Abstract
The association between systolic blood pressure (SBP) and cognitive function is controversial in elderly adults. In addition, few studies focused on the cumulative effect of SBP. We aimed to investigate the association between cumulative SBP exposure and cognitive function among middle-aged and elderly adults.
The analysis was based on the Asymptomatic Polyvascular Abnormalities Community (APAC) study. The primary predictor was the cumulative SBP calculated by consecutive SBP values measured through baseline (2006–2007) up to the fourth examination (2012–2013). The cognitive function was estimated by mini-mental state examination (MMSE) in the fourth examination. Linear regression and logistic regression analyses were used to investigate the association between cumulative SBP and cognitive function.
Among 2211 participants (41.4% female, aged 40–94 years), 167 (7.55%) were diagnosed with cognitive impairment (MMSE score < 24). Higher cumulative exposure to SBP (per SD increment) was independently associated with poor cognitive performance after controlling for multiple factors (P < 0.001). We observed nondifferential association between men and women. However, higher cumulative SBP in the adults aged ≥60 years had a stronger association with poor cognitive performance compared with that in adults aged 40 to 60 years.
Greater exposure to cumulative SBP is associated with worse cognitive performance among middle-aged and elderly adults. This association is similar between men and women, but stronger in elderly adults.
Keywords: cognitive function, cumulative systolic blood pressure, mini-mental state examination
1. Introduction
The number of demented patients will grow from 24.3 million people in 2000 to 81.1 million by 2040, with 60% living in developing countries.[1] The increasing number of demented patients will bring the aging society of China a huge socioeconomic burden. To investigate modifiable risk factors of cognitive impairment is important for preventing the incidence of dementia.
However, the cognitive impairment might be a vascular impairment with neurodegenerative consequences.[2] And many cross-sectional and longitudinal studies all suggested that cardiovascular risk factors accounted for the incidence of cognitive impairment.[3–6] Systolic blood pressure (SBP) is a traditional cardiovascular risk factor. Several studies report that high level of SBP in midlife is associated with future cognitive impairment risk, whereas studies on the association for SBP in late-life give mixed results.[3,6,7] Some studies observed that high level of SBP was inversely related to the prevalence of cognitive impairment in elderly people.[7] The relationship between SBP and cognitive impairment is complex.
Furthermore, few studies research the cumulative effect of SBP in relation to cognitive function because single measures of SBP is limited to reflect the longitudinal variation and cumulative burden associated with elevated SBP level, but cumulative SBP can capture both the duration and intensity of SBP over several years.[8] In this study, we tried to investigate the relationship between cumulative SBP and cognitive function among middle-aged and elderly adults.
2. Methods
2.1. Study design and population
The data in this study were obtained from the Symptomatic Polyvascular Abnormalities Community (APAC) study, which is a prospective, community-based study, aimed to investigate the epidemiology of asymptomatic polyvascular abnormalities in Chinese adults. The methods of participant recruitment and selection have been described in detail elsewhere.[9] Briefly, a total of 5440 participants eventually enrolled from the Kailuan study,[10] following these inclusion criteria: (1) older than 40 years; (2) no history of coronary disease, transient ischemic attack, or stroke, at baseline; (3) absence of neurologic deficits suggesting previous stroke. From June 2006 to October 2007 (baseline), all participants underwent questionnaire surveys, clinical examinations and laboratory tests. And they updated these health records every 2 years. In the fourth follow-up examination (2012–2013), they underwent some further measurements including the cognitive function assessment. This study was approved by the Ethics Committee of Beijing Tiantan Hospital and Kailuan General Hospital, in compliance with the Declaration of Helsinki. All participants have signed informed consent.
2.2. Measurement of indicators
Questionnaires were administered in person by well-trained investigators at each examination. Information obtained included demographic data, medical history, behavior and habits, smoking status, physical activity, and so on. Smoking was defined as at least 1 cigarette per day for >1 year. Drinking was defined as intake of at least 100 mL of liquor (with ≥ 50% alcoholicity) per day for >1 year. Physical exercise was defined as aerobic exercise, such as walking, jogging, swimming, and so on, with ≥ 3 times per week, and ≥ 30 minutes every time. The body mass index (BMI) was calculated as body weight (kg) divided by the square of body height (m2). Hypertension was defined as self-reported history of hypertension, or SBP ≥140 mm Hg or diastolic blood pressure (DBP) ≥90 mm Hg. Diabetes was defined as a fasting blood glucose (FBG) level ≥ 7.0 mmol/L or self-reported history of diabetes. Hyperlipidemia was defined as low-density lipoprotein-cholesterol (LDL-c) ≥130 mg/dL, or high-density lipoprotein <40 mg/dL, or triglycerides (TG) ≥150 mg/dL, or self-reported history of hyperlipidemia. The collection of fasting blood specimens has been described previously.[9,11]
2.3. Calculation of cumulative SBP
Blood pressure (BP) was measured in the office. Before measuring, participants were forbade to smoke or drink tea or coffee for 30 minutes, and requested to rest in a chair for 15 minutes. We measured right brachial artery BP using a calibrated mercury sphygmomanometer for 3 times, and each measurement separated by 1 to 2 minute interval. Then, we took the average value as the final BP.
Cumulative SBP is defined as the sum of products of average BP in 2 consecutive follow-up checkups multiplied by the follow-up interval. The formula is: cumulative SBP = [(SBP06 + SBP08) /2 × time06–08] + [(SBP08 + SBP10) /2 × time08–10] + [(SBP10 + SBP12) /2 × time10–12]. “SBP06, SBP08, SBP10, SBP12” respectively represented the SBP measured in 2006–2007, 2008–2009, 2010–2011, and 2012–2013, and “‘time” represented the follow-up interval between the 2 measurements of SBP. We stratified participants into 4 groups according to cumulative SBP [12]: <744 mm Hg(120 mm Hg × 6.2 y), 744–806 mm Hg (≥120–130 mm Hg × 6.2 y), 806–868 mm Hg (130–140 mm Hg × 6.2 y), ≥ 868 mm Hg (≥140 mm Hg × 6.2 y). The “6.2 y” represented the average follow-up interval.
2.4. Assessment of cognitive function
Cognitive function was measured using the Mini-Mental State Examination (MMSE) in the fourth follow-up examination from 2012 to 2013. The MMSE assesses orientation to time and place, attention, calculation and construction, learning, language, and memory, with a range of 0 to 30.[13] Higher scores indicate better performance. A score of <24 was defined as cognitive impairment in the present study.
2.5. Statistical analyses
Statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC). Data are described as means ± SD for continuous variables and frequencies or percentages for categorical variables. Baseline variables among different groups of cumulative SBP were compared by the chi-square test for categorical variables and ANOVA or the Kruskal–Wallis test for continuous variables. The association between cumulative SBP and MMSE score was analyzed using a multivariate linear regression. The association between cumulative SBP and cognitive impairment (MMSE score < 24) was analyzed using a multiple logistic regression analysis. In order to adjust for all potential confounders, 3 models were used. Model 1 was the unadjusted. Model 2 was adjusted for age and gender. Model 3 was further adjusted for age, gender, SBP at baseline, BMI, FBG, LDL-c, HDL-c, TC, TG, C-reactive protein (CRP), smoking, drinking, physical activity, hypertension, diabetes, hyperlipidemia, antihypertensive treatment, hypoglycemic treatment, and lipid-lowering treatment. The multivariate linear regression analysis using Model 3 was performed stratified by the gender and the age (40–60 years and ≥ 60 years), respectively. A P-value <0.05 (2-sided) was considered significant.
3. Results
From the APAC study, we excluded 1835 participants for any time without BP data in the 4 physical examinations, and 1394 participants without MMSE measurements or with incomplete MMSE information in the fourth examinations. Eventually, 2211 participants (41.4% female, aged 40–94 years) were eligible for inclusion in this study.
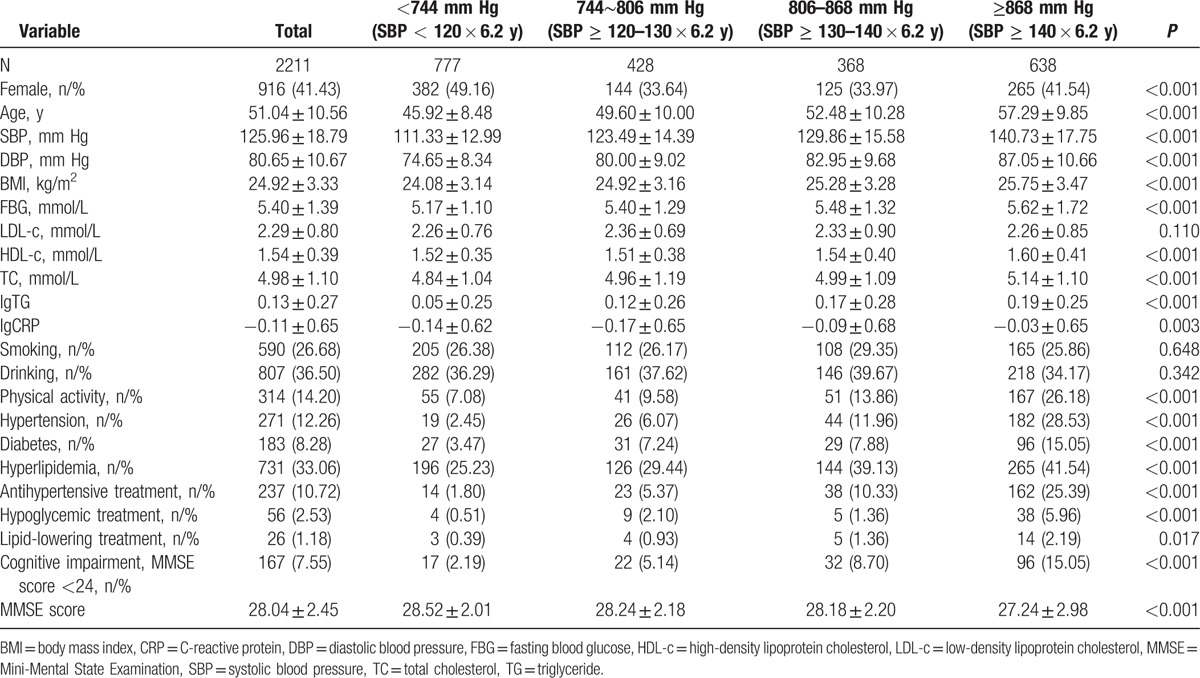
Table 1 showed the demographic and baseline characteristics for different cumulative SBP groups. For the 2211 participants, the mean age was 51.04 ± 10.56 years. Participants in the group of cumulative SBP ≥ 868 mm Hg were older and have a higher SBP, DBP, BMI, FBG, HDL-c, and TC (all P < 0.001). Different cumulative SBP groups differed significantly by gender, physical activity, hypertension, diabetes, hyperlipidemia, antihypertensive treatment, hypoglycemic treatment, and lipid-lowering treatment (all P < 0.05). Participants in the higher cumulative SBP group performed with lower MMSE score (P < 0.001). Furthermore, the rate of cognitive impairment showed higher in the higher cumulative SBP groups (P < 0.001).
Table 1.
Demographic and baseline characteristics of different groups of cumulative systolic blood pressure.
Using the Spearman rank correlation test, cumulative SBP negatively correlated with MMSE score (r = –0.144, P < 0.001). The multivariate linear regression analysis showed that cumulative SBP was independently negatively correlated with MMSE score, after adjustment of age, gender, SBP at baseline, BMI, FBG, LDL-c, HDL-c, TC, TG, CRP, smoking, drinking, physical activity, hypertension, diabetes, hyperlipidemia, antihypertensive treatment, hypoglycemic treatment, and lipid-lowering treatment (β = −0.36, P < 0.001; Table 2). Gender showed no interaction terms with cumulative SBP (P- interaction = 0.613). However, we observed the significant age interaction for cumulative SBP (P-interaction < 0.001). In the stratified analysis by age, cumulative SBP has a stronger association with MMSE score in participants ≥ 60 years than that in those aged 40–60 years (≥ 60 years, β = −1.01, 95% CI: −1.49 to −0.51, P < 0.001; 40–60 years, β = −0.45, 95% CI: −0.60 to −0.31, P < 0.001). For every SD (129.09 mm Hg) increases, the MMSE score decreased by 1.01 in participants ≥ 60 years, and 0.45 in participants aged 40 to 60 years.
Table 2.
Multivariate linear regression analysis of association between cumulative systolic blood pressure∗ and Mini-Mental State Examination score.
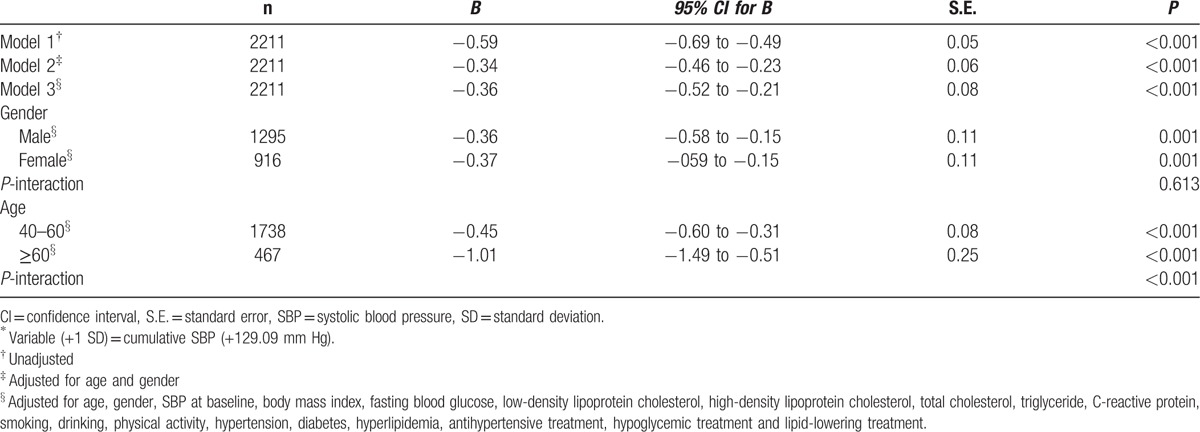
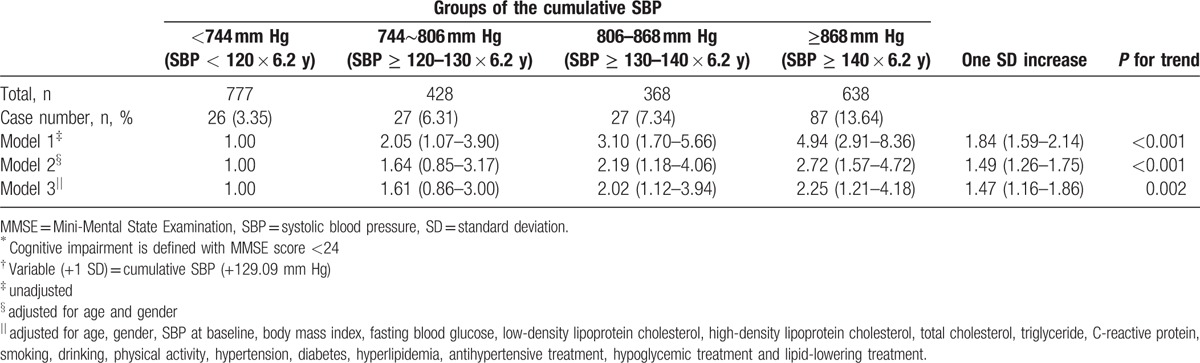
Table 3 shows multiple logistic regression analysis of associations between cumulative SBP and cognitive impairment (MMSE score < 24). Cumulative SBP was positively associated with incident cognitive impairment in logistic models unadjusted or adjusted only for age and gender (Model 1 and Model 2). The association was also positive and statistically significant after adjustment of all the same confounders used in the linear regression Model 3 (OR: 1.47; 95% CI: 1.16∼1.86; P< 0.05). The risk of cognitive impairment increases by 1.47 times for every SD (129.09 mm Hg) increases in cumulative SBP. Compared with the group of cumulative SBP <744 mm Hg, groups of higher cumulative SBP had the higher risk of cognitive impairment (P for trend = 0.002).
Table 3.
Odds ratios and 95% confident intervals of cognitive impairment∗ according to the cumulative systolic blood pressure† in multiple stepwise logistic regression analysis.
4. Discussion
In this study, we found that there was a significant relationship between cumulative SBP and cognitive function among middle-aged and elderly adults. In particular, greater exposure to cumulative SBP was associated with worse cognitive performance in a period of mean 6 years. This association was similar between men and women, and was stronger in adults ≥ 60 years.
A number of studies have reported the association between SBP and cognitive function.[3,6,14–16] The most consistent findings have been between elevated SBP and cognitive impairment in middle-aged populations. Several longitudinal, population-based studies have shown that high SBP in midlife modestly or significantly increased the risk of dementia.[3,6,14] Studies on long-term trajectories of SBP also suggested that adults who developed dementia had a steep increase in SBP from midlife to late life.[16,17] In the present study, we further confirmed the association among middle-aged and elderly people in a Chinese community-based cohort. Moreover, we repeated measures of SBP and evaluated the cumulative effect of SBP. Our findings emphasized the effect of duration of SBP exposure on cognitive function.
Previous studies that investigated the association between SBP and cognitive function have been less consistent in elderly populations. A longitudinal study reported that the high level of SBP significantly increased the risk of dementia in the 70-year-old study population.[6] Similarly, results of our study suggested that higher cumulative SBP was associated with future cognitive impairment; in addition, the association was stronger in elderly adults than that in middle-aged adults. However, a previous cross-sectional study observed that the high level of SBP was inversely related to the prevalence of dementia in elderly people.[7] Studies on long-term trajectories of SBP also revealed that adults who developed dementia had a decrease in SBP in late life and before the dementia diagnosis.[16,17] Reasons for such results are explained as that the low BP is probably a complication of dementia process,[7] and the BP closer to the age of dementia onset may just reflect the natural neurodegenerative processes.[8,15] The accurate acknowledge for the relationship of SBP and cognitive function needs to be updated by further investigations.
A potential difference of effect by gender in association between BP and cognitive function has been reported.[18,19] Thus, several studies that investigated this association included women and men separately.[6,16,17] Most of these studies have found that high SBP is associated with cognitive impairment in both men and women, and some studies suggested a stronger association in men.[5] A prospective study that consisted of postmenopausal women reported that high BP was not independently associated with cognitive impairment.[20] In our study, we found a similar association of cumulative SBP and cognitive function between men and women.
The mechanism of higher BP in relation with cognitive impairment was hypothesized based on some longitudinal neurological imaging studies. It was demonstrated that higher BP may accelerate the risk of structural brain changes, including both atrophy and infarcts, which were associated with the cognitive impairment.[21,22] Additionally, both atrophic and ischemic changes were driven by altered BP control beginning in midlife,[22] suggesting that the impairment of BP for cognitive function is a long-term process. Moreover, the impact of elevated BP on vascular dementia was also explained by subcortical white matter damage.[23,24] Studies have found that systemic hypertension related to accrual of white matter hyperintensity and functional decline.[25,26]
There are some limitations to consider. First, the Kailuan cohort is based on the community located in Tangshan, which is a representative industrial city in the north of China. The results may not be as generalizable to the whole Chinese population or other race group. Second, the follow-up period in our study is shorter compared with previous studies.[4,5,23] Dementia mostly occurs in old age, but the process to dementia starts early in adulthood.[22] Longitudinal studies of the cumulative SBP in relation to cognitive function are needed. Third, the number of adults aged ≥ 60 years was small, which might result in low statistical power. Fourth, we took BP measurement in the office, and did not perform the ambulatory BP monitoring. However, ambulatory BP is a better predictor of cognitive impairment than the office BP measured at a time point. But we still have some strengths. In the present study, we used the cumulative value to accurately capture the longitudinal exposure of SBP, and confirmed the relationship of cumulative SBP and cognitive function. The results indicated that long-term efforts made to reduce the SBP are necessary for delaying or preventing the development of cognitive impairment.
5. Conclusions
Among the middle-aged and elderly people in China, greater exposure to cumulative SBP is associated with worse cognitive performance. And this association is similar between men and women, but stronger in elderly adults. This emphasizes the importance of long-term controlling SBP level to prevent cognitive impairment, especially in the elderly adults.
Acknowledgments
The authors thank the participants and their relatives in the study and the members of the survey teams in the 11 regional hospitals of the Kailuan Medical Group, as well as to the project development and management teams in Beijing Tiantan hospital and the Kailuan Group.
Footnotes
Abbreviations: APAC = Asymptomatic Polyvascular Abnormalities Community, BMI = body mass index, BP = blood pressure, CRP = C-reactive protein, DBP = diastolic blood pressure, FBG = fasting blood glucose, HDL-c = high-density lipoprotein cholesterol, LDL-c = low-density lipoprotein cholesterol, MMSE = Mini-Mental State Examination, SBP = systolic blood pressure, TC = total cholesterol, TG = triglycerides.
JL, YH and GC equally contributed to this study.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005;366:2112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tolppanen AM, Solomon A, Soininen H, et al. Midlife vascular risk factors and Alzheimer's disease: evidence from epidemiological studies. J Alzheimers Dis 2012;32:531–40. [DOI] [PubMed] [Google Scholar]
- [3].Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ 2001;322:1447–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Whitmer RA, Sidney S, Selby J, et al. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 2005;64:277–81. [DOI] [PubMed] [Google Scholar]
- [5].Kimm H, Lee PH, Shin YJ, et al. Mid-life and late-life vascular risk factors and dementia in Korean men and women. Arch Gerontol Geriatr 2011;52:e117–22. [DOI] [PubMed] [Google Scholar]
- [6].Ronnemaa E, Zethelius B, Lannfelt L, et al. Vascular risk factors and dementia: 40-year follow-up of a population-based cohort. Dement Geriatr Cogn Disord 2011;31:460–6. [DOI] [PubMed] [Google Scholar]
- [7].Guo Z, Viitanen M, Fratiglioni L, et al. Low blood pressure and dementia in elderly people: the Kungsholmen project. BMJ 1996;312:805–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yaffe K, Vittinghoff E, Pletcher MJ, et al. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation 2014;129:1560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhou Y, Li Y, Xu L, et al. Asymptomatic polyvascular abnormalities in community (APAC) study in China: objectives, design and baseline characteristics. PLoS One 2013;8:e84685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang A, Chen S, Wang C, et al. Resting heart rate and risk of cardiovascular diseases and all-cause death: the Kailuan study. PLoS One 2014;9:e110985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wu J, Zhang Q, Yang H, et al. Association between non-high-density-lipoprotein-cholesterol levels and the prevalence of asymptomatic intracranial arterial stenosis. PLoS One 2013;8:e65229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zemaitis P, Liu K, Jacobs DR, Jr, et al. Cumulative systolic BP and changes in urine albumin-to-creatinine ratios in nondiabetic participants of the multi-ethnic study of atherosclerosis. Clin J Am Soc Nephrol 2014;9:1922–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- [14].Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging 2000;21:49–55. [DOI] [PubMed] [Google Scholar]
- [15].Power MC, Weuve J, Gagne JJ, et al. The association between blood pressure and incident Alzheimer disease: a systematic review and meta-analysis. Epidemiology 2011;22:646–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stewart R, Xue QL, Masaki K, et al. Change in blood pressure and incident dementia: a 32-year prospective study. Hypertension 2009;54:233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Joas E, Backman K, Gustafson D, et al. Blood pressure trajectories from midlife to late life in relation to dementia in women followed for 37 years. Hypertension 2012;59:796–801. [DOI] [PubMed] [Google Scholar]
- [18].Dufouil C, Seshadri S, Chene G. Cardiovascular risk profile in women and dementia. J Alzheimers Dis 2014;42suppl 4:S353–63. [DOI] [PubMed] [Google Scholar]
- [19].Chene G, Beiser A, Au R, et al. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement 2015;11:310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Johnson KC, Margolis KL, Espeland MA, et al. A prospective study of the effect of hypertension and baseline blood pressure on cognitive decline and dementia in postmenopausal women: the Women's Health Initiative Memory Study. J Am Geriatr Soc 2008;56:1449–58. [DOI] [PubMed] [Google Scholar]
- [21].Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 2011;77:461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Knopman DS, Penman AD, Catellier DJ, et al. Vascular risk factors and longitudinal changes on brain MRI: the ARIC study. Neurology 2011;76:1879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Venkat P, Chopp M, Chen J. Models and mechanisms of vascular dementia. Exp Neurol 2015;272:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol 2014;88:640–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].White WB, Marfatia R, Schmidt J, et al. INtensive versus standard ambulatory blood pressure lowering to prevent functional DeclINe in the ElderlY (INFINITY). Am Heart J 2013;165:258–65.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Abraham HM, Wolfson L, Moscufo N, et al. Cardiovascular risk factors and small vessel disease of the brain: blood pressure, white matter lesions, and functional decline in older persons. J Cereb Blood Flow Metab 2016;36:132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]


