Abstract
GP-2, a 75-kDa glycoprotein, was isolated from dog pancreatic zymogen granule membranes (ZGMs). In a carbohydrate-shift strategy, N-terminal and internal peptide sequences were obtained on glycosylated and deglycosylated forms of GP-2, respectively, by gas-phase sequencing. Sets of mixed oligonucleotides and the polymerase chain reaction were used to obtain a double-stranded cDNA probe, which was used to isolate overlapping cDNA clones from a dog pancreatic cDNA library. The sequence of these clones revealed an open reading frame that encodes a protein of 509 amino acids, eight N-linked oligosaccharide attachment sites, and an N-terminal signal sequence absent from the mature form of GP-2 associated with ZGMs. The C terminus shows a 20-residue hydrophobic transmembrane domain preceded by a decapeptide containing potential phosphatidylinositol-glycan attachment sites. GP-2 completely released from ZGMs by exogenous phospholipase C showed similar immunochemical properties and electrophoretic mobilities compared to the form associated with ZGMs. A similar form of GP-2 was released from zymogen granules permeabilized with saponin and incubated in the absence of added phospholipase C. Kinetic analysis of GP-2 release at 0 degrees C and 37 degrees C suggested the presence of a granule enzyme responsible for endogenous release of GP-2 to granule contents and into the apical medium. The data indicate that GP-2 is a phosphatidylinositol-glycan-linked membrane protein released from the membrane of mature zymogen granules by an enzymatic mechanism. The cDNA structure presented here thus encodes both membrane-bound and free forms of GP-2.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Leavitt J., Saavedra R. A., Hood L. E., Kent S. B. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis after in situ protease digestion on nitrocellulose. Proc Natl Acad Sci U S A. 1987 Oct;84(20):6970–6974. doi: 10.1073/pnas.84.20.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Fukuoka S., Scheele G. A. Rapid and selective cloning of monitor peptide, a novel cholecystokinin-releasing peptide, using minimal amino acid sequence and the polymerase chain reaction. Pancreas. 1990;5(1):1–7. doi: 10.1097/00006676-199001000-00001. [DOI] [PubMed] [Google Scholar]
- Fukuoka S., Scheele G. Nucleotide sequence encoding the major glycoprotein (GP2) of rat pancreatic secretory (zymogen) granule membranes. Nucleic Acids Res. 1990 Oct 11;18(19):5900–5900. doi: 10.1093/nar/18.19.5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., van der Ley P. A., Scheffer R. C. Use of colloidal gold particles in double-labeling immunoelectron microscopy of ultrathin frozen tissue sections. J Cell Biol. 1981 Jun;89(3):653–665. doi: 10.1083/jcb.89.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B., Kelly R. B. Two distinct intracellular pathways transport secretory and membrane glycoproteins to the surface of pituitary tumor cells. Cell. 1982 Jan;28(1):51–59. doi: 10.1016/0092-8674(82)90374-9. [DOI] [PubMed] [Google Scholar]
- HOKIN L. E. Isolation of the zymogen granules of dog pancreas and a study of their properties. Biochim Biophys Acta. 1955 Nov;18(3):379–388. doi: 10.1016/0006-3002(55)90101-3. [DOI] [PubMed] [Google Scholar]
- Hansen L. J., Reddy M. K., Reddy J. K. Comparison of secretory protein and membrane composition of secretory granules isolated from normal and neoplastic pancreatic acinar cells of rats. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4379–4383. doi: 10.1073/pnas.80.14.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havinga J. R., Strous G. J., Poort C. Biosynthesis of the major glycoprotein associated with zymogen-granule membranes in the pancreas. Eur J Biochem. 1983 Jun 15;133(2):449–454. doi: 10.1111/j.1432-1033.1983.tb07484.x. [DOI] [PubMed] [Google Scholar]
- Havinga J. R., Strous G. J., Poort C. Intracellular transport of the major glycoprotein of zymogen granule membranes in the rat pancreas. Demonstration of high turnover at the plasma membrane. Eur J Biochem. 1984 Oct 1;144(1):177–183. doi: 10.1111/j.1432-1033.1984.tb08446.x. [DOI] [PubMed] [Google Scholar]
- LeBel D., Beattie M. Identification of the proteins exposed on the cytoplasmic surface of the pancreatic zymogen granule. Biochim Biophys Acta. 1984 Feb 15;769(3):622–624. doi: 10.1016/0005-2736(84)90061-0. [DOI] [PubMed] [Google Scholar]
- LeBel D., Beattie M. The integral and peripheral proteins of the zymogen granule membrane. Biochim Biophys Acta. 1984 Feb 15;769(3):611–621. doi: 10.1016/0005-2736(84)90060-9. [DOI] [PubMed] [Google Scholar]
- LeBel D., Beattie M. The major protein of pancreatic zymogen granule membranes (GP-2) is anchored via covalent bonds to phosphatidylinositol. Biochem Biophys Res Commun. 1988 Jul 29;154(2):818–823. doi: 10.1016/0006-291x(88)90213-6. [DOI] [PubMed] [Google Scholar]
- Lisanti M. P., Caras I. W., Davitz M. A., Rodriguez-Boulan E. A glycophospholipid membrane anchor acts as an apical targeting signal in polarized epithelial cells. J Cell Biol. 1989 Nov;109(5):2145–2156. doi: 10.1083/jcb.109.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G. Glycosyl-phosphatidylinositol: a versatile anchor for cell surface proteins. FASEB J. 1989 Mar;3(5):1600–1608. doi: 10.1096/fasebj.3.5.2522071. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Ronzio R. A. Comparative analysis of zymogen granule membrane polypeptides. Biochem Biophys Res Commun. 1972 Oct 17;49(2):377–382. doi: 10.1016/0006-291x(72)90421-4. [DOI] [PubMed] [Google Scholar]
- Masterson W. J., Doering T. L., Hart G. W., Englund P. T. A novel pathway for glycan assembly: biosynthesis of the glycosyl-phosphatidylinositol anchor of the trypanosome variant surface glycoprotein. Cell. 1989 Mar 10;56(5):793–800. doi: 10.1016/0092-8674(89)90684-3. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mayor S., Menon A. K., Cross G. A. Glycolipid precursors for the membrane anchor of Trypanosoma brucei variant surface glycoproteins. II. Lipid structures of phosphatidylinositol-specific phospholipase C sensitive and resistant glycolipids. J Biol Chem. 1990 Apr 15;265(11):6174–6181. [PubMed] [Google Scholar]
- Meldolesi J., Jamieson J. D., Palade G. E. Composition of cellular membranes in the pancreas of the guinea pig. I. Isolation of membrane fractions. J Cell Biol. 1971 Apr;49(1):109–129. doi: 10.1083/jcb.49.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micanovic R., Gerber L. D., Berger J., Kodukula K., Udenfriend S. Selectivity of the cleavage/attachment site of phosphatidylinositol-glycan-anchored membrane proteins determined by site-specific mutagenesis at Asp-484 of placental alkaline phosphatase. Proc Natl Acad Sci U S A. 1990 Jan;87(1):157–161. doi: 10.1073/pnas.87.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
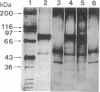
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
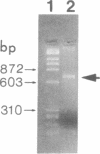
- Noda M., Yoon K., Rodan G. A., Koppel D. E. High lateral mobility of endogenous and transfected alkaline phosphatase: a phosphatidylinositol-anchored membrane protein. J Cell Biol. 1987 Oct;105(4):1671–1677. doi: 10.1083/jcb.105.4.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Pancholi V., Fischetti V. A. Identification of an endogenous membrane anchor-cleaving enzyme for group A streptococcal M protein. Its implication for the attachment of surface proteins in gram-positive bacteria. J Exp Med. 1989 Dec 1;170(6):2119–2133. doi: 10.1084/jem.170.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pâquet M. R., St-Jean P., Roberge M., Beaudoin A. R. Isolation of zymogen granules from rat pancreas and characterization of their membrane proteins. Eur J Cell Biol. 1982 Aug;28(1):20–26. [PubMed] [Google Scholar]
- Rindler M. J., Hoops T. C. The pancreatic membrane protein GP-2 localizes specifically to secretory granules and is shed into the pancreatic juice as a protein aggregate. Eur J Cell Biol. 1990 Oct;53(1):154–163. [PubMed] [Google Scholar]
- Ronzio R. A., Kronquist K. E., Lewis D. S., MacDonald R. J., Mohrlok S. H., O'Donnell J. J., Jr Glycoprotein synthesis in the adult rat pancreas. IV. Subcellular distribution of membrane glycoproteins. Biochim Biophys Acta. 1978 Mar 21;508(1):65–84. doi: 10.1016/0005-2736(78)90189-x. [DOI] [PubMed] [Google Scholar]
- Sarkar P., Sengupta D., Basu S., Maitra U. Nucleotide sequence of a major class-III phage-T3 RNA-polymerase promoter located at 98.0% of phage-T3 genetic map. Gene. 1985;33(3):351–355. doi: 10.1016/0378-1119(85)90243-4. [DOI] [PubMed] [Google Scholar]
- Scheele G. A., Palade G. E., Tartakoff A. M. Cell fractionation studies on the guinea pig pancreas. Redistribution of exocrine proteins during tissue homogenization. J Cell Biol. 1978 Jul;78(1):110–130. doi: 10.1083/jcb.78.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G., Simons K. Lipid polarity and sorting in epithelial cells. J Cell Biochem. 1988 Jan;36(1):51–58. doi: 10.1002/jcb.240360106. [DOI] [PubMed] [Google Scholar]