Asymmetrical distribution of lipids in the cell membrane is a fundamental feature of all eukaryotic cells. The most important negatively charged phospholipids phosphatidylserine (PS) and phosphatidylinositol species reside almost exclusively in the cytoplasmic leaflet, where they serve as essential co-factors for many membrane-bound enzymes including protein kinase C, phosphatase PTEN, tyrosine kinase c-Src, and MARCKS.1 Clusters of basic amino acid residues draw the proteins via electrostatic interactions to the lipid, enabling them to exert their function at the cytosolic membrane face.
Calcium-dependent and independent pathways can promote PS translocation to the outer membrane leaflet. Platforms for assembly of procoagulant enzyme complexes are thus generated on activated platelets. Surface exposed PS can also be recognized by cellular receptors and mediate important cell-cell interactions.2 Particularly prominent is the role of PS as an “eat-me” signal for macrophages on apoptotic cells.
In contrast, no biological role has hitherto been assigned to translocated PS in the cell itself.
What if the phospholipid were also to control the action of membrane-anchored surface proteins, analogous to and as a counterpart of its function at the cytosolic face?
Our investigation originated in the observation that PS-translocation regularly accompanied the activation of the Disintegrin and Metalloproteinase 17 (ADAM17).3 This evolutionarily conserved, vitally important protease was originally identified as the TNF-a releasing enzyme. Today, ADAM17 is known to be involved in the shedding of an increasing number of cell surface proteins including the EGFR ligand TGF-α, TNF receptor 1, and L-selectin. Very diverse biological processes are thus regulated by a single protease. A remarkably broad and heterogeneous spectrum of stimuli have been found to activate the enzyme, whereupon substrate cleavage occurs at sites located very close to the cell membrane surface.
It was found that triggers of ADAM17 activation provoked, while respective inhibitors suppressed PS-exposure in all the cell models tested. Did this correlation reflect a causal connection? Independent lines of evidence converged to generate an affirmative answer.
Scott syndrome is a rare bleeding disorder caused by the incapacity of blood cells to expose PS in response to intracellular Ca2+-elevation. The defect is due to a missense mutation in the calcium-dependent PS scramblase Anoctamin-6.4 While calcium influx provoked rapid PS exposure and loss of L-selectin in normal B-cells, Scott lymphocytes responded neither with PS exposure nor with substrate shedding. Because expression of caspase-dependent scramblases remains unaltered in Scott lymphocytes, we tested whether induction of apoptosis would lead to PS exposure and L-selectin release. This was indeed the case.
As a direct corollary, experiments were performed with Raji cells that have a blunted caspase-dependent PS-exposure capacity.5 Here, PS-externalization and TNFR1 shedding were absent upon apoptosis induction.
The possible mechanism underlying ADAM17 activation by PS was investigated. Should PS be directly interacting with the protease, its soluble head group ortho-phosphorylserine (OPS) might act as a competitive inhibitor. Indeed, ADAM17-dependent substrate shedding was reduced in the presence of OPS in the cell systems tested.
The membrane proximal domain (MPD) represented a likely candidate for interaction with PS because of its proximity to the membrane surface, and recombinant MPD was then found to bind to PS but not to PC liposomes. NMR-spectroscopy localized the PS-interaction site to a cluster of basic amino acids R625/K626/K628. Mutation of these amino acids to glycines abolished PS-binding capacity. When the corresponding ADAM17 mutant was transfected into ADAM10/ADAM17-double deficient cells, it was no longer able to cleave its physiological substrate TGF-α. However, the cells did express ADAM17 on their surface and the mutated protease was still capable of cleaving a soluble peptide substrate in the culture medium. A key finding was thus made that abrogation of PS-binding selectively affected the release of cell membrane bound substrates but not the bona-fide enzymatic activity of the protease.
The ADAM17 stalk region, also named CANDIS (Conserved ADAM-SeventeeN Dynamic Interaction Sequence), has recently been found to represent a membrane-interacting amphipathic helix that is also required for sheddase function.6 The combined data now lead to a 2-step model for ADAM17-mediated shedding which shares similarities with the activation of intracellular proteins including the accessory HIV-1 protein Nef. It associates with membranes in a biphasic process.7 A fast protein-lipid interaction driven by electrostatic attraction of a basic amino acid cluster to PS is followed by formation of an amphipathic helix that intercalates into the bilayer and stabilizes membrane-binding. Analogously, externalized PS interacts electrostatically with ADAM17 MPD, and this is accompanied by association of the amphipathic CANDIS-helix with the external membrane leaflet (Fig. 1).
Figure 1.
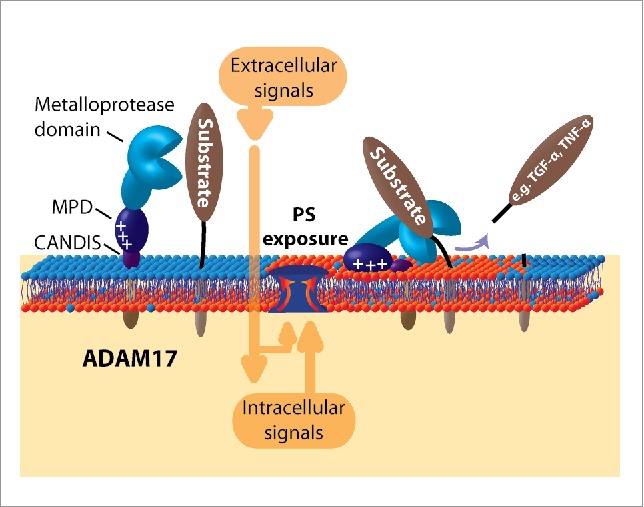
Role of PS-exposure for ADAM17 function. Many cellular stimuli provoke transient translocation of PS. Electrostatic interaction with the membrane proximal domain (MPD) of ADAM17 together with membrane association of the neighboring CANDIS-helix then enable substrate processing to take place.
Cells expend considerable amounts of energy to uphold PS-asymmetry in the membrane. Irreversible disruption of PS-homeostasis as occurring during platelet activation and apoptosis bears well known consequences. In contrast, the possibility that transient PS-externalization may be biologically relevant has been voiced quite seldomly and mainly in the context of immune cell activation. Perhaps, transient externalization of PS in defined membrane micro/nanodomains is a common event that will allow cells to respond rapidly and flexibly to myriad stimuli. Delineation of the events underlying upregulation of ADAM17 function may pave the way to this new field of research.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Mulgrew-Nesbitt A, Diraviyam K, Wang J, Singh S, Murray P, Li Z, Rogers L, Mirkovic N, Murray D. The role of electrostatics in protein–membrane interactions.. Biochim Biophys Acta 2006; 1761:812-26; PMID:16928468; http://dx.doi.org/ 10.1016/j.bbalip.2006.07.002 [DOI] [PubMed] [Google Scholar]
- [2].Bevers EM, Williamson PL. Getting to the outer leaflet: Physiology of phosphatidylserine exposure at the plasma membrane. Physiol Rev 2016; 96:605-45; PMID:26936867; http://doi.org/ 10.1152/physrev.00020.2015 [DOI] [PubMed] [Google Scholar]
- [3].Sommer A, Kordowski F, Büch J, Maretzky T, Evers A, Andrä J, Düsterhöft S, Michalek M, Lorenzen I, Somasundaram P, et al. Phosphatidylserine exposure is required for ADAM17 sheddase function.. Nat Commun 2016; 7:11523; PMID:27161080; http://dx.doi.org/ 10.1038/ncomms11523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Suzuki J, Umeda M, Sims PJ, Nagata S. Calcium-dependent phospholipid scrambling by TMEM16F.. Nature 2010; 468:834-8; PMID:21107324; http://dx.doi.org/ 10.1038/nature09583 [DOI] [PubMed] [Google Scholar]
- [5].Suzuki J, Denning DP, Imanishi E, Horvitz HR, Nagata S. Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells.. Science 2013; 341:403-6; PMID:23845944; http://dx.doi.org/ 10.1126/science.1236758 [DOI] [PubMed] [Google Scholar]
- [6].Dusterhoft S, Michalek M, Kordowski F, Oldefest M, Sommer A, Röseler, Reiss K, Grötzinger J, Lorenzen I. Extracellular juxtamembrane segment of ADAM17 interacts with membranes and is essential for its shedding activity.. Biochemistry 2015; 54:5791-801; PMID:26348730; http://dx.doi.org/ 10.1021/acs.biochem.5b00497 [DOI] [PubMed] [Google Scholar]
- [7].Gerlach H, Laumann V, Martens S, Becker CFW, Goody RS, Geyer M. HIV-1 Nef membrane association depends on charge, curvature, composition and sequence.. Nat Chem Biol 2010; 6:46-53; PMID:19935658; http://dx.doi.org/ 10.1038/nchembio.268 [DOI] [PubMed] [Google Scholar]


