Abstract
Key points
Vascular brain lesions and atherosclerosis are two similar conditions that are characterized by increased inflammation and oxidative stress.
Non‐invasive imaging in a murine model of atherosclerosis showed vascular brain damage and peripheral inflammation.
In this study, exercise training reduced magnetic resonance imaging‐detected abnormalities, insulin resistance and markers of oxidative stress and inflammation in old ApoE−/− mice.
Our results demonstrate the protective effect of exercise on neurovascular damage in the ageing brain of ApoE−/− mice.
Abstract
Vascular brain lesions, present in advanced atherosclerosis, share pathological hallmarks with peripheral vascular lesions, such as increased inflammation and oxidative stress. Physical activity reduces these peripheral risk factors, but its cerebrovascular effect is less documented, especially by non‐invasive imaging. Through a combination of in vivo and post‐mortem techniques, we aimed to characterize vascular brain damage in old ApoE−/− mice fed a high‐cholesterol (HC) diet with dietary controlled intake. We then sought to determine the beneficial effects of exercise training on oxidative stress and inflammation in the brain as a treatment option in an ageing atherosclerosis mouse model. Using in vivo magnetic resonance imaging (MRI) and biological markers of oxidative stress and inflammation, we evaluated the occurrence of vascular abnormalities in the brain of HC‐diet fed ApoE−/− mice >70 weeks old, its association with local and systemic oxidative stress and inflammation, and whether both can be modulated by exercise. Exercise training significantly reduced both MRI‐detected abnormalities (present in 71% of untrained vs. 14% of trained mice) and oxidative stress (lipid peroxidation, 9.1 ± 1.4 vs. 5.2 ± 0.9 μmol mg−1; P < 0.01) and inflammation (interleukin‐1β, 226.8 ± 27.1 vs. 182.5 ± 21.5 pg mg−1; P < 0.05) in the brain, and the mortality rate. Exercise also decreased peripheral insulin resistance, oxidative stress and inflammation, but significant associations were seen only within brain markers. Highly localized vascular brain damage is a frequent finding in this ageing atherosclerosis model, and exercise is able to reduce this outcome and improve lifespan. In vivo MRI evaluated both the neurovascular damage and the protective effect of exercise.
Key points
Vascular brain lesions and atherosclerosis are two similar conditions that are characterized by increased inflammation and oxidative stress.
Non‐invasive imaging in a murine model of atherosclerosis showed vascular brain damage and peripheral inflammation.
In this study, exercise training reduced magnetic resonance imaging‐detected abnormalities, insulin resistance and markers of oxidative stress and inflammation in old ApoE−/− mice.
Our results demonstrate the protective effect of exercise on neurovascular damage in the ageing brain of ApoE−/− mice.
Abbreviations
- AOPP
advanced oxidation protein products
- BBB
blood–brain barrier
- BCA
bicinchoninic acid
- CS
citrate synthase
- FRAP
ferric reducing antioxidant power
- GPX
glutathione peroxidase
- HF/HC
high fat/high cholesterol
- IL‐1β
interleukin‐1β
- IL‐4
interleukin‐4
- MDA
malondialdehyde
- MRI
magnetic resonance imaging
- NF‐κB
nuclear factor‐κB
- NOx
nitric oxide metabolites
- O‐ApoE‐ExT
old ApoE−/− exercise trained
- O‐ApoE‐UT
old ApoE−/− untrained
- O‐C57‐ ExT
old C57 mice exercise trained
- O‐C57‐UT
old C57 mice untrained
- SOD
superoxide dismutase
- TNFα
tumour necrosis factor‐α
- TPTZ
2,4,6‐Tris(2‐pyridyl)‐s‐triazine
- USPIO
ultrasmall iron oxide nanoparticles
- VWR
voluntary wheel running
- Y‐ApoE‐UT
young ApoE−/− mice untrained
Introduction
Vascular brain lesions develop in a similar manner to those of atherosclerosis as both conditions are characterized by an increased inflammation and oxidative stress. The burden of dementia in the aged population and the recent investigations linking high‐risk cardiovascular factors and neurodegenerative diseases incites translational investigations of altered cerebrovascular function in high‐risk ageing patients. Relevant imaging biomarkers are therefore being developed for non‐invasive evaluation of lipid‐induced peripheral and neurovascular dysfunction, inflammation and oxidative stress (Briley‐Saebo et al. 2012). In the context of ageing and disturbed lipid trafficking, the brain is highly susceptible to reactive oxygen species‐induced damage because of its high rate of oxidative metabolism and relatively low levels of antioxidant enzymes (Coyle & Puttfarcken, 1993). It is possible that blood–brain barrier (BBB) permeability (Hafezi‐Moghadam et al. 2007; Zlokovic, 2008), a cholesterol‐rich diet and the macrophage infiltration that is common in atherosclerosis could also affect the brain vessel function in the ageing process (Casserly & Topol, 2004; Saleh et al. 2004). Therefore, risk factors for atherosclerosis could be involved in the development of inflammatory conditions in the brain and, ultimately, lead to cerebrovascular ischaemia or haemorrhage (Dutta et al. 2012).
Exercise training has been well documented in the amelioration of oxidative stress and inflammation in cardiovascular disease (Lee et al. 2011). Exercise training is able to reduce oxidative stress by increasing antioxidant capabilities and by maintaining nitric oxide metabolism (Chirico et al. 2012). Exercise training could also be an impetus for an anti‐inflammatory environment because aerobic exercise has been associated with lower circulating levels of pro‐inflammatory markers (Lesniewski et al. 2011). Exercise is also connected with improvement in general metabolic conditions, such as lipid dysfunction and insulin resistance. As shown by Pellegrin et al. (2009 a,b), the beneficial effects of exercise training on atherosclerosis have been established in young ApoE−/− mice.
In old ApoE−/− mice fed a high‐fat, high‐cholesterol (HF/HC) diet, acute events such as paralysis, hemiplegia and sudden death have been observed (ENC and VP, unpublished observations), which are highly suggestive of cerebrovascular diseases. We used aortic and brain magnetic resonance imaging (MRI) to characterize these observations further and to highlight the combination of systemic and local effects of exercise in this advanced vascular and metabolic model (Drake et al. 2011). Indeed, a recent review suggested that heart or brain infarcts could be potentiated by a whole‐body inflammatory network (Nahrendorf et al. 2015).
We hypothesized that voluntary wheel running (VWR) could ameliorate vascular‐induced abnormalities in the brain and decrease inflammation; two conditions that suggest a high risk of stroke or other neurovascular complications.
Methods
Ethical approval
All animal procedures conformed to the European regulation for animal use, and this study was approved by the local ethics committee of our institution (University Claude Bernard Lyon 1). All the authors understand the ethical principles under which The Journal of Physiology operates. In addition, the present work complies with the animal ethics checklist of The Journal of Physiology. Finally, we have taken all the steps to minimize the animals’ pain and suffering for each methodological procedure where animals were involved, as detailed below.
In brief, five groups of animals were studied: old untrained and exercise‐trained ApoE−/− mice (C57BL/6 background; Charles‐River, France; O‐ApoE‐UT and O‐ApoE‐ExT, respectively), young untrained ApoE−/− mice (Y‐ApoE‐UT) and old untrained and exercise‐trained C57BL/6 (O‐C57‐UT and O‐C57‐ExT, respectively). Using in vivo MRI, the ascending aorta and brain were imaged, and contrast agents (iron oxide nanoparticles, P‐904 and gadolinium Gd‐DOTA; Guerbet, Aulnay‐sous‐Bois, France) were used to quantify macrophage infiltration and vascular permeability. At the end of the study, plasma measurements and tissue samples were taken. Total blood cholesterol, insulin, oxidative stress markers [advanced oxidation protein products (AOPP), malondialdehyde (MDA) and nitrotyrosine], antioxidant markers [catalase, glutathione peroxidase (GPX), superoxide dismutase (SOD), ferric reducing antioxidant power (FRAP) and nitric oxide metabolites (NOx)] and inflammatory markers [tumour necrosis factor‐α (TNFα), interleukin‐1β (IL‐1β) and nuclear factor‐κB (NF‐κB)/p65] were measured. Brain samples were stained with standard Haematoxylin and Eosin, F4/80 immunostaining for macrophages and IgG immunostaining for BBB permeability.
Animals
Male and female ApoE−/− mice (C57BL/6 background; Charles‐River, France) were fed a HF/HC diet (western diet; 21% fat, 0.15% cholesterol; U8220 version 153; SAFE, Augy, France) starting at 8 weeks of age, and control male and female C57BL/6 mice (Charles‐River, France) were fed a normal diet (Teklad Global 16% Protein rodent diet with 12% fat; Harlan, Gannat, France). All animals were maintained on a 12 h–12 h light–dark cycle and were supplied with food (limited at 20 g week−1 per animal, equivalent to 955 kcal week−1 for ApoE−/− mice and 688 kcal week−1 for C57BL/6 mice) and water ad libitum. After careful maintenance of health conditions for 1 year (Guerbet, Animal Care Unit), at 60 ± 1 weeks old both ApoE−/− and C57 mice were randomly divided into two activity groups (untrained, UT; and exercise trained, ExT). Mice in the exercise‐trained group (O‐ApoE‐ExT and O‐C57‐ExT) were individually housed in cages equipped with a 12.5 cm metal running wheel (HAGEN‐61700, Montreal, QC, Canada) and digital magnetic counter (model BC906; Sigma Sport, Neustadt, Germany), whereas the untrained (O‐ApoE‐UT and O‐C57‐UT) groups had a standard cage. Male and female young‐adult ApoE−/− mice (Y‐ApoE‐UT; aged 10 weeks) fed the same high‐fat diet (starting at 8 weeks of age; fed the diet for 14 weeks in total) served as age controls. During the 12 weeks of training, the distance run and the general health (i.e. tumours, skin irritations) of the mice were noted three times a week. The exclusion criterion was overall poor health of the animal (i.e. tumours, skin irritations). A follow‐up was performed daily by the technician in the animal facility to evaluate pain on the basis of external physical appearance, weight loss (with respect to food and water ingestion), assessable clinical signs (in particular, increase in respiratory frequency), change in behaviour (in particular, prostration) and non‐response to external stimuli.
Magnetic resonance imaging protocol
Mice were randomly selected to undergo the imaging protocol. The mice were anaesthetized by inhalation of isoflurane (2% for induction and 1% to maintain anaesthesia; Tem Sega, Lormont, France). Cardiac and respiratory rates were monitored throughout the session, and body temperature was maintained using a circulating heated water blanket at 37 ± 1°C. Magnetic resonance image acquisition was performed on a 4.7 T Bruker magnet (Ettingen, Germany). The total duration of the MRI protocol was <2 h. The protocol was then repeated 48 h after the injection of ultrasmall iron oxide nanoparticles (USPIO; P904, 1 mmol Fe kg−1; Guerbet) for vessel wall inflammation assessment, followed by the acquisition of brain images (Fig. 1).
Figure 1. Magnetic resonance imaging (MRI) protocol .
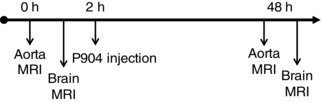
A pre‐contrast imaging (0 h) protocol was performed on the aorta, followed immediately by brain imaging with gadolinium injection. An ultrasmall iron oxide nanoparticles (USPIO) contrast agent, P904 (Guerbet, Aulnay‐sous‐Bois, France), was then injected. Forty‐eight hours later (48 h), an identical post‐USPIO aortic imaging protocol was performed for assessment of inflammation. This was followed immediately by brain inflammation imaging.
Magnetic resonance imaging of the aorta
For imaging of the ascending aorta, double cardiac and respiratory gated acquisitions were obtained as previously described (Sigovan et al. 2012) with a homemade gating system developed in Matlab (The MathWorks Inc., Natick, MA. USA). Electrocardiographic signals were collected via three electrodes placed on the paws, and respiratory signals were collected via a pressure sensor placed on the abdomen.
The ascending and descending aorta was identified using reference axial slices. A bright‐blood cine‐mode FLASH sequence was used to locate the aortic arch. The reference slices were acquired with a gradient echo (GE) sequence with the following parameters: repetition time (TR) / echo time (TE) = one R–R interval/6.7 ms; field of view = 3.8 cm × 3.8 cm; matrix = 256 × 256; bandwidth, 25 kHz; and slice thickness = 1.1 mm. The oblique slice was placed perpendicular to the ascending aorta, directly above the sinus to avoid flow artifacts.
A black‐blood multigradient echo sequence was used to image USPIO accumulation using the same slice number and positioning, spatial resolution, partial echo acquisition, and the following parameters: minimal repetition time, 742 ms (achieved by setting the gating system between three and five R–R intervals depending on the animal's heart rate); four echoes; bandwidth, 79.3 kHz; and number of averages, 2. The sequence was performed twice with two different values of TE: 3.1 ms, followed by 4.0 ms. The eight echo images acquired were interleaved to allow a better sampling of the T2* decay curve.
Magnetic resonance imaging of the brain
For brain imaging, a birdcage coil of 72 mm i.d. was used for radiofrequency transmission and a surface coil anatomically shaped to the mouse head for reception (Rapid Biomedical, Wurzburg, Germany).
Brain T2‐weighted spin‐echo images were acquired using a Rapid Imaging with Refocused Echoes (RARE) sequence in both axial and coronal planes. T2 RARE and T2* multi gradient echo (MGE) sequence positioned using standard MRI brain anatomical references for careful pre‐ and post‐contrast registration were acquired. The RARE sequence was used with the following parameters: TR/TE = 4000 /69 ms; field of view = 2 cm × 2 cm; matrix = 256 × 256; slice thickness = 1 mm; RARE factor = 8; and number of slices = 15. The MGE sequence was used with the following parameters: TR/first TE = 1500/2.6 ms; flip angle = 75 deg; field of view = 2 cm × 2 cm; matrix = 256 × 192; slice thickness = 1 mm; 12 echoes and echo interval = 3.5 ms; and number of slices = 15.
In order to characterize the neurovascular lesions, an extended brain MRI protocol was performed in a separate set of old untrained ApoE−/− mice fed the HF/HC diet (n = 10) and old C57BL/6 mice. Neurovascular lesions and iron deposits were assessed respectively by baseline T2 and T2* imaging. T2* quantification was obtained using a multislice multi‐echo gradient echo sequence. For BBB permeability assessment, a T1‐weighted MGE sequence with identical geometrical parameters was acquired before and 10 min after injection of gadolinium chelate (Gd‐DOTA, 0.1 mmol kg−1; Guerbet, Aulnay‐sous‐Bois, France) with the following parameters: TR/TE = 124/2.8 ms; field of view = 2 cm × 2 cm; matrix = 256 × 192; slice thickness = 1 mm; and number of slices = 15. This was followed by USPIO injection and the 48 h post‐USPIO T2/T2* imaging.
Analysis of MRI
For brain analysis, areas of interest on pre‐USPIO T2/T2* images and post‐gadolinium T1 images were first visually categorized based on the size of abnormal areas and the number of slices affected. The blinded investigators scored changes in pre‐ and post‐contrast (48 h post‐USPIO) T2/T2* images and gadolinium leakage on T1‐weighted images. Briefly, a score of 1–4 was given for abnormalities seen on pre‐contrast images and a score of 1–4 was given for changes seen on post‐contrast images (see Table 1 for scoring assessment).
Table 1.
Visual lesion characteristics
| Score | Pre‐contrast image | Post‐contrast image |
|---|---|---|
| 1 | No abnormality | No observable change from pre‐contrast |
| 2 | Small lesions (>10 pixels) on ≤2 slices | Change in SNR and increase in dark region size from pre‐contrast on ≤2 slices |
| 3 | >2 slices or medium‐sized lesion (10 < pixels < 20) on ≤2 slices | Medium change in SNR and increase in dark region size from pre‐contrast on ≤2 slices |
| 4 | Large lesion (>20 pixels) on >2 slices | Major change in SNR and increase in dark region size from pre‐contrast on >2 slices |
Abbreviations: SNR, signal‐to‐noise ratio
For analysis of the aortic arch, inner and outer vessel wall contours were delineated and vessel wall area calculated. T2* mapping of pre‐ and post‐USPIO series was performed using Matlab (The MathWorks Inc.,) on interleaved multi‐slice‐multi‐echo (MSME) images. The vessel wall regions of interest were used for the analysis of aortic T2* on both pre‐ and post‐contrast images as described by Sigovan et al. (2010).
Dissections
After the second imaging session, mice were anaesthetized by an i.p. injection of pentobarbital (50 mg kg−1; Dolethal; Vétoquinol, Lure, France) and blood was collected by cardiac puncture. Mice were killed by exsanguination with a 0.9% NaCl transcardial perfusion for 70 s. The brain, heart, ascending–descending aorta, liver and soleus were removed. Sections to be used for biological assays were stored at −80°C until assessment.
Immunohistochemistry
Brain samples were harvested and fixed in a paraformaldehyde solution for 1 h followed by sucrose for 24 h and preserved at −80°C until processing. Four successive 15‐μm‐thick sections for three MRI locations were assessed with standard Haematoxylin and Eosin staining, F4/80 immunostaining for macrophages and IgG immunostaining for BBB permeability, as previously described (Wiart et al. 2007). A DeadEnd™ Colourimetric TUNEL System kit (Promega, San Luis Obispo, CA, USA) was used to detect DNA fragmentation (apoptosis), F4/80 antibody (#MF48000; Caltag Medsystems, Buckingham, UK) for macrophages and anti‐mouse IgG (3A9044‐2ML; Sigma) for BBB permeability.
Biological analysis
All tissues were kept frozen and homogenized with a 10% v/w buffer (1× PBS plus 0.5 mm EDTA). Homogenates were centrifuged at 4°C for 4 min at 1500g for protein content and MDA analysis, and again at 4°C for 10 min at 12,000g for the remaining analyses. Supernatants were frozen at −80°C. Protein concentrations were determined spectrophotometrically (Biophotometre; Eppendorf, Hamburg, Germany) using a bicinchoninic acid (BCA) kit according to the instructions (Sigma). The protein concentration was adjusted to 1000 μg ml−1 for brain, heart and liver and to 100 μg ml−1 for aorta and soleus. All the biological assays were read on a microplate reader (Tecan Infinite M200, Mannedorf, Switzerland).
Markers of metabolism, enzymatic activity, oxidative stress and inflammation
Citrate synthase (CS)
To determine metabolic muscle adaptations to physical activity, skeletal muscle CS activity was determined using soleus muscle homogenate by the method of Shepherd & Garland (1969). Briefly, the citrate synthesis rate from acetyl coenzyme A and oxaloacetate was determined according to a coupling reaction between coenzyme A and DTNB [5,5′‐dithiobis(2‐nitrobenzoic acid)]. This coupling reaction was spectrophotometrically measured at 412 nm for 45 s.
Advanced oxidation protein products
Advanced oxidation protein products were determined in plasma, aorta, heart, liver and brain supernatant using the semi‐automated method developed by Witko‐Sarsat et al. (1996), as previously described (Pialoux et al. 2009 b). Briefly, AOPP were measured by spectrometry and were calibrated with a chloramine‐T solution that absorbs at 340 nm in the presence of potassium iodide. The absorbance of the reaction was immediately read at 340 nm on the microplate reader against a blank containing 200 μl of PBS. The AOPP activity was expressed as micromoles per litre of chloramine‐T equivalents.
Catalase
Catalase activity in the plasma, heart and brain were determined by the method of Johansson & Borg (1988), using hydrogen peroxide (H2O2) as a substrate and formaldehyde as a standard. Catalase activity was determined by the rate of formation of formaldehyde, read at 540 nm for 20 min, induced by the reaction of methanol and H2O2 using catalase as the enzyme.
Ferric reducing antioxidant power
Plasma FRAP was measured by spectrophotometry using the method of Benzie & Strain (1996). Briefly, the FRAP concentration was calculated using an aqueous solution of a known Fe2+ concentration (FeSO4.7H2O) as the standard. Plasma was mixed with a FRAP working solution at 37°C, containing buffer acetate, 2,4,6‐Tris(2‐pyridyl)‐s‐triazine (TPTZ) and ferric choloride (FeCl3.6H2O). The Fe2+–TPZT complex subsequently formed was read at 593 nm after 4 min.
Glutathione peroxidase
Glutathione peroxidase in the plasma, heart, liver and brain was determined by the modified method of Paglia & Valentine (1967), using hydrogen peroxide (H2O2) as a substrate. Glutathione peroxidase was determined by the rate of oxidation of NADPH to NADP+ after addition of glutathione reductase (GR), reduced glutathione (GSH) and NADPH. The NADPH extinction was read at 340 nm for 5 min.
Malondialdehyde
Although the MDA assay has methodological limitations (Lefèvre et al. 1998), it is the most common lipid peroxidation marker and it is still a widely used marker of oxidative stress. Concentrations of plasma, heart, liver and brain MDA were determined as thiobarbituric acid reactive substances by a modification of the method of Ohkawa et al. (1979), as previously described (Pialoux et al. 2009 a). The pink chromogen of the thiobarbituric acid–MDA complex formed after 1 h at 100°C was extracted with n‐butanol, and its absorbance was measured at 532 nm by spectrophotometry. The MDA concentration was calculated using 1,1,3,3‐tetraethoxypropane as standard.
Nitrite and nitrate (NOx)
The end‐products of endothelial nitric oxide, nitrites and nitrates, were measured in the plasma, based on methods previously described (Misko et al. 1993). The sum of nitrite and nitrate in the plasma (NOx) is considered an index of nitric oxide production (Hebbel et al. 1982). Briefly, the fluorometric quantification of nitrite/nitrate is based upon the reaction of nitrite with 2,3‐diaminonaphthalene to form the fluorescent product, 1‐(H)‐naphthotriazole and read at 450 nm after excitation at 365 nm. Nitrates are converted to nitrite by nitrate reductase.
Nitrotyrosine
Concentrations of plasma nitrotyrosine, as the end‐product of protein nitration by ONOO·−, were measured by a competitive enzyme‐linked immunosorbent assay as previously described (Galiñanes & Matata, 2002) using precoated nitrated bovine serum albumin microplates and subsequent incubations and washings with anti‐nitrotyrosine and anti‐rabbit IgG–HRP conjugate. The concentration of nitrotyrosine was then calculated using nitrated bovin serum albumin as the standard.
Superoxide dismutase
The quantitative determination of SOD activity was performed on the plasma, aorta, heart, liver and brain using the method of Beauchamp & Fridovich (1971), slightly modified by Oberley & Spitz (1985). The SOD activity was determined by the degree of inhibition of the reaction between superoxide radicals, produced by a hypoxanthine–xanthine oxidase system, and nitroblue tetrazolium. The formazan blue subsequently formed was read at 560 nm for 5 min.
Cholesterol and metabolic measurements
Total blood cholesterol was assessed using an Amplex Red Cholesterol Assay Kit as instructed by Invitrogen (Carlsbad, CA, USA). One week before MRI, an intraperitoneal insulin tolerance test was performed on mice fasted for 6 h. Mice were injected i.p. with 0.75 mU (g body weight)−1 of insulin. Blood was taken by tail puncture immediately before and at 15, 30, 45 and 60 min time points after injection for measurement of blood glucose.
All reagents used for biochemical assays were purchased from Sigma‐Aldrich.
Inflammatory markers
Tumour necrosis factor‐α (BD Biosciences, San Jose, CA, USA) and IL‐1β (RayBiotech, Inc., Norcross, GA, USA) were assessed in plasma, aorta and brain supernatant, and interleukin‐4 (IL‐4; Abcam, Cambridge, UK) in brain supernatant using a commercially available mouse enzyme‐linked immunosorbent assay kit, according to the manufacturer's instructions. The NF‐κB/p65 activity (Imgenex, San Diego, CA, USA) was assessed in plasma according to the manufacturer's instructions.
Statistics
Statistical analyses were conducted using Statistica (version 8.0; Statsoft, Tulsa, OK, USA). Results are presented as means ± SD. For each parameter, a minimum of seven mice per group was used. Statistical comparisons between five groups (O‐ApoE‐UT, O‐ApoE‐ExT, Y‐ApoE‐UT, O‐C57‐UT and O‐C57‐ExT) were performed by one‐way ANOVA followed by Bonferroni post hoc test. Pearson's coefficient correlations were used to determine the associations between plasma markers, brain markers and distance run. A logrank test was used for survival curve analysis. Statistical significance was determined by a P value of <0.05. All the animals that did not complete the study were excluded from all the analyses carried out except for the calculation of the survival rate.
Results
Animal characteristics and general effects of exercise
Ninety‐one old ApoE−/−, 20 young‐adult ApoE−/− and 16 old C57BL/6 (O‐C57) mice were originally included in the protocol. These animals were vulnerable to cerebrovascular disease, especially in the older cohorts, and a number of animals died prior to any biological assessment. The majority of these animals died spontaneously; however, seven mice died after signs of mono‐ or hemiplegia (all being old ApoE−/− mice, representing 13% of all deaths of this group), a possible symptom of cerebrovascular disease. Of the mice that died during the protocol, 29 were in the O‐ApoE‐UT group, 10 were in the O‐ApoE‐ExT group (Fig. 2), one in the O‐C57‐UT and one in the O‐C57‐ExT group. An additional six mice died during the insulin resistance test or MRI, and three mice were excluded at autopsy because of large tumours. After the induction and training period, 43 old ApoE−/− mice (19 O‐ApoE‐UT, 72.4 ± 2.4 weeks; and 24 O‐ApoE‐ExT, 71.8 ± 1.9 weeks) and 20 young‐adult mice (Y‐ApoE‐UT, 20 ± 0 weeks) were used for biological assessment (Table 2). The O‐ApoE‐ExT mice ran 17.8 ± 15.3 km week−1, whereas the O‐C57‐ExT mice ran 16.2 ± 8.8 km week−1. The training effect was supported by higher CS activity in the soleus of both O‐ApoE‐ExT and O‐C57‐ExT mice compared with the corresponding UT mice (see Table 2). Despite higher plasma cholesterol concentrations in all ApoE−/− mice compared with old C57 mice (independently of training), Y‐ApoE‐UT mice had higher insulin sensitivity than O‐ApoE‐UT mice (Table 3). The training effect was also evident on both insulin sensitivity and plasma cholesterol concentrations in the O‐ApoE‐ExT vs. the corresponding untrained mice (see Table 2). Importantly, the O‐ApoE‐ExT mice had a significantly higher survival rate compared with the O‐ApoE‐UT mice (77 vs. 49%; P = 0.03; Fig. 2), Exercise training was therefore able to decrease the mortality rate of the old ApoE−/− mice. Both O‐C57‐UT and O‐C57‐ExT mice had a survival rate of 87%, which was significantly higher than the old ApoE−/− mice, confirming the pathological state of our old ApoE−/− mice.
Figure 2.
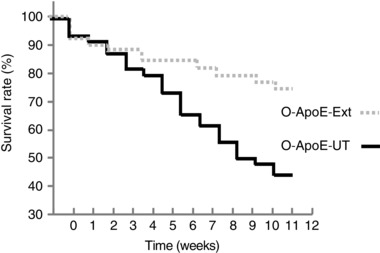
Survival rate for old untrained (O‐ApoE‐UT) and exercise‐trained (O‐ApoE‐ExT) ApoE−/− mice (P = 0.03)
Table 2.
Number of mice at inclusion, that died spontaneously, that died during experimental procedures, excluded after dissection for large tumours and used for biological assessments
| Y‐ApoE‐UT | O‐ApoE‐UT | O‐ApoE‐ExT | O‐C57‐UT | O‐C57‐ExT | |
|---|---|---|---|---|---|
| Mice at inclusion (n) | 20 | 53 | 38 | 8 | 8 |
| Mice that died spontaneously (n) | 0 | 29 | 10 | 1 | 1 |
| Mice that died during ITT and MRI (n) | 0 | 3 | 3 | 0 | 0 |
| Mice excluded after dissection for large tumours (n) | 0 | 2 | 1 | 0 | 0 |
| Mice used for biological assessments (n) | 20 | 19 | 24 | 7 | 7 |
Abbreviations: ITT, insulin tolerance test; MRI, magnetic resonance imaging; O‐C57‐ExT, old C57BL/6 exercise trained; O‐C57‐UT, old C57BL/6 untrained; O‐ApoE‐ExT, old ApoE−/− exercise trained; O‐ApoE‐UT, old ApoE−/− untrained; and Y‐ApoE‐UT, young‐adult ApoE−/− untrained.
Table 3.
Effect of age and exercise training on body weight, citrate synthase activity, cholesterol and insulin resistance
| Parameter | Y‐ApoE‐UT | O‐ApoE‐UT | O‐ApoE‐ExT | O‐C57‐UT | O‐C57‐ExT |
|---|---|---|---|---|---|
| Body weight (g) | 32.7 ± 1.5 | 40.2 ± 1.5* | 38.5 ± 1.5* | 27.2 ± 1.2 | 27.7 ± 1.1 |
| Citrate synthase activity (nmol min−1 mg−1) | 3.68 ± 0.2 | 6.76 ± 0.8* | 8.50 ± 0.2*† | 4.92 ± 0.42* | 8.44 ± 0.86*† |
| Cholesterol (mmol l−1) | 22.0 ± 4.1 | 18.2 ± 2.0 | 15.5 ± 1.2*† | 6.2 ± 0.6‡ | 5.0 ± 0.4*‡ |
| Insulin resistance (%) | −20.7 ± 8.1 | −11.6 ± 2.2* | −22.3 ± 2.8† | −24.9 ± 2.4 | −26.5 ± 1.9 |
Insulin resistance was estimated as the percentage change of glycaemia at 30 min (vs. baseline) during an insulin tolerance test. Groups are as in Table 2. *Significantly different (P < 0.05) from young. †Significantly different (P < 0.05) from corresponding untrained. ‡Significantly different (P < 0.05) from corresponding ApoE−/−.
Presence of multiple neurovascular lesions in old sedentary ApoE−/− mice on the HF/HC diet
As observed by in vivo MRI and histology, there were significant abnormalities in the brain vasculature of O‐ApoE‐UT mice (Fig. 3), whereas O‐C57‐UT mice did not show such abnormalities as indicated by significantly lower scoring between old O‐ApoE‐UT and O‐C57‐UT for both post‐USPIO T2 and post‐gadolinium T1 images (Fig. 3). On pre‐contrast images, several dark areas on T2 images and T2* images indicated iron accumulation in a large number of O‐ApoE‐UT mice at the same periventricular location (71% of O‐ApoE‐UT vs. 0% for O‐C57‐UT). Post‐gadolinium T1 images indicated the presence of periventricular BBB leakage and endothelial permeability in O‐ApoE‐UT mice (gadolinium score of 2.7 ± 0.80 for O‐C57‐UT mice vs. 1.42 ± 0.23 for O‐C57‐UT, P < 0.05; Fig. 3). Comparing the pre‐ and post‐USPIO T2 images (Fig. 3), it was evident that there was also an accumulation of iron oxide nanoparticles. Given that circulating iron oxide nanoparticles would have been cleared from the circulation, this suggests the presence of macrophages and phagocytic activity in the same area. These signs of neuro‐inflammation observed on both post‐USPIO T2* and T2 images were confirmed by histology (Figs 4 and 6). Disorganized brain parenchyma was seen in the middle ventral zone, but was not related to an active apoptotic process (TUNEL negative on immunohistochemistry; data not shown). There was also an anatomical correspondence between this abnormal area in both T2/T2* images and post‐gadolinium T1 images, which was histologically confirmed respectively by positive staining for IgG and F4/80. This would indicate BBB leakage (endothelial permeability). There was also some evidence of vesicular aggregates, which could indicate foam cell development (data not shown).
Figure 3. Brain magnetic resonance imaging of old untrained C57 and trained and untrained ApoE−/− mice .
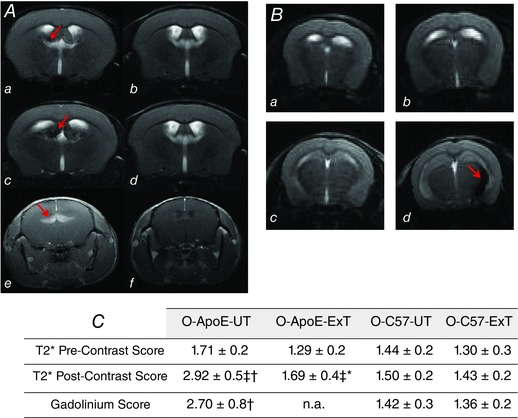
A, brain MRI in old untrained ApoE−/− (O‐ApoE‐UT, left panel) vs. old untrained C57 mice (O‐C57‐UT, right panels; pre‐USPIO T2 MRI, a and b; and 48 h post‐USPIO T2 MRI, c and d; post‐gadolinium T1 MRI, e and f). In O‐ApoE‐UT mice, red arrows indicate a hypointense region, suggesting inflammation, in a and c, and bright zone in e, suggesting BBB leakage. B, brain T2 MRI (pre‐USPIO, a and b; and 48h‐post‐USPIO, c and d) in untrained (O‐ApoE‐UT, left panels) vs. trained old ApoE−/− mice (O‐ApoE‐Ext, right panels) showing a hypointense region (red arrow in d) demonstrating inflammation in the left periventricular fornix fimbria in the O‐ApoE‐UT mouse, and a normal image in the O‐ApoE‐ExT (d). C, brain pre‐ and post‐USPIO T2* scores and post‐gadolinium T1 score for old sedentary and trained ApoE−/− mice (O‐ApoE‐UT and O‐ApoE‐ExT, respectively) and old sedentary and trained C57 mice (O‐C57‐UT and O‐C57‐ExT, respectively). *Significantly different (P < 0.05) O‐ApoE‐ExT vs. O‐ApoE‐UT. †Significantly different (P < 0.05) O‐C57‐UT vs. O‐ApoE‐UT. ‡Significantly different (P < 0.05) from pre‐contrast.
Figure 4. Brain magnetic resonance images of an old untrained ApoE−/− mouse .
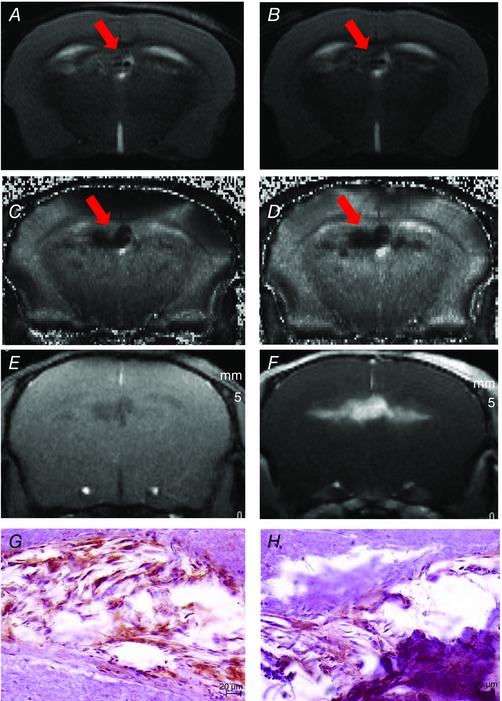
Pre‐ (A) and post‐USPIO (B) T2 images, both showing hyposignal and heterogeneous regions around the choroid plexus representative of vascular sequelae (A, arrow) and inflammation (B, arrow). Pre‐ (C) and post‐USPIO (D) T2* maps, with an increase of the hyposignal region on post‐USPIO, suggesting iron deposits (C, arrow) and phagocytic activity (D, arrow). Pre‐ (E) and post‐gadolinium (F) T1 images, the enhancing bright zone showing BBB leakage in the same area (F). G, positive F4/80 staining, confirming macrophages in this area. H, positive IgG staining in the same locations, confirming MRI findings of blood–brain barrier leakage.
Figure 6. Histological staining of brain of old untrained ApoE−/− and C7 mice .
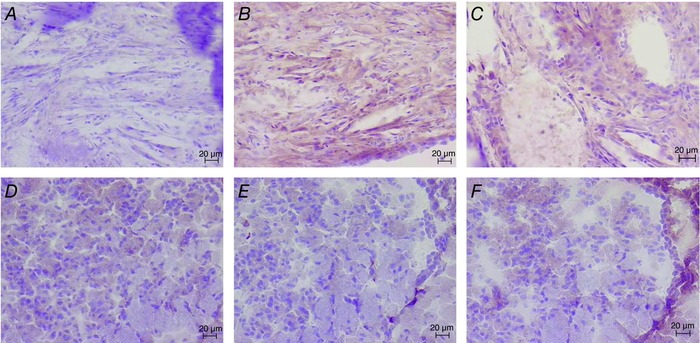
Negative control (A), positive F4/80 (B) and positive IgG (C) for an old ApoE−/− untrained mouse (left side, at the level of the fornix fimbriae, original magification ×20). Negative control (D), positive F4/80 (E) and positive IgG (F) for an old C57 untrained mouse (left side, at the level of the fornix fimbriae, original magification ×20).
Brain MRI in trained vs. untrained old ApoE−/− mice
There were significantly more abnormalities in the brain vasculature of O‐ApoE‐UT (n = 7; 71% of mice) compared with O‐ApoE‐ExT mice (n = 7, 14% of mice). Comparing the pre‐USPIO images (respective scores, 1.71 ± 0.19 vs. 1.29 ± 0.19; P = 0.12) with post‐USPIO images, there was more USPIO accumulation in O‐ApoE‐UT mice (vs. O‐ApoE‐ExT), suggesting phagocytic activity and inflammation (Figs 3 and 4) as indicated by the significantly higher post‐USPIO T2* score in O‐ApoE‐UT mice compared with O‐ApoE‐ExT mice (2.92 ± 0.49 vs. 1.69 ± 0.44; P < 0.05).
Old ApoE−/− mice expressed higher brain oxidative stress and inflammation than old C57 and young ApoE−/− mice
Malondialdehyde, AOPP, Il‐1β and TNFα were significantly lower and FRAP significantly higher in O‐C57‐UT than in O‐ApoE‐UT mice (P < 0.01), confirming the brain pathological state of our old ApoE−/− mice (Table 4). Superoxide dismutase and GPX were not different between O‐C57‐UT and O‐ApoE‐UT groups (Table 4). Brain concentrations of the oxidative stress marker MDA and the pro‐inflammatory markers TNFα and IL‐1β were significantly higher in both O‐ApoE‐UT and O‐ApoE‐ExT compared with Y‐ApoE‐UT mice (P < 0.01). On the contrary, SOD and GPX (see Table 4) and IL‐4 (O‐ApoE‐UT, 2.34 ± 0.83 pg mg−1 vs. Y‐ApoE‐UT, 5.04 ± 3.26 pg mg−1; P < 0.05) were decreased with age in ApoE−/− mice.
Table 4.
Effect of age and exercise training on brain markers of inflammation and oxidative stress
| Marker | Y‐ApoE‐UT | O‐ApoE‐UT | O‐ApoE‐ExT | O‐C57‐UT | O‐C57‐ExT |
|---|---|---|---|---|---|
| MDA (μmol mg−1) | 3.8 ± 0.6 | 9.1 ± 1.4* | 5.2 ± 0.9*† | 1.6 ± 1.3‡ | 0.7 ± 0.3‡ |
| TNFα (pg mg−1) | 47.4 ± 3.7 | 137.9 ± 20.8* | 114.1 ± 8.9*† | 36.6 ± 4.0‡ | 31.2 ± 4.7*‡ |
| IL‐1β (pg mg−1) | 127.7 ± 18.7 | 226.8 ± 27.1* | 182.5 ± 21.5*† | 84.5 ± 19.7‡ | 88.3 ± 13.2‡ |
| AOPP (μmol mg−1) | 14.5 ± 1.1 | 16.1 ± 1.4 | 12.7 ± 1.2† | 3.1 ± 1.1†‡ | 4.8 ± 2.5†‡ |
| Catalase (μmol min−1 mg−1) | 174.1 ± 14.6 | 65.5 ± 6.3* | 90.2 ± 11.5*† | 33.7 ± 10.4*‡ | 46.3.2 ± 29.3*‡ |
| GPX (μmol min−1 mg−1) | 168.0 ± 11.4 | 74.3 ± 1.5* | 74.7 ± 12.0* | 77.0 ± 13.9* | 73.9 ± 10.1* |
| FRAP (μmol mg−1) | 62.2 ± 4.1 | 56.9 ± 5.2 | 50.7 ± 4.3 | 70.1 ± 15.3*‡ | 65.4 ± 29.4 |
| SOD (μmol min−1 mg−1) | 9.56 ± 0.8 | 6.96 ± 1.1* | 7.16 ± 0.9* | 7.39 ± 3.20 | 6.33 ± 2.55* |
Abbreviations: AOPP, protein oxidation; FRAP, ferric reducing antioxidant power; GPX, catalase and SOD, antioxidant enzymes activities; IL‐1β, interleukin‐1β; MDA, lipid peroxidation; and TNFα: tumour necrosis factor‐α. Groups are as in Table 2. *Significantly different (P < 0.05) from young. †Significantly different (P < 0.05) from corresponding untrained. ‡Significantly different (P < 0.05) from corresponding ApoE−/−.
Exercise‐induced changes in markers of oxidative stress and inflammation in the brain of old ApoE−/− mice (Table 4)
In the brain, MDA and AOPP were decreased in response to exercise training (P < 0.01 and P < 0.05, respectively). Moreover, there was an increase in brain catalase (P < 0.05) and a decrease in Il‐1β and TNFα in O‐ApoE‐ExT compared with O‐ApoE‐UT mice (P < 0.05). Brain concentrations of IL‐4 were significantly higher in O‐ApoE‐ExT compared with O‐ApoE‐UT mice (5.70 ± 3.87 vs. 2.34 ± 0.83 pg mg−1 for O‐ApoE‐ExT and O‐ApoE‐UT, respectively; P < 0.05). The FRAP, GPX and SOD were not significantly affected by exercise in old ApoE−/− mice.
Magnetic resonance imaging of the aorta and biological vessel wall response
The O‐ApoE‐UT mice had a larger vessel wall area than the Y‐ApoE‐UT mice (4.02 ± 0.22 vs. 2.66 ± 0.03 mm2; P < 0.01). In the O‐ApoE‐ExT mice, vessel wall area was reduced compared with O‐ApoE‐UT mice (see Fig. 5). Concerning T2* measurements, both pre‐ and post‐USPIO values in O‐ApoE‐UT mice were lower than in Y‐ApoE‐UT mice, confirming more complex plaque composition and more inflammatory activity, respectively (Mihai et al. 2011). The O‐ApoE‐ExT mice had an increase in pre‐USPIO T2* measurement compared with O‐ApoE‐UT mice, suggesting a less complex plaque composition (see Fig. 5). Post‐USPIO T2* was lower than pre‐contrast values for all the groups, indicating the presence of iron particles and phagocytic activity in the vessel wall. In the aorta, both O‐ApoE‐ExT and O‐ApoE‐UT mice had more TNFα, IL‐1β and AOPP than Y‐ApoE‐UT mice, whereas only O‐ApoE‐UT mice had more SOD than Y‐ApoE‐UT mice. The O‐ApoE‐UT mice had more TNFα, IL‐1β, AOPP and SOD in the aorta than O‐ApoE‐ExtT mice (see Table 5; P < 0.05). The AOPP, Il‐1β and TNFα in the aorta were much lower in old C57 than in old ApoE−/− mice independent of exercise training (P < 0.01), which strengthens the atherosclerotic phenotype seen in the aorta of old ApoE−/− mice.
Figure 5. Aorta magnetic resonance imaging of old and young untrained and old trained ApoE−/− mice .
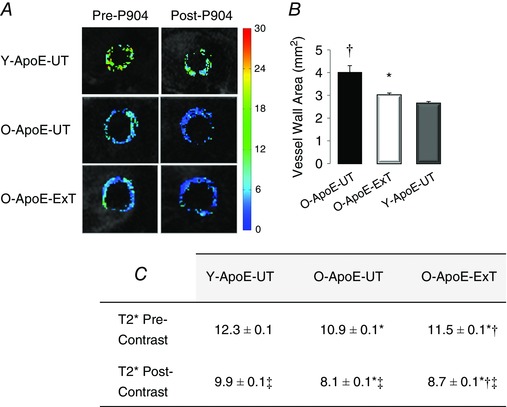
A, pre‐ and post‐USPIO T2* maps of the ascending aorta in a young untrained (Y‐ApoE‐UT), an old untrained (O‐ApoE‐UT) and an old trained (O‐ApoE‐Ext) ApoE−/− mouse. B, ascending aorta vessel wall area measurements. C, vessel wall pre‐ and post‐USPIO T2* measurements. In O‐ApoE‐UT mice, vessel wall area is significantly larger, and pre‐ and post‐USPIO T2* significantly lower, representative of advanced and complex atherosclerotic lesions with inflammatory activity. *Significantly different (P < 0.05) from old ApoE−/− mice (O‐ApoE‐UT). †Significantly different (P < 0.05) from young ApoE−/− mice (Y‐ApoE‐UT). ‡Significantly different (P < 0.05) from pre‐contrast.
Table 5.
Effect of age and exercise training on aortic TNFα, IL‐1β, AOPP and SOD
| Marker | Y‐ApoE‐UT | O‐ApoE‐UT | O‐ApoE‐ExT | O‐C57‐UT | O‐C57‐ExT |
|---|---|---|---|---|---|
| TNFα (pg mg−1) | 6.7 ± 1.7 | 11.8 ± 1.2* | 9.6 ± 1.5*† | 0.5 ± 0.3 | 2.5 ± 1.3 |
| IL‐1β (pg mg−1) | 16.7 ± 4.9 | 47.8 ± 17.6* | 28.0 ± 10.7*† | 0.9 ± 0.2‡ | 1.0 ± 0.2‡ |
| AOPP (μmol mg−1) | 21.6 ± 1.4 | 159.5 ± 25.2* | 122.3 ± 20.9*† | 8.4 ± 2.7‡ | 9.1 ± 2.9‡ |
| SOD (μmol min−1 mg−1) | 9.27 ± 2.1 | 26.2 ± 1.7* | 6.7 ± 2.0† | 7.79 ± 1.7‡ | 5.59 ± 1.8 |
Abbreviations: AOPP, protein oxidation; IL‐1β, interleukin‐1β; SOD, superoxide dismutase activity; and TNFα, tumour necrosis factor‐α. Groups are as in Table 2. *Significantly different (P < 0.05) from young. †Significantly different (P < 0.05) from corresponding untrained. ‡Significantly different (P < 0.05) from corresponding ApoE−/−.
Heart markers
Heart concentrations of AOPP were higher in O‐ApoE‐UT compared with Y‐ApoE‐UT mice. The O‐ApoE‐UT mice had lower GPX and SOD than Y‐ApoE‐UT mice. Advanced oxidation protein products and antioxidant enzymes (SOD, GPX and catalase) were higher in old C57 compared with old ApoE−/− mice (Table 6). The O‐ApoE‐ExT mice had higher activities of SOD and lower AOPP than O‐ApoE‐UT mice (see Table 6).
Table 6.
Effect of age and exercise training on heart and liver markers of oxidative stress and antioxidants
| Marker | Y‐ApoE‐UT | O‐ApoE‐UT | O‐ApoE‐ExT | O‐C57‐UT | O‐C57‐ExT |
|---|---|---|---|---|---|
| AOPP in heart (μmol mg−1) | 28.5 ± 2.4 | 75.3 ± 5.8* | 68.4 ± 5.6*† | 152.7 ± 14.4‡ | 138.9 ± 6.1‡ |
| Catalase in heart (μmol min−1 mg−1) | 831 ± 76 | 2492 ± 436* | 2033 ± 400*† | 4955 ± 508‡ | 4266 ± 449‡ |
| GPX in heart (μmol min−1 mg−1) | 451 ± 11 | 322.7 ± 7* | 338 ± 7* | 834 ± 87‡ | 781 ± 83‡ |
| SOD in heart (μmol min−1 mg−1) | 32.7 ± 4.5 | 24.1 ± 2.8* | 32.2 ± 2.4† | 48.0 ± 6.6‡ | 44.9 ± 7.9‡ |
| AOPP in liver (μmol mg−1) | 14.8 ± 5.3 | 41.4 ± 10.9* | 36.0 ± 9.1*† | 6.6 ± 2.0‡ | 9.3 ± 3.4‡ |
| MDA in liver (μmol mg−1) | 0.75 ± 0.42 | 4.11 ± 2.08* | 2.37 ± 1.42*† | 1.87 ± 0.45‡ | 2.37 ± 1.47 |
| SOD in liver μmol min−1 mg−1) | 0.92 ± 0.12 | 0.71 ± 0.26 | 0.83 ± 0.13† | 0.75 ± 0.24 | 0.79 ± 0.21 |
| GPX in liver μmol min−1 mg−1) | 14.8 ± 12.9 | 6.0 ± 4.5* | 27.4 ± 9.6*† | 10.9 ± 1.5 | 22.7 ± 6.6 |
Abbreviations: AOPP, protein oxidation; FRAP, ferric reducing antioxidant power; GPX, catalase and SOD, antioxidant enzymes activities; and MDA, malondialdehyde. Groups are as in Table 2. *Significantly different (P < 0.05) from young. †Significantly different (P < 0.05) from corresponding untrained. ‡Significantly different (P < 0.05) from corresponding ApoE−/−.
Liver markers
Liver concentrations of AOPP and MDA were higher in O‐ApoE‐UT compared with both Y‐ApoE‐UT and O‐C57‐UT mice, whereas liver GPX activity was lower in O‐ApoE‐UT than in Y‐ApoE‐UT mice. In addition, O‐ApoE‐ExT mice had higher activities of SOD and GPX and lower AOPP and MDA than O‐ApoE‐UT mice (see Table 6).
Systemic oxidative stress and inflammation is higher in old ApoE−/− compared with old C57 mice
Nitrotyrosine and AOPP were higher (P < 0.05), whereas NOx (P < 0.05) was lower in old ApoE−/− compared with old C57 mice independently of training (Table 7). Glutathione peroxidase was higher in O‐C57‐ExT than in O‐ApoE‐UT mice (P < 0.01).
Table 7.
Effect of age and exercise training on plasma markers of oxidative stress, antioxidants and inflammation
| Marker | Y‐ApoE‐UT | O‐ApoE‐UT | O‐ApoE‐ExT | O‐C57‐UT | O‐C57‐ExT |
|---|---|---|---|---|---|
| AOPP (μmol l−1) | 202.6 ± 9.1 | 152.4 ± 11.8* | 131.0 ± 10.7* | 15.3 ± 2.6‡ | 13.3 ± 2.7‡ |
| GPX (μmol l−1 min−1) | 123.0 ± 17.7 | 113.4 ± 14.5 | 102.4 ± 13.7 | 109.3 ± 15.2 | 227.0 ± 62.8† |
| Nitrotyrosine (nmol l−1) | 25.6 ± 3.8 | 50.6 ± 11.0* | 66.4 ± 8.9* | 36.4 ± 4.0‡ | 26.4 ± 5.2‡ |
| NOx (μmol l−1) | 22.3 ± 1.3 | 22.6 ± 1.0 | 27.3 ± 1.1*† | 28.3 ± 2.4‡ | 35.7 ± 3.2*†‡ |
| SOD (μmol ml−1 min−1) | 37.1 ± 1.0 | 32.3 ± 1.4* | 36.7 ± 1.2 | 27.3 ± 1.8 | 33.3 ± 3.1† |
| TNFα (pg ml−1) | 82.3 ± 7.9 | 42.3 ± 8.9* | 40.8 ± 6.7* | 46.3 ± 5.6 | 72.4 ± 16.4† |
| IL‐1β (pg ml−1) | 157.6 ± 14.7 | 118.2 ± 6.8* | 114.2 ± 8.2* | 111.5 ± 22.7 | 105.4 ± 12.8 |
Abbreviations: AOPP, protein oxidation; FRAP, ferric reducing antioxidant power; GPX and SOD, antioxidant enzymes activities; IL‐1β, interleukin‐1β; nitrotyrosine, protein nitration; NOx, nitric oxide metabolism (nitrites plus nitrates); and TNFα, tumour necrosis factor‐α. Groups are as in Table 2. *Significantly different (P < 0.05) from young. †Significantly different (P < 0.05) from corresponding untrained. ‡Significantly different (P < 0.05) from corresponding ApoE−/−.
Exercise‐induced changes in systemic oxidative stress and inflammation
Plasma MDA decreased in response to exercise training in the old ApoE−/− mice (23.3 ± 1.8 vs. 17.9 ± 1.7 μmol l−1 for O‐ApoE‐UT and O‐ApoE‐ExT, respectively; P < 0.05), whereas AOPP showed a trend to increase (P = 0.09; Table 7). The plasma volume collected from for O‐C57‐ExT and O‐C57‐U mice was insufficient to allow plasma MDA analysis for these two groups of mice. Antioxidant markers NOx and SOD were higher in O‐ApoE‐ExT than in O‐ApoE‐UT mice (P < 0.05 and P = 0.11, respectively; Table 7). Plasma AOPP were higher in O‐ApoE‐UT compared with Y‐ApoE‐UT mice (P < 0.01; see Table 7), whereas MDA was lower (23.3 ± 1.8 vs. 8.8 ± 0.6 μmol l−1 for O‐ApoE‐UT and Y‐ApoE‐UT, respectively; P < 0.01). The pro‐inflammatory markers TNFα and IL‐1β were also significantly different in old ApoE−/− mice compared with young‐adult mice (P < 0.001). Interestingly, NOx, SOD and GPX were improved in the trained old C57 mice (vs. untrained). On the contrary, in ApoE−/− mice fed the HF/HC diet, NF‐κB was not significantly affected by age or exercise training (Y‐ApoE‐ExT, 97.4 ± 13.6 pg ml−1; O‐ApoE‐UT, 102.3 ± 15.6 pg ml−1; and O‐ApoE‐ExT, 84.1 ± 12.2 pg ml−1).
Correlations
Inflammation and oxidative stress markers were correlated within the brain (see Table 8). However, none of the brain markers was correlated with corresponding plasma (see Table 9), aorta, heart or liver markers.
Table 8.
Correlations between brain markers of oxidative stress and inflammation in young and old ApoE−/− mice
| Variable | Correlated with variable | n | Pearson's correlation | P value |
|---|---|---|---|---|
| MDA in brain | IL‐1β in brain | 26 | 0.429 | 0.029 |
| AOPP in brain | IL‐1β in brain | 26 | 0.764 | 0.000 |
| Catalase in brain | FRAP in brain | 27 | 0.453 | 0.018 |
| SOD in brain | 27 | 0.573 | 0.002 | |
| GPX in brain | 26 | 0.505 | 0.009 | |
| TNFα in brain | IL‐1β in brain | 27 | 0.508 | 0.007 |
| FRAP in brain | SOD in brain | 27 | 0.552 | 0.003 |
| GPX in brain | 26 | 0.515 | 0.007 | |
| SOD in brain | GPX in brain | 26 | 0.683 | 0.000 |
Abbreviations: AOPP, protein oxidation; FRAP, ferric reducing antioxidant power; GPX and SOD, antioxidant enzyme activities; IL‐1β, interleukin‐1β; MDA, malondialdehyde; and TNFα, tumour necrosis factor‐α.
Table 9.
Lack of correlations between brain and plasma markers of oxidative stress and inflammation
| Variable | Correlated with variable | n | Pearson's correlation | P value |
|---|---|---|---|---|
| TNFα in plasma | TNFα in brain | 21 | −0.1818 | 0.430 |
| IL‐1β in plasma | IL‐1β in brain | 22 | 0.1700 | 0.449 |
| AOPP in plasma | AOPP in brain | 42 | 0.1482 | 0.349 |
| MDA in plasma | MDA in brain | 27 | −0.0811 | 0.688 |
| SOD in plasma | SOD in brain | 27 | 0.1836 | 0.359 |
Abbreviations: AOPP, protein oxidation; IL‐1β, interleukin‐1β; MDA, malondialdehyde; SOD, superoxide dismutase; and TNFα, tumour necrosis factor‐α.
Discussion
Vascular brain lesions share similar pathological features with atherosclerosis, such as increased inflammation and oxidative stress (Dutta et al. 2012). In the present study, we found that compared with age‐matched C57 mice fed a normal diet and with young ApoE−/− mice, old ApoE−/− mice fed a HF/HC diet exhibited increases in brain abnormalities and BBB permeability in specific periventricular areas, as confirmed by both MRI and histology. These results were associated with an increase of both inflammation and oxidative stress markers in the brain in the old ApoE−/− mice. All these phenotypic changes are likely to be responsible for the low survival (49% over 12 weeks) of sedentary ApoE−/− mice compared with C57BL6 mice (87%), which confirms the literature (Rowlatt et al. 1976; Blackwell et al. 1995; Moghadasian et al. 2001). More importantly, we found that in ageing ApoE−/− mice, exercise training, possibly via its ability to lower oxidative stress and inflammation, reduced brain macrophage infiltration, limited inflammation and oxidative stress in the brain, and improved metabolic conditions, thereby improving health status and life expectancy.
Inflammation and oxidative stress in old ApoE−/− mice fed HF/HC diet
Oxidative stress in the cerebral vasculature plays a crucial role in the pathogenesis of ischaemic brain injury [such as a compromised BBB (Hafezi‐Moghadam et al. 2007; ElAli et al. 2011) and macrophage accumulation], especially in the aged ApoE−/− mouse model (Coyle & Puttfarcken, 1993). Disruption of the BBB, as measured here in vivo by the extent of gadolinium leakage in T1‐weighted MRI, is a sign of endothelial permeability (Gloor et al. 2001). During conditions of inflammation, macrophages are able to cross the BBB and infiltrate the CNS parenchyma, as suggested by iron oxide nanoparticle‐enhanced MRI in stroke patients and models (Nighoghossian et al. 2007; Wiart et al. 2007). In our study, the location of gadolinium leakage corresponded anatomically to the presence of macrophages detected in vivo after injection of iron nanoparticles by T2/T2*‐weighted MRI and post mortem by histology. The iron accumulation observed on pre‐contrast T2* in some old mice might have resulted from repeated microhaemorrhage, and could possibly explain the observed symptoms of hemi‐ and monoplegia. It should be acknowledged that non‐invasive MRI with nanoparticles or gadolinium agents has previously demonstrated effectiveness in evaluation of endothelial permeability or in mapping atherosclerotic vascular territories in ApoE−/− mice fed with a high‐fat diet (Phinikaridou et al. 2012), as well as in other experimental cerebral models (Wiart et al. 2007; Koffie et al. 2011). We have shown that combined brain and vascular inflammation imaging provides biomarkers of central and vascular metabolic dysfunction and inflammation in this mouse model. In addition, our study is also the first to demonstrate that such imaging is sufficiently sensitive to detect a beneficial effect of exercise training.
In this advanced atherosclerosis model, our study confirms the presence of multi‐organ inflammation, as previously described in cardiovascular disease (Nahrendorf et al. 2015), as well as oxidative stress. Elevated marker concentrations were found in the aortic wall, brain, heart and liver. As they were not correlated, it can be hypothesized that local microenvironments drive specific pathological consequences. Indeed, brain inflammation was far more pronounced and was associated with severe functional impairments in periventricular areas, i.e. BBB damage and high phagocytic activity in the untrained ApoE−/− mice. Also, the observation of motor deficiency cases and the increased mortality rate in the untrained group are likely to be driven by these brain abnormalities shown by MRI. Local vicious circles of inflammation may be nourished further by systemic and distant inflammatory foci.
Effects of exercise training
We also showed that even in an advanced atherosclerotic mouse model, VWR was able to reduce risk factors for atherosclerosis and cerebrovascular damage by altering macrophage accumulation, oxidative stress, inflammation and metabolic parameters. Our mice ran 17.8 km week−1, similar to the age‐matched C57 mice (16.2 km week−1). These weekly distances are also close to those reported by Soto et al. (2015; 23 km week−1), who used voluntary wheel running in ApoE−/− mice receiving a standard diet. These distances are much greater than a forced treadmill running protocol, which is usually between 4 and 6 km week−1. As mice are naturally active, VWR provides a stress‐free way to exercise, as opposed to forced treadmill training and swimming, which may induce a stress response (Moraska et al. 2000). The total running distance with VWR is often superior to forced exercise, and it has been shown to produce cardiovascular adaptations, such as heart and left ventricular hypertrophy, and an increase in muscle oxidative capacity (Aufradet et al. 2012). Although the distance run by the mice in the present study was lower than healthy C57 mice (Aufradet et al. 2012), it was sufficient to increase muscle CS activity, a commonly used marker of an adaptation to habitual exercise (Sexton, 1995). Voluntary wheel running was also shown to be of moderate intensity rather than high intensity as can be seen in treadmill running.
In addition, VWR, regardless of exact distance, was able to induce a positive effect and reduced overall mortality compared with sedentary old animals. This is consistent with previous reports that physical inactivity is an independent predictor of mortality in animals (Laufs et al. 2005) and humans (Szostak & Laurant, 2011), whereas physical fitness is associated with preserved brain health (Weuve et al. 2004; Podewils et al. 2005). A previous study found that VWR was sufficient to extend survival and decrease neuronal damage after a short episode of forebrain ischaemia (Stummer et al. 1994). In treadmill‐trained rats, induced brain injury was also less severe as a consequence of improved brain integrity (Ding et al. 2006) and BBB function (Davis et al. 2007). On brain MRI, old trained ApoE−/− mice showed a significantly decreased score of BBB leakage at the locations where this dysfunction was observed in their untrained counterparts. Beneficial effects of exercise training on neurovascular damage to the brain via an increase in the number of pericytes has been reported recently in ApoE−/− mice (Soto et al. 2015). These adaptive effects may be the result of repetitive cerebrovascular shear stress induced by each bout of running training and a long‐term increase in cerebral blood flow, both leading to a decrease in oxidative stress and endothelial dysfunction in the brain (Viboolvorakul & Patumraj, 2014). In this context, exercise training was also shown to restore the impaired nitric oxide synthase‐dependent responses of cerebral arterioles in diabetic rats (Mayhan et al. 2011) and the impaired dilator responses of cerebral arterioles in rats submitted to chronic exposure to nicotine (Mayhan et al. 2010). In both studies, beneficial effects of exercise were associated with an increase in SOD and subsequent decrease in superoxide content. In our study, although we did not find any effect of exercise on the brain SOD and GPX activities, lipid peroxidation was reduced, suggesting that the content of reactive oxygen species might be reduced. Given that antioxidant therapies have been shown to reduce infarct volume and neurological impairment in different murine models of ischaemic stroke (Majid, 2014), it could be hypothesized that the beneficial effect of exercise that we observed in the brain could be drawn partly from a decrease in oxidative stress.
In contrast to a recent study by Soto et al. (2015), our mice were fed a HC/HF diet and were indeed more pathological than those fed a normal chow diet, as confirmed by the mortality of our old ApoE−/− mice (65% in 12 weeks at 70 weeks) compared with the 18‐ to 24‐month‐old mice used by Soto et al. (2015), which suggests a longer life expectancy. The HF/HC diet in ApoE−/− mice was previously shown to induce major changes at the level of the neurovascular unit (Badaut et al. 2012). Interestingly, this change included lipid droplet accumulation and BBB leakage at the same location as in our old ApoE−/− mice.
Additionally, exercise training reduced pro‐inflammatory markers, which could be attributable to a concomitant increase in anti‐inflammatory cascades (Pedersen, 2006; Szostak & Laurant, 2011), as we found for brain IL‐4. Exercise was also shown to reduce brain IL‐1β in a mouse model of Alzheimer's disease (Nichol et al. 2008) and brain inflammation in response to stroke (Ding et al. 2005), possibly through an increase in anti‐inflammatory pathways at the level of the neurovascular unit.
Better brain health has been shown to be related to systemic improvement of cardiovascular health, lipid–cholesterol balance and inflammation (Pedersen, 2006). Here, beyond its effects on the brain, exercise training was able to improve the overall effects of atherosclerosis in the old ApoE−/− mice. More specifically, O‐ApoE‐ExT mice expressed lower oxidative stress and inflammation in the aorta than their sedentary counterparts. Magnetic resonance imaging revealed a decrease in vessel wall size in the mice that ran compared with sedentary mice, in agreement with others (Shimada et al. 2007; Pellegrin et al. 2009 b; Kadoglou et al. 2011). In a study by Pellegrin et al. (2009 b), swimming‐trained ApoE−/− mice showed a decrease in macrophage accumulation, along with an increase in smooth muscle cell content, suggesting a more stable plaque. Exercise‐trained mice had higher T2* values, suggesting a more stable plaque (Sigovan et al. 2010). Nevertheless, in future studies, measurement of biological markers of plaque stability in aortic tissue, such as metalloproteinases activity, should confirm this hypothesis.
In old ApoE−/− mice, training decreased oxidative stress markers in the plasma, heart and liver, in addition to the brain and aorta, suggesting that exercise may have a whole‐body effect on oxidative stress in this model.
Compared with old ApoE−/− mice, oxidative stress is very low in the old C57 mice (between five and 10 times lower than in old ApoE−/− mice in the different organs). This suggests that C57 mice at the age of 70 weeks might have maintained their pro‐oxidant–antioxidant balance. We also think that further reduction of oxidative stress could be more detrimental than beneficial. Indeed, reactive oxygen species and lipid peroxidation end‐products (such as MDA) also have a role in the regulation and modulation of antioxidant cell signalling and gene expression, and a decrease of such products, when they are not in excess, might limit physiological adaptations (Niki, 2009).
Finally, although we report clear differences between O‐ApoE‐ExT and O‐ApoE‐UT mice regarding brain and aorta that suggest beneficial effects of exercise training, a longitudinal follow‐up study of aortic and brain inflammation by MRI might be one of the perspectives of the present study to confirm our results.
In conclusion, we found that cardiovascular disease risk factors, which involve chronic systemic oxidative stress and inflammation, are associated with neurovascular lesions in old ApoE−/− mice at specific ‘at‐risk’ locations. All together, our results demonstrate that 12 weeks of moderate‐intensity physical activity was able to improve survival in aged ApoE−/− mice and decrease the extent of the neurovascular damage present in this dyslipidaemic aged mouse model. The VWR protocol decreased both brain disorders (BBB leakage and macrophage accumulation) and aortic plaque size, and increased aortic plaque stabilization. The decrease in oxidative stress and inflammation directly at the brain level as a result of exercise training could be responsible for the neuroprotective effect and the reduced prevalence of lesions. Taken together, these results demonstrate the benefits of exercise training in a model of atherosclerosis. Finally, on the basis of the present study, non‐invasive imaging, such as MRI, appears to be an essential tool, as follows: (i) to evaluate neurovascular risk in the brain of atherosclerotic patients; and (ii) to measure therapeutic intervention, such as physical exercise, with both a site‐specific location and combined imaging biomarkers to evaluate the effect of exercise on BBB integrity and reduction of neuro‐inflammation. More advanced hybrid molecular imaging techniques, such as positron emission tomography–MRI and targeted nanoparticles (Briley‐Saebo et al. 2012), may provide longitudinal follow‐up and new therapeutic options for these complex, high‐risk cardiovascular profiles.
Additional information
Competing interests
None declared.
Author contributions
E.N.C., V.P. and E.C.‐S. designed the study and drafted the manuscript. E.N.C., V.D.C., F.C., A.G., D.P., B.T., J.R., V.P. and E.C.‐S. analysed the data. All authors interpreted the data and revised the manuscript critically for important intellectual content. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This study was supported by the Institut Universitaire de France and the French Ministry of Research for PhD students.
Translational perspective
Vascular brain lesions share pathological hallmarks with atherosclerosis, such as increased inflammation and oxidative stress.
We showed that an ageing model of atherosclerosis (old ApoE−/− mice on a high‐fat, high‐cholesterol diet) presented with vascular brain damage and that these neurovascular disorders were associated with peripheral inflammation. Interestingly, exercise training was able to reduce these pathological outcomes.
Our findings suggest that non‐invasive imaging could be used to evaluate neurovascular risk in atherosclerosis and that regular physical activity might reduce these risks.
Acknowledgements
Thanks to Guerbet for supplying the contrast agent and the old ApoE−/− mice; to Radu Bolbos and Jean‐Baptiste Langlois at the Centre d'Exploration et de Recherche Médicale par Emission de Positons (CERMEP) imaging platform for brain acquisitions; to Andrew Fowler and Monica Sigovan for Matlab developments for MRI analysis; and to Charles Vilallonga for brain immunohistochemistry analyses.
V. Pialoux and E. Canet‐Soulas contributed equally to the supervision of this work.
This is an Editor's Choice article from the 1 December 2016 issue.
References
- Aufradet E, Bessaad A, Alsaid H, Schäfer F, Sigovan M, De Souza G, Chirico E, Martin C & Canet‐Soulas E (2012). In vivo cardiac anatomical and functional effects of wheel running in mice by magnetic resonance imaging. Exp Biol Med (Maywood) 237, 263–270. [DOI] [PubMed] [Google Scholar]
- Badaut J, Copin J‐C, Fukuda AM, Gasche Y, Schaller K & da Silva RF (2012). Increase of arginase activity in old apolipoprotein‐E deficient mice under Western diet associated with changes in neurovascular unit. J Neuroinflammation 9, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C & Fridovich I (1971). Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44, 276–287. [DOI] [PubMed] [Google Scholar]
- Benzie IF & Strain JJ (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239, 70–76. [DOI] [PubMed] [Google Scholar]
- Blackwell BN, Bucci TJ, Hart RW & Turturro A (1995). Longevity, body weight, and neoplasia in ad libitum‐fed and diet‐restricted C57BL6 mice fed NIH‐31 open formula diet. Toxicol Pathol 23, 570–582. [DOI] [PubMed] [Google Scholar]
- Briley‐Saebo KC, Nguyen TH, Saeboe AM, Cho Y‐S, Ryu SK, Volkova ER, Volkava E, Dickson S, Leibundgut G, Wiesner P, Weisner P, Green S, Casanada F, Miller YI, Shaw W, Witztum JL, Fayad ZA & Tsimikas S (2012). In vivo detection of oxidation‐specific epitopes in atherosclerotic lesions using biocompatible manganese molecular magnetic imaging probes. J Am Coll Cardiol 59, 616–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casserly I & Topol EJ (2004). Convergence of atherosclerosis and Alzheimer's disease: inflammation, cholesterol, and misfolded proteins. Lancet 363, 1139–1146. [DOI] [PubMed] [Google Scholar]
- Chirico EN, Martin C, Faes C, Feasson L, Oyonno‐Engelle S, Aufradet E, Dubouchaud H, Francina A, Canet‐Soulas E, Thiriet P, Messonnier L & Pialoux V (2012). Exercise training blunts oxidative stress in sickle cell trait carriers. J Appl Physiol 112, 1445–1453. [DOI] [PubMed] [Google Scholar]
- Coyle J & Puttfarcken P (1993). Oxidative stress, glutamate, and neurodegenerative disorders. Science 262, 689–695. [DOI] [PubMed] [Google Scholar]
- Davis W, Mahale S, Carranza A, Cox B, Hayes K, Jimenez D & Ding Y (2007). Exercise pre‐conditioning ameliorates blood–brain barrier dysfunction in stroke by enhancing basal lamina. Neurol Res 29, 382–387. [DOI] [PubMed] [Google Scholar]
- Ding Y‐H, Ding Y, Li J, Bessert DA & Rafols JA (2006). Exercise pre‐conditioning strengthens brain microvascular integrity in a rat stroke model. Neurol Res 28, 184–189. [DOI] [PubMed] [Google Scholar]
- Ding Y‐H, Young CN, Luan X, Li J, Rafols JA, Clark JC, McAllister JP 2nd & Ding Y (2005). Exercise preconditioning ameliorates inflammatory injury in ischemic rats during reperfusion. Acta Neuropathol 109, 237–246. [DOI] [PubMed] [Google Scholar]
- Drake C, Boutin H, Jones MS, Denes A, McColl BW, Selvarajah JR, Hulme S, Georgiou RF, Hinz R, Gerhard A, Vail A, Prenant C, Julyan P, Maroy R, Brown G, Smigova A, Herholz K, Kassiou M, Crossman D, Francis S, Proctor SD, Russell JC, Hopkins SJ, Tyrrell PJ, Rothwell NJ & Allan SM (2011). Brain inflammation is induced by co‐morbidities and risk factors for stroke. Brain Behav Immun 25, 1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R & Nahrendorf M (2012). Myocardial infarction accelerates atherosclerosis. Nature 487, 325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElAli A, Doeppner TR, Zechariah A & Hermann DM (2011). Increased blood–brain barrier permeability and brain edema after focal cerebral ischemia induced by hyperlipidemia role of lipid peroxidation and calpain‐1/2, matrix metalloproteinase‐2/9, and RhoA overactivation. Stroke 42, 3238–3244. [DOI] [PubMed] [Google Scholar]
- Galiñanes M & Matata BM (2002). Protein nitration is predominantly mediated by a peroxynitrite‐dependent pathway in cultured human leucocytes. Biochem J 367, 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor SM, Wachtel M, Bolliger MF, Ishihara H, Landmann R & Frei K (2001). Molecular and cellular permeability control at the blood–brain barrier. Brain Res Brain Res Rev 36, 258–264. [DOI] [PubMed] [Google Scholar]
- Hafezi‐Moghadam A, Thomas KL & Wagner DD (2007). ApoE deficiency leads to a progressive age‐dependent blood‐brain barrier leakage. Am J Physiol Cell Physiol 292, C1256–C1262. [DOI] [PubMed] [Google Scholar]
- Hebbel RP, Eaton JW, Balasingam M & Steinberg MH (1982). Spontaneous oxygen radical generation by sickle erythrocytes. J Clin Invest 70, 1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson LH & Borg LA (1988). A spectrophotometric method for determination of catalase activity in small tissue samples. Anal Biochem 174, 331–336. [DOI] [PubMed] [Google Scholar]
- Kadoglou NPE, Kostomitsopoulos N, Kapelouzou A, Moustardas P, Katsimpoulas M, Giagini A, Dede E, Boudoulas H, Konstantinides S, Karayannacos PE & Liapis CD (2011). Effects of exercise training on the severity and composition of atherosclerotic plaque in apoE‐deficient mice. J Vasc Res 48, 347–356. [DOI] [PubMed] [Google Scholar]
- Koffie RM, Farrar CT, Saidi L‐J, William CM, Hyman BT & Spires‐Jones TL (2011). Nanoparticles enhance brain delivery of blood–brain barrier‐impermeable probes for in vivo optical and magnetic resonance imaging. Proc Natl Acad Sci USA 108, 18837–18842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs U, Wassmann S, Czech T, Münzel T, Eisenhauer M, Böhm M & Nickenig G (2005). Physical inactivity increases oxidative stress, endothelial dysfunction, and atherosclerosis. Arterioscler Thromb Vasc Biol 25, 809–814. [DOI] [PubMed] [Google Scholar]
- Lee S, Park Y, Zuidema MY, Hannink M & Zhang C (2011). Effects of interventions on oxidative stress and inflammation of cardiovascular diseases. World J Cardiol 3, 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre G, Beljean‐Leymarie M, Beyerle F, Bonnefont‐Rousselot D, Cristol JP, Thérond P & Torreilles J (1998). [Evaluation of lipid peroxidation by measuring thiobarbituric acid reactive substances]. Ann Biol Clin (Paris) 56, 305–319. [PubMed] [Google Scholar]
- Lesniewski LA, Durrant JR, Connell ML, Henson GD, Black AD, Donato AJ & Seals DR (2011). Aerobic exercise reverses arterial inflammation with aging in mice. Am J Physiol Heart Circ Physiol 301, H1025–H1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majid A (2014). Neuroprotection in stroke: past, present, and future. Int Sch Res Notices 2014, e515716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhan WG, Arrick DM, Patel KP & Sun H (2011). Exercise training normalizes impaired NOS‐dependent responses of cerebral arterioles in type 1 diabetic rats. Am J Physiol Heart Circ Physiol 300, H1013–H1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhan WG, Arrick DM, Sun H & Patel KP (2010). Exercise training restores impaired dilator responses of cerebral arterioles during chronic exposure to nicotine. J Appl Physiol 109, 1109–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihai G, He X, Zhang X, McCarthy B, Tran T, Pennell M, Blank J, Simonetti OP, Jackson RD & Raman SV (2011). Design and rationale for the study of changes in iron and atherosclerosis risk in perimenopause. J Clin Exp Cardiolog 2, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko TP, Schilling RJ, Salvemini D, Moore WM & Currie MG (1993). A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem 214, 11–16. [DOI] [PubMed] [Google Scholar]
- Moghadasian MH, McManus BM, Nguyen LB, Shefer S, Nadji M, Godin DV, Green TJ, Hill J, Yang Y, Scudamore CH & Frohlich JJ (2001). Pathophysiology of apolipoprotein E deficiency in mice: relevance to apo E‐related disorders in humans. FASEB J 15, 2623–2630. [DOI] [PubMed] [Google Scholar]
- Moraska A, Deak T, Spencer RL, Roth D & Fleshner M (2000). Treadmill running produces both positive and negative physiological adaptations in Sprague‐Dawley rats. Am J Physiol Regul Integr Comp Physiol 279, R1321–R1329. [DOI] [PubMed] [Google Scholar]
- Nahrendorf M, Frantz S, Swirski FK, Mulder WJM, Randolph G, Ertl G, Ntziachristos V, Piek JJ, Stroes ES, Schwaiger M, Mann DL & Fayad ZA (2015). Imaging systemic inflammatory networks in ischemic heart disease. J Am Coll Cardiol 65, 1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol KE, Poon WW, Parachikova AI, Cribbs DH, Glabe CG & Cotman CW (2008). Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J Neuroinflammation 5, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nighoghossian N, Wiart M, Cakmak S, Berthezène Y, Derex L, Cho T‐H, Nemoz C, Chapuis F, Tisserand G‐L, Pialat J‐B, Trouillas P, Froment J‐C & Hermier M (2007). Inflammatory response after ischemic stroke: a USPIO‐enhanced MRI study in patients. Stroke 38, 303–307. [DOI] [PubMed] [Google Scholar]
- Niki E (2009). Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med 47, 469–484. [DOI] [PubMed] [Google Scholar]
- Oberley L & Spitz D (1985). Nitroblue tetrazolium In Handbook of Methods for Oxygen Radical Research, ed Greenwald RA. CRC Press, Boca Raton. [Google Scholar]
- Ohkawa H, Ohishi N & Yagi K (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95, 351–358. [DOI] [PubMed] [Google Scholar]
- Paglia DE & Valentine WN (1967). Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70, 158–169. [PubMed] [Google Scholar]
- Pedersen BK (2006). The anti‐inflammatory effect of exercise: its role in diabetes and cardiovascular disease control. Essays Biochem 42, 105–117. [DOI] [PubMed] [Google Scholar]
- Pellegrin M, Alonso F, Aubert J‐F, Bouzourene K, Braunersreuther V, Mach F, Haefliger J‐A, Hayoz D, Berthelot A, Nussberger J, Laurant P & Mazzolai L (2009. a). Swimming prevents vulnerable atherosclerotic plaque development in hypertensive 2‐kidney, 1‐clip mice by modulating angiotensin II type 1 receptor expression independently from hemodynamic changes. Hypertension 53, 782–789. [DOI] [PubMed] [Google Scholar]
- Pellegrin M, Miguet‐Alfonsi C, Bouzourene K, Aubert J‐F, Deckert V, Berthelot A, Mazzolai L & Laurant P (2009. b). Long‐term exercise stabilizes atherosclerotic plaque in ApoE knockout mice. Med Sci Sports Exerc 41, 2128–2135. [DOI] [PubMed] [Google Scholar]
- Phinikaridou A, Andia ME, Protti A, Indermuehle A, Shah A, Smith A, Warley A & Botnar RM (2012). Noninvasive magnetic resonance imaging evaluation of endothelial permeability in murine atherosclerosis using an albumin‐binding contrast agent. Circulation 126, 707–719. [DOI] [PubMed] [Google Scholar]
- Pialoux V, Brown AD, Leigh R, Friedenreich CM & Poulin MJ (2009. a). Effect of cardiorespiratory fitness on vascular regulation and oxidative stress in postmenopausal women. Hypertension 54, 1014–1020. [DOI] [PubMed] [Google Scholar]
- Pialoux V, Mounier R, Brown AD, Steinback CD, Rawling JM & Poulin MJ (2009. b). Relationship between oxidative stress and HIF‐1α mRNA during sustained hypoxia in humans. Free Radic Biol Med 46, 321–326. [DOI] [PubMed] [Google Scholar]
- Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M & Lyketsos CG (2005). Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol 161, 639–651. [DOI] [PubMed] [Google Scholar]
- Rowlatt C, Chesterman FC & Sheriff MU (1976). Lifespan, age changes and tumour incidence in an ageing C57BL mouse colony. Lab Anim 10, 419–442. [DOI] [PubMed] [Google Scholar]
- Saleh A, Schroeter M, Jonkmanns C, Hartung H, Mödder U & Jander S (2004). In vivo MRI of brain inflammation in human ischaemic stroke. Brain 127, 1670–1677. [DOI] [PubMed] [Google Scholar]
- Sexton WL (1995). Vascular adaptations in rat hindlimb skeletal muscle after voluntary running‐wheel exercise. J Appl Physiol 79, 287–296. [DOI] [PubMed] [Google Scholar]
- Shepherd D & Garland PB (1969). The kinetic properties of citrate synthase from rat liver mitochondria. Biochem J 114, 597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K, Kishimoto C, Okabe T, Hattori M, Murayama T, Yokode M & Kita T (2007). Exercise training reduces severity of atherosclerosis in apolipoprotein E knockout mice via nitric oxide. Circ J 71, 1147–1151. [DOI] [PubMed] [Google Scholar]
- Sigovan M, Bessaad A, Alsaid H, Lancelot E, Corot C, Neyran B, Provost N, Majd Z, Breisse M & Canet‐Soulas E (2010). Assessment of age modulated vascular inflammation in ApoE−/− mice by USPIO‐enhanced magnetic resonance imaging. Invest Radiol 45, 702–707. [DOI] [PubMed] [Google Scholar]
- Sigovan M, Kaye E, Lancelot E, Corot C, Provost N, Majd Z, Breisse M & Canet‐Soulas E (2012). Anti‐inflammatory drug evaluation in ApoE−/− mice by ultrasmall superparamagnetic iron oxide‐enhanced magnetic resonance imaging. Invest Radiol 47, 546–552. [DOI] [PubMed] [Google Scholar]
- Soto I, Graham LC, Richter HJ, Simeone SN, Radell JE, Grabowska W, Funkhouser WK, Howell MC & Howell GR (2015). APOE stabilization by exercise prevents aging neurovascular dysfunction and complement induction. PLoS Biol 13, e1002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stummer W, Weber K, Tranmer B, Baethmann A & Kempski O (1994). Reduced mortality and brain damage after locomotor activity in gerbil forebrain ischemia. Stroke 25, 1862–1869. [DOI] [PubMed] [Google Scholar]
- Szostak J & Laurant P (2011). The forgotten face of regular physical exercise: a ‘natural’ anti‐atherogenic activity. Clin Sci 121, 91–106. [DOI] [PubMed] [Google Scholar]
- Viboolvorakul S & Patumraj S (2014). Exercise training could improve age‐related changes in cerebral blood flow and capillary vascularity through the upregulation of VEGF and eNOS. Biomed Res Int 2014, 230791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J, Kang JH, Manson JE, Breteler MMB, Ware JH & Grodstein F (2004). Physical activity, including walking, and cognitive function in older women. JAMA 292, 1454–1461. [DOI] [PubMed] [Google Scholar]
- Wiart M, Davoust N, Pialat J‐B, Desestret V, Moucharrafie S, Cho TH, Mutin M, Langlois JB, Beuf O, Honnorat J, Nighoghossian N & Berthezène Y (2007). MRI monitoring of neuroinflammation in mouse focal ischemia. Stroke 38, 131–137. [DOI] [PubMed] [Google Scholar]
- Witko‐Sarsat V1, Friedlander M, Capeillère‐Blandin C, Nguyen‐Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps‐Latscha B (1996). Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int 49, 1304–1313. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV (2008). The blood‐brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178–201. [DOI] [PubMed] [Google Scholar]


