Abstract
Many epidemiologic, clinical, and experimental findings point to sex differences in myofascial pain in view of the fact that adult women tend to have more myofascial problems with respect to men. It is possible that one of the stimuli to sensitization of fascial nociceptors could come from hormonal factors such as estrogen and relaxin, that are involved in extracellular matrix and collagen remodeling and thus contribute to functions of myofascial tissue. Immunohistochemical and molecular investigations (real-time PCR analysis) of relaxin receptor 1 (RXFP1) and estrogen receptor-alpha (ERα) localization were carried out on samples of human fascia collected from 8 volunteers patients during orthopedic surgery (all females, between 42 and 70 yrs, divided into pre- and post-menopausal groups), and in fibroblasts isolated from deep fascia, to examine both protein and RNA expression levels. We can assume that the two sex hormone receptors analyzed are expressed in all the human fascial districts examined and in fascial fibroblasts culture cells, to a lesser degree in the post-menopausal with respect to the pre-menopausal women. Hormone receptor expression was concentrated in the fibroblasts, and RXFP1 was also evident in blood vessels and nerves. Our results are the first demonstrating that the fibroblasts located within different districts of the muscular fasciae express sex hormone receptors and can help to explain the link between hormonal factors and myofascial pain. It is known, in fact, that estrogen and relaxin play a key role in extracellular matrix remodeling by inhibiting fibrosis and inflammatory activities, both important factors affecting fascial stiffness and sensitization of fascial nociceptors.
Key words: Fascia, fascial fibroblasts, estrogen, relaxin, immunostaining
Introduction
It is well known that sex hormones affect the connective tissues, above all the ligaments. Moalli et al.1 demonstrated that type I collagen in the arcus tendineous fasciae pelvis decreased in post-menopausal women, possibly compromising the tissue’s tensile strength and increasing susceptibility to anterior vaginal wall prolapse. Petrofsky and Lee2 demonstrated plantar fascia elasticity changes during the menstrual cycle, suggesting a possible role of the sexual hormones in increasing the elasticity of human connective tissue. In 1984, a prospective study by Möller-Nielsen and Hammar3 showed that women soccer players were more susceptible to traumatic injuries during premenstrual and menstrual periods with respect to other periods of their menstrual cycle. Their study also disclosed that women using contraceptive pills had a lower rate of traumatic injuries (P<0.05) with respect non-pill users. Following publication of that study, many authors set out to examine how sex hormones affect ligaments and cartilage. Konopka et al.4 demonstrated, for example, that female collegiate athletes whose serum relaxin concentrations were higher than 6.0 pg/mL had more than 4 times increased risk for anterior cruciate ligament (ACL) tears. Sex steroid receptors have been localized in many fasciae of the pelvic floor,5,6 suggesting that an alteration in ERα in these fasciae could play an important role in the pathophysiology of prolapse and stress urinary incontinence.7
Sex receptors have also been found in the human female ACL.8,9 Konopka et al.4 demonstrated, for example, that relaxin-2 significantly upregulated intracellular processes in female ACL cells, but no effect was observed in the cells of males. Relaxin increased metalloproteases (MMP1 and MMP3) and decreased Alpha-smooth muscle actin (αSMA) and Type I and III collagen expression, which may act to alter the structural integrity of ligaments over time. These alterations may affect the load bearing properties of female ACL and contribute to non-contact ACL injuries.10 Circulating levels of relaxin and the detection of relaxin receptor concurrent with MMPs in multiple tissues of the trapeziometacarpal joint have supported the hypothesis that relaxin contributes to the cascade of joint destruction.11 In view of the fact that sex hormone receptors have been found both in pelvic fasciae and in knee ligaments, we hypothesized that estrogen and relaxin receptors could also be expressed in various muscular fasciae. The current study aimed thus to investigate the expression and the localization of relaxin receptor 1 (RXFP1) and estrogen receptor-alpha (ERα) in different women fascial districts and in isolated fascial fibroblasts. The work is a preliminary study examining, for the first time, sex hormone receptor expression in muscle fascia and any potential correlation with pre- or post- menopausal periods. Its findings could contribute to our understanding of hormonal influences on myofascial properties and to explaining the sex differences noted in the prevalence of myofascial pain.
Materials and Methods
This study was approved by the Institutional Ethics Review Board (approval no. 3722/AO/16) whose ethical regulations regarding research conducted on human tissues were carefully followed. Written informed consent was obtained from all of the volunteer donors.
Samples of fascia that were a few millimeters wide were collected from 8 volunteers, females patients, average age 56±10 (range 42-70), who were undergoing elective surgical procedures at the Orthopedic Clinic of Padua University. The samples were collected from: the crural fascia of the leg (2 samples: one from a pre- and one from a post-menopausal volunteer), the rectus sheath of the abdomen (2 samples: one from a pre- and one from a post-menopausal volunteer) and the fascia lata of the thigh (4 samples: one from a pre- and three from post-menopausal volunteers). The samples were transported to the laboratory in phosphate buffered saline (PBS) within a few hours of their collection.
Each sample was divided into three sections: two were maintained in PBS and used fresh for cell isolation and real-time PCR, the third one was formalin-fixed for histology. The protocols used for each sample are described below.
Cell isolation and culture
Fascia was digested with Collagenase B 0.1% in Hank’s Balanced Salt Solution (HBSS) overnight and fibroblast cells were isolated and characterized by immunohistochemical staining with anti-Fibroblast Surface Protein [1B10] antibody (1:100, Mouse monoclonal antibodies; Abcam, Cambridge, UK), as previously described in one of our previous works.12 Cell cultures were maintained at 37°C, 95% humidity and 5% CO2, in DMEM 1g/L glucose, 10% FBS and 1% penicillin-streptomycin antibiotic, and used from passage 3rd to 9th.
Immunocytochemistry and immunohistochemistry
Human fascia specimens were fixed in 10% formalin solution overnight, dehydrated in graded ethanol, embedded in paraffin and cut into 6 μm-thick sections. To detect relaxin receptor 1 (RXFP1) and estrogen receptor-alpha (ERα), dewaxed sections were treated with Tris-EDTA pH 9.0 buffer for 15 min at 95°C, rinsed in water, and then washed in PBS. Isolated cells from fascia were plated (200 cells/mm2 in 24-multiwells containing a glass coverslip) and allowed to attach for 48 h at 37°C. Cells were then washed in PBS, fixed for 10 min with 2% paraformaldehyde in PBS pH 7.4 and then washed three times in PBS. Afterwards, the protocol described below was used for all the samples (tissues and cells).
Endogen peroxidase were blocked with 1.5% H2O2 in PBS for 10 min at room temperature. Samples were pre-incubated with a permeabilizing buffer (0.2% Tween-20 in PBS) for 60 min at room temperature and then were incubated in rabbit polyclonal antibodies diluted 1:200 (cell samples) and 1:400 (tissues) for anti-RXFP1 Receptor (Sigma-Aldrich, St. Louis, MO, USA), and 1:100 for Estrogen Receptor alpha (GeneTex Inc., Irvine, CA, USA). The primary antibodies were diluted in the same preincubation buffer and incubated overnight at 4°C. After repeated PBS washings, samples were incubated with the ready-to-use secondary antibody Advance HRP Detection System (Dako Corp., Carpinteria, CA, USA), and washed in PBS buffer. The reaction was then developed with 3,3’-diaminobenzidine (Liquid DAB + substrate Chromogen System kit, Dako) and stopped with distilled water.
Negative controls were similarly treated omitting the primary antibody, confirming the specificity of the immunostaining. Samples of human adrenal gland sample for ERα,13 and human skin and adrenal gland for RXFP1 were used as positive controls as indicated in the datasheet. Nuclei in tissue sections were counterstained with ready-to-use hematoxylin (Dako).
Images were acquired with the Leica DMR microscope (Leica Microsystems, Wetzlar, Germany; objectives 20X and 40X, Leica) and analyzed using Image J software.
Real-time PCR
Cells isolated from human fascia at the third passage were seeded (3×104 cells/well) on a 24-well plate to examine the expression of mRNA for ERα and RXFP1, and differences in the fascia samples. Following 48 h of incubation in medium, the cells were harvested in RNA lysis buffer and total RNA was extracted with the SV Total RNA Isolation System (Promega Corp., Madison, WI, USA) and purified. Tissue specimens were homogenized in RNA lysis buffer, and total RNA was extracted following the same protocol used for the cells. To remove genomic DNA contamination, a DNAse treatment was also carried out during RNA extraction. The quantity and integrity of the RNA extracted were systematically checked using a Nanodrop 2000C spectrophotometer (Thermo Scientific, Waltham, MA, USA), and RNA was then transcribed into cDNA. Realtime PCR was carried out in an I-Cycler iQ detection system (BioRad Laboratories, Milan, Italy), utilizing the primers listed in Table 1, and a starting amount of 30 ng of cDNA. The PCR program included a denaturation step at 95°C for 3 min, 40 cycles of three amplification steps (15s 95°C - 15s 60°C - 15s 72°C) and melting curve (60-90°C with a heating rate of 0.5°C/10 s).
Table 1.
Sense and antisense sequences used as RT-PCR primers to detect RXFP1, ERα and RPS18 expression.
| Sequence name | Acc. number | BP | Primers |
|---|---|---|---|
| RPS18 F142 | NM_022551 | 111 | 5’-ATT AAG GGT GTG GGC CGA AG -3’ |
| RPS18 R252 | 5’-GGT GAT CAC ACG TTC CAC CT -3’ | ||
| RXFP1 F1381 | NM_001253727 | 132 | 5’- AGT CTC TCA GCC TAG AAG GGA-3’ |
| RXFP1 R1512 | 5’-TAC AGC TGC GAA CAT GTG GT -3’ | ||
| ERα F1693 | NM_000125.3 | 112 | 5’-ATC CAC CTG ATG GCC AAG-3’ |
| ERα R1804 | 5’-GCT CCA TGC CTT TGT TAC TCA -3’ |
The fluorescence signal threshold was calculated during the exponential phase, and the fraction number of PCR cycles required to reach the threshold (cycle threshold, Ct) was determined. Ct values decreased linearly with increasing input target quantity. All the samples were amplified in duplicate and RPS18 (Ribosomal Protein S18) expression was used as a reference.14 The specificity of the amplification was tested at the end of each run by applying melting curve analysis, using the I-Cycler sequencing Software ver. 3.0.
Despite the small size of the group of patients analyzed (8 women), we divided the samples into two groups (pre- and post-menopausal) in order to compare the expression of the sex hormone receptors during two distinct hormonal periods: pre- and postmenopause.
Results
The typical fascial organization of longitudinally oriented collagen fibers and elongated fibroblasts was evident in all the samples (Figures 1 and 2), with different degrees of cellularisation depending on the district: for example, few fibroblasts were evident in the crural fascia of the leg (Figure 1F), while many and elongated cells were found in the rectus sheath of the abdomen (Figure 1C).
Figure 1.
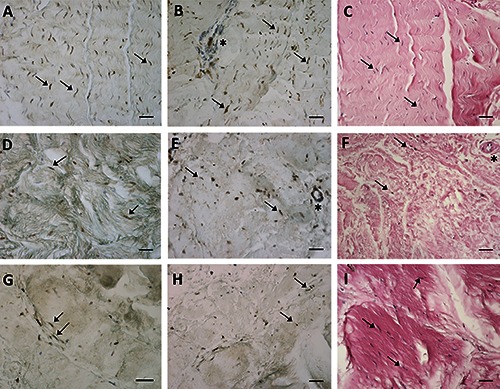
ERα (A,D,G) expression, RXFP1 (B,E,H) expression and H&E staining (C,F,I) of paraffin sections from samples collected from three different districts of human fascia of three women patients: the rectus sheath of abdomen (A-C), the crural fascia of the leg (D-F), the fascia lata of the thigh (G-I). Arrows indicate some fibroblasts (in A,D,G positive for ERα and in B,E,H positive for RXFP1); asterisks show blood vessels, positive for RXFP1 in B,E. Scale bars: 50 μm.
Figure 2.
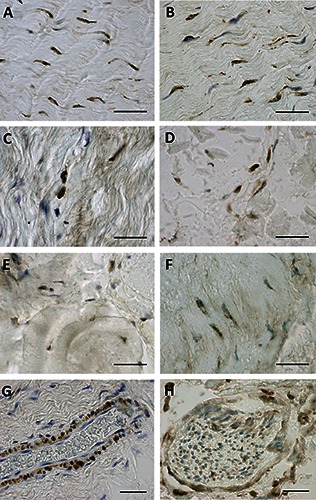
ERα (A,C,E) and RXFP1 (B,D,F) expression of paraffin sections of the rectus sheath of the abdomen (A,B), the crural fascia of the leg (C,D), and the fascia lata of the thigh (E,F). G and H show a RXFP1 positive blood vessel and nerve, respectively. Scale bars: 50 μm.
Figure 1 demonstrates the positive ERα (panels A, D, G) and RXFP1 (panels B, E, H) expression noted in most of the fascial fibroblasts in the samples of the pre-menopausal women, although with different degrees of reactivity. The relaxin receptor was also evident in blood vessels (Figure 1 B,E; Figure 2G) and nerves (Figure 2H). Those same samples at higher magnification are shown in Figure 2. The same expression was also evident in the post-menopausal women (data not shown). Positive tissue controls uncovered positive cells in zona glomerulosa of the human adrenal gland (ERα and RXFP1) and in the spinous layer of human epidermis (RXFP1) (data not shown). These results were also confirmed in cells isolated from the same patients’ fascia tissues described above (Figure 3), that we characterized as fibroblasts, as we described in one of our previous works:12 all the cells were positive for the hormone receptors analyzed (ERα, Figure 3 A,D,G; RXFP1, Figure 3 B,E,H), although with different distributions and degrees of reactivity depending on the fascial district of origin. The cells isolated from crural fascia (Figure 3 D-F) were, for example, smaller and slightly less elongated with respect to the fibroblasts of the abdominal fascia (Figure 3 A-C), and reacted showing lower positivity. Positivity was nevertheless localized in the cytoplasm of the cells and also in several nuclei and nuclear membranes in all of the samples (leg, abdomen and thigh).
Figure 3.
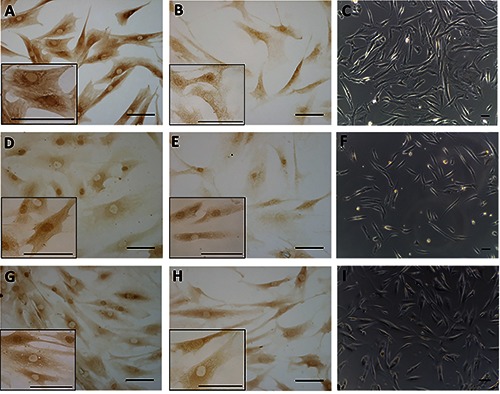
ERα (A,D,G) and RXFP1 (B,E,H) receptor expression in fibroblasts isolated from three different fascial districts: the rectus sheath of the abdomen (A-C), the crural fascia of the leg (D-F), and the fascia lata of the thigh (G-I). Higher magnification images are shown in the insets. Bright-field images are shown in C,F,I. Scale bars: 100 μm.
The sex hormone receptors expression was also confirmed and analyzed by means of RT-PCR experiments that were carried out in the cells and tissue samples of the pre- and post-menopausal women, using the same amount of starting cDNA. The specificity of the reactions and the absence of false amplicons were verified by monitoring if all the samples had the same specific melting temperature for each amplified sequence (data not shown). The results confirmed that ERα and RXFP1 were both expressed in the fibroblasts and fascial tissues in both the pre- and post-menopausal women (Table 2), although with a lower expression compared to the housekeeping gene RPS18, as indicated by the higher Ct values. The expression of the two receptors was lower in the post-menopausal women, with the exception of the RXFP1 expression in the fascial fibroblast cells: ERα had a mean Ct of 28±0.1 in the pre-menopausal fascial tissues, and of 32±0.4 in the post-menopausal ones; the Ct of RXFP1 was 32.5±1.5 (pre-) and 34.8±0.1 (post-menopausal). The expression values of the three genes examined showed a lower variability in the cell samples, and there were no significant differences in RXFP1 expressions (29.8±1.6 in the pre-menopausal cell samples and 29.6±1.3 in post-menopausal ones). To summarize, we can affirm that relaxin was significantly expressed in the walls of the blood vessels supplying fascia and also in the fibroblasts, mainly at the cytoplasmic level, in all of the fascial districts examined. The estrogen receptor showed an evident expression in the fascial fibroblasts, both in the cytoplasm and in some nuclear membranes as well as in some cell nuclei.
Table 2.
Real time PCR Ct values in fascia tissues samples and in fascial fibroblasts in the two groups of patients: pre-menopausal and post-menopausal women. The data represent mean ± standard deviation of at least two samples of different fascial districts in triplicate.
| Ct fascial tissue | Ct fibroblast cells | |||||
|---|---|---|---|---|---|---|
| RXFP1 | ERα | RPS18 | RXFP1 | ERα | RPS18 | |
| Premenopausal women | 32.5±1.5 | 28±0.1 | 18.7±2.1 | 29.8±1.6 | 28.3±0.2 | 15.5±0.2 |
| Postmenopausal women | 34.8±0.1 | 32±0.4 | 19.6±0.2 | 29.6±1.3 | 30.1±0 | 16±0.4 |
Discussion
This is the first study demonstrating that all fibroblasts from different districts of the muscular fasciae express sex hormone receptors. Real time RT-PCR uncovered ERα and RXFP1 expression in all analyzed women fascia, with a lower gene expression of both investigated receptors in the post-menopausal women with respect to pre-menopausal ones (Table 2). Samples of fascia were collected from three different districts (leg, abdomen and thigh) of 8 women. To exclude variability within patients, we averaged the Ct values resulting from real time PCR experiments, subdividing the results into two groups: pre- and post-menopausal. As expected, expression resulted lower in the latter, especially with regard to ERα expression, according with the decrease in estrogen hormone levels in post-menopausal women. The current study showed that the expression of sex hormone receptors was prevalently localized in the fibroblast cells, in all the samples examined. More specifically, in the tissues not all the fibroblasts are positive, whereas the isolated and expanded cells showed homogeneous results. This could be explained by the fact that tissue is composed of a pool of different cell types (fibroblasts, vascular cells, mast cells, adipocytes, etc.) with a variability of expressions, while cell cultures isolated from the fascia are made up of an homogeneous type of cell.12 Relaxin receptors are expressed not only in fibroblast cells,15,16 but also in the nerves and in blood vessels (as shown in Figure 1 B,E and Figure 2 G,H). This evidence could help to explain the variability of RXFP1 expression: the expression is dependent on the composition and structure of fascial tissue and on the number of vessel cells in the starting homogenate. Also the housekeeping gene RPS18 in PCR experiments showed a variability of expression between cells and fascial tissue (18.7±2.1 and 19.6±0.2 in pre- and post- menopausal fascial tissues subgroups, respectively; 15.5±0.2 and 16±0.4 in the corresponding isolated cells) that could be justified by the different starting materials (pools of cells and isolated fibroblasts), as indicated by the literature.17
The presence of sex hormone receptors in fascial tissue could help to explain sex differences in the prevalence of myofascial pain.18 It is well known that relaxin is a multifunctional factor which contributes to collagen tissue remodeling by inhibiting fibrosis and inflammatory activities19 and that a longer duration of estrogen deficiency is associated with increased fibrosis, as demonstrated for the liver20 and the heart.21 In this respect, estrogen and estrogen receptor beta (ERβ) inhibit fibrosis reducing TGFβ expression, connective tissue growth factor production and function, matrix metalloproteinases 2 and 9 expression and activity, the conversion of fibroblasts to myofibroblasts and the production of collagens I and III. All these factors are also important to define fascial remodeling and fascial tension. If fascial stiffness is increased, the nociceptors within the fascia could be sensitized causing the underlying muscles to be stiffer.22 Lee et al. demonstrated that women who were taking oral contraceptives presented significantly lower ACL elasticity with respect to their non oral contraceptive counterparts.23 In addition, knee flexion extension hysteresis is significantly higher in oral contraceptive pill users (P<0.05). Eiling et al.24 reported that musculotendinous stiffness of the lower limbs of young (between 16 and 18 years of age) female net-ball players was significantly lower at week 3 (ovulatory phase) with respect to weeks 1 and 2 (8.7 and 4.5%, respectively).
These data are to be deepened, studying the differences on the basis of age and gender and presence of myofascial pain, not yet analyzed in this work, and investigating also the expression of other receptors isoforms, such as estrogen receptor-beta, and the expression of other proteins, such as progesterone receptor and G protein-coupled estrogen receptor 1 (GPER), that binds to and is activated by the female sex hormone estradiol, in turn activating cellular signaling pathways25. However, the current work, which is the first demonstrating sex hormone receptor expression in fascial fibroblasts, helps to explain how hormonal factors are linked to myofascial pain. If verified, this new concept may lead the way to novel pharmaceutical or mechanical approaches that could complement existing treatments of myofascial pain.
References
- 1.Moalli PA, Talarico LC, Sung VW, Klingensmith WL, Shand SH, Meyn LA, et al. Impact of menopause on collagen subtypes in the arcus tendineous fasciae pelvis. Am J Obstet Gynecol 2004;190:620-7. [DOI] [PubMed] [Google Scholar]
- 2.Petrofsky J, Lee H. Greater reduction of balance as a result of increased plantar fascia elasticity at ovulation during the menstrual cycle. Tohoku J Exp Med 2015; 237:219-26. [DOI] [PubMed] [Google Scholar]
- 3.Möller-Nielsen J, Hammar M. Women’s soccer injuries in relation to the menstrual cycle and oral contraceptive use. Med Sci Sports Exerc 1989;21:126-9. [PubMed] [Google Scholar]
- 4.Konopka JA, DeBaun MR, Chang W, Dragoo JL. The intracellular effect of relaxin on female anterior cruciate ligament cells. Am J Sports Med 2016; 20:1-9. [DOI] [PubMed] [Google Scholar]
- 5.Bai SW, Jung YW, Kwon HS, Yoon JM, Shin JS, Kim SK, et al. The role of estrogen receptor, progesterone receptor and p53 in development of stress urinary incontinence. Yonsei Med J 2004;45:885-90. [DOI] [PubMed] [Google Scholar]
- 6.Grigoriadis C, Hassiakos D, Bakas P, Tympa A, Panoulis C, Creatsas G, et al. Effect of gonadal steroid receptors alterations on the pathophysiology of pelvic organ prolapse and urinary incontinence. Minerva Ginecol 2016;68:37-42. [PubMed] [Google Scholar]
- 7.Lu Y, Lang JH, Zhu L. [Estrogen receptors in pelvic floor for female stress urinary incontinence].[Article in Chinese]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2007;29:402-6. [PubMed] [Google Scholar]
- 8.Dragoo JL, Lee RS, Benhaim P, Finerman GA, Hame SL. Relaxin receptors in the human female anterior cruciate ligament. Am J Sports Med. 2003,31:577-84. [DOI] [PubMed] [Google Scholar]
- 9.Galey S, Konieczko EM, Arnold CA, Cooney TE. Immunohistological detection of relaxin binding to anterior cruciate ligaments. Orthopedics 2003;26:1201-4. [DOI] [PubMed] [Google Scholar]
- 10.Dragoo JL, Padrez K, Workman R, Lindsey DP. The effect of relaxin on the female anterior cruciate ligament: Analysis of mechanical properties in an animal model. Knee 2009;16:69-72. [DOI] [PubMed] [Google Scholar]
- 11.Clifton KB, Rodner C, Wolf JM. Detection of relaxin receptor in the dorsoradial ligament, synovium, and articular cartilage of the trapeziometacarpal joint. J Orthop Res 2014;32:1061-7. [DOI] [PubMed] [Google Scholar]
- 12.Fede C, Albertin G, Petrelli L, Sfriso MM, Biz C, De Caro R, et al. Expression of the endocannabinoid receptors in human fascial tissue. Eur J Histochem 2016;60:2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caroccia B, Seccia MT, Campos AG, Gioco F, Kuppusamy M, Ceolotto G, et al. GPER-1 and estrogen receptor-β ligands modulate aldosterone synthesis. Endocrinology 2014;155:4296-304. [DOI] [PubMed] [Google Scholar]
- 14.Rienzo M, Schiano C, Casamassimi A, Grimaldi V, Infante T, Napoli C. Identification of valid reference housekeeping genes for gene expression analysis in tumor neovascularization studies. Clin Transl Oncol 2013;15:211-8. [DOI] [PubMed] [Google Scholar]
- 15.Novak J, Parry LJ, Matthews JE, Kerchner LJ, Indovina K, Hanley-Yanez K, et al. Evidence for local relaxin ligand-receptor expression and function in arteries. FASEB J. 2006;20 2352-62. [DOI] [PubMed] [Google Scholar]
- 16.Bathgate RA, Samuel CS, Burazin TC, Gundlach AL, Tregear GW. Relaxin: new peptides, receptors and novel actions. Trends Endocrinol Metab 2003;14:207-13. [DOI] [PubMed] [Google Scholar]
- 17.Thellin O, Zorzi W, Lakaye B, De Borman B, Coumans B, Hennen G, et al. Housekeeping genes as internal standards: use and limits. J Biotechnol 1999;75:291-5. [DOI] [PubMed] [Google Scholar]
- 18.Rollman GB, Lautenbacher S. Sex differences in musculoskeletal pain. Clin J Pain 2001;17:20-4. [DOI] [PubMed] [Google Scholar]
- 19.Kang YM, Choi YR, Yun CO, Park JO, Suk KS, Kim HS, et al. Down-regulation of collagen synthesis and matrix metalloproteinase expression in myofibroblasts from Dupuytren nodule using adenovirus-mediated relaxin gene therapy. J Orthop Res 2014;32:515-23. [DOI] [PubMed] [Google Scholar]
- 20.Klair JS, Yang JD, Abdelmalek MF, Guy CD, Gill RM, Yates K, et al. A longer duration of estrogen deficiency increases fibrosis risk among postmenopausal women with nonalcoholic fatty liver disease. Hepatology 2016;64:85-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedram A, Razandi M, Narayanan R, Levin ER. Estrogen receptor beta signals to inhibition of cardiac fibrosis. Mol Cell Endocrinol 2016;434:57-68. [DOI] [PubMed] [Google Scholar]
- 22.Schleip R, Naylor IL, Ursu D, Melzer W, Zorn A, Wilke HJ, et al. Passive muscle stiffness may be influenced by active contractility of intramuscular connective tissue. Med Hypotheses 2006;66:66-71. [DOI] [PubMed] [Google Scholar]
- 23.Lee H, Petrofsky JS, Daher N, Berk L, Laymon M. Differences in anterior cruciate ligament elasticity and force for knee flexion in women: oral contraceptive users versus non-oral contraceptive users. Eur J Appl Physiol 2014;114:285-94. [DOI] [PubMed] [Google Scholar]
- 24.Eiling E, Bryant AL, Petersen W, Murphy A, Hohmann E. Effects of menstrual-cycle hormone fluctuations on musculotendinous stiffness and knee joint laxity. Knee Surg Sports Traumatol Arthrosc 2007;15: 126-32. [DOI] [PubMed] [Google Scholar]
- 25.Prossnitz ER, Arterburn JB, Sklar LA. GPR30: A G protein-coupled receptor for estrogen. Mol Cell Endocrinol 2007;265-266:138-42. [DOI] [PMC free article] [PubMed] [Google Scholar]


