The integration of extracellular growth and proliferative ques with cell division is sensed and regulated by the D-type cyclins in concert with a catalytic subunit, either CDK4/6. Mammalian cells encode 3 D cyclins, D1-3, that are expressed in a tissue restricted pattern. While the innate biochemical activity of each cyclin, its capacity to activate either CDK4 or CDK6, is largely indistinguishable in vitro allowing for considerable functional redundancy, the generation of mice ablated for expression of individual D cyclins has revealed unique functional and regulatory characteristics. While the knowledge provided by genetic manipulation is provocative, molecular insights into unique biochemical properties of each of the 3 D-type cyclins have been sparse. Work by DeMicco et al describes mechanisms that regulate cyclin D3 accumulation during B and T lymphocyte maturation and provide clues to the importance of lineage-specific pathways.1
Significant progress has been made with regard to the elucidation of how extracellular cues regulate cyclin D protein accumulation and biological functions during the past 20 years; however, much of this has focused on cyclin D1 due to its frequent overexpression in human cancer. With our current view that all cyclins are not created alike, the need to explore the regulation of cyclins D2 and D3 has come to the forefront. With respect to unique function, cyclin D3 is specifically required for B- and T-lymphocyte proliferation.2 B- and T-lymphocyte maturation, unlike many other cell lineages, depends on programmed DNA damage in the form of double strand DNA breaks (DSBs), a process necessary for the genesis of immunoglobulin molecules and mature T-cell receptor repertoire respectively. The ability process such a program requires that lymphocytes also have a mechanism to prevent progression from G1 phase to S-phase transition prior to resolution of DSBs. The absence of such a mechanism would result in low fidelity DNA replication leading to cell apoptosis, immune failure and/or cancer. The efforts of DeMicco et al reveal that both immature T- and B-lymphocytes downregulate cyclin D3 in response to DSBs. The striking aspect of this regulation is that while both utilize the checkpoint kinase, Ataxia telangiectasia mutated (ATM), to coordinate the damage response with cyclin D3 repression, the underlying mechanisms of suppression are distinct in the respective cell types (Fig. 1).
Figure 1.
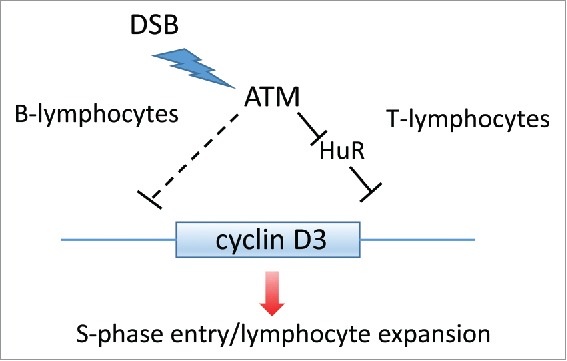
Lineage-dependent regulation of cyclin D3 accumulation.
DNA damage dependent regulation of D-type cyclin accumulation is not without precedence. Cyclin D1 undergoes rapid, ubiquitin-dependent degradation in response to genotoxic stress and the precise molecular mechanism depends upon cell cycle stage.3,4 Strikingly, no alterations in cyclin D3 degradation were observed in either lymphocyte lineage arguing that constitutive turnover is sufficient. Rather, cyclin D3 loss in immature T-lymphocytes reflects inhibition of protein synthesis. Cyclin D3 protein synthesis in lymphocytes is in part regulated by the RNA binding protein HuR; HuR binding to AU-rich sequences in the cyclin D3 3′UTR increases translational efficiency (Fig. 1).5 Mechanistically, DSB-dependent ATM activation triggers the release HuR from CCND3 thereby reducing cyclin D3 synthesis.1 ATM-dependent regulation of HuR likely reflects Chk2-dependent phosphorylation of the RNA binding domain of HuR which reduces its affinity for RNA.6
In contrast to immature T-lymphocytes, immature B-lymphocytes signal the transcriptional repression of the CCND3 gene (Fig. 1) through a yet to be defined ATM-dependent mechanism. Since DNA damage requires an acute and immediate cellular response, transcription regulation of CCND3 seems paradoxical. However, the relative instability of CCND3 mRNA likely enables this mechanism. Transcriptional repression can be an inefficient checkpoint mechanism, as the “stop” signal remains dependent upon mRNA and protein degradation. The relative instability of cyclin D3 mRNA and protein should be essential for the efficacy of this signal. Logic dictates that transcriptional regulation in B-lymphocytes versus regulation of protein synthesis T-lymphocytes will contribute to altering rates of recovery from genotoxic damage or the kinetics of maturation and lymphocyte amplification during an immune challenge. Regardless of the precise mechanism, reduced cyclin D3 is essential for inhibition of unscheduled S-phase entry.1
Although individual D-type cyclins clearly exhibit overlapping functions through their combinatorial activation of either CDK4 or 6, accumulating evidence is revealing unique, cell type specific regulatory mechanisms. While one aspect of lineage-specific regulation of a given cycle likely reflects inherent signaling specificity within a given cell type, the dependence of such cells on a cyclin D3-dependent kinase suggests unique functional characteristics. The work of DeMicco et al supports and adds to previous work suggesting a model wherein lymphocytes are uniquely dependent upon the cyclin D3-dependent kinase 2,7 and highlight the importance of further investigation into its regulation and biological function.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].DeMicco A, Reich T, Arya R, Rivera-Reyes A, Fisher MR, Bassing CH. Lymphocyte lineage-specific and developmental stage specific mechanisms suppress cyclin D3 expression in response to DNA double strand breaks. Cell Cycle 2016. Jun 21; [Epub ahead of print]; PMID:27327568; http://dx. doi.org/ 10.1080/15384101.2016.1198861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sicinska E, Aifantis I, Le Cam L, Swat W, Borowski C, Yu Q, Ferrando AA, Levin SD, Geng Y, von Boehmer H, et al.. Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell 2003; 4:451-61; PMID:14706337; http://dx.doi.org/ 10.1016/S1535-6108(03)00301-5 [DOI] [PubMed] [Google Scholar]
- [3].Pontano LL, Aggarwal P, Barbash O, Brown EJ, Bassing CH, Diehl JA. Genotoxic stress-induced cyclin D1 phosphorylation and proteolysis are required for genomic stability. Mol Cell Biol 2008; 28:7245-58; PMID:18809569; http://dx.doi.org/ 10.1128/MCB.01085-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Agami R, Bernards R. Distinct initiation and maintenance mechanisms cooperate to induce G1 cell cycle arrest in response to DNA damage. Cell 2000; 102:55-66; PMID:10929713; http://dx.doi.org/ 10.1016/S0092-8674(00)00010-6 [DOI] [PubMed] [Google Scholar]
- [5].Rodriguez PC, Hernandez CP, Morrow K, Sierra R, Zabaleta J, Wyczechowska DD, Ochoa AC. L-arginine deprivation regulates cyclin D3 mRNA stability in human T cells by controlling HuR expression. J Immunol 2010; 185:5198-204; PMID:20889542; http://dx.doi.org/ 10.4049/jimmunol.1001224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Abdelmohsen K, Pullmann R Jr, Lal A, Kim HH, Galban S, Yang X, Blethrow JD, Walker M, Shubert J, Gillespie DA, et al.. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell 2007; 25:543-57; PMID:17317627; http://dx.doi.org/ 10.1016/j.molcel.2007.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cooper AB, Sawai CM, Sicinska E, Powers SE, Sicinski P, Clark MR, Aifantis I. A unique function for cyclin D3 in early B cell development. Nat Immunol 2006; 7:489-97; PMID:16582912; http://dx.doi.org/ 10.1038/ni1324 [DOI] [PubMed] [Google Scholar]


