Supplemental Digital Content is available in the text
Keywords: decentralization, diagnostic accuracy, dried blood spots, HIV-1, point-of-care, viral load
Abstract
The use of dried blood spots (DBS) instead of plasma as a specimen type for HIV-1 viral load (VL) testing facilitates the decentralization of specimen collection and can increase access to VL testing in resource-limited settings. The performance of DBS for VL testing is lower, however, when compared to the gold standard sample type plasma. In this diagnostic accuracy study, we evaluated 3 VL assays with DBS.
Participants were recruited between August 2012 and April 2015. Both plasma and DBS specimens were prepared and tested for HIV-1 VL with the Roche CAP/CTM HIV-1 test v2.0, the Abbott RealTime HIV-1, and the bioMérieux NucliSENS EasyQ HIV-1 v2.0. Sensitivity and specificity to detect treatment failure at a threshold of 1000 cps/mL with DBS were determined.
A total of 272 HIV-positive patients and 51 HIV-negative people were recruited in the study. The mean difference or bias between plasma and DBS VL was <0.5 log cps/mL with all 3 assays but >25% of the specimens differed by >0.5 log cps/mL.
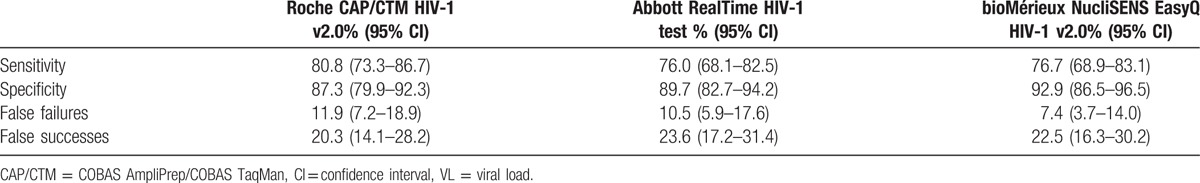
All 3 assays had comparable sensitivities around 80% and specificities around 90%. Upward misclassification rates were around 10%, but downward misclassification rates ranged from 20.3% to 23.6%. Differences in between assays were not statistically significant (P > 0.1).
The 3 VL assays evaluated had suboptimal performance with DBS but still performed better than immunological or clinical monitoring. Even after the introduction of the much-anticipated point-of-care VL devices, it is expected that DBS will remain important as a complementary option for supporting access to VL monitoring, particularly in rural, resource-limited settings. Manufacturers should accelerate efforts to develop more reliable, sensitive and specific methods to test VL on DBS specimens.
1. Introduction
The World Health Organization currently recommends viral load (VL) monitoring as the preferred approach to determining the effectiveness of antiretroviral therapy (ART) for people living with HIV.[1] Routine VL monitoring allows for earlier detection of treatment failure compared to clinical or immunological monitoring, thereby reducing morbidity and mortality, avoiding accumulation of resistance mutations, and identifying patients in need of adherence support.[2–7] In resource-limited settings, however, access to viral load monitoring is generally limited.[8] Barriers to VL testing in resource-limited settings include the high cost, the need for substantial laboratory infrastructure, efficient specimen transport systems, and implementation of quality assurance measures.[3,9–11] As a result, most people living with HIV in resource-limited settings, where the needs are greatest, lack access to VL testing,[12] and clinical and immunological monitoring is still commonly used, despite reduced accuracy for identifying treatment failure.[8]
The complexity of specimen collection, storage, and transport for VL testing is mainly related to the use of blood plasma. In resource-limited settings, dried blood spots (DBS) are being introduced as an alternative specimen type to plasma. DBS can be collected using capillary blood from a finger prick, carry no risk of infection, and can be stored and transported at ambient temperature, thus avoiding the need for phlebotomists, cold chain, and same-day transport to a VL testing facility. Using DBS instead of plasma as a specimen type facilitates the decentralization of specimen collection while maintaining high-throughput centralized laboratory testing.[13–15]
Nevertheless, most commercially available VL assays have not been validated for use with DBS and concise locked-down manufacturer protocols for specimen preparation, storage, and processing are often lacking. Several studies comparing DBS with plasma for VL testing have been published but due to the lack of standardization in specimen preparation, storage and processing, these studies are difficult to compare.[16,17] In addition, most evaluations have been carried out either on a single or on 2 different assays, limiting the possibility to compare across assays. There is need for more reliable, standardized, quality assured, and validated methods of HIV-1 VL testing on DBS.
This study summarizes the results of a diagnostic accuracy study with 3 commercially available WHO prequalified HIV-1 VL assays on DBS specimens.
2. Methods
2.1. Study population and setting
Participants were recruited at the Institute of Tropical Medicine (ITM) in Antwerp, Belgium, and the University Hospital in Ghent, Belgium. All consenting HIV-positive patients ≥18 years presenting for VL monitoring as well as people seeking an HIV test between August 2012 and April 2015 were eligible to participate.
2.2. Study procedure
Venous whole blood was collected in EDTA vacutainers, DBS specimens were spotted, and plasma was prepared from the remaining blood by centrifugation at 1100 × g for 20 minutes. Plasma was stored at −80°C until testing. Plasma specimens were tested for VL with the Roche COBAS AmpliPrep/COBAS TaqMan (CAP/CTM) HIV-1 test v2.0 (Branchburg, NJ) at the AIDS Reference Laboratories of the ITM in Antwerp and the University Hospital in Ghent. Results from this assay were used as the reference results in this study. All DBS specimens were tested at the ITM in Antwerp with the Roche CAP/CTM, the Abbott RealTime HIV-1 (Des Plaines, IL), and the bioMérieux NucliSENS EasyQ HIV-1 v2.0 (Marcy-l’Etoile, France).
2.3. Dried blood spot preparation
DBS were prepared by spotting 5 times 70 μL (Roche and Abbott) or 50 μL (bioMérieux) of EDTA venous whole blood on Whatman 903 filter paper cards (Whatman, Maidstone, UK). The blood was left to dry overnight, packed in separate zip-lock bags with desiccant sachets and a humidity indicator card, and stored at −20°C until testing.
2.4. Dried blood spot processing
Each manufacturer supplied different DBS processing instructions.
2.4.1. Roche: (Free virus elution “FVE” protocol)
One entire blood spot was cut out with individual scissors, transferred to an S-tube, 1000 μL of sterile phosphate buffered saline (PBS) was added and incubated at room temperature for 1 hour without shaking. After incubation, tubes were gently tapped to homogenize the solution and then loaded on the CAP instrument without removing the filter paper.
2.4.2. Abbott
One circle of 12 mm was punched out with a punching device (bioMérieux, Marcy-l’Etoile, France), transferred to an empty master mix tube, 1300 μL of Abbott mWash 1 buffer[18] was added and incubated at room temperature for 20 minutes without shaking. After the incubation, tubes were loaded on the m2000sp instrument without removing the filter paper.
2.4.3. bioMérieux
Two circles of 12 mm were punched out with a punching device (bioMérieux, Marcy-l’Etoile, France), transferred to a 15 mL tube containing 2 mL lysis buffer and incubated at room temperature for 30 minutes on a roller-mixer. After incubation, the tubes were centrifuged for 15 seconds and all liquid was transferred to the disposables and loaded on the EasyMAG instrument.
2.5. Outcome measures
Bias, sensitivity, specificity, and misclassification rates at a threshold of 1000 cps/mL were used as outcome measures. The threshold of 1000 cps/mL was chosen in accordance with the current WHO guidelines for defining treatment failure.[1] In addition, sensitivity and specificity were determined using the respective limit of detection of each assay. For each of the 3 assays, DBS VL results were compared to an accepted reference value. Here, the reference value was defined as the result obtained using 1.0 mL of EDTA plasma as a specimen with the Roche CAP/CTM.
2.6. Data analysis
To compensate for the fact that DBS contains whole blood instead of plasma, with ∼50% red blood cells, Roche recommends that DBS results be multiplied by 2, whereas bioMérieux recommended using a factor of 2.29 for comparison with plasma. Abbott has incorporated such a conversion factor in the assay software itself.
The Roche CAP/CTM, the Abbott RealTime, and NucliSENS EasyQ assays report results in cps/mL, where 1 copy corresponds to 1.7, 1.74 and 2.08 international units respectively. Therefore, Abbott and bioMérieux results were corrected with a factor of 1.02 (=1.74/1.7) and 1.22 (=2.08/1.7) respectively before comparison with Roche results.
Sensitivities and specificities were compared with a 2-sided Chi-squared test in Graphpad Prism 7.0 (La Jolla, CA). P-values <0.05 were considered significant.
Ethics approval was obtained from the Institutional Review Board of the ITM and the University Hospital in Antwerp (Wilrijkstraat 10, 2650 Edegem Belgium).
3. Results
A total of 272 HIV-positive patients and 51 HIV-negative people were recruited in the study. The average age was 44.0 years (IQR: 32.2 – 49.6) and 79.1% were male. Fifty-one had an undetectable plasma VL, 75 had a detectable VL < 1000 cps/mL, and 146 had a VL > 1000 cps/mL. Subtype information was available for 64% of patients, of which 35% were infected with subtype B, 46% with recombinant forms, and 19% with other subtypes.
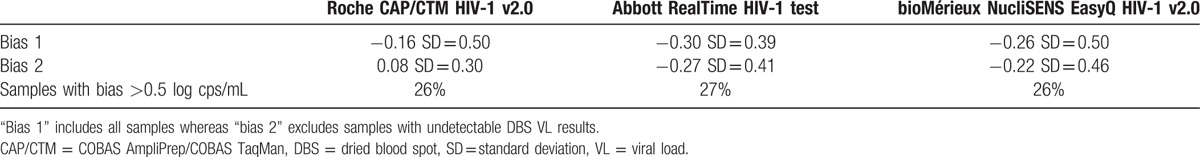
Bias between the results on DBS and the reference value was calculated for specimens with a plasma viral load > 1000 cps/mL. For specimens with an undetectable VL on DBS, an arbitrary value was assigned, corresponding to half of the limit of detection. Similarly, for specimens with a detectable VL below the limit of quantification (LOQ), half of the LOQ was assigned.[19] In Table 1, the resulting bias is named “bias 1.” Bias was also calculated after excluding all specimens with an undetectable viral load on DBS and was named “bias 2.” All biases were <0.5 log cps/mL. Nevertheless, 26%, 27%, and 26% differed by >0.5 log cps/mL on Roche, Abbott and bioMérieux, respectively.
Table 1.
Bias (in log cps/mL) between dried blood spot and plasma viral load results for samples with a plasma viral load >1000 cps/mL.
Sensitivities, specificities, and percentages of false treatment failures and successes were calculated at a threshold of 1000 cps/mL. All 3 assays had sensitivities around 80% and specificities around 90% (Table 2). Upward misclassification rates were around 10%, but downward misclassification rates ranged from 20.3% to 23.6% (Table 2). Differences in between assays were not statistically significant (P > 0.1).
Table 2.
Sensitivity, specificity, false failures, and false successes for dried blood spot VL testing as compared to plasma VL testing.
DBS specimens from 51 HIV negative people were tested. None gave a positive signal when tested with bioMérieux. However, 5 had a detectable result below the LOQ when tested with Roche and 2 had a detectable result above 1000 cps/mL when tested with Abbott. When retested, none of the 5 were detectable with Roche, whereas 1 of the 2 remained detectable above 1000 cps/mL with Abbott.
All plasma and DBS VL results are listed in a table as Supplemental Digital Content 1.
4. Discussion
In this study, the mean difference between plasma and DBS VL was <0.5 log cps/mL, but 26% (Roche and bioMérieux) and 27% (Abbott) of individual specimens did differ by >0.5 log cps/mL. Sensitivities and specificities to detect treatment failure at a threshold of 1000 cps/mL were comparable across the 3 assays and similar to previously published studies.[16] The false positive results on Roche and Abbott when testing HIV negative DBS specimens are an issue of concern. Overall, the results of this study suggest a need to improve the sensitivity of VL testing on DBS.
We found that DBS VL systematically underestimated plasma VL, resulting in many false treatment successes. In clinical practice, this means that when DBS is used to test VL, a small but important proportion of treatment failures would go unnoticed. Especially in settings where patients are monitored just once per year, this is an issue of concern. VL assays should be further optimized for better performance with DBS with a focus on increasing its sensitivity. Further research and development will need to determine whether this is best done by increasing input blood volume, improving blood elution from the filter paper, or by applying better correction factors to account for, for example, hematocrit.
Despite these concerns, the results of our study show that DBS VL for adults performs better than immunological or clinical monitoring. A systematic review of the performance of immunologic and clinical criteria for detecting virological failure found that sensitivity was 55% for immunological criteria and 11% for clinical criteria.[8] This suggests that in settings where DBS is the only viable means to monitor VL, it will still provide a more accurate assessment of treatment success than immunological or clinical monitoring alone. Based on the findings of a large systematic review of the performance of DBS, which included the results of this study, the latest WHO guidelines for antiretroviral therapy recommend the use of DBS as an option for improving access to VL monitoring.[1]
This study has a number of strengths and limitations. Strengths include the fact that all evaluations were done in the same laboratory, on the same patient specimens by the same 2 operators whom had several years of experience with VL testing. However, the operators were not blind to the results of the reference test. As this evaluation used blood specimens collected from routine monitoring visits, limited volumes were available and all DBS results obtained with 3 different assays were compared to plasma results obtained from the Roche CAP/CTM v2.0 assay only. Inherent differences between the different assays warrant caution when comparing data between assays.[20–24]
In addition, the VL assays from Roche and the protocol in use at the time for Abbott had not been fully validated by the manufacturers for DBS and no official instructions for use were available except for the NucliSENS EasyQ v2.0 assay. Since then, Roche has adapted its DBS processing instructions and Abbott has obtained CE approval and WHO prequalification status for a revised DBS protocol.
Another limitation of this evaluation was that DBS specimens were stored at −20°C, whereas ambient temperature would have been more relevant to the context in which DBS will be used. This was necessary because of the length of time needed to reach a large enough sample size of the different VL categories before specimens could be tested. This may have affected the results for the Roche assay, for which the recently developed FVE protocol was used. According to the manufacturer, DBS specimens should never be frozen or refrigerated for extended periods of time when using FVE, as this might release cell-associated viral RNA and over-estimate the results. Finally, these results are limited to adults. There is a need to also evaluate DBS to increase access to VL monitoring in children, given the very low sensitivity and specificity of clinical and immunological monitoring.
In conclusion, the 3 VL assays evaluated had sub-optimal performance with DBS, although performance was better than immunological or clinical monitoring. Even after the introduction of the much-anticipated point-of-care VL devices, it is expected that DBS will remain important as a complementary option for supporting access to VL monitoring, particularly in rural, resource-limited settings. Manufacturers should accelerate efforts to develop more reliable, sensitive, and specific methods to test VL on DBS specimens.
Supplementary Material
Acknowledgments
The authors would like to thank the AIDS Reference Laboratory of the University Hospital Ghent, Belgium, for their help with the specimen collection.
Footnotes
Abbreviations: ART = antiretroviral therapy, CAP/CTM = COBAS AmpliPrep/COBAS TaqMan, DBS = dried blood spot, ITM = Institute of Tropical Medicine, LOQ = limit of quantification, VL = viral load.
Funding: This work was funded by the World Health Organization.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].WHO Guidelines Approved by the Guidelines Review Committee. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva: World Health Organization; 2016. [PubMed] [Google Scholar]
- [2].Anderson AM, Bartlett JA. Changing antiretroviral therapy in the setting of virologic relapse: review of the current literature. Current HIV/AIDS Rep 2006;3:79–85. [DOI] [PubMed] [Google Scholar]
- [3].Calmy A, Ford N, Hirschel B, et al. HIV viral load monitoring in resource-limited regions: optional or necessary? Clin Infect Dis 2007;44:128–34. [DOI] [PubMed] [Google Scholar]
- [4].Hosseinipour MC, van Oosterhout JJ, Weigel R, et al. The public health approach to identify antiretroviral therapy failure: high-level nucleoside reverse transcriptase inhibitor resistance among Malawians failing first-line antiretroviral therapy. AIDS (London, England) 2009;23:1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Keiser O, Tweya H, Braitstein P, et al. Mortality after failure of antiretroviral therapy in sub-Saharan Africa. Trop Med Int Health 2010;15:251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kumarasamy N, Madhavan V, Venkatesh KK, et al. High frequency of clinically significant mutations after first-line generic highly active antiretroviral therapy failure: implications for second-line options in resource-limited settings. Clin Infect Dis 2009;49:306–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pujades-Rodriguez M, O’Brien D, Humblet P, et al. Second-line antiretroviral therapy in resource-limited settings: the experience of Medecins Sans Frontieres. AIDS (London, England) 2008;22:1305–12. [DOI] [PubMed] [Google Scholar]
- [8].Rutherford GW, Anglemyer A, Easterbrook PJ, et al. Predicting treatment failure in adults and children on antiretroviral therapy: a systematic review of the performance characteristics of the 2010 WHO immunologic and clinical criteria for virologic failure. AIDS (London, England) 2014;28suppl 2:S161–169. [DOI] [PubMed] [Google Scholar]
- [9].Roberts T, Bygrave H, Fajardo E, et al. Challenges and opportunities for the implementation of virological testing in resource-limited settings. J Int AIDS Soc 2012;15:17324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Roberts T, Cohn J, Bonner K, et al. Scale-up of routine viral load testing in resource-poor settings: current and future implementation challenges. Clin Infect Dis 2016;62:1043–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rutstein SE, Golin CE, Wheeler SB, et al. On the front line of HIV virological monitoring: barriers and facilitators from a provider perspective in resource-limited settings. AIDS Care 2016;28:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].UNAIDS, Médecins Sans Frontières. Speed up scale-up: strategies, tools and policies to get the best HIV treatment to more people sooner. Available at: https://www.msfaccess.org/sites/default/files/MSF_assets/HIV_AIDS/Docs/AIDS_report_SpeedUpScaleUp_ENG_2012.pdf Accessed 19/05/2016. [Google Scholar]
- [13].Boillot F, Serrano L, Muwonga J, et al. Implementation and operational research: programmatic feasibility of dried blood spots for the virological follow-up of patients on antiretroviral treatment in Nord Kivu, Democratic Republic of the Congo. J Acquir Immune Defic Syndr (1999) 2016;71:e9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Erba F, Brambilla D, Ceffa S, et al. Measurement of viral load by the automated Abbott real-time HIV-1 assay using dried blood spots collected and processed in Malawi and Mozambique. South African Med J 2015;105:1036–8. [DOI] [PubMed] [Google Scholar]
- [15].Johannessen A, Troseid M, Calmy A. Dried blood spots can expand access to virological monitoring of HIV treatment in resource-limited settings. J Antimicrob Chemother 2009;64:1126–9. [DOI] [PubMed] [Google Scholar]
- [16].Johannessen A. Dried blood spots in HIV monitoring: applications in resource-limited settings. Bioanalysis 2010;2:1893–908. [DOI] [PubMed] [Google Scholar]
- [17].Smit PW, Sollis KA, Fiscus S, et al. Systematic review of the use of dried blood spots for monitoring HIV viral load and for early infant diagnosis. PloS One 2014;9:e86461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Abbott RealTime HIV-1 Package insert, Available at: https://www.abbottmolecular.com/en-us/staticAssets/pdfs/us/realtime-hiv-1-package-insert.pdf Accessed 19/05/2016. [Google Scholar]
- [19].Hong F, Aga E, Cillo AR, et al. Novel assays for measurement of total cell-associated HIV-1 DNA and RNA. J Clin Microbiol 2016;54:902–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Amendola A, Marsella P, Bloisi M, et al. Ability of two commercially available assays (Abbott RealTime HIV-1 and Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 Version 2.0) to quantify low HIV-1 RNA Levels (<1,000 copies/milliliter): comparison with clinical samples and NIBSC working reagent for nucleic acid testing assays. J Clin Microbiol 2014;52:2019–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Saune K, Delaugerre C, Raymond S, et al. Analytical sensitivity of three real-time PCR assays for measuring subtype B HIV-1 RNA. J Clin Virol 2013;57:80–3. [DOI] [PubMed] [Google Scholar]
- [22].Scott L, Carmona S, Stevens W. Performance of the new Roche Cobas AmpliPrep-Cobas TaqMan version 2.0 human immunodeficiency virus type 1 assay. J Clin Microbiol 2009;47:3400–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sire JM, Vray M, Merzouk M, et al. Comparative RNA quantification of HIV-1 group M and non-M with the Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 v2.0 and Abbott Real-Time HIV-1 PCR assays. J Acquir Immune Defic Syndr (1999) 2011;56:239–43. [DOI] [PubMed] [Google Scholar]
- [24].Sollis KA, Smit PW, Fiscus S, et al. Systematic review of the performance of HIV viral load technologies on plasma samples. PloS One 2014;9:e85869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


