Supplemental Digital Content is available in the text
Keywords: introducer method, percutaneous endoscopic gastrostomy, portable endoscopy, ultrathin transnasal endoscopy
Abstract
Background:
A portable disposable ultrathin endoscope (DUE) with high visual quality and maneuverability would reduce the need for expensive facilities and emergency endoscopy could be available anywhere. It would increase patient satisfaction, prevent unnecessary sedation, and reduce infection. Our aim was to evaluate the usefulness of portable DUE in performing percutaneous endoscopic gastrostomy (PEG).
Methods:
We prospectively enrolled patients who underwent PEG under DUE guidance and compared them with historical controls who underwent PEG under conventional ultrathin endoscopy (CUE) guidance. The primary outcomes were successful stomach visualization and PEG tube insertion.
Results:
Twenty-five patients (19 male) were enrolled and compared with 25 gender and indication-matched controls. The most common indications for PEG were aspiration due to stroke or brain injury, dementia, and head and neck cancer. Entrance into the stomach was achieved in 92.0% (23/25) and 96% (24/25) in the DUE and CUE groups, and PEG was performed in 91.3% (21/23) and 95.8% (23/24), respectively. The mean insertion time for the DUE and CUE groups were 22.7 ± 9.3 minutes and 17.1 ± 5.7 minutes (P = 0.044). The 3 cases of failure to reach the stomach in both groups were caused by esophageal blockage. The 3 cases of failed PEG tube insertion were caused by poor visualization of the insertion site. Bleeding and pneumoperitoneum occurred in 1 and 2 patients in the DUE group. One case of fever was noted in the CUE group. All adverse events were conservatively managed.
Conclusions:
Our study shows that portable DUE in facilities without endoscopy equipment may be clinically feasible.
1. Introduction
Attempts have been made to develop a portable gastrointestinal endoscope.[1,2] The advantages of a portable endoscope with high visual quality and maneuverability are evident; equipment with high cost and space requirements would be unnecessary and emergency endoscopy could be available anywhere. The development of a portable disposable ultrathin endoscope (DUE) would increase patient satisfaction, prevent unnecessary sedation, and reduce infection.[3–5]
Percutaneous endoscopic gastrostomy (PEG) is commonly performed to provide long-term enteral feeding.[6,7] PEG via the introducer method is feasible, safe, and can be performed when the pull-through technique is difficult.[8] This method has the advantage of reducing postprocedural peristomal infection.[9,10] The use of an ultrathin endoscope improves the patient's comfort, reduces cardiopulmonary risk and has a high success rate.[3,4,10] PEG insertion via the introducer method requires accurate gastric visualization, making it a potential test procedure for DUE evaluation.
The E.G.Scan™ II (IntroMedic Co., Ltd, Seoul, S. Korea) is a portable DUE. It allows esophagoscopy in outpatient clinics and is safe and well-tolerated.[2] Its effectiveness has been demonstrated in esophageal disorders and gastrointestinal bleeding.[2,11–13] The advantages of this system include portability and disposability. The E.G.Scan™ II has an air insufflation function which allows visualization of the gastric walls and external compression site. Our aim was to verify if PEG can be performed under portable DUE guidance.
2. Methods
2.1. Patients
This was a prospective, open-labeled study conducted at Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea from August 2013 to December 2014. Consecutive patients referred for PEG were enrolled and compared with gender and indication-matched historical controls who had undergone PEG under conventional ultrathin endoscope (CUE) guidance. Patients who had a history of esophageal or gastric surgery, were at high risk of gastric bleeding, had mechanical ileus, had a history of PEG insertion, or did not provide written informed consent were excluded. This study was approved by the institutional review board of Seoul St. Mary's Hospital (KC13DISI0255) and registered with Clinicaltrial.gov (NCT02183207).
2.2. Portable DUE
The stomach was visualized during PEG by the E.G.Scan™ II, which comprises a disposable probe, controller, and viewing program. The disposable probe specifications of this system are as follows: an optic capsule with a 6-mm diameter and 3- to 50-mm view depth, light source of 4 white light-emitting diodes, a 3.5-mm-diameter tube with a length of 109 mm and weight of 42.5 g, a 125° view angle, and up- and down-bending angles of 160° each. The controller has free, capture, and air insertion functions. The processor is able to displace room air at a rate of 5 L/minute with a maximal air pressure of 0.45 kg/cm2. A real-time view is available via a viewing program that can record both video and still images and be set up in normal desk or laptop computers. In the present study, images were recorded at 30 frames per second with a 400 × 400-pixel resolution (Fig. 1).
Figure 1.
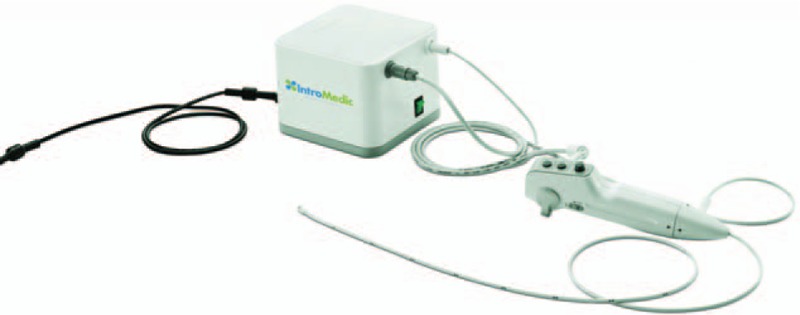
Portable disposable ultrathin endoscopy system.
2.3. PEG via the introducer method
All PEG procedures were performed by either B.M.K. or L.C.H., both certified as endoscopy experts by the Korean Society of Gastrointestinal Endoscopy. All procedures were performed free of preprocedural antibiotic administration unless the patient was taking antibiotics for another underlying disease. The procedures were performed at the endoscopy center of Seoul St. Mary's Hospital under continuous patient monitoring. PEG was carried out using an introducer PEG kit (Cliny PEG Kit; Create Medic, Yokohama, Japan) according to previously described methods.[9] Before insertion of the endoscope, 0.01 to 0.03 mg/kg of midazolam and 50 mg of pethidine were administered intravenously. Upon verification of mild to moderate sedation, 2% lidocaine jelly was applied to the nasal cavities and the DUE was inserted via the transnasal route. Upon entering the stomach, optimal PEG tube placement at the anterior wall of the lower body was determined by depressing the abdominal wall with a finger. About 10 mL of 1% lidocaine was subcutaneously injected into the region marked for PEG tube insertion. The stomach was punctured with a double-lumen gastropexy device under DUE visualization. Two sutures were placed 2 to 3 cm apart, and a 5-mm incision was made between the 2 suture points. A trocar with a plastic peel-away sheath was inserted into the stomach through the incision site. The trocar was verified with the DUE; the trocar was then removed, and a 15-Fr PEG tube was inserted via the peel-away sheath. The balloon at the end of the PEG tube was inflated with 5 mL of sterile water, and the PEG tube was pulled until appropriate approximation of the balloon to the gastric wall was achieved (Fig. 2, see Video, Supplemental Digital Content 1 which shows the PEG insertion). The peel-away sheath was removed and the retaining plate put in place. If the PEG tube insertion failed, the DUE was changed to a conventional ultrathin endoscope (CUE) (GIF-XP260N; Olympus Optical Co., Ltd, Tokyo, Japan). All cases had the gastric insertion state verified by CUE, immediately after completion of PEG insertion.
Figure 2.
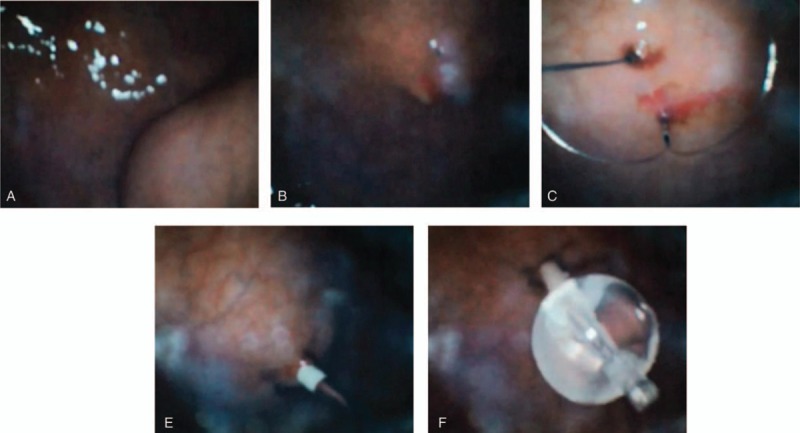
Percutaneous endoscopic gastrostomy under portable disposable ultrathin endoscopy guidance. (A) Palpation of the abdominal wall to verify the insertion site at the anterior side of the lower gastric body. (B) Insertion of the pexy device. (C) Snaring of the first suture line. (D) Insertion of the trocar. (E). Insertion and ballooning of the gastrostomy tube.
2.4. Follow-up
Enteral nutrition was initiated through the inserted PEG tube in all patients 1 day after the procedure. Laboratory parameters including the hemoglobin level, white blood cell count, and C-reactive protein level were measured on the morning of the procedure and the day afterward. On the morning after the procedure, chest and abdominal radiographs were taken to check for signs of adverse events. Body temperature was checked every 6 to 8 hours for 3 days after the procedure or until discharge. The PEG site was checked by the medical team or the home-visiting nurse for 30 days; peristomal infection was defined as pus or pus-like discharge at the insertion site.
3. Results
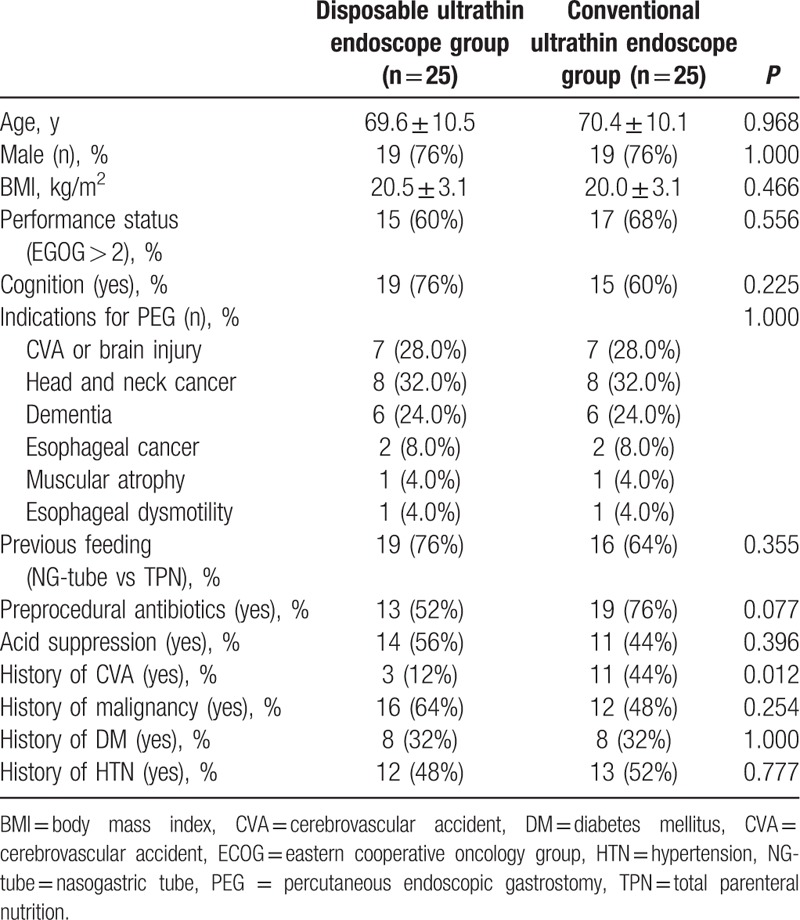
Twenty-five patients were enrolled in the DUE group and compared with 25 controls. The baseline characteristics are shown in Table 1. Successful gastric insertion was achieved in 23 patients in the DUE (92.0%) and 24 in the CUE group (96.0%). Of these, PEG insertion was successfully performed in 21 patients in the DUE (91.3%) and 23 in the CUE group (95.8%). Mean PEG insertion time was 22.7 ± 9.3 minutes (9–35 minutes) in the DUE and 17.1 ± 5.7 minutes (8–26 minutes) in the CUE group (P = 0.044).
Table 1.
Baseline characteristics of study subjects.
There were 3 cases of failed gastric insertion, 2 in the DUE and 1 in the CUE group. All were in esophageal cancer patients with a blocked lumen. PEG failed despite gastric insertion in 3 cases, all due to failure in identifying the insertion site. In the DUE group, the DUE was changed to a stiffer and brighter CUE, and PEGs were successfully achieved. In the CUE group, all failed cases were successfully converted to percutaneous gastrostomy insertion under fluoroscopy guidance.
There were 3 complications in the DUE group: 1 case of bleeding and 2 cases of pneumoperitoneum. Postprocedural bleeding occurred in an 89-year-old man who underwent PEG for aspiration due to dementia. Fresh blood was noted in the PEG tube 12 hours after insertion with a fall in the hemoglobin level from 9.6 to 6.3 mg/dL. Emergency endoscopy was performed, which found the insertion site at the anterior wall of the lower body and oozing some fresh blood. The PEG balloon was pulled up to compress the bleeding site, resulting in reduction of the oozing. After conservative treatment, enteral feeding via the PEG tube commenced on the third day without any further events.
There were 2 cases of pneumoperitoneum discovered by plain chest and abdominal X-rays. In 1 case, 1 pexy suture was cut during trocar insertion. Although the other suture was pulled up during the procedure, X-rays showed the presence of pneumoperitoneum. The other case of pneumoperitoneum was discovered by routine follow-up X-rays after the procedure. Neither patient complained of any abdominal symptoms, and no fever or peritoneal signs developed. Enteral feeding was started on the second day after PEG tube insertion. The pneumoperitoneum resolved in 2 weeks in 1 patient and 3 weeks in the other without any further events.
The CUE group had one case of fever due to a liver abscess. This was determined to be nonrelated to PEG insertion and was conservatively managed with antibiotics.
4. Discussion
The results of this study show that PEG via the introducer method may be possible under portable DUE-guided visualization. Successful visualization of the PEG tube insertion site at the anterior wall of the lower body was achieved in 91.3% (21/23) of the cases in which the DUE reached the stomach. These results suggest that endoscopic examination of the stomach could be achieved via a portable DUE.
This study also verified several advantages of the E.G.Scan™ II system. First, the anterior wall of the lower body, external compression of the gastric wall, and insertion of the pexy and trocar could be verified through the DUE. Thus, evaluation of the stomach could be possible through a DUE. Second, PEG was successful in 91.3% of the attempted cases in which the DUE reached the stomach. Successful endoscopy via a disposable portable system suggests that this procedure could be performed in centers without dedicated endoscopy and disinfection facilities such as outpatient clinics, nursing care centers, emergency rooms, and field hospitals. Third, though we did not compare the cost-effectiveness between DUE and CUE, the DUE has the potential to be financially more attractive. The DUE probe costs around $100 while a CUE costs about $700.[13,14] In case of facility costs, the DUE processing system costs about $5000 (company marketing information) while the added cost of an upper gastrointestinal endoscope, video endoscope processors/light source, and automated endoscope reprocessors range from $49,000 to 117,000.[15,16] Considering the substantial investment required for conventional endoscopy facilities, we believe that the portable and inexpensive nature of the DUE has merit in locations where funds are lacking.
In the DUE group, the stomach was not reached in 2 cases and the PEG tube insertion site was unable to be identified in another 2 cases. These occurrences suggest that improvements should be made to the E.G.Scan™ II system. The cases of failure to reach the stomach were due to esophageal cancer blocking the esophageal lumen. The DUE is very flexible, similar to a conventional nasogastric tube. As such, it is difficult for the DUE to pass through narrow obstructions, which resulted in the failure to reach the stomach in our study. Because PEG tube insertion was successfully accomplished under conversion to a CUE, which is much stiffer than a DUE, we believe that improvements in the stiffness of the DUE would result in better outcomes. One case of failure was due to the low light for visualization and the lack of a channel for suction. Although the DUE has 2 light settings, 1 bright and 1 dark, the bright setting was insufficient for observing the opposite (anterior) wall of the stomach. The patient in this case was obese and had a large stomach. When the stomach was fully distended, the pexy site could not be verified. If the light source had been brighter or a suction channel was present to reduce the distended stomach, PEG should have been possible, as was done after conversion to the CUE. The other case of failure was due to loss of gastric spatial orientation during the study. The DUE does not have up–down and left–right reference points. Because of its flexible, the scope readily rotates during insertion through the nasal and oral cavities. Upon reaching the stomach, it may be difficult to accurately assess the anatomical position. This patient had a history of a head and neck cancer operation that led to anatomical changes in the mouth and pharynx. Gastric insertion required twisting of the scope which resulted in loss of spatial orientation. This case was also successfully managed after conversion to the CUE.
There were 3 cases of adverse events in the DUE group: 1 of bleeding and 2 of pneumoperitoneum. Emergency endoscopy revealed the PEG tube insertion site at the anterior wall of the lower body, the ideal location site, where the risk of bleeding from the epiploic arteries is low. There were no signs of gastric trauma at or opposite the pexy and trocar sites. We believe that the bleeding was not due to poor visibility, but that it may have occurred regardless of type the PEG tube insertion performed. The 2 other adverse events in our study were asymptomatic pneumoperitoneum. Pneumoperitoneum has been reported in 5% to 50% of patients who underwent PEG.[17–21] While surgical intervention should be considered in patients with clinical signs of intraabdominal adverse events,[17,22] asymptomatic “benign pneumoperitoneum” after PEG is relatively common and should not warrant further intervention.[18–21]
The current portable DUE system requires some improvements. First, as mentioned above, the scope is too flexible, resulting in difficulties in scope manipulation such as passing through areas of resistance or providing torsion and rotation. Second, visibility is limited by both low light power and the lack of a cleansing channel. Insertion of the DUE through the nasal cavity can result in mucus material covering the lens. We found that copious application of a clear jelly to the lens before insertion somewhat mitigated this problem. However, too much mucus resulted in scope withdrawal and reinsertion, which could lead to loss of gastric spatial orientation. Increasing the light power or changing the light source to an optic cable as well as adding a water channel for cleansing would help to increase visibility. Third, the lack of a suction channel caused problems in controlling the gastric distension. Moreover, bloating could develop if the procedure fails or takes too long to complete. Successful insertion of the PEG tube may result in fewer bloating symptoms because PEG tubes have been used for gastrointestinal decompression.[23]
Attempts to develop a portable endoscope for evaluation of the gastrointestinal tract have been partially successful.[1,2] Most such studies have involved the esophageal tract because it is easy to access and does not require high optical quality.[1,2,12] However, the use of a portable endoscope with high visual quality and easy maneuverability has advantages: dedicated equipment with high cost and space requirements would not be needed, the endoscope would be available in cases of emergency, and examinations could be performed without moving patients. To date, studies beyond the esophagus have been limited to checking for gastrointestinal bleeding and the present study.[13] A recent study carried out with the previous version of the DUE reported that high-quality images of the duodenum were obtainable.[2] PEG was successfully carried out with the added improvements to this version, such as air insufflation and the increased bending angle. Further improvements including increased stiffness, a brighter light source, and the presence of water/suction channels are underway; these improvements would allow for the performance of true portable diagnostic endoscopy regardless of the surroundings. This would have the potential to save space and costs, limit the need to move patients, and reduce the risk of infection caused by repeated scope use.
In conclusion, PEG via the introducer method under portable DUE guidance is feasible and relatively safe, opening up the possibility of the use of the portable DUE in facilities without endoscopy equipment. Further advancements should be made to the DUE in terms of the scope stiffness, light source, and water/suction channels to improve safety and success rates. We believe that this study opens up the possibility of portable DUE becoming a clinically useful tool to endoscopists.
Acknowledgment
We would also like to thank nurses Ae Kyung Gu, Ho Jin Jang, and Won Jin Choi for their assistance with the PEG tube insertions.
This research was supported by the Medical Device Development Center under the direction of the Korea Evaluation Institute of Industrial Technology (Grant number 10049769) and the program of Global Research and Development Center through the National Research Foundation of Korea, funded by the Ministry of Science, ICT and Future Planning (Grant number NRF-2011-00316441).
Supplementary Material
Footnotes
Abbreviations: CUE = conventional ultrathin endoscope, DUE = disposable ultrathin endoscope, PEG = percutaneous endoscopic gastrostomy.
Funding: This study was supported by the Medical Device Development Center under the direction of the Korea Evaluation Institute of Industrial Technology (grant number 10049769).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Mokhashi MS, Wildi SM, Glenn TF, et al. A prospective, blinded study of diagnostic esophagoscopy with a superthin, stand-alone, battery-powered esophagoscope. Am J Gastroenterol 2003;98:2383–9. [DOI] [PubMed] [Google Scholar]
- [2].Aedo MR, Zavala-Gonzalez MA, Meixueiro-Daza A, et al. Accuracy of transnasal endoscopy with a disposable esophagoscope compared to conventional endoscopy. World J Gastrointest Endosc 2014;6:128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yuki M, Amano Y, Komazawa Y, et al. Unsedated transnasal small-caliber esophagogastroduodenoscopy in elderly and bedridden patients. World J Gastroenterol 2009;15:5586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vitale MA, Villotti G, D’Alba L, et al. Unsedated transnasal percutaneous endoscopic gastrostomy placement in selected patients. Endoscopy 2005;37:48–51. [DOI] [PubMed] [Google Scholar]
- [5].Jin P, Wang X, Yu DL, et al. Safety and efficacy of a novel disposable sheathed gastroscopic system in clinical practice. J Gastroenterol Hepatol 2014;29:757–61. [DOI] [PubMed] [Google Scholar]
- [6].Gomes CA, Jr, Lustosa SA, Matos D, et al. Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for adults with swallowing disturbances. Cochrane Database Syst Rev 2012;3:Cd008096. [DOI] [PubMed] [Google Scholar]
- [7].Loser C, Aschl G, Hebuterne X, et al. ESPEN guidelines on artificial enteral nutrition—percutaneous endoscopic gastrostomy (PEG). Clin Nutr 2005;24:848–61. [DOI] [PubMed] [Google Scholar]
- [8].Dormann AJ, Wejda B, Kahl S, et al. Long-term results with a new introducer method with gastropexy for percutaneous endoscopic gastrostomy. Am J Gastroenterol 2006;101:1229–34. [DOI] [PubMed] [Google Scholar]
- [9].Lim CH, Park JM, Cho YK, et al. Successful control of peristomal infection by introducer-type percutaneous endoscopic gastrostomy: a retrospective historical control study. Dig Dis Sci 2011;56:2024–9. [DOI] [PubMed] [Google Scholar]
- [10].Lee HS, Lim CH, Park EY, et al. Usefulness of the introducer method for percutaneous endoscopic gastrostomy using ultrathin transnasal endoscopy. Surg Endosc 2014;28:603–6. [DOI] [PubMed] [Google Scholar]
- [11].Choi JH, Choi JH, Lee YJ, et al. Comparison of a novel bedside portable endoscopy device with nasogastric aspiration for identifying upper gastrointestinal bleeding. World J Gastroenterol 2014;20:8221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lim CH, Choi MG, Baeg MK, et al. Novel disposable transnasal endoscopy for assessment of esophageal motor function. J Clin Gastroenterol 2014;48:402–6. [DOI] [PubMed] [Google Scholar]
- [13].Cho JH, Kim HM, Lee S, et al. A pilot study of single-use endoscopy in screening acute gastrointestinal bleeding. World J Gastroenterol 2013;19:103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012;143:1179–87. e1–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Desilets D, Kaul V, Tierney WM, et al. Automated endoscope reprocessors. Gastrointest Endosc 2010;72:675–80. [DOI] [PubMed] [Google Scholar]
- [16].Varadarajulu S, Banerjee S, Barth BA, et al. GI endoscopes. Gastrointest Endosc 2011;74:1.e6–6.e6. [DOI] [PubMed] [Google Scholar]
- [17].Blum CA, Selander C, Ruddy JM, et al. The incidence and clinical significance of pneumoperitoneum after percutaneous endoscopic gastrostomy: a review of 722 cases. Am Surg 2009;75:39–43. [PubMed] [Google Scholar]
- [18].Dulabon GR, Abrams JE, Rutherford EJ. The incidence and significance of free air after percutaneous endoscopic gastrostomy. Am Surg 2002;68:590–3. [PubMed] [Google Scholar]
- [19].Alley JB, Corneille MG, Stewart RM, et al. Pneumoperitoneum after percutaneous endoscopic gastrostomy in patients in the intensive care unit. Am Surg 2007;73:765–7. discussion 768. [PubMed] [Google Scholar]
- [20].Wiesen AJ, Sideridis K, Fernandes A, et al. True incidence and clinical significance of pneumoperitoneum after PEG placement: a prospective study. Gastrointest Endosc 2006;64:886–9. [DOI] [PubMed] [Google Scholar]
- [21].Rahnemai-Azar AA, Rahnemaiazar AA, Naghshizadian R, et al. Percutaneous endoscopic gastrostomy: indications, technique, complications and management. World J Gastroenterol 2014;20:7739–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nazarian A, Cross W, Kowdley GC. Pneumoperitoneum after percutaneous endoscopic gastrostomy among adults in the intensive care unit: incidence, predictive factors, and clinical significance. Am Surg 2012;78:591–4. [PubMed] [Google Scholar]
- [23].Holm AN, Baron TH. Palliative use of percutaneous endoscopic gastrostomy and percutaneous endoscopic cecostomy tubes. Gastrointest Endosc Clin N Am 2007;17:795–803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


