Abstract
To determine which polysomnography parameters are associated with severity of hypertension.
This retrospective study collected data on all patients admitted to our urban, academic center in Beijing with hypertension who had undergone polysomnograms (PSG) and were diagnosed with obstructive sleep apnea (OSA) (apnea–hyponea index [AHI] ≥5/hour). We then compared polysomnographic parameters (AHI, oxygen desaturation index [ODI], lowest oxygen saturation [LOS], and mean apnea–hypopnea duration [MAD]) by hypertension severity in this cohort.
There were 596 subjects who met entry criteria. Age, sex distribution, body mass index (BMI), history of current smoking and alcohol were similar among groups. Subjects with longer MAD suffered from more severe hypertension (P = 0.011). There were no relationship between AHI, ODI, and LOS and hypertension in our cohort. There were no significant differences in age, sex, BMI, history of current smoking and alcohol use between hypertension groups. MAD had a small but significant independent association (odds ratio [OR] = 1.072, 95% confidence interval [CI] 1.019–1.128, P = 0.007) with moderate to severe hypertension, using logistic regression analysis that accounted for age, sex, BMI, history of current smoking and alcohol, AHI, and LOS.
Chinese inpatients with longer MAD by PSG face higher odds of moderate to severe hypertension. The mechanism of these effects may be due to aggravated nocturnal hypoxaemia and hypercapnia, as well as disturbed sleep architecture. These results suggest that additional information available in the polysomnogram, such as MAD, should be considered when evaluating OSA patients.
Keywords: Disease evaluation, Hypertension, Mean apnea–hypopnea duration, Obstructive sleep apnea syndrome
1. Introduction
Obstructive sleep apnea (OSA) leads to systemic hypertension in animal models[1] and human epidemiological studies in the general population, and in OSA patients.[2–4] Although part of this association may be mediated by co-existing risk factors, such as obesity, a large body of evidence supports an independent role of OSA in the pathogenesis of hypertension, although some find this link to be controversial.[5,6] A recent and large study[7] found an increased hazard ratio for incident hypertension in patients with OSA compared with control subjects, and the association between OSA and hypertension remained independent of confounders including obesity and age (1889 participants, 12.2 years of median follow up). Furthermore, follow-up of this patient cohort revealed a dose–response relationship between the severity of OSA and the cumulative incidence of hypertension. Supporting this idea, several randomized trials have shown that treatment with continuos positive airway pressure for obstructive sleep apnea reduces systemic blood pressure.[8] Thus, relatively robust evidence implicates OSA as a factor in the development of hypertension.
Pathophysiological studies have extensively investigated numerous factors affecting hypertension, including large and small artery remodeling and functional changes,[9] sympathetic nervous system alterations[10] and the related change of the molecular pathways such as G-protein-coupled receptor kinases.[11–13] Given the independent link between OSA and hypertension, several mechanisms may be responsible.[14] All of these pathophysiologic processes (autonomic alterations,[15] endothelial dysfunction,[16] and inflammation[17]) occur during apneic episodes where the reduction in arterial oxygen pressure and hypercapnia occur. However, the severity of hypertension and the severity of OSA do not correlate perfectly, indicating that some physiologic parameters in OSA may be reflective of the underlying connection more than others. Apnea–hypopnea index (AHI), currently used for the estimation of the severity of OSA, does not contain information on the duration aspect of the breathing cessations and related oxygen desaturations. Obviously, longer apnea–hypopnea duration and deeper desaturation may have more severe consequences than shorter and shallower ones. To our knowledge, only one pilot study (n = 9 with OSA) investigated the effect of apnea duration on arterial blood pressure.[18] This study showed that as apnea duration increased from 10 seconds to greater than 30 seconds, the mean percentage rise in blood pressure increased from 14% to 26% for amplitude and 14% to 23% for area, respectively. They suggested that the apnea duration has a measurable effect on the degree of rise in arterial blood pressure during apnea episodes (P = 0.0002).[18]
The goal of this study was to confirm the effect of mean apnea–hypopnea duration (MAD) on hypertension. To do so, we studied a cohort of patients (n = 596) recruited from Beijing Anzhen Hospital, in Beijing, where OSA and hypertension are prevalent.
2. Methods
2.1. Study population
In this single center, cross-sectional analysis, data for patients who were hospitalized in Beijing Anzhen Hospital between 2012 and 2015 were extracted from the hospital information system and each patient was individually reviewed to determine whether they met all the inclusion and exclusion criteria. During this period, 1299 of these in patients underwent polysomnography (PSG) in our certified Sleep Medicine Laboratory (see below). We included patients with untreated OSA by PSG (AHI ≥5/h) with comorbid hypertension diagnosed by 24-hour BP monitoring in our hypertension clinic, either prior to admission or during their hospitalization. Using these data, we classified the hypertension stage as follows: hypertension stage I: blood pressure (BP) in the range 140 to 159 mm Hg systolic and/or 90 to 99 mm Hg diastolic; hypertension stage II: BP in the range 160 to 179 mm Hg systolic and/or 100 to 109 mm Hg diastolic; hypertension stage III: BP more than 180 mm Hg systolic and/or 110 mm Hg diastolic.
We excluded subjects with an AHI < 5/h, chronic obstructive pulmonary disease (COPD), chronic bronchitis, restrictive lung diseases, respiratory failure, aortic coarctation, cushing syndrome, pheochromocytoma, primary aldosteronism, renal parenchymal disease, renovascular hypertension, thyroid disorders, and those who could not give a complete medical history.
After screening, a total of 596 subjects meeting these criteria were included in our analysis (Fig. 1). All subjects gave written, informed consent for the records to be used for our research, and the Institutional Review Board of Beijing Anzhen Hospital approved the study.
Figure 1.
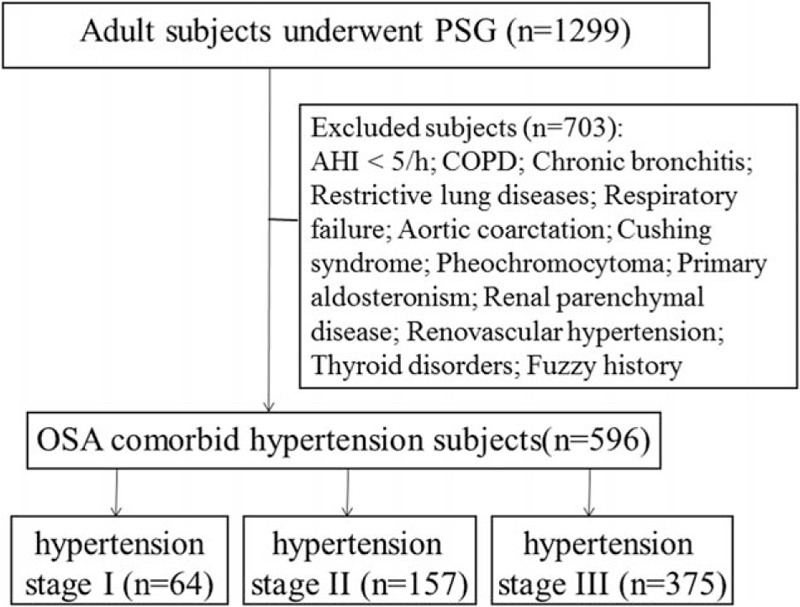
Study design.
2.2. Classification of OSA
Sleep evaluations were performed by a trained specialist in Sleep Medicine at Beijing Anzhen Hospital Sleep Center during admission. We recorded each patient's diagnostic PSG parameters, which included measures of the electromyogram, electro-oculogram, electroencephalogram, electrocardiogram, pulse oximetry, naso-oral airflow, and thoracoabdominal excursions. Sleep, arousals, and periodic legs movements were scored by American Academy of Sleep Medicine standards.[19] Respiratory events were scored manually as follows: apneas were identified when the airflow amplitude on the nasal cannula was <10% of baseline and no flow occurred on the oral thermistor. Hypopneas were identified when airflow amplitude was reduced by 30% from baseline and the event was followed by 4% O2 desaturation.[19] AHI was defined as the sum of apneas and hypopneas divided by total sleep time as suggested by the recent criteria of the American Academy of Sleep Medicine.[19] OSA is the most common type of sleep apnea and is caused by complete or partial obstructions of the upper airway. It is characterized by repetitive episodes of shallow or paused breathing during sleep, despite effort to breathe, and is associated with a reduction in blood oxygen saturation and other physiologic derangements. We collected data on coexisting conditions, morning blood pressure and BMI of each person at the time of PSG. The personnel collecting these data were different from the one collecting data from the PSG and confirming the sleep diagnoses, and also from those who collected the BP data.
2.3. Statistical analysis
Characteristics of the study population and laboratory tests results are presented as mean ± SD and count (with percentages). Analysis of variance (ANOVA) or chi-square tests were used to compare demographics and PSG data of the three groups according to the status of hypertension. Multivariate logistic regression analysis was used to assess the independent risk factors associated with the status of hypertension. We also determined the correlation between MAD and other polysomnographic findings using linear regression analysis. A P value <0.05 was considered to be statistically significant. Tests were two-tailed. All analyses were performed using SPSS 19.0 software for Windows (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Patient characteristics and association of sleep parameters
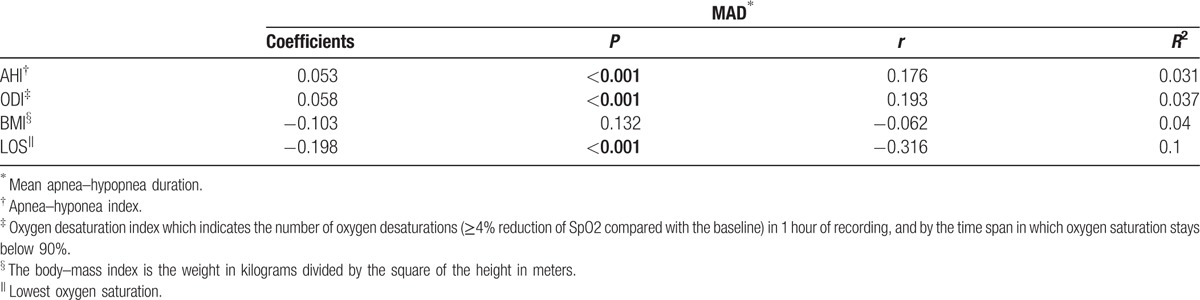
We included 596 who met entry criteria. Table 1 shows the linear regression analyses between MAD and AHI, MAD and ODI, MAD and BMI, MAD and LOS for the entire study cohort. There were no statistically significant correlation between MAD and BMI (P = 0.132). A small but statistically significant correlation existed between MAD and AHI (r = 0.176, P < 0.001) and ODI (r = 0.193, P < 0.001). A stronger but statistically significant correlation existed between MAD and LOS (r = −0.316, P < 0.001).
Table 1.
Linear regression model for MAD and polysomnographic findings.
3.2. Hypertension grade
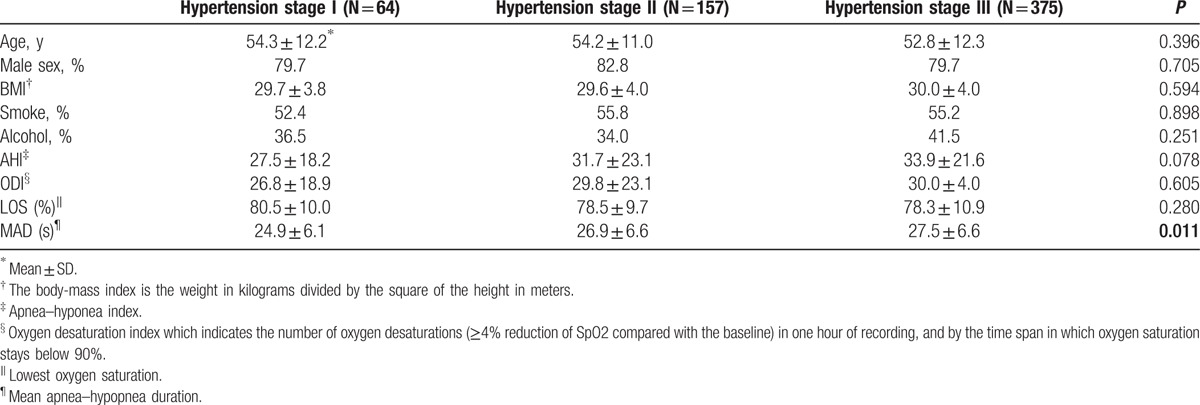
Table 2 shows the main demographic characteristics and polysomnographic findings of the subjects according to hypertension grade. Age, sex, BMI, history of current smoking and alcohol were similar in three hypertension groups. Patients with longer MAD (24.9 ± 6.1 seconds vs. 26.9 ± 6.6 seconds vs. 27.5 ± 6.6 seconds, P = 0.011) exhibited more severe hypertension. However, there was no association between hypertension grade and AHI, ODI and LOS.
Table 2.
Study population.
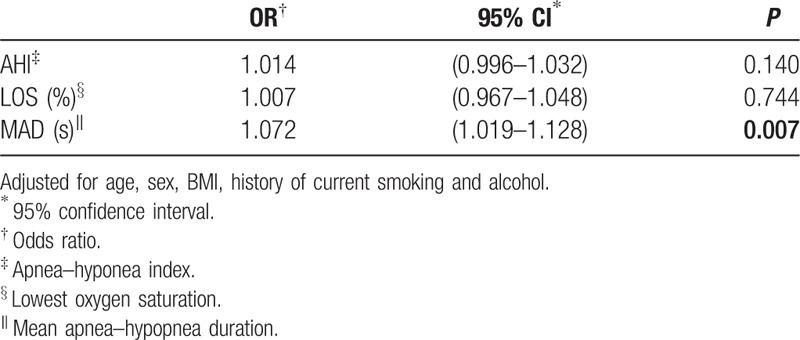
Among the 3 different severity hypertension groups, patients with hypertension stage I had a significant shorter MAD than patients at hypertension stage II (P = 0.039) and hypertension stage III (P = 0.003). However, no significant difference of MAD was found between hypertension stage II and hypertension stage III (P = 0.302). So, we defined hypertension stage I as mild hypertension group and hypertension stage II and III as moderate-severe hypertension group. After adjusting for age, sex, BMI, history of current smoking and alcohol, AHI and LOS were not associated with the severity of hypertension (Table 3). However, increased MAD was significantly associated with moderate to severe hypertention (OR = 1.072, 95% CI 1.019–1.128, P = 0.007).
Table 3.
Independent factors associated with severity of hypertension by multivariate logistic regression analysis.
4. Discussion
We found that OSA patients with longer MAD have worse hypertension than patients with shorter MAD. Interestingly, we did not find a statistically significant difference of AHI among different severity of hypertension groups. Our data clearly demonstrate that MAD rather than AHI have a greater impact on the severity of hypertension in Chinese adults. To our knowledge, this large study is the first to examine this topic.
Several studies have reported overall variations in blood pressure during apnea episodes and found that there is a progressive increase in blood pressure during apnea–hypopnea episode followed by a rapid decrease below the baseline following apnea termination.[18,20] They suggest that the severity of hemodynamic changes due apnea–hypopnea may be correlated to the duration of apnea–hypopnea episodes. Even though apnea is defined as cessation of breathing for a minimum of 10 seconds, the duration of apnea episodes can vary anywhere from 10 seconds to over a minute and may not be reflected in the AHI and ODI scores. The lengthening of apnea and hypopnea events may even lead to a decrease in AHI and ODI indices. So, a focus on AHI may be an imperfect way to evaluate the severity of OSA, especially regarding effects on other physiologic systems. Some researchers proposed novel parameters[21,22] which exploit the information related to the duration and morphology of the sleep disordered breathing events to evaluate severity of sleep disordered breathing and diagnose OSA. These investigators take the aspect of the varying apnea and hypopnea event durations into consideration as novel parameters versus traditional AHI. There is data to suggest that patients with similar AHI may in fact suffer from OSA of very different severities.[22] Novel parameters in the PSG may bring more comprehensive view to the individual estimation of the severity of OSA, but need additional study to determine their role in sleep physiology and its relationship to comorbidities. MAD appears to be one such useful parameter.
The mechanisms of how longer MAD affects blood pressure may include both short- and long-term effects (Fig. 2). In the short-term, this may be due to repetitive occurrence of complete or partial interruptions in airflow resulting from upper airway occlusion, when blood oxygen saturation can drop to dangerously low levels, especially in the longer events, eventually leading to arousals from sleep resulting in sleep fragmentation. Additionally, for a longer duration apnea–hypopnea, the amount of CO2 accumulated in the body and the amount of oxygen desaturation will be higher compared with that of a shorter duration apnea. The combined effects of hypoxia[23] and hypercapnia work synergistically[24] and the disturbed sleep structure[25,26] can increase sympathetic nerve activity. Hence, for a longer duration apnea, the degree of sympathetic activation may be higher which in turn can lead to more elevated blood pressure. In the long-term, the sleep fragmentation in OSA elicits events such as constriction of blood vessels due to increased sympathetic activity and the severer nocturnal hypoxaemia of OSA. Longer MAD also triggers systemic inflammation[17,27] and metabolic dysregulation,[28] which can lead to short-term and long-term rises in arterial blood pressure eventually leading to chronic hypertension.[29]
Figure 2.
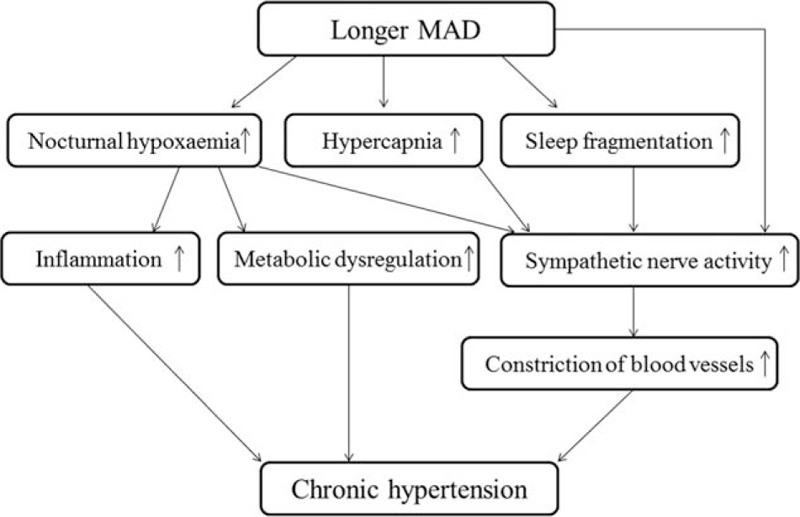
The mechanisms of how longer MAD affects blood pressure. MAD = mean apnea–hypopnea duration.
Prior studies have shown the importance of MAD in the assessment of OSA diagnosis and treatment. For example, increased MAD and lower nocturnal oxygen saturation are associated with excessive daytime sleepiness.[30] Conversely, patients with excessive daytime sleepiness have longer MAD and lower saturation levels, which supports the assumption that longer and deeper events have more severe physiological consequences. Another finding that supports the fact that AHI does not capture all aspects of sleep problems in OSA is the weak correlation reported between the improvement of AHI and sleepiness score after surgical treatments.[31] The most obvious example to illustrate this is nasal surgery. Meen and Chandra[32] reported no improvement in AHI after nasal surgery, but consistent improvement in subjective symptoms of OSA such as sleepiness and overall quality of life. A single randomized controlled trial that compared septoplasty and sham surgery found no significant decrease in AHI for either group. However, those who underwent septoplasty reported reduced sleepiness.[33] In the research of Nakata et al,[34] MAD was significantly reduced (33.5 ± 7.3 vs. 28.8 ± 7.4, P < 0.05) after the nasal surgery, and also nocturnal oxygenation, sleep quality, and daytime sleepiness were improved. These studies justify further study of additional measures of sleep, including MAD, in understanding how OSA affects other body systems.
Here, we found a potential relationship between MAD on the severity of hypertension. Notably, AHI and ODI showed no such relationship. OSA is a complex medical health problem with severe cardiovascular consequences[35] and the physiological stress and the mechanisms behind it are complex. The classic AHI might not contain all the necessary information needed to diagnose the severity of OSA and to evaluate the need for treatment, so new evaluation systems need to be established. This field also needs an evaluation system that includes the grading and staging of the disease. And, it needs more animal experiments and clinical prospective studies to explore the effect of MAD on hypertension.
Although the current study is the largest to date in its field, the retrospective nature of the investigation is an important limitation. The lack of prospective data collection could have introduced bias into the data because patients were excluded for lack of sufficient data (the main reason of the lack of control group) or missing part of the original 24-hour BP monitoring recordings only accurate hypertension grade were retained. Therefore, we cannot provide BP levels and P values for which patients with longer MAD exhibited more severe hypertension. We limited our analyses to inpatients, so our findings may not apply to outpatients or other cohorts. We also included only patients with PSG and hypertension data, thus we need to replicate these findings in a prospective cohort, ideally in the general population. Nevertheless, these data provide a first step in examining how OSA relates to hypertension.
5. Conclusion
In conclusion, OSA patients with longer MAD have worse hypertension, a finding that appears to be more specific than with AHI. These results suggest that additional information, such as MAD, should be considered when evaluating OSA patients, especially those with comorbid hypertension. Animal experiments and clinical prospective studies are needed to determine whether MAD can affect the occurrence and development of hypertension and underlying mechanisms.
Acknowledgments
The authors would like to thank all of the staff for their help in extracting data from Beijing Anzhen Hospital Sleep Center. We also thank the staff from Beijing Anzhen Hospital Cardiovascular Department for their assistance with subjects. Dr. J.M. Pinto (University of Chicago) provided helpful advice and editorial input.
Footnotes
Abbreviations: AHI = apnea–hyponea index, BMI = body mass index, BP = blood pressure, LOS = lowest oxygen saturation, MAD = mean apnea–hypopnea duration, ODI = oxygen desaturation index, OSA = obstructive sleep apnea, PSG = polysomnograms.
Funding: The current study is funded by International Science & Technology Cooperation Program of China (NO. 2015DFA3016). The sponsor had no role in the design or conduct of this research. This work was also supported by a High End Visiting Foreign Experts Program (20151100504).
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
- [1].Dumitrascu R, Heitmann J, Seeger W, et al. Obstructive sleep apnea, oxidative stress and cardiovascular disease: lessons from animal studies. Oxid Med Cell Longevity 2013;2013:234631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000;342:1378–84. [DOI] [PubMed] [Google Scholar]
- [3].Goff EA, O’Driscoll DM, Simonds AK, et al. The cardiovascular response to arousal from sleep decreases with age in healthy adults. Sleep 2008;31:1009–17. [PMC free article] [PubMed] [Google Scholar]
- [4].Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 2011;58:811–7. [DOI] [PubMed] [Google Scholar]
- [5].Cano-Pumarega I, Duran-Cantolla J, Aizpuru F, et al. Obstructive sleep apnea and systemic hypertension: longitudinal study in the general population: the Vitoria Sleep Cohort. Am J Respir Crit Care Med 2011;184:1299–304. [DOI] [PubMed] [Google Scholar]
- [6].O’Connor GT, Caffo B, Newman AB, et al. Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med 2009;179:1159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012;307:2169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Montesi SB, Edwards BA, Malhotra A, et al. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med 2012;8:587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Laurent S, Boutouyrie P. The structural factor of hypertension: large and small artery alterations. Circ Res 2015;116:1007–21. [DOI] [PubMed] [Google Scholar]
- [10].Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res 2015;116:976–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Izzo R, Cipolletta E, Ciccarelli M, et al. Enhanced GRK2 expression and desensitization of betaAR vasodilatation in hypertensive patients. Clin Transl Sci 2008;1:215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Santulli G, Iaccarino G. Adrenergic signaling in heart failure and cardiovascular aging. Maturitas 2016;93:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Santulli G, Trimarco B, Iaccarino G. G-protein-coupled receptor kinase 2 and hypertension: molecular insights and pathophysiological mechanisms. High Blood Press Cardiovasc Prev 2013;20:5–12. [DOI] [PubMed] [Google Scholar]
- [14].Parati G, Lombardi C, Hedner J, et al. Recommendations for the management of patients with obstructive sleep apnoea and hypertension. Eur Respir J 2013;41:523–38. [DOI] [PubMed] [Google Scholar]
- [15].Idiaquez J, Santos I, Santin J, et al. Neurobehavioral and autonomic alterations in adults with obstructive sleep apnea. Sleep Med 2014;15:1319–23. [DOI] [PubMed] [Google Scholar]
- [16].Minville C, Hilleret MN, Tamisier R, et al. Nonalcoholic fatty liver disease, nocturnal hypoxia, and endothelial function in patients with sleep apnea. Chest 2014;145:525–33. [DOI] [PubMed] [Google Scholar]
- [17].Vitulano N, Di Marco Berardino A, Re A, et al. Obstructive sleep apnea and heart disease: the biomarkers point of view. Front Biosci 2013;5:588–99. [DOI] [PubMed] [Google Scholar]
- [18].Alex R, Manchikatla S, Machiraju K, et al. Effect of apnea duration on apnea induced variations in cerebral blood flow velocity and arterial blood pressure. Conf Proc IEEE Eng Med Biol Soc 2014;2014:270–3. [DOI] [PubMed] [Google Scholar]
- [19].Thornton AT, Singh P, Ruehland WR, et al. AASM criteria for scoring respiratory events: interaction between apnea sensor and hypopnea definition. Sleep 2012;35:425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kario K. Obstructive sleep apnea syndrome and hypertension: ambulatory blood pressure. Hypertens Res 2009;32:428–32. [DOI] [PubMed] [Google Scholar]
- [21].Kulkas A, Tiihonen P, Eskola K, et al. Novel parameters for evaluating severity of sleep disordered breathing and for supporting diagnosis of sleep apnea-hypopnea syndrome. J Med Eng Technol 2013;37:135–43. [DOI] [PubMed] [Google Scholar]
- [22].Kulkas A, Tiihonen P, Julkunen P, et al. Novel parameters indicate significant differences in severity of obstructive sleep apnea with patients having similar apnea-hypopnea index. Med Biol Eng Comput 2013;51:697–708. [DOI] [PubMed] [Google Scholar]
- [23].Cutler MJ, Swift NM, Keller DM, et al. Hypoxia-mediated prolonged elevation of sympathetic nerve activity after periods of intermittent hypoxic apnea. J Appl Physiol 2004;96:754–61. [DOI] [PubMed] [Google Scholar]
- [24].Somers VK, Mark AL, Abboud FM. Sympathetic activation by hypoxia and hypercapnia—implications for sleep apnea. Clin Exp Hypertens 1988;10:413–22. [DOI] [PubMed] [Google Scholar]
- [25].Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand 2003;177:385–90. [DOI] [PubMed] [Google Scholar]
- [26].Fung MM, Peters K, Redline S, et al. Decreased slow wave sleep increases risk of developing hypertension in elderly men. Hypertension 2011;58:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wu H, Yuan X, Wang L, et al. The relationship between obstructive sleep apnea hypopnea syndrome and inflammatory markers and quality of life in subjects with acute coronary syndrome. Respirat Care 2016;61:1207–16. [DOI] [PubMed] [Google Scholar]
- [28].Drager LF, Togeiro SM, Polotsky VY, et al. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol 2013;62:569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pauletto P, Rattazzi M. Inflammation and hypertension: the search for a link. Nephrol Dial Transplant 2006;21:850–3. [DOI] [PubMed] [Google Scholar]
- [30].Mediano O, Barcelo A, de la Pena M, et al. Daytime sleepiness and polysomnographic variables in sleep apnoea patients. Eur Respir J 2007;30:110–3. [DOI] [PubMed] [Google Scholar]
- [31].Smith DF, Cohen AP, Ishman SL. Surgical management of OSA in adults. Chest 2015;147:1681–90. [DOI] [PubMed] [Google Scholar]
- [32].Meen EK, Chandra RK. The role of the nose in sleep-disordered breathing. Am J Rhinol Allergy 2013;27:213–20. [DOI] [PubMed] [Google Scholar]
- [33].Koutsourelakis I, Georgoulopoulos G, Perraki E, et al. Randomised trial of nasal surgery for fixed nasal obstruction in obstructive sleep apnoea. Eur Respir J 2008;31:110–7. [DOI] [PubMed] [Google Scholar]
- [34].Nakata S, Noda A, Yasuma F, et al. Effects of nasal surgery on sleep quality in obstructive sleep apnea syndrome with nasal obstruction. Am J Rhinol 2008;22:59–63. [DOI] [PubMed] [Google Scholar]
- [35].Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009;373:82–93. [DOI] [PubMed] [Google Scholar]


