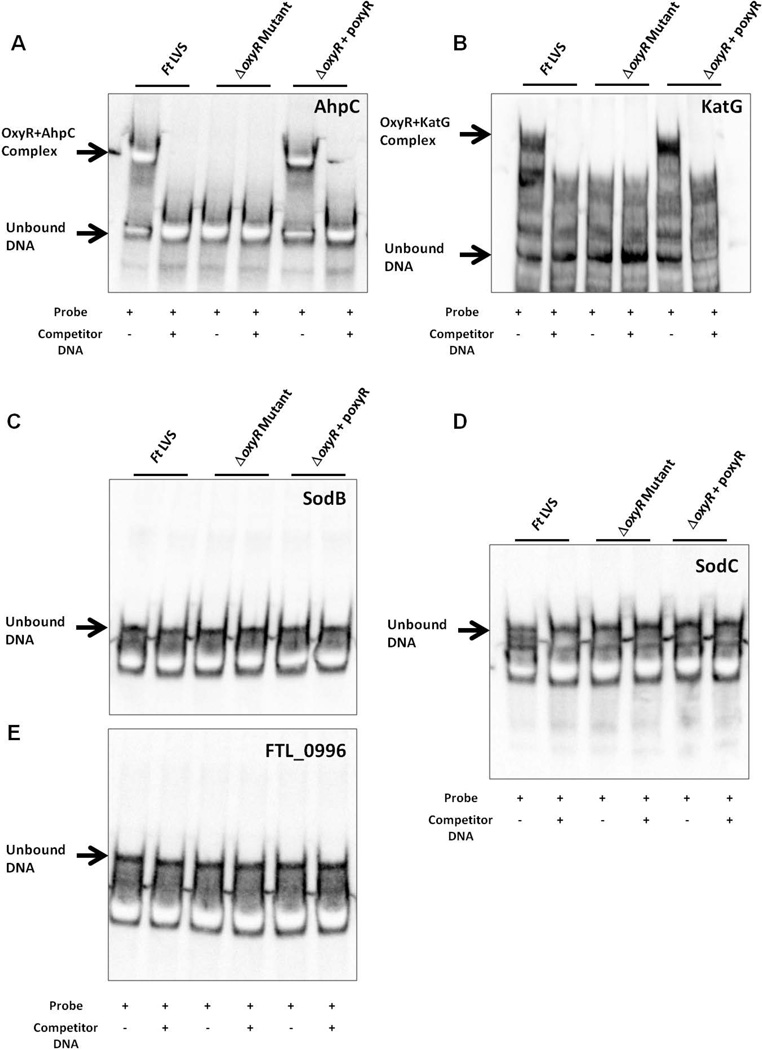
Next, we performed an electrophoretic mobility shift assay (EMSA) to confirm the binding of OxyR to the promoter regions of the ahpC, katG, and sodB genes. We used biotin-labeled DNA probes from the regions identified in Fig. 7 and whole-cell lysates from the wild-type F. tularensis LVS, the ΔoxyR mutant and the transcomplemented strains for EMSA. The unlabeled fragments from the same regions were used as competitor DNA. The activity of the ΔoxyR mutant lysate was confirmed by the binding of an OxyR-independent transcriptional regulator, PmrA1, to its cognate pmrA promoter region (Fig. S3A and B). Consistent with our ChIP results, we observed a mobility shift as a result of the binding of OxyR to the promoter regions of the ahpC and katG genes in lysates from the wild-type F. tularensis LVS or the transcomplemented strain, but not in lysates from the ΔoxyR mutant. The addition of unlabeled competitor DNA to the F. tularensis LVS or the transcomplemented strain lysates prevented the mobility shift and confirmed the specificity of the binding of OxyR to the ahpC and katG gene promoter regions (Fig. 8A and B). However, we failed to detect any mobility shift when EMSA was performed with the binding region identified for the sodB gene (Fig. 8C). Similarly, no binding of OxyR to the upstream promoter regions of either the sodC or FTL_0996 genes was observed (Fig. 8D and E). Collectively, these results demonstrate that OxyR regulates the transcription of the ahpC and katG genes by binding to their promoter regions.
Figure 8. OxyR interacts with the putative promoter regions of primary antioxidant genes of F. tularensis.
Electrophoretic mobility shift assay (EMSA) with the promoter regions (identified in Fig. 7) for ahpC (A); katG (B); sodB (C); sodC (D) and FTL_0996 (E) of F. tularensis. EMSA was performed using bacterial lysates from the F. tularensis (Ft) LVS, the ΔoxyR mutant and the transcomplemented strain (ΔoxyR+poxyR) with a LightShift Chemiluminescent EMSA kit, as described in the Experimental Procedures section. Biotinylated promoter regions were used as the probes, whereas unlabeled promoter regions were used as controls. The results are representative of two independent experiments.
Discussion
OxyR, SoxR, PerR and RopS serve as primary regulators of oxidative stress in bacteria (Chiang and Schellhorn, 2012). Additionally, the regulators NorR and IscR are also induced in response to oxidative stress to mitigate RNS and the formation of iron-sulfur (Fe-S) clusters (Gardner et al., 2003). The activation of these regulators also results in the induction and activation of other oxidative stress regulators, such as Fur and Zur, which are involved in the homeostatic control of intracellular iron and zinc levels, respectively, to minimize oxidative stress (Imlay, 2015). OxyR, a member of the LysR family of transcriptional regulators (Schell, 1993a), regulates the expression of genes required for protection against H2O2 toxicity, heat stress, oxidation-mediated cell damage, and phagocyte-mediated killing (Staudinger et al., 2002). Most bacteria, with the exception of Deinococcus radiodurans, possess a single copy of the oxyR gene (Yin et al., 2010). OxyR positively regulates the expression of the catalase and ahpC genes in gamma-proteobacteria, such as E. coli, Haemophilus, Pseudomonas, Salmonella and Yersinia. In beta-proteobacteria, such as Burkholderia and Neisseria, the catalase gene is regulated both positively and negatively by OxyR (Loprasert et al., 2003; Tseng et al., 2003). In addition to OxyR, PerR also serves as a modulator of the oxidative stress response induced by H2O2 (Rea et al., 2005). SoxR, on the other hand, protects against O2− radicals and is induced in response to O2−-generating compounds, NO, or high levels of H2O2 (Nunoshiba et al., 1992; Manchado et al., 2000). SoxR homologs are present in alpha- and gamma-proteobacteria and serve as regulators of efflux pump genes and Sods. However, SoxR homologs are absent in Bacteroidetes and Actinobacteria (Ohara et al., 2006). OxyR- and SoxR-dependent responses are induced during the exponential phase, whereas RpoS-dependent oxidative stress responses are induced during the stationary phase of bacterial growth (Eisenstark et al., 1995).
This study investigated the role of OxyR of F. tularensis in tularemia pathogenesis and the regulation of oxidative stress responses. As observed for other bacterial pathogens, sequence analysis of F. tularensis OxyR reveals two conserved cysteine residues (Cys199 and Cys208) that are required for the oxidant-dependent activation of OxyR. However, it exhibits only 36% homology with the well-characterized OxyR of E. coli. The oxyR gene in F. tularensis is transcribed divergently from the ahpC gene. Our results demonstrate that OxyR regulates the genes encoding the primary antioxidant enzymes katG, ahpC and sodB in response to H2O2-induced oxidative stress in F. tularensis. These results also indicate that similar to Bacteroidetes and Actinobacteria, in the absence of SoxR homologs, OxyR regulates the expression of SodB in F. tularensis. Furthermore, despite an identical genomic organization of the oxyR gene between Francisella and M. tuberculosis, OxyR does not regulate catalase or the ahpC gene due to mutations in the OxyR protein in M. tuberculosis (Deretic et al., 1995; Sherman et al., 1995)..
A previous study identified an oxyR mutant by screening transposon mutants of F. novicida in Drosophila melanogaster. It was reported that the oxyR mutant of F. novicida is highly sensitive to H2O2 and had attenuated virulence in flies; however, the virulence of the oxyR mutant is restored in flies that cannot synthesize melanin and therefore fail to produce ROS (Moule et al., 2010). The results from our study extend this observation in F. tularensis and demonstrate that OxyR is required to resist oxidative stress caused by superoxide-generating compounds, organic peroxides, and H2O2 under acellular growth conditions. The loss of oxyR does not result in the loss of viability of the ΔoxyR mutant grown in the absence of oxidative stress, but it does contribute to its survival at acidic pH. Exposure of the bacterial cells to acidic pH induces highly toxic ROS, including OH− radicals by Fenton chemistry (Mols and Abee, 2011). Our results suggest that OxyR protects F. tularensis against acid stress, primarily by regulating the production of the antioxidant enzymes involved in the neutralization of ROS. During macrophage infection, the Francisella-containing phagosomes are not acidified, and thus OxyR may not be required to overcome acidic pH in macrophages. However, F. tularensis causes gastrointestinal (GI) infections and must survive the acidic pH of the stomach. We speculate that OxyR may contribute to the survival of F. tularensis in the GI tract. An acid-sensitive oxyR mutant of Klebsiella pneumoniae deficient in GI tract colonization has been reported (Hennequin and Forestier, 2009).
The results of this study demonstrate that the ΔoxyR mutant of F. tularensis is deficient in survival and growth in macrophages and A549 lung epithelial cells. However, the intramacrophage replication and virulence of the ΔoxyR mutant is rescued in gp91phox−/− BMDMs or mice, indicating a role for NADPH-oxidase-dependent ROS in diminishing its fitness. The NADPH-oxidase-dependent bacterial killing in the phagosomes is rapid and accomplished quickly following bacterial uptake (Vazquez-Torres et al., 2000). Nearly equal numbers of viable wild-type F. tularensis LVS or ΔoxyR mutant bacteria were recovered from the infected macrophages after 4 hrs of infection. These results indicate that NADPH-oxidase-dependent killing of the ΔoxyR mutant bacteria may not occur during its phagosomal residence. However, the numbers of the ΔoxyR mutant bacteria recovered at 24 hrs were similar to those observed after 4 hrs of infection. These observations suggest that the enhanced sensitivity of the ΔoxyR mutant to oxidative stress makes it susceptible to low levels of NADPH-oxidase-dependent ROS in the phagosomes, thereby reducing its fitness and ability to escape from the phagosomes. Furthermore, the reduced phagosomal escape may be associated with reduced growth in the presence of physiological levels of ROS in the macrophage/epithelial cell cytosol. Indeed, our TEM results demonstrate that the ΔoxyR mutant remains trapped in the phagosomes for up to 6 hrs post-infection, indicating its reduced fitness to escape the phagosomes. The attenuated growth of the ΔoxyR mutant in A549 lung epithelial cells further supports the notion that the ΔoxyR mutant is indeed sensitive to physiological levels of ROS encountered in the host cell cytoplasm.
The mechanism(s) of regulation of oxidative stress responses in F. tularensis remains unknown to date. In Francisella, very few factors regulate gene expression, and reported evidence suggests that gene regulation occurs at the level of transcription. The well-characterized transcriptional regulators in Francisella include mglA (Charity et al., 2007; Guina et al., 2007), fevR/pigR (Wehrly et al., 2009; Charity et al., 2007; Brotcke and Monack, 2008; Brotcke et al., 2006), response regulator pmrA (Sammons-Jackson et al., 2008; Bell et al., 2010), Fur (Ramakrishnan et al., 2008; Deng et al., 2006; Kiss et al., 2008), qseC (Weiss et al., 2007), the sensor kinase kdpD, and the sigma factors rpoD and rpoS (Grall et al., 2009). Initially, it was speculated that H2O2-induced oxidative stress responses in F. tularensis are regulated by the Francisella Pathogenicity Island protein IglC (Lenco et al., 2005). However, the ΔiglC mutant did not show sensitivity to H2O2, and with the exception of AhpC, did not reveal differential induction of oxidative stress response proteins when exposed to H2O2. These results do not support the notion that IglC is a regulator of oxidative stress responses (Andersson et al., 2006). The role of the transcriptional regulator MglA has been widely studied in both F. novicida and the F. tularensis LVS. In addition to the regulation of the genes encoded on the FPI, it has been reported that MglA also regulates several stress response proteins involved in heat shock and oxidative stress resistance. Moreover, the mglA mutant exhibits increased sensitivity towards the superoxide-generating compound paraquat; however, it is resistant to H2O2 (Guina et al., 2007). The enhanced H2O2 resistance has been attributed to increased amounts of KatG in the mglA mutant (Guina et al., 2007). A later study investigated the mechanism of the resistance of mglA mutant to H2O2. It was reported that increased oxidation in the mglA mutant under aerobic growth conditions results in a compensatory increase in KatG activity that ultimately leads to H2O2 resistance. However, under microaerobic conditions, the mglA mutant is as susceptible to H2O2 as the wild-type F. tularensis LVS (Honn et al., 2012). Additionally, H2O2 resistance in the mglA mutant was suggested to be due to a concomitant upregulation of KatG and downregulation of the genes involved in iron sequestration (Honn et al., 2012). The exact mechanism through which MglA regulates the expression of KatG remains elusive. Recently, it has been demonstrated that MglA and stringent starvation protein A (SspA) form a complex and work in concert with the transcriptional activator PigR. The MglA-SspA complex interacts with all the F. tularensis promoters; however, a sequence-specific motif recognized by PigR determines their expression (Ramsey et al., 2015). Although the MglA-SspA complex binds to the oxyR promoter, it is not regulated by the MglA-SspA-PigR complex (Ramsey et al., 2015) ruling out the possibility that MglA may drive the OxyR-dependent transcription of the oxidative stress response genes, including katG. Considering these observations and the results obtained from the present study, it is possible that MglA may not have a direct role in the regulation of oxidative stress response genes per se. However, an exaggerated oxidative environment in the mglA mutant may result in the activation of OxyR, which in turn may upregulate the expression of KatG and AhpC observed in the mglA mutant. Clarification of this notion will require further studies.
This study established a mechanism of regulation of the classical antioxidant enzyme genes of F. tularensis. The OxyR targets ahpC and katG are positively regulated by the direct binding of OxyR to their putative upstream promoter regions, as revealed by in vivo ChIP and in vitro EMSA. However, despite a smaller magnitude of binding of OxyR to the promoter region of the sodB gene in the ChIP assay, the EMSA failed to validate this finding. This variation may be due to differences in the amounts of oxidized OxyR required, or due to a lack of additional regulatory components required for optimal binding to the sodB gene promoter of F. tularensis. Most importantly, the results of this study demonstrate OxyR as a global regulator of oxidative stress response in F. tularensis. A differential induction of the proteins involved in oxidative stress resistance, transcription, translation, transport, metabolism, and other cellular functions was observed in the ΔoxyR mutant subjected to oxidative stress conditions induced by H2O2. The differential expression of proteins involved in a multitude of cellular functions indicates that the OxyR-dependent oxidative stress response is mediated both by suppressing as well as elevating the levels of proteins involved in transcription, translation, transport functions, and metabolism. The suppression of proteins involved in cellular functions apparently help Francisella to slow down metabolically intensive processes to minimize the oxidative stress caused by aerobic respiration and rapid bacterial growth. In contrast, OxyR facilitates the efficient utilization of the limited resources available to Francisella during the conditions of oxidative stress by activating crucial cellular mechanisms, including the primary antioxidant enzymes. The OxyR function, especially in E. coli, is intimately linked with the activation of iron utilization genes, such as those belonging to the fur operon (Zheng et al., 1999). Surprisingly, none of the proteins involved in iron utilization were differentially expressed in the ΔoxyR mutant, indicating that the regulation of the fur operon may be independent of OxyR in F. tularensis. A similar observation has also been recorded for Pseudomonas aeruginosa, where OxyR has not been shown to be required for the expression of fur genes (Wei et al., 2012). The mechanism of regulation of the sodC gene of F. tularensis remained unanswered in this study. However, based on the observations from other bacterial pathogens, it is possible that the transcription of sodC may be regulated by RpoS. It has been reported that RpoS-dependent expression of sodC in E. coli and Salmonella occurs in response to oxidative stress during the stationary phase of growth (Gort et al., 1999). Moreover, the oxyR regulon is not functionally characterized in F. tularensis SchuS4. Previous studies have reported differences in the magnitude of oxidant resistance between the F. tularensis LVS and SchuS4 strains (Lindgren et al., 2007). However, the identical genomic organization as well as nucleotide and amino acid sequences of the oxyR gene in the F. tularensis LVS and SchuS4 strains suggests a similar regulatory role for OxyR in the latter strain.
In conclusion, this study describes OxyR as an important virulence factor of F. tularensis. Most importantly, this study provides unique insights into a mechanism of OxyR-dependent regulation of the oxidative stress response in F. tularensis. An understanding of these unique pathogenic mechanisms is essential for the development of effective therapeutics and prophylactics against this important biothreat agent.
Experimental Procedures
Bacterial strains and culture conditions
The bacterial strains used in this study are listed in Table 1. The F. tularensis subsp. holarctica live vaccine strain (LVS) (ATCC 29684; American Type Culture Collection, Rockville, MD) was obtained from BEI Resources, Manassas, VA. F. tularensis cultures were grown on Mueller Hinton (MH) chocolate agar plates (BD Biosciences, San Jose, CA) or modified MH-chocolate agar (MMH) (Bakshi et al., 2006) supplemented with IsoVitaleX at 37°C with 5% CO2 or in MH broth (BD Biosciences, San Jose, CA) supplemented with ferric pyrophosphate and IsoVitaleX (BD Biosciences, San Jose, CA) at 37°C with shaking (160 rpm). Active mid-log-phase bacteria grown in MH broth were harvested and stored at −80°C. The Escherichia coli DH5-α strain was used for cloning experiments. E. coli cultures were grown in Luria-Bertani (LB) broth or on LB agar plates. When necessary, kanamycin (25 µg/mL) or hygromycin (200 µg/mL) was included in the broth and agar media for the selection of transformants, mutants or transcomplemented strains.
Table 1.
List of bacterial strains, plasmid vectors and primers used in this study.
| Francisella Strains | Genotype | Source |
|---|---|---|
| F. tularensis LVS | Wild-type strain | ATCC |
| ΔoxyR mutant | Deletion mutant of F. tularensis LVS oxyR gene |
This study |
| oxyR tag strain | F. tularensis LVS, oxyR-VSV-G fusion, Kanr | This study |
| rpoC tag strain | F. tularensis LVS, rpoC-VSV-G fusion, Kanr | This study |
|
oxyR transcomplement (ΔoxyR + poxyR) |
F. tularensis LVS, ΔoxyR pMM08(pMP822+oxyR), Hygror |
This study |
| E. coli Strains | ||
| DH5α | F– Φ80lacZΔM15 Δ(lacZYA-argF) U169 recA1 endA1 hsdR17 (rK–, mK+) phoA supE44 λ– thi-1 gyrA96 relA1 |
Invitrogen |
| ME004 | DH5α with pMP822, Hygror | (LoVullo et al., 2009) |
| ME011 | DH5α with pJC84, Kanr | This Study |
| ME014 | DH5α with pMM05, Kanr | This Study |
| ME017 | DH5α with pMM08, Hygror | This study |
| ME022 | DH5α with pKL02, Kanr | (Ramsey et al., 2015) |
| ME024 | DH5α with pMM12, Kanr | This study |
| Plasmids | ||
| pMP822 | E. coli-Francisella shuttle vector, Hygror | (LoVullo et al., 2006) |
| pJC84 | E. coli-Francisella suicide vector, Kanr | (Wehrly et al., 2009) |
| pKL02 | rpoC-VSV-G tag integration vector | (Ramsey et al., 2015) |
| pMM05 | pJC84 + fused flanking fragment of oxyR gene, Kanr |
This study |
| pMM08 | pMP822 + oxyR, Hygror | This study |
| pMM12 | pKL02+oxyR-VSV-G, Kanr | This study |
| Primers | Sequence | Purpose |
| oxyR deletion construct | ||
| oxyR upstream fragment | ||
| MP159 | 5’-CAAggatccCCAGCTACAGACTTAAGATAAGCA-3’ | F-primer with a BamHI site at the 5’ end |
| MP160 | 5’-AGTACGTGTATTCATAACTAAATC-3’ | R-primer |
| oxyR downstream fragment | ||
| MP162 | 5’ -GATTTAGTTATGAATACACGTACTTAAGCTCACATAAA TATCATCCAA-3’ |
F-primer |
| MP163 | 5’-TGATgtcgacTACCATGTCAGGTTTAGCTGAGGT-3’ | R-Primer with a SalI site |
| oxyR deletion mutant screening | ||
| MP037 | 5’-CCGGATCCATGAAATTTGAATTACCAAAAC-3’ | F-primer for sodB as a control |
| MP038 | 5’-CGCTGCAGCTAATCAGCGAATTGCTCAGAAAC-3’ | R-primer for sodB as a control |
| MP233 | 5’-CGCACAGAGTTTAAGAGTTTGATC-3’ | F-primer for oxyR |
| MP234 | 5’-CTATTGGCGCATTTCCAACT-3’ | R-primer for oxyR |
| oxyR complementation construct | ||
| MP253 | 5’-CAAggatccATGAATACACGTACTCTTAAATAT-3’ | F-primer for oxyR with a BamHI site |
| MP254 | 5’-TGATctcgagTTAATGATTATTTGAAATTATTTT-3’ | R-primer for oxyR with an XhoI site |
| Transcriptional analysis | ||
| tul4 (Internal control) | ||
| MP029 | 5’-TCGCAGGTTTAGCGAGCTGTTCTA-3’ | F-primer |
| MP030 | 5’-ACAGCAGCAGCTTGCTCAGTAGTA-3’ | R-primer |
| Peroxidase/Catalase (FTL_1504, katG) | ||
| MP077 | 5’-CCTGCCAAATAAAGTTTTGCTC-3’ | F-primer |
| MP078 | 5’-AGCTCACCAATGGACTCCTAC-3’ | R-primer |
| Superoxide dismutase [Fe](FTL_1791, sodB) | ||
| MP101 | 5’-GGCGGAATATTTAATAACGCTGC-3’ | F-primer |
| MP102 | 5’-GTGCTCCCAAACATCAAAAG-3’ | R-primer |
| Superoxide dismutase (Cu-Zn) precursor (FTL_0380, sodC) | ||
| MP103 | 5’-TGTCAATACTCATAAAGAGGTTG-3’ | F-primer |
| MP104 | 5’-AGTTGCTGTACCATCTGCGTTA-3’ | R-primer |
| AhpC/TSA family protein (FTL_1015, ahpC) | ||
| MP258 | 5’-TTGTATTCTCATTACCAGGAGCA-3’ | F-primer |
| MP259 | 5’-ACAATCATTGCATAGCGCCA-3’ | R-primer |
| FTL_0996 | ||
| MP264 | 5’-CAGCTAAGCTAAAAGAGCTTGGTG-3’ | F-primer |
| MP265 | 5’-CTACCATTCTGATAACTTCATCCA-3’ | R-primer |
| oxyR-VSV-G epitope tag construct | ||
| MP316 | 5’-CAA gtcgacTCTCCCTGCTATCAAACAAGAAC-3’ | F-primer for oxyR with a SalI site |
| MP317 | 5’-TGAT gcggccgcATGATTATTTGAAATTATTTTAGCG-3’ | R-primer for oxyR with a NotI site |
| MP322 | 5’-TATGCTTCCGGCTCGTATGTTGTG-3’ | Sequencing primer |
| ChIP Analysis (qPCR) | ||
| katG locus | ||
| MP326 | 5’-GAAAAGAACATGAAAGGTTGGAG-3’ | F- primer for ChIP region 1 |
| MP327 | 5’-AGTGTTCCTCAAACCATTGATTA-3’ | R-primer for ChIP region 1 |
| MP330 | 5’-TCTTTTGATGCTCTATATCACTG-3’ | F- primer for ChIP region 2 |
| MP331 | 5’-AGCCATAACTAAGGATGTTATGC-3’ | R-primer for ChIP region 2 |
| MP332 | 5’-TGATTGATAATAGAACCTACCCCT-3’*** | F- primer for ChIP region 3 |
| MP333 | 5’-GCAAATTTTGACAAAGAAGCTC-3’ | R-primer for ChIP region 3 |
| MP334 | 5’-GAGCTTCTTTGTCAAAATTTGCA-3’*** | F- primer for ChIP region 4 |
| MP335 | 5’-CATTCCAGACATTCCTAAAGCAG-3’ | R-primer for ChIP region 4 |
| MP338 | 5’-AAACTGGGGACTATCACCTGAAGA-3’ | F-primer for ChIP region 5 |
| MP339 | 5’-TGCTTGCTTGACTTTATCCTCTG-3’ | R-primer for ChIP region 5 |
| sodB locus | ||
| MP340 | 5’-TCGCCAGATTCATTCATTTC-3’ | F- primer for ChIP region 1 |
| MP341 | 5’-ATGCAGCAACGGCAATTAGA-3’ | R-primer for ChIP region 1 |
| MP342 | 5’-CTGGAGTGTACATTTTTGCTTCC-3’*** | F- primer for ChIP region 2 |
| MP343 | 5’-TCAACAGCGTAAGGTAGTTTTGG-3’ | R-primer for ChIP region 2 |
| MP346 | 5’-CGCTGCTCAAGTTTTTAATCATAC-3’ | F-primer for ChIP region 3 |
| MP347 | 5’-TCTCTGTTAATGGGCAACCA-3’ | R-primer for ChIP region 3 |
| ahpC locus | ||
| MP348 | 5’-TGGTAGTTTTCAGTAGGAGTTGC-3’ | F-primer for ChIP region 1 |
| MP349 | 5’-TTGCTGAGCTACTCTTAGAGAATG-3’ | R-primer for ChIP region 1 |
| MP352 | 5’-TGACAAAACATTTTTCTGATGCTG-3’*** | F-primer for ChIP region 2 |
| MP353 | 5’-GCCAATACCCTCATCTCTTACTCT-3’ | R-primer for ChIP region 2 |
| MP356 | 5’-CGGTAAACGATAGCTTTGTTATGA-3’ | F-primer for ChIP region 3 |
| MP357 | 5’- ACAATCATTGCATAGCGCCA-3’ | R-primer for ChIP region 3 |
| fopA coding region (internal control) | ||
| MP358 | 5’-TGCTGGTTGGGCAAATCTA-3’ | F-primer |
| MP359 | 5’-TGTAGTCGCACCATTATCCTGA-3’ | R-primer |
| sodC locus | ||
| MP360 | 5’-TCTGCATGTCTTCTTTAGGGAT-3’ | F-primer for ChIP region 1 |
| MP361 | 5’-GGAATAATCATAGGCAAGGCATC-3’ | R-primer for ChIP region 1 |
| MP362 | 5’-GCGTATCAGCTAAAGTGATAATCG-3’*** | F-primer for ChIP region 2 |
| MP363 | 5’-CAAGTGAAAGCATACTCATTCCAC-3’ | R-primer for ChIP region 2 |
| MP364 | 5’-CACCATATATTCATGATGGTAACC-3’ | F-primer for ChIP region 3 |
| MP365 | 5’-CCCGCTAGCTCTTCTAGAGAATTA-3’ | R-primer for ChIP region 3 |
| FTL_0996 locus | ||
| MP366 | 5’-TGACATTCTCGAGGCTTTAGGTT-3’ | F-primer for ChIP region 1 |
| MP367 | 5’-CAAACACCTAAAATAGCTGCTGA-3’ | R-primer for ChIP region 1 |
| MP368 | 5’-TGCGATAGTACCATCACAATCAA-3’*** | F-primer for ChIP region 2 |
| MP369 | 5’-ACCTAAAACAGCTGGTGCATTG-3’ | R-primer for ChIP region 2 |
| MP370 | 5’-CAGCTAAGCTAAAAGAGCTTGGTG-3’ | F-primer for ChIP region 3 |
| MP371 | 5’-CATTAACCACTTGATGACGCAC-3’ | R-primer for ChIP region 3 |
| PmrA EMSA | ||
| MP402 | 5’-GGGAGCTAGTGAGAGGAATTTTT-3’*** | F- Primer for PmrA promoter |
| MP403 | 5’-CCAAATGAAGATCATCTTCAGCC-3’ | R- Primer for PmrA promoter |
These primers were labeled with biotin at the 5’ end for the EMSA experiments.
Construction of F. tularensis deletion mutants and transcomplementation strains
The plasmids and primers used for the generation of mutants and transcomplemented strains in this study are listed in Table 1. To generate in-frame gene deletion mutants of F. tularensis, a suicide vector, pJC84 (Wehrly et al., 2009), allowing for SacB-assisted allelic replacement in Francisella was used. For F. tularensis oxyR gene deletion, a 5’ 1214 bp fragment upstream of the start codon of the oxyR (FTL_1014) gene and its first 5 codons, as well as a 3’ 1272 bp fragment containing the oxyR gene stop codon and its downstream region were amplified by PCR. These 5’ and 3’ fragments were fused by overlapping extension PCR, and fused fragments were cloned into the pJC84 vector at the BamHI and SalI sites, resulting in the pMM05 plasmid. pMM05 was used to transform the F. tularensis LVS by electroporation, as published previously (Maier et al., 2004). The kanamycin-resistant colonies obtained after 3–5 days of incubation were serially diluted and plated on MMH agar with 8% sucrose and incubated at 37°C with 5% CO2 for 2–3 days. Sucrose-resistant clones were re-plated on kanamycin-containing MMH agar plates to verify the loss of kanamycin resistance. The sucrose-resistant and kanamycin-sensitive clones were screened for oxyR gene deletion using oxyR gene-specific primers or primer pairs flanking the oxyR gene (Table 1). To further confirm oxyR gene deletion, a duplex colony PCR was performed using primers specific for the oxyR gene. sodB gene primers were used as internal controls. This improved gene deletion strategy preserved the frame of the downstream gene(s) and prevented any polar effects resulting from gene deletion. To further confirm, DNA sequencing was performed on the oxyR gene deletion mutants (ΔoxyR) to verify that the deletion of oxyR did not disrupt the reading frame.
To transcomplement the ΔoxyR gene deletion mutant, the oxyR gene was amplified by PCR. The amplified fragment was digested with the restriction enzymes BamHI and XhoI and cloned into the E. coli-Francisella shuttle vector pMP822 (LoVullo et al., 2009). The resulting plasmid, pMM08, was verified by PCR and DNA sequencing, electroporated into the ΔoxyR mutant, and selected on MMH agar supplemented with 200 µg/mL hygromycin. The transcomplementation was confirmed by PCR.
Susceptibility of the ΔoxyR mutant to oxidants
The wild-type F. tularensis LVS, the ΔoxyR mutant, and the transcomplemented strain were tested for their susceptibilities to detergents, superoxide-generating compounds, and peroxides. For disc diffusion assays, bacterial suspensions adjusted to an OD600 of 2.0 were spread uniformly on MH-chocolate agar plates with a spreader. Sterile paper discs with sodium dodecyl sulfate (SDS; 750 µg/disk), sodium deoxycholate (100 µg/disk), Triton X-100 (2.5% solution), ethidium bromide (5 µg/disk), menadione (0.156 µg/mL), tert-butyl hydroperoxide (TBH; 21.9% solution), and cumene hydroperoxide (CHP 1.25% solution) in a 5 µL volume were impregnated on plates. The plates were incubated for 48 hrs, and zones of inhibition around the discs were measured. Growth curves were generated by diluting the bacterial strains grown on MH-chocolate agar plates to an OD600 of 0.2 (corresponds to 1×109 CFU/mL) and treating with menadione (1.56 µM), TBH (17.48 µM), and hydrogen peroxide (H2O2, 500 µM and 1 mM). The OD600 was measured at 4 h intervals. The susceptibility of the ΔoxyR mutant to H2O2 was also confirmed by performing bacterial killing assays. Equal numbers of bacteria (1×109 CFU/mL) were exposed to 750 µM H2O2. The numbers of viable bacteria were determined after 1 and 3 hrs of incubation by plating serial dilutions on MH-chocolate agar plates. Bacterial colonies were counted after 48 hrs, expressed as CFU/mL and compared with those obtained for the wild-type F. tularensis LVS or the transcomplemented strain.
Cell culture assays
A gentamicin protection assay was performed to determine the role of the ΔoxyR mutant in intracellular survival (Mahawar et al., 2012). Briefly, the murine macrophage cell line RAW264.7 (from an already existing collection), BMDMs isolated from gp91Phox−/− or wild-type C57BL/6 mice (according to approved protocol), and A549 Type II alveolar epithelial cells were infected with the F. tularensis LVS, the ΔoxyR mutant, or the transcomplemented strain at a multiplicity of infection (MOI) of 100. Two hrs after infection, the macrophages or epithelial cells were treated with gentamicin (100 µg/mL) for 2 hrs to kill all the extracellular bacteria. Medium containing gentamicin was then replaced with medium without antibiotics, followed by incubation at 37°C in 5% CO2. The macrophages were lysed at 4 and 24 hrs post-infection, while the epithelial cells were lysed at 4, 8, 12 and 24 hrs post-infection with 0.1% sodium deoxycholate. The cell lysates were serially diluted in sterile PBS and plated on MH-chocolate agar plates for bacterial enumeration. The results were expressed as CFU/mL.
Transmission electron microscopy (TEM)
For TEM, RAW264.7 cells were infected with the F. tularensis LVS or the ΔoxyR mutant at an MOI of 100 for 1 and 6 hrs. The cells were fixed in 2.5% glutaraldehyde, processed following standard protocol for sectioning, and viewed by Hitachi HT 7700 TEM. For quantitation of the phagosomal and cytosolic bacteria, at least 100 bacteria were counted in randomly selected sections (14–20 independent sections) of the macrophages at 61,000× magnification.
Mouse experiments
All mouse experiments were conducted in the Animal Resource Facility of New York Medical College according to the approved IACUC protocols. Briefly, deeply anesthetized wild-type C57BL/6 or gp91Phox−/− mice (Jackson Laboratories) were infected intranasally with 1×104 of the F. tularensis LVS or 1×104 and 1×105 CFU of the ΔoxyR mutant and observed for morbidity and mortality for a period of 21 days. The results are expressed as Kaplan-Meier survival curves, and statistical significance was determined by log-rank test.
Quantitative proteomic analysis
To investigate the global effects of oxyR gene deletion on protein expression, iTRAQ analysis was performed. The wild-type F. tularensis LVS and the isogenic ΔoxyR mutant were grown to an OD600 of 0.6 in 12 mL MH broth. The cells were pelleted by centrifugation, re-suspended in the same volume of MH broth, and divided equally into two tubes. H2O2 (1 mM) was added to one tube, while the second tube was kept as an untreated control. Both tubes were incubated for 2 hrs at 37°C. The bacterial cells were centrifuged, washed once with 1× PBS, and resuspended in 200 µl lysis buffer (10 mM Tris-Cl, pH 7.5; 0.1% SDS; 0.5% sodium deoxycholate; 0.5% Triton X-100; 0.5 mM EDTA; 0.1 mM DTT) containing protease inhibitors (Sigma). The bacterial cell lysates were sonicated in a water bath sonicator and were processed for iTraq analysis using ABSCIEX iTraq reagents at the Center for Functional Genomics (CFG), SUNY Albany, as described previously with slight modifications (Ross, Huang et al. 2004; Luo, Ning et al. 2009). Briefly, total protein extracts were precipitated by trichloroacetic acid followed by acetone wash. The resulting protein precipitates were re-suspended in 400 µl of sample preparation buffer (100 mM Tris-HCl; 7 M urea; 2 M thiourea; 0.4% SDS; 5 mM tributylphosphine, pH 8.3) followed by iodoacetamide alkylation. The protein concentrations were determined with a MicroBCA protein assay kit (Pierce, Rockford, IL). The protein mixtures were then diluted 10-fold in 50 mM Tris-HCl, pH 8.5. Modified trypsin (Sigma) was added to a final substrate-to-enzyme ratio of 30:1, and the trypsin digests were incubated overnight at 37°C. The resulting peptides were cleaned up using a Discovery DSC-18 Cartridge (Sigma). Equal amounts (100 µg) of sample were labeled with 8-plex iTRAQ reagent (ABSCIEX) according to the manufacturer's instructions. Briefly, after desalting on a C18 cartridge, the peptide mixtures were lyophilized and re-suspended in 30 µl of 0.5 M triethylammonium bicarbonate (TEAB), pH 8.5. The appropriate iTRAQ reagent (dissolved in 70 µl isopropanol) was added, allowed to react for 2 hrs at room temperature, and then quenched with 10 µl of 1 M Tris, pH 8.5. The iTRAQ-labeled peptide mixtures were then concentrated, mixed and acidified to a total volume of 8.0 mL, followed by an off-line cation exchange chromatography. A total of 30 fractions were collected, and the samples were dried by a speed-vacuum prior to RP-LC-MS/MS analysis. The on-line nano-LC ESI QqTOF MS analysis was conducted as described previously (Luo et al., 2009). The data were analyzed using Paragon Algorithm against the database within ProteinPilot v4.5 software with trypsin as the digesting agent, cysteine alkylation, an ID focus of biological modifications, and other default settings (ABSCIEX). Proteins designated as being significantly differentially produced were quantified in at least three spectra (allowing the generation of a P-value), had a fold change of more than 1.3 or less than 0.7 from two independent experiments performed with two biological replicates each, and had a P-value of <0.05. A log2 ratio of the fold change in protein levels (ΔoxyR Mutant/F. tularensis LVS) of 0.11 or higher indicated an increase, while a ratio of less than −0.15 indicated a decrease in the protein levels.
Transcriptional analysis
The wild-type F. tularensis LVS, the ΔoxyR mutant and the transcomplemented strain were grown to an OD600 of 0.5 (~2.5×109 CFU/mL) at 37°C in 10 mL MH broth. The bacterial cultures were divided equally into two tubes. A final concentration of 1 mM H2O2 was added to the first tube, while the second tube of culture was left untreated. After incubation for an additional 2 hrs, both the treated and untreated bacterial cells were pelleted, and the total RNA was isolated using a Purelink RNA Mini Kit (Ambion). The contaminating DNA from the RNA preparations was removed using on-Column Purelink DNase treatment. cDNA was synthesized using an iScript cDNA Synthesis Kit (Ambion). Quantitative real-time PCR (qRT-PCR) was performed using iQ SYBR Green Supermix (BioRad) to quantitate the gene transcripts. The amount of target gene amplification was normalized to a Tul4 internal control. The relative mRNA levels of the target genes are presented as averages of three biological replicates with standard deviation (SD). The primer sequences used for qPCR are shown in Table 1.
Construction of F. tularensis oxyR-vesicular stomatitis virus glycoprotein (VSV-G) tagged strain
The F. tularensis LVS oxyR-VSV-G strain, which expresses an OxyR fusion protein with a C-terminal VSV-G tag, was constructed to determine the in vivo binding of OxyR to putative promoter regions of genes coding for antioxidant enzymes. A tag-integration vector, pKL02, containing a cloned RpoC-VSV-G tag protein (Ramsey et al., 2015) was used. A DNA fragment specifying the 5’ end of the oxyR gene of the F. tularensis LVS minus the stop codon engineered to have SalI site and a 3’ region engineered to have a NotI site was amplified by PCR using the primer combinations shown in Table 1. The PCR fragment was cloned into pKL02 at the SalI and NotI sites, replacing the rpoC gene in pKL02 and fusing oxyR in frame with the VSV-G tag sequence. The resulting vector, pMM012, and the parental vector, pKL02, were used to transform the wild-type F. tularensis LVS by electroporation and selected for integration on MH-chocolate agar containing 25 µg/mL kanamycin. The resulting F. tularensis LVS strains expressing oxyR-VSV-G under the native oxyR promoter or expressing rpoC-VSVG were verified by PCR and DNA sequencing (Fig. S4).
Chromatin immunoprecipitation (ChIP) assay
ChIP was performed to demonstrate the binding of OxyR to the promoter regions of the antioxidant enzyme genes of F. tularensis. Cells of the wild-type, oxyR-VSV-G, and rpoC-VSV-G F. tularensis strains were grown in 50 mL MH broth at 37°C with shaking. When an OD600 of 0.4 was achieved, cultures of the rpoC-VSV-G strain were treated with a final concentration of 50 µg/mL rifampicin (Sigma) for 30 min. All the treated and untreated cultures were then cross-linked with 1% formaldehyde for 30 min, followed by 5 min incubation with 250 mM glycine to prevent further crosslinking. The bacterial cells were washed thrice with 1× PBS, resuspended in lysis buffer (20 mM HEPES pH 7.9; 50 mM KCl; 0.5 mM DTT; 10% glycerol) with protease inhibitors (Sigma), and sonicated in sonicator water bath to lyse the cells and shear their chromosomal DNA. After the cell debris was removed by centrifugation, the supernatant was adjusted for the salt concentration and immunoprecipitated overnight at 4°C using anti-VSV-G-agarose beads (Sigma). A 50 µl aliquot of supernatant was diluted in 200 µL TE + 1% SDS for use as an input control. The immunoprecipitates were washed five times with IPP150 buffer (10 mM Tris HCl, pH 8.0; 150 mM NaCl; 0.1% NP40), twice with TE buffer (10 mM Tris, pH 7.4; 1 mM EDTA, pH 8.0), and subsequently eluted with 150 µL elution buffer (50 mM Tris-HCl, pH 8; 10 mM EDTA; 1% SDS) and 100 µL TE + 1% SDS buffer, respectively. The eluted and input samples were incubated overnight at 65°C to reverse the crosslinking. The DNA was purified using a PCR purification kit (Qiagen). The ChIP and input samples were analyzed for specific DNA fragments by qRT-PCR using the primers detailed in Table 1. The qRT-PCR values were normalized to the inputs and a fopA coding region internal control. The ChIP assays were also performed with the wild-type F. tularensis LVS (mock) and rpoC-VSV-G (positive control) strains for comparison. The results are expressed as relative enrichments of the detected fragments.
Electrophoretic mobility shift assay (EMSA)
EMSA was performed using a LightShift Chemiluminescent EMSA kit (Thermo Scientific). The promoter DNA probe was generated from wild-type F. tularensis LVS genomic DNA by PCR amplification with a 5’ biotin-labeled forward primer from IDT (Integrated DNA Technologies) and an unlabeled reverse primer. The competitor DNA was amplified with the same unlabeled primer pairs. Both the biotin-labeled probe and the competitor DNA were purified with a PCR purification kit (Invitrogen) and used in the binding reaction. The primer sequences for the promoters of five genes, including ahpC (MP352/MP353, amplifying a 239 bp fragment encompassing −179 to + 60 relative to the start site of the ahpC ORF), katG (MP332/MP335, amplifying a 508 bp fragment encompassing −505 to +3 relative to the start site of the katG ORF), sodB (MP342/MP343, amplifying a 186 bp fragment from −148 to +38b relative to the start site of the sodB coding sequence), sodC (MP362/MP363, amplifying a 264 bp fragment from –218 to +46 relative to the start site of the sodC gene), FTL_0996 (MP368/MP369, amplifying a 212 bp fragment from −158 to +54 relative to the start site of the FTL_0996 coding sequence) and pmrA (FTL_0552; MP402/MP403, amplifying a 505 bp fragment covering the entire upstream intergenic region of the pmrA gene) are shown in Table 1. The binding of the transcriptional regulator PmrA1 (Sammons-Jackson et al., 2008) to its promoter region was used as a positive control to test the activity of the ΔoxyR mutant lysates (Table 1). The primer pairs were similar to those used for ChIP detection, except that the forward primers were labeled with biotin at the 5’ end to be used as a probe in the EMSA experiments. The wild-type F. tularensis LVS, the ΔoxyR mutant, and the transcomplemented strain were grown to an OD600 of 0.5 in 10 mL MH broth at 37°C with shaking, centrifuged, washed once with PBS, and resuspended in 250 µl TE buffer (10 mM Tris, pH 7.4, 1 mM EDTA, pH 8.0) containing protease inhibitors (Sigma). Bacterial cell suspensions were sonicated in a sonicator water bath to lyse the cells and extract the proteins. The cell lysates were centrifuged for 15 min at 4000×g at 4°C to pellet the insoluble fraction. The supernatants containing soluble proteins were used for the binding assay. The protein concentrations were determined using BioRad reagent. One nanogram of biotin-labeled probe alone or in combination with 30 ng of specific competitor DNA was incubated with 5 µg whole-cell protein extracts for 20 min at room temperature in 20 µl of reaction buffer as per the instructions of the EMSA kit (Thermo Scientific). The reaction mixture was loaded onto 5% TBE non-denaturing Ready Gel (BioRad), electrophoresed at room temperature in 0.5% TBE buffer (45 mM Tris-borate, 1 mM EDTA, pH 8.3), and transferred to a Hybond-N+ nylon membrane (Amersham). The biotin-labeled DNA on the membrane was probed with streptavidin-horseradish peroxidase conjugate and detected by chemiluminescent substrate according to the manufacturer’s protocol. The DNA bands were visualized on a Chemidoc XRS system (BioRad), and digital images were captured.
Western blot analysis
The F. tularensis LVS and the ΔoxyR mutant strain were grown at 37°C with shaking in 10 mL MH broth to an OD600 of approximately 0.5. Aliquots were collected and centrifuged at 4000×g for 10 min. The bacterial cell pellets were resuspended in 200 µl lysis buffer [200 mM Tris-HCl, pH 8.0; 320 mM (NH4)2SO4; 5 mM MgCl2; 10 mM EDTA; 10 mM EGTA; 20% glycerol; 1 mM dithiothreitol (DTT); protease and phosphatase inhibitors]. The protein concentrations of the cell lysates were determined with BioRad reagent. Five micrograms of protein from each sample was run on a 10% SDS-PAGE gel, transferred to a polyvinylidene difluoride membrane (Millipore) and probed with anti-KatG (1:20000) (kindly provided by Dr. Karsten Hazlett, Albany Medical College, Albany NY) and secondary monoclonal antibodies (anti-rabbit immunoglobulin, IgG, 1:5000) conjugated to horseradish peroxidase (Amersham). The protein bands on the membrane were visualized using Supersignal West Pico chemiluminescent substrate (Thermo Scientific) on a Chemidoc XRS system (BioRad) and quantitated.
Statistical analysis
The results are expressed as the means ± S.E.M. or S.D. Statistical significance between the groups was determined by one-way ANOVA followed by Tukey-Kramer and Multiple Comparison tests or by Student’s t test. The survival data were analyzed by a log-rank test and presented as Kaplan-Meier survival curves. Differences between the experimental groups were considered statistically significant at a P < 0.05 level.
Supplementary Material
Acknowledgments
This work was supported, in whole or in part, by National Institutes of Health Grants R15AI107698 (MM) and P01AI056320, R56AI101109 (CSB). The authors thank Dr. Simon Dove, Division of Infectious Diseases, Boston Children's Hospital, Harvard Medical School for kindly providing pKL02, the rpoC-VSV-G tag integration vector. We also thank Dr. Zbigniew Darzynkiewicz, New York Medical College for providing A549 cells.
Footnotes
No financial conflicts of interest exist regarding the contents of the manuscript and its authors.
Author Contributions
MM and CSB conceived and designed the experiments. ZM, VCR, SMR, YJ, SV and CSB conducted the experiments. ZM, CSB and MM analyzed the data. CSB and MM wrote the manuscript.
Reference List
- Allen LA. Interview with Dr. Lee-Ann Allen regarding Pivotal Advance: Francisella tularensis LVS evades killing by human neutrophils via inhibition of the respiratory burst and phagosome escape. Interview by Helene F. Rosenberg. J Leukoc Biol. 2006;80:1222–1223. doi: 10.1189/jlb.1306287. [DOI] [PubMed] [Google Scholar]
- Andersson H, Hartmanova B, Ryden P, Noppa L, Naslund L, Sjostedt A. A microarray analysis of the murine macrophage response to infection with Francisella tularensis LVS. J Med Microbiol. 2006;55:1023–1033. doi: 10.1099/jmm.0.46553-0. [DOI] [PubMed] [Google Scholar]
- Bakshi CS, Malik M, Regan K, Melendez JA, Metzger DW, Pavlov VM, Sellati TJ. Superoxide dismutase B gene (sodB)-deficient mutants of Francisella tularensis demonstrate hypersensitivity to oxidative stress and attenuated virulence. J Bacteriol. 2006;188:6443–6448. doi: 10.1128/JB.00266-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell BL, Mohapatra NP, Gunn JS. Regulation of virulence gene transcripts by the Francisella novicida orphan response regulator PmrA: role of phosphorylation and evidence of MglA/SspA interaction. Infect Immun. 2010;78:2189–2198. doi: 10.1128/IAI.00021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binesse J, Lindgren H, Lindgren L, Conlan W, Sjostedt A. Roles of reactive oxygen species-degrading enzymes of Francisella tularensis SCHU S4. Infect Immun. 2015;83:2255–2263. doi: 10.1128/IAI.02488-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotcke A, Monack DM. Identification of fevR, a novel regulator of virulence gene expression in Francisella novicida. Infect Immun. 2008;76:3473–3480. doi: 10.1128/IAI.00430-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotcke A, Weiss DS, Kim CC, Chain P, Malfatti S, Garcia E, Monack DM. Identification of MglA-regulated genes reveals novel virulence factors in Francisella tularensis. Infect Immun. 2006;74:6642–6655. doi: 10.1128/IAI.01250-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charity JC, Costante-Hamm MM, Balon EL, Boyd DH, Rubin EJ, Dove SL. Twin RNA polymerase-associated proteins control virulence gene expression in Francisella tularensis. PLoS Pathog. 2007;3:e84. doi: 10.1371/journal.ppat.0030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase JC, Celli J, Bosio CM. Direct and indirect impairment of human dendritic cell function by virulent Francisella tularensis Schu S4. Infect Immun. 2009;77:180–195. doi: 10.1128/IAI.00879-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang SM, Schellhorn HE. Regulators of oxidative stress response genes in Escherichia coli and their functional conservation in bacteria. Arch Biochem Biophys. 2012;525:161–169. doi: 10.1016/j.abb.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Deng K, Blick RJ, Liu W, Hansen EJ. Identification of Francisella tularensis genes affected by iron limitation. Infect Immun. 2006;74:4224–4236. doi: 10.1128/IAI.01975-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, et al. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285:2763–2773. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- Deretic V, Philipp W, Dhandayuthapani S, Mudd MH, Curcic R, Garbe T, et al. Mycobacterium tuberculosis is a natural mutant with an inactivated oxidative-stress regulatory gene: implications for sensitivity to isoniazid. Mol Microbiol. 1995;17:889–900. doi: 10.1111/j.1365-2958.1995.mmi_17050889.x. [DOI] [PubMed] [Google Scholar]
- Dieppedale J, Sobral D, Dupuis M, Dubail I, Klimentova J, Stulik J, et al. Identification of a putative chaperone involved in stress resistance and virulence in Francisella tularensis. Infect Immun. 2011;79:1428–1439. doi: 10.1128/IAI.01012-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstark A, Yallaly P, Ivanova A, Miller C. Genetic mechanisms involved in cellular recovery from oxidative stress. Arch Insect Biochem Physiol. 1995;29:159–173. doi: 10.1002/arch.940290206. [DOI] [PubMed] [Google Scholar]
- Fortier AH, Slayter MV, Ziemba R, Meltzer MS, Nacy CA. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect Immun. 1991;59:2922–2928. doi: 10.1128/iai.59.9.2922-2928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner AM, Gessner CR, Gardner PR. Regulation of the nitric oxide reduction operon (norRVW) in Escherichia coli. Role of NorR and sigma54 in the nitric oxide stress response. J Biol Chem. 2003;278:10081–10086. doi: 10.1074/jbc.M212462200. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Flecha B, Demple B. Transcriptional regulation of the Escherichia coli oxyR gene as a function of cell growth. J Bacteriol. 1997;179:6181–6186. doi: 10.1128/jb.179.19.6181-6186.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gort AS, Ferber DM, Imlay JA. The regulation and role of the periplasmic copper, zinc superoxide dismutase of Escherichia coli. Mol Microbiol. 1999;32:179–191. doi: 10.1046/j.1365-2958.1999.01343.x. [DOI] [PubMed] [Google Scholar]
- Grall N, Livny J, Waldor M, Barel M, Charbit A, Meibom KL. Pivotal role of the Francisella tularensis heat-shock sigma factor RpoH. Microbiology. 2009;155:2560–2572. doi: 10.1099/mic.0.029058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guina T, Radulovic D, Bahrami AJ, Bolton DL, Rohmer L, Jones-Isaac KA, et al. MglA regulates Francisella tularensis subsp. novicida (Francisella novicida) response to starvation and oxidative stress. J Bacteriol. 2007;189:6580–6586. doi: 10.1128/JB.00809-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennequin C, Forestier C. oxyR, a LysR-type regulator involved in Klebsiella pneumoniae mucosal and abiotic colonization. Infect Immun. 2009;77:5449–5457. doi: 10.1128/IAI.00837-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honn M, Lindgren H, Sjostedt A. The role of MglA for adaptation to oxidative stress of Francisella tularensis LVS. BMC Microbiol. 2012;12:14. doi: 10.1186/1471-2180-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieva R, Roncarati D, Metruccio MM, Seib KL, Scarlato V, Delany I. OxyR tightly regulates catalase expression in Neisseria meningitidis through both repression and activation mechanisms. Mol Microbiol. 2008;70:1152–1165. doi: 10.1111/j.1365-2958.2008.06468.x. [DOI] [PubMed] [Google Scholar]
- Imlay JA. Transcription Factors That Defend Bacteria Against Reactive Oxygen Species. Annu Rev Microbiol. 2015;69:93–108. doi: 10.1146/annurev-micro-091014-104322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagielski T, Bakula Z, Roeske K, Kaminski M, Napiorkowska A, ugustynowicz-Kopec E, et al. Mutation profiling for detection of isoniazid resistance in Mycobacterium tuberculosis clinical isolates. J Antimicrob Chemother. 2015;70:3214–3221. doi: 10.1093/jac/dkv253. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Dong Y, Luo Q, Li N, Wu G, Gao H. Protection from oxidative stress relies mainly on derepression of OxyR-dependent KatB and Dps in Shewanella oneidensis. J Bacteriol. 2014;196:445–458. doi: 10.1128/JB.01077-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Cho Y, Jang IA, Park W. Molecular mechanism involved in the response to hydrogen peroxide stress in Acinetobacter oleivorans DR1. Appl Microbiol Biotechnol. 2015;99:10611–10626. doi: 10.1007/s00253-015-6914-5. [DOI] [PubMed] [Google Scholar]
- Kim JS, Holmes RK. Characterization of OxyR as a negative transcriptional regulator that represses catalase production in Corynebacterium diphtheriae. PLoS One. 2012;7:e31709. doi: 10.1371/journal.pone.0031709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss K, Liu W, Huntley JF, Norgard MV, Hansen EJ. Characterization of fig operon mutants of Francisella novicida U112. FEMS Microbiol Lett. 2008;285:270–277. doi: 10.1111/j.1574-6968.2008.01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson P, Oyston PC, Chain P, Chu MC, Duffield M, Fuxelius HH, et al. The complete genome sequence of Francisella tularensis, the causative agent of tularemia. Nat Genet. 2005;37:153–159. doi: 10.1038/ng1499. [DOI] [PubMed] [Google Scholar]
- Lee BY, Horwitz MA, Clemens DL. Identification, recombinant expression, immunolocalization in macrophages, and T-cell responsiveness of the major extracellular proteins of Francisella tularensis. Infect Immun. 2006;74:4002–4013. doi: 10.1128/IAI.00257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Lee SM, Mukhopadhyay P, Kim SJ, Lee SC, Ahn WS, et al. Redox regulation of OxyR requires specific disulfide bond formation involving a rapid kinetic reaction path. Nat Struct Mol Biol. 2004;11:1179–1185. doi: 10.1038/nsmb856. [DOI] [PubMed] [Google Scholar]
- Lenco J, Pavkova I, Hubalek M, Stulik J. Insights into the oxidative stress response in Francisella tularensis LVS and its mutant DeltaiglC1+2 by proteomics analysis. FEMS Microbiol Lett. 2005;246:47–54. doi: 10.1016/j.femsle.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Lindgren H, Shen H, Zingmark C, Golovliov I, Conlan W, Sjostedt A. Resistance of Francisella tularensis strains against reactive nitrogen and oxygen species with special reference to the role of KatG. Infect Immun. 2007;75:1303–1309. doi: 10.1128/IAI.01717-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn AC, Jones CL, Napier BA, Bina JE, Weiss DS. Macrophage replication screen identifies a novel Francisella hydroperoxide resistance protein involved in virulence. PLoS One. 2011;6:e24201. doi: 10.1371/journal.pone.0024201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loprasert S, Whangsuk W, Sallabhan R, Mongkolsuk S. Regulation of the katG-dpsA operon and the importance of KatG in survival of Burkholderia pseudomallei exposed to oxidative stress. FEBS Lett. 2003;542:17–21. doi: 10.1016/s0014-5793(03)00328-4. [DOI] [PubMed] [Google Scholar]
- LoVullo ED, Sherrill LA, Pavelka MS., Jr Improved shuttle vectors for Francisella tularensis genetics. FEMS Microbiol Lett. 2009;291:95–102. doi: 10.1111/j.1574-6968.2008.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoVullo ED, Sherrill LA, Perez LL, Pavelka MS., Jr Genetic tools for highly pathogenic Francisella tularensis subsp. tularensis. Microbiology. 2006;152:3425–3435. doi: 10.1099/mic.0.29121-0. [DOI] [PubMed] [Google Scholar]
- Luo Y, Yang C, Jin C, Xie R, Wang F, McKeehan WL. Novel phosphotyrosine targets of FGFR2IIIb signaling. Cell Signal. 2009;21:1370–1378. doi: 10.1016/j.cellsig.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Banik S, Rane H, Mora VT, Rabadi SM, Doyle CR, et al. EmrA1 membrane fusion protein of Francisella tularensis LVS is required for resistance to oxidative stress, intramacrophage survival and virulence in mice. Mol Microbiol. 2014;91:976–995. doi: 10.1111/mmi.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahawar M, Atianand MK, Dotson RJ, Mora V, Rabadi SM, Metzger DW, et al. Identification of a novel Francisella tularensis factor required for intramacrophage survival and subversion of innate immune response. J Biol Chem. 2012;287:25216–25229. doi: 10.1074/jbc.M112.367672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier TM, Havig A, Casey M, Nano FE, Frank DW, Zahrt TC. Construction and characterization of a highly efficient Francisella shuttle plasmid. Appl Environ Microbiol. 2004;70:7511–7519. doi: 10.1128/AEM.70.12.7511-7519.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchado M, Michan C, Pueyo C. Hydrogen peroxide activates the SoxRS regulon in vivo. J Bacteriol. 2000;182:6842–6844. doi: 10.1128/jb.182.23.6842-6844.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meireles DA, Alegria TG, Alves SV, Arantes CR, Netto LE. A 14.7 kDa protein from Francisella tularensis subsp. novicida (named FTN_1133), involved in the response to oxidative stress induced by organic peroxides, is not endowed with thiol-dependent peroxidase activity. PLoS One. 2014;9:e99492. doi: 10.1371/journal.pone.0099492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melillo AA, Bakshi CS, Melendez JA. Francisella tularensis antioxidants harness reactive oxygen species to restrict macrophage signaling and cytokine production. J Biol Chem. 2010;285:27553–27560. doi: 10.1074/jbc.M110.144394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melillo AA, Mahawar M, Sellati TJ, Malik M, Metzger DW, Melendez JA, Bakshi CS. Identification of Francisella tularensis live vaccine strain CuZn superoxide dismutase as critical for resistance to extracellularly generated reactive oxygen species. J Bacteriol. 2009;191:6447–6456. doi: 10.1128/JB.00534-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mols M, Abee T. Primary and secondary oxidative stress in Bacillus. Environ Microbiol. 2011;13:1387–1394. doi: 10.1111/j.1462-2920.2011.02433.x. [DOI] [PubMed] [Google Scholar]
- Moule MG, Monack DM, Schneider DS. Reciprocal analysis of Francisella novicida infections of a Drosophila melanogaster model reveal host-pathogen conflicts mediated by reactive oxygen and imd-regulated innate immune response. PLoS Pathog. 2010;6:e1001065. doi: 10.1371/journal.ppat.1001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunoshiba T, Hidalgo E, mabile Cuevas CF, Demple B. Two-stage control of an oxidative stress regulon: the Escherichia coli SoxR protein triggers redox-inducible expression of the soxS regulatory gene. J Bacteriol. 1992;174:6054–6060. doi: 10.1128/jb.174.19.6054-6060.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara N, Kikuchi Y, Shoji M, Naito M, Nakayama K. Superoxide dismutase-encoding gene of the obligate anaerobe Porphyromonas gingivalis is regulated by the redox-sensing transcription activator OxyR. Microbiology. 2006;152:955–966. doi: 10.1099/mic.0.28537-0. [DOI] [PubMed] [Google Scholar]
- Pechous RD, McCarthy TR, Zahrt TC. Working toward the future: insights into Francisella tularensis pathogenesis and vaccine development. Microbiol Mol Biol Rev. 2009;73:684–711. doi: 10.1128/MMBR.00028-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan G, Meeker A, Dragulev B. fslE is necessary for siderophore-mediated iron acquisition in Francisella tularensis Schu S4. J Bacteriol. 2008;190:5353–5361. doi: 10.1128/JB.00181-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KM, Osborne ML, Vvedenskaya IO, Su C, Nickels BE, Dove SL. Ubiquitous promoter-localization of essential virulence regulators in Francisella tularensis. PLoS Pathog. 2015;11:e1004793. doi: 10.1371/journal.ppat.1004793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea R, Hill C, Gahan CG. Listeria monocytogenes PerR mutants display a small-colony phenotype, increased sensitivity to hydrogen peroxide, and significantly reduced murine virulence. Appl Environ Microbiol. 2005;71:8314–8322. doi: 10.1128/AEM.71.12.8314-8322.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammons-Jackson WL, McClelland K, Manch-Citron JN, Metzger DW, Bakshi CS, Garcia E, et al. Generation and characterization of an attenuated mutant in a response regulator gene of Francisella tularensis live vaccine strain (LVS) DNA Cell Biol. 2008;27:387–403. doi: 10.1089/dna.2007.0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santic M, Molmeret M, Klose KE, Abu KY. Francisella tularensis travels a novel, twisted road within macrophages. Trends Microbiol. 2006;14:37–44. doi: 10.1016/j.tim.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Schell MA. Molecular biology of the LysR family of transcriptional regulators. Annu Rev Microbiol. 1993a;47:597–626. doi: 10.1146/annurev.mi.47.100193.003121. [DOI] [PubMed] [Google Scholar]
- Schell MA. Molecular biology of the LysR family of transcriptional regulators. Annu Rev Microbiol. 1993b;47:597–626. doi: 10.1146/annurev.mi.47.100193.003121. [DOI] [PubMed] [Google Scholar]
- Sherman DR, Sabo PJ, Hickey MJ, Arain TM, Mahairas GG, Yuan Y, et al. Disparate responses to oxidative stress in saprophytic and pathogenic mycobacteria. Proc Natl Acad Sci U S A. 1995;92:6625–6629. doi: 10.1073/pnas.92.14.6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Wan F, Mao Y, Gao H. Unraveling the Mechanism for the Viability Deficiency of Shewanella oneidensis oxyR Null Mutant. J Bacteriol. 2015;197:2179–2189. doi: 10.1128/JB.00154-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostedt A. Intracellular survival mechanisms of Francisella tularensis, a stealth pathogen. Microbes Infect. 2006;8:561–567. doi: 10.1016/j.micinf.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Staudinger BJ, Oberdoerster MA, Lewis PJ, Rosen H. mRNA expression profiles for Escherichia coli ingested by normal and phagocyte oxidase-deficient human neutrophils. J Clin Invest. 2002;110:1151–1163. doi: 10.1172/JCI15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz G, Imlay JA. Oxidative stress. Curr Opin Microbiol. 1999;2:188–194. doi: 10.1016/s1369-5274(99)80033-2. [DOI] [PubMed] [Google Scholar]
- Tseng HJ, McEwan AG, Apicella MA, Jennings MP. OxyR acts as a repressor of catalase expression in Neisseria gonorrhoeae. Infect Immun. 2003;71:550–556. doi: 10.1128/IAI.71.1.550-556.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Torres A, Jones-Carson J, Mastroeni P, Ischiropoulos H, Fang FC. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J Exp Med. 2000;192:227–236. doi: 10.1084/jem.192.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vi-Dor Y, Yaniv H. The activity of catalase in Pasteurella tularensis. J Bacteriol. 1952;63:751–757. doi: 10.1128/jb.63.6.751-757.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrly TD, Chong A, Virtaneva K, Sturdevant DE, Child R, Edwards JA, et al. Intracellular biology and virulence determinants of Francisella tularensis revealed by transcriptional profiling inside macrophages. Cell Microbiol. 2009;11:1128–1150. doi: 10.1111/j.1462-5822.2009.01316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Minh PN, Dotsch A, Hildebrand F, Panmanee W, Elfarash A, et al. Global regulation of gene expression by OxyR in an important human opportunistic pathogen. Nucleic Acids Res. 2012;40:4320–4333. doi: 10.1093/nar/gks017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss DS, Brotcke A, Henry T, Margolis JJ, Chan K, Monack DM. In vivo negative selection screen identifies genes required for Francisella virulence. Proc Natl Acad Sci U S A. 2007;104:6037–6042. doi: 10.1073/pnas.0609675104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Wang L, Lu H, Xu G, Chen H, Zhan H, et al. DRA0336, another OxyR homolog, involved in the antioxidation mechanisms in Deinococcus radiodurans. J Microbiol. 2010;48:473–479. doi: 10.1007/s12275-010-0043-8. [DOI] [PubMed] [Google Scholar]
- Zheng M, Doan B, Schneider TD, Storz G. OxyR and SoxRS regulation of fur. J Bacteriol. 1999;181:4639–4643. doi: 10.1128/jb.181.15.4639-4643.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.