Abstract
The aim of the study was to evaluate dynamic contrast-enhanced breast magnetic resonance imaging (DCE-MRI) features for the prediction of early and late recurrences in patients with breast cancer.
Of 1030 breast cancer patients who underwent surgery at our hospital from January 2007 to July 2011, 83 recurrent breast cancer patients were enrolled in this study. We compared MRI features (background parenchymal enhancement [BPE], internal enhancement, adjacent vessel sign, whole-breast vascularity, initial enhancement pattern, kinetic curve types, and quantitative kinetic parameters) and clinico-pathologic variables (age, stage, histologic grade, nuclear grade, existence of lymphovascular invasion and extensive intraductal carcinoma component, and immunohistochemical profiles) between patients with early (≤2.5 years after surgery) and late recurrence (>2.5 years after surgery). Cox proportional hazard regression analysis was performed to evaluate independent risk factors for early and late recurrence.
On breast MRI, prominent ipsilateral whole-breast vascularity was independently associated with early recurrence (hazard ratio [HR], 2.86; 95% confidence intervals [CI], 1.39–5.88) and moderate or marked BPE (HR, 2.08; 95% CI, 1.04–4.18) and rim enhancement (HR, 2.14; 95% CI, 1.00–4.59) were independently associated with late recurrence. Clinico-pathologic variables independently associated with early recurrence included negative estrogen receptor (HR, 0.53; 95% CI, 0.29–0.96), whereas T2 stage (HR, 2.08; 95% CI, 1.04–4.16) and nuclear grade III (HR, 2.54; 95% CI, 1.29–4.98) were associated with late recurrence.
In DCE-MRI, prominent ipsilateral whole-breast vascularity, moderate or marked BPE, and rim enhancement could be useful for predicting recurrence timing in patients with breast cancer.
Keywords: breast cancer, image enhancement, magnetic resonance imaging, neoplasm recurrence
1. Introduction
Breast cancer is one of the most common causes of cancer-related death in women worldwide.[1] Despite advances in early diagnosis, treatment, and biomarker identification of this cancer, ∼20% to 30% of breast cancer patients experience recurrence and substantially worse overall survival.[2,3] Therefore, it is critical to identify risk factors that can predict breast cancer recurrence.
Angiogenesis is a requirement of neoplastic growth, progression, and metastasis. For breast cancer, contrast-enhanced breast magnetic resonance imaging (MRI) is a good imaging modality to depict tumor angiogenesis because the contrast enhancement pattern of the tumor correlates with microvessel density characteristic of tumor angiogenesis.[4,5] In addition, dynamic contrast-enhanced MRI (DCE-MRI) features such as peripheral rim enhancement, faster enhancement, and washout curve type are correlated with overall recurrence and overall survival of breast cancer patients.[6,7]
Determining predictive factors for early and late recurrences of breast cancer has become increasingly important, considering that ∼70% of early recurrence occurs within 3 years of diagnosis and patients with early recurrence experience shorter median survival and more aggressive course than those with late recurrence.[8] Additionally, understanding when breast cancer may recur has relevance for clinicians in selecting adjuvant treatment options.[8–10] For example, in estrogen receptor (ER)-positive breast cancers, which are associated with late recurrence, selection of tamoxifen-aromatase inhibitor therapy as adjuvant endocrine therapy can improve disease-free survival and overall survival.[11] Patients with breast cancer showing features associated with early recurrence would require chemotherapy.[12] Previous studies have found that clinico-pathologic factors such as N stage, histologic grade, nuclear grade, p53, Ki-67, human epidermal growth factor receptor 2 (HER-2), and ER are associated with the time of recurrence.[13,14] To the best of our knowledge, however, DCE-MRI features associated with the timing of breast cancer recurrence have not yet been investigated.
The aim of the current study was to evaluate DCE-MRI features for the prediction of early and late recurrences in patients with breast cancer. We hypothesized that identifying DCE-MRI features predictive of early and late recurrence may help clinicians establish protocol for follow-up and predict prognosis in patients with recurrent breast cancers even before surgery.
2. Materials and methods
2.1. Study population
This retrospective study was approved by the institutional review board of our hospital, and the requirement for informed consent was waived. From January 2007 to July 2011, 1030 consecutive women underwent completion of curative surgery for breast cancer at our hospital. After surgery, adjuvant therapy such as radiation therapy, chemotherapy, or hormonal therapy was administered according to the patient's condition and clinical and molecular characteristics of the tumor. Among this population, a total of 602 women were enrolled in this study (Fig. 1). The inclusion criteria were as follows: (a) patients had pathologically confirmed breast cancer; (b) patients had no history of cancer in the breast or at any other site; (c) patients had preoperative breast MR imaging available; and (d) patients had no distant metastasis at the time of diagnosis. The remaining 428 women who underwent excisional or vacuum-assisted biopsy prior to preoperative MR imaging (n = 163), had received neoadjuvant chemotherapy (n = 92), had clinical or pathological T4 cancer (n = 81), or were lost to follow-up (n = 92) were excluded from this study.
Figure 1.
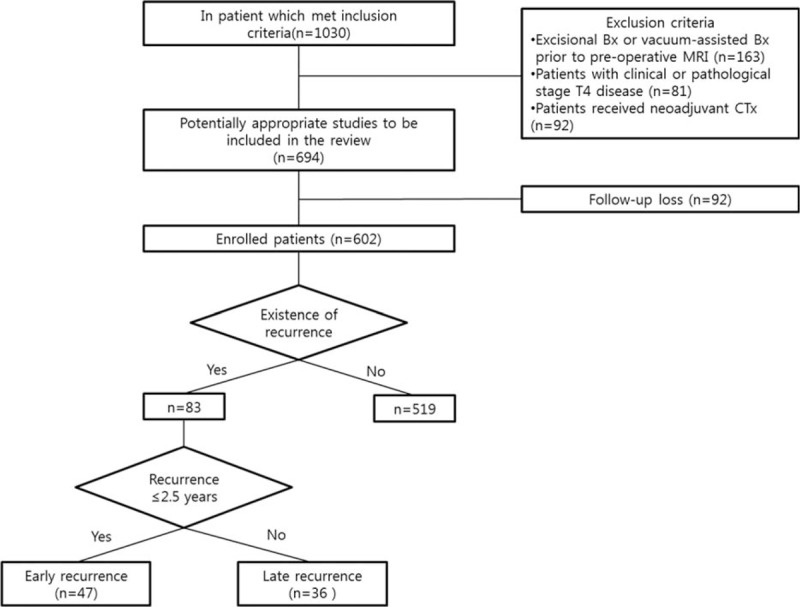
Flowchart of 1030 patients with breast cancer. Bx = biopsy, CTx = chemotherapy.
Breast cancer recurrence was defined as either loco-regional (limited to the ipsilateral breast or chest wall and/or axillary, infraclavicular, or supraclavicular lymph nodes) or distant (metastasis to other parts of the body). Follow-up was scheduled every 6 months during the first 2 years after surgery and annually beginning the third year as part of the routine clinical standard of care at our institution. All patients were examined via bilateral mammography and bilateral whole-breast ultrasonography using hand-held ultrasound for the surveillance of loco-regional recurrence. Chest radiography, chest computed tomography, bone scan, and/or whole-body fluorine 18 fluorodeoxyglucose positron emission tomography were performed for the surveillance of distant metastases. Once lesions suspicious for recurrence were detected on the follow-up imaging, fine-needle aspiration, core needle biopsy, vacuum-assisted biopsy, or excisional biopsy was performed to confirm the recurrence. If no histopathological result was obtained, follow-up imaging was performed to evaluate interval growth or additional lesions suspicious for recurrence.
2.2. Breast MRI examination technique
All breast MRI examinations were performed using a 1.5-T system (Symphony; Siemens Healthcare, Erlangen, Germany) with a dedicated breast coil. Fat-suppressed T2-weighted spin-echo sagittal images were obtained by using the following image parameters: repetition time ms/echo time ms, 2900/150; matrix, 320 × 240; field of view, 200 × 200 mm; section thickness, 3 mm without gaps. Subsequently, a 3-dimensional fast low-angle shot series was acquired (repetition time ms/echo time ms, 11.0/4.5; matrix, 320 × 221; flip angle, 25°; field of view, 200 × 200 mm; section thickness, 2 mm without gaps) was performed with 1 precontrast and 5 postcontrast dynamic series obtained immediately after intravenous administration of a bolus injection of 0.1 mmol/kg gadobutrol (Gadovist; Schering AG, Berlin, Germany) followed by a 20-mL saline flush at an injection rate of 3 mL/s using an automatic injector. Standard subtraction images were obtained by subtracting the precontrast images from the second dynamic series (or early peak) of postcontrast images on a pixel-by-pixel basis. In addition, maximum-intensity-projection reconstructions were applied to the subtraction images.
2.3. Breast MRI analysis
Two radiologists with 3 and 5 years of experience in breast MR imaging blinded to clinical information other than breast cancer independently reviewed DCE-MRI of the index lesion on a picture archiving and communication system workstation monitor (m-view; Marotech, Seoul, South Korea). In patients with multifocal or multicentric disease, the largest focus/center was evaluated. The radiologists analyzed background parenchymal enhancement (BPE) (minimal/mild or moderate/marked) and internal enhancement (nonrim or rim) of the index cancer according to the second edition of Breast Imaging Reporting and Data System (BI-RADS) MRI lexicon.[15] For adjacent vessel signs on subtracted images, the presence of vessels either entering the enhancing lesion or in contact with the lesion edge was accepted as a positive adjacent vessel sign.[16] Whole-breast vascularity of the ipsilateral breast with cancer was compared with that of the contralateral breast at each maximum-intensity-projection images on the basis of the number of vessels that were 3 cm or longer in length and 2 mm or larger in maximal transverse diameter.[17] The degree of vascularity difference was classified as “prominent” if the number of vessels in the ipsilateral breast was 3 or more than that of the contralateral breast; “moderate” if higher by 2; “mild” if higher by 1; and “not increased” if the number of vessels in the ipsilateral breast was the same as or lower than that of the contralateral breast.[18] Time-intensity curves of index cancers were retrospectively generated by each radiologist with dedicated DCE-MRI software (Mean curve: Siemens Healthcare, Erlangen, Germany). A region of interest (ROI) was placed on the fastest-enhancing area or the area showing the most suspicious washout curve pattern of the lesion. For kinetic curve assessment, the initial enhancement pattern and kinetic curve pattern were classified as slow, medium, or rapid and as persistent, plateau, or washout, respectively.[15] In cases in which there was discordance between the radiologists’ assessments of DCE-MRI features of BPE, internal enhancement, adjacent vessel sign, whole-breast vascularity, and kinetic curve pattern, consensus was reached.
Quantitative kinetic parameters were derived from time-intensity curve images. For each curve, the initial enhancement percentage (E1), peak enhancement percentage (Epeak), and time to peak enhancement (TTP) were measured as follows:[19]E1 = 100×(S1 – S0)/S0, Epeak = 100 × (Speak – S0)/S0, where E1 is the initial percentage enhancement, Epeak is the peak percentage enhancement, S1 is the signal intensity in the ROI at the first contrast-enhanced point, Speak is the peak signal intensity, and S0 is the unenhanced signal intensity in the ROI. Time to peak enhancement is the time in seconds between injection of contrast material and the peak of the signal intensity-time curve. The signal enhancement ratio (SER) was calculated as a measure of washout as follows:[20] SER = (S1 – S0)/(Slast – S0), where Slast is the signal intensity in the ROI at the last point of contrast enhancement.
2.4. Clinico-pathologic features
After review of patient medical records, we compiled data on patient age, tumor staging, axillary node status, histologic grade, nuclear grade, existence of lymphovascular invasion and an extensive intraductal carcinoma component, and ER, progesterone receptor (PR), Ki-67, HER-2, p53, and cytokeratin-5 expression. ER and PR positivity was defined as the presence of 10% or more positively stained nuclei in 10 high-power fields. Ki-67 labeling was defined as negative (<14%) or positive (≥14%). The intensity of HER-2 staining was semiquantitatively scored as 0, 1 +, 2 +, or 3 +. Tumors with a 3 + score were classified as HER-2-positive and tumors with a 0 or 1+ score were classified as HER-2-negative. In tumors with a 2+ score, gene amplification with fluorescence in situ hybridization was used to determine HER-2 status. HER-2 expression was considered positive if the ratio of HER-2 gene chromosome 17 signals was >2.2. The molecular subtype of the tumor was classified into luminal (hormonal receptor positive and HER-2 negative), triple negative (normal receptor negative and HER-2 negative), and HER-2 enriched (HER-2 positive).
2.5. Breast cancer recurrence
The medical records of all patients were reviewed to document early and late recurrence of breast cancer. Recurrence time was calculated from the date of surgery to the date of first recurrence diagnosis. Patients with recurrence were divided into 2 groups according to the time of recurrence: early (≤2.5 years after surgery) and late (>2.5 years after surgery). The cut-off of 2.5 years was selected for stratification because it is widely recognized that the peak time to recurrence for breast cancer is 2 to 3 years after diagnosis[21,22]
2.6. Statistical analysis
The demographic characteristics was compared between the group of loss to follow-up (n = 92) and the enrolled group (n = 602). To compare DCE-MRI and clinico-pathologic features between early and late recurrence and clinico-pathologic features between the group of loss to follow-up and the enrolled group, χ2 or Fisher's exact test was used for categorical variables and the Wilcoxon signed rank test was used for continuous variables. The recurrence hazard rate was calculated by a kernel smoothing method utilizing the Nelson–Aalen estimates. Recurrence-free survival was measured from the date of surgery to the earliest date of recurrence. All observations of patients without cancer recurrence were censored on the date of the last follow-up or the date of nonbreast cancer death. Univariate analysis was performed to compare DCE-MRI and clinico-pathologic features between no recurrence and early or late recurrence groups using the log-rank test. Univariate and multivariate Cox proportional hazard regression analyses were performed to evaluate independent risk factors for disease progression. All continuous covariates except age were logarithmically transformed to minimize the influence of extreme observations. Variables with P values < 0.2 on univariate analysis were entered as input variables for multivariate models. The area under the receiver operating characteristic (ROC) curves (AUC) was calculated to measure and compare the predictability of recurrence using DCE-MRI features and clinico-pathologic factors selected in multivariate analyses. All statistical analyses were performed using SAS (version 9.3; SAS Institute, Cary, NC). Two-tailed P < 0.05 was considered statistically significant.
3. Results
3.1. General characteristics of patients and survival outcomes
In demographic characteristics, no significant difference was found between the group of loss to follow-up and the enrolled group except patient age. The mean age of the follow-up loss group (54.5 ± 11.8 years) was significantly higher than that of enrolled group (50.9 ± 10.4 years) (P = 0.003). Among the 602 women eligible for analysis in this study, breast cancer recurred in 83 (13.8%) during the follow-up period (median 62.3 months; range, 7.2–99.6 months): early recurrence in 47 (median 16.5 months; range, 4.0–30.0 months) and late recurrence in 36 (median 61.4 months; range 31.2–93.6 months). The mean age at the time of diagnosis was 50.9 years (range 26–80 years). Median recurrence-free survival was 58.9 months (range 4.0–99.6 months), and the median overall survival was 59.9 months (range 5.8–99.6 months). The shape of the annual recurrence hazard curve over time reveals the dynamics of recurrence (Fig. 2).
Figure 2.
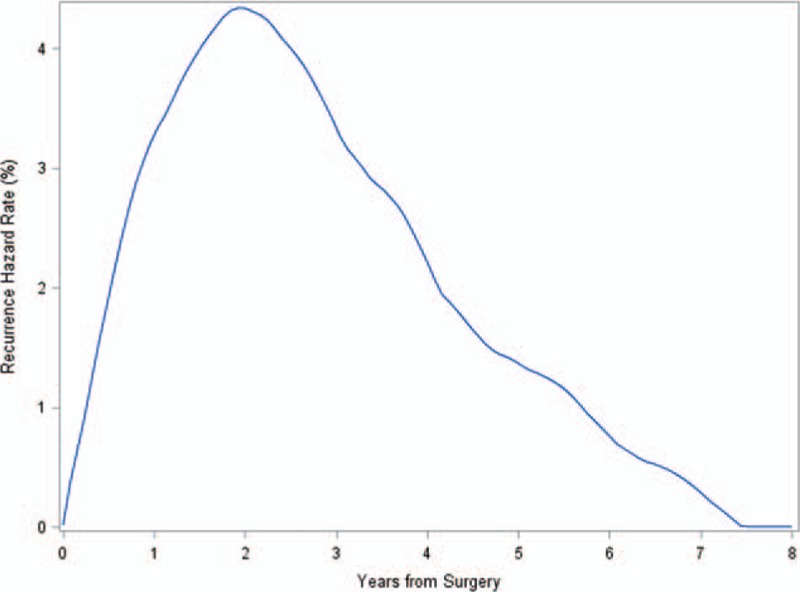
The annual recurrence hazard rate for 602 breast cancer patients. The hazard rates described demonstrate hazard of recurrence for each 1-year interval.
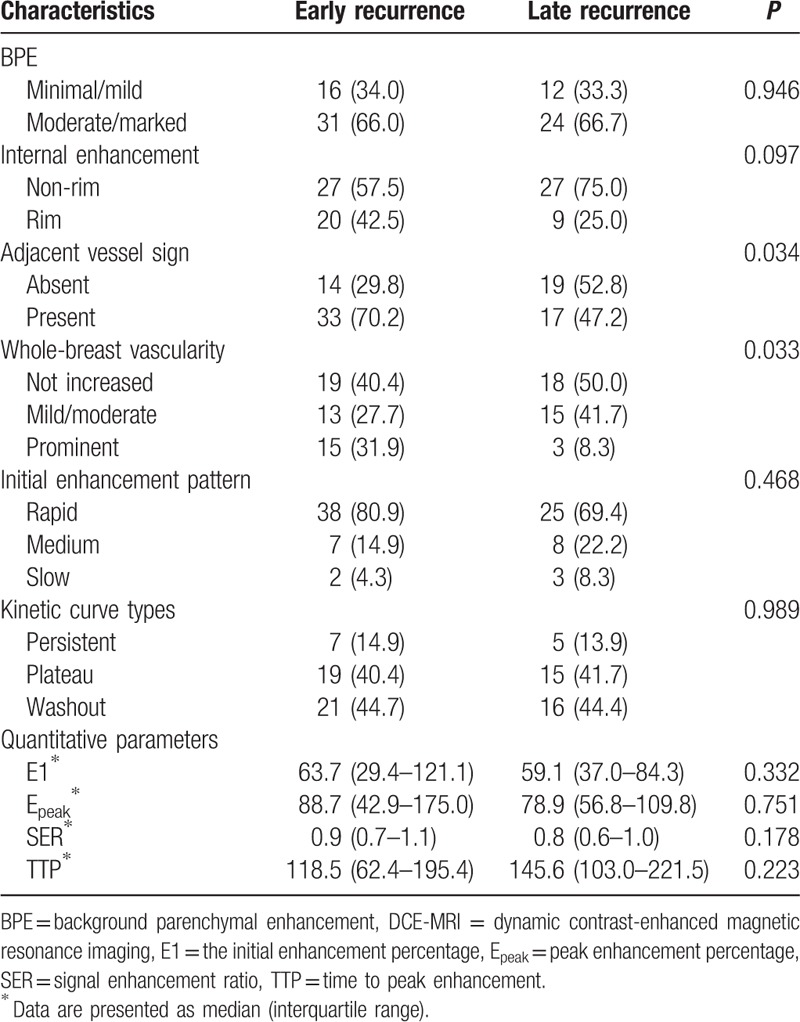
For clinico-pathologic variables (Table 1), only HER-2 status significantly differed between early and late recurrence. The proportion of negative HER-2 tumors in early recurrence (70.2%) and positive HER-2 tumors in late recurrence (55.6%) was significantly higher (P = 0.018). For DCE-MRI features (Table 2), adjacent vessel sign and whole-breast vascularity significantly differed between early and late recurrence. The proportion of adjacent vessel sign in early recurrence (70.2%) was higher than that in late recurrence (47.2%) (P = 0.034). The proportion of prominent increased whole-breast vascularity in early recurrence (31.9%) was higher than that of late recurrence (8.3%) (P = 0.033).
Table 1.
Comparison of clinico-pathologic characteristics between early and late recurrence.
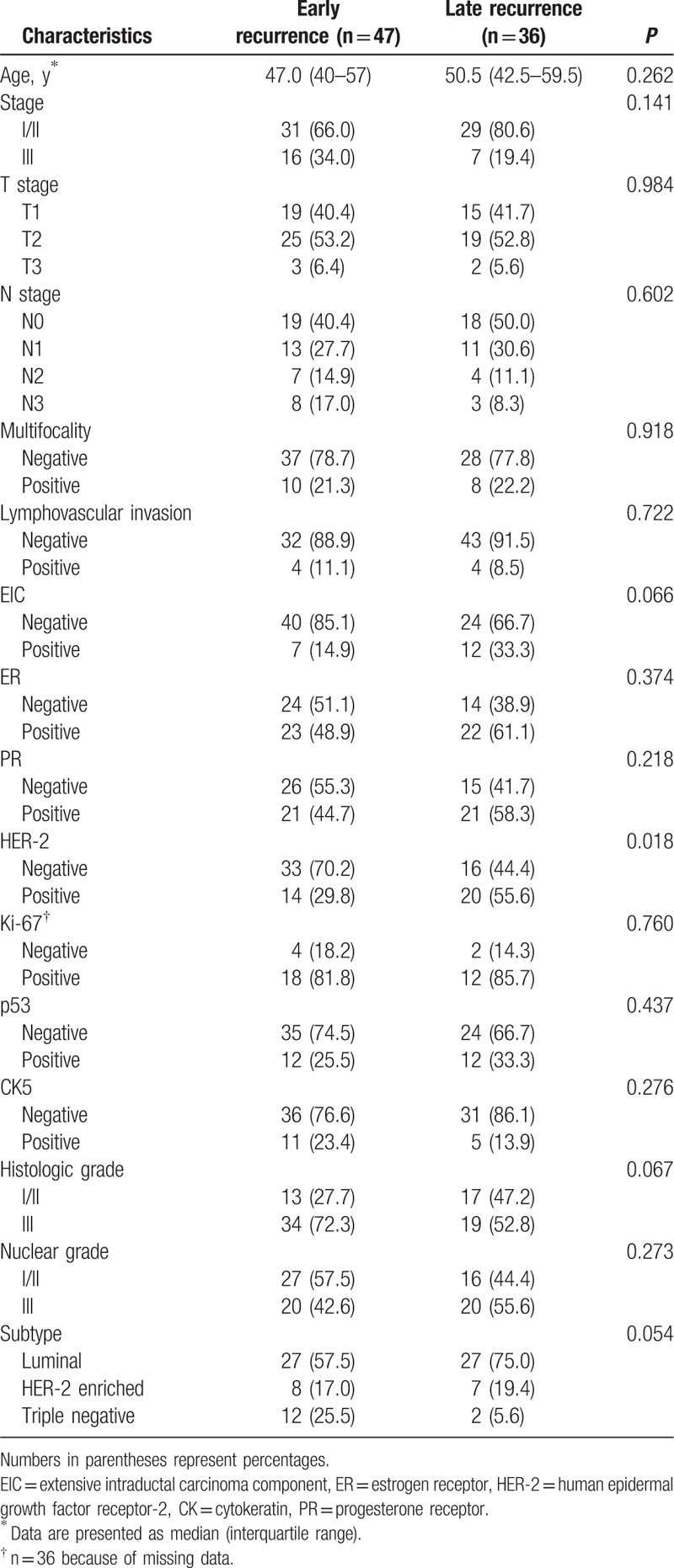
Table 2.
Comparison of DCE-MRI characteristics between early and late recurrence.
3.2. Recurrence-free survival analysis: univariate
Among the tested clinico-pathologic variables (Table 3), stage III, T stage ≥ T2, N stage ≥ N2, ER negativity, PR negativity, Ki-67 positivity, histologic grade III, and nuclear grade III were significantly associated with overall recurrence. Regarding the time of recurrence, younger age, stage III, T stage ≥ T2, N stage ≥ N2, ER negativity, PR negativity, Ki-67 positivity, histologic grade III, and triple-negative cancer were significant predictors of early recurrence. T2 stage, HER-2 positivity, Ki-67 positivity, and nuclear grade III were significantly associated with late recurrence. Among the tested DCE-MRI features (Table 4), moderate or marked BPE (hazard ratio [HR], 2.13; 95% confidence interval [CI], 1.35–3.37), rim enhancement (HR, 3.56; 95% CI, 2.27–5.60), and prominent increased ipsilateral whole-breast vascularity (HR, 4.44; 95% CI, 2.52–7.80) were significantly associated with overall recurrence. Longer TTP (HR, 0.40; 95% CI; 0.27–0.59) was protective against overall recurrence. Moderate or marked BPE, rim enhancement, higher E1, and shorter TTP were significantly associated with both early and late recurrence. Prominent increased ipsilateral whole-breast vascularity was significantly associated with early recurrence.
Table 3.
Univariate Cox proportional hazard analysis of clinico-pathologic variables.
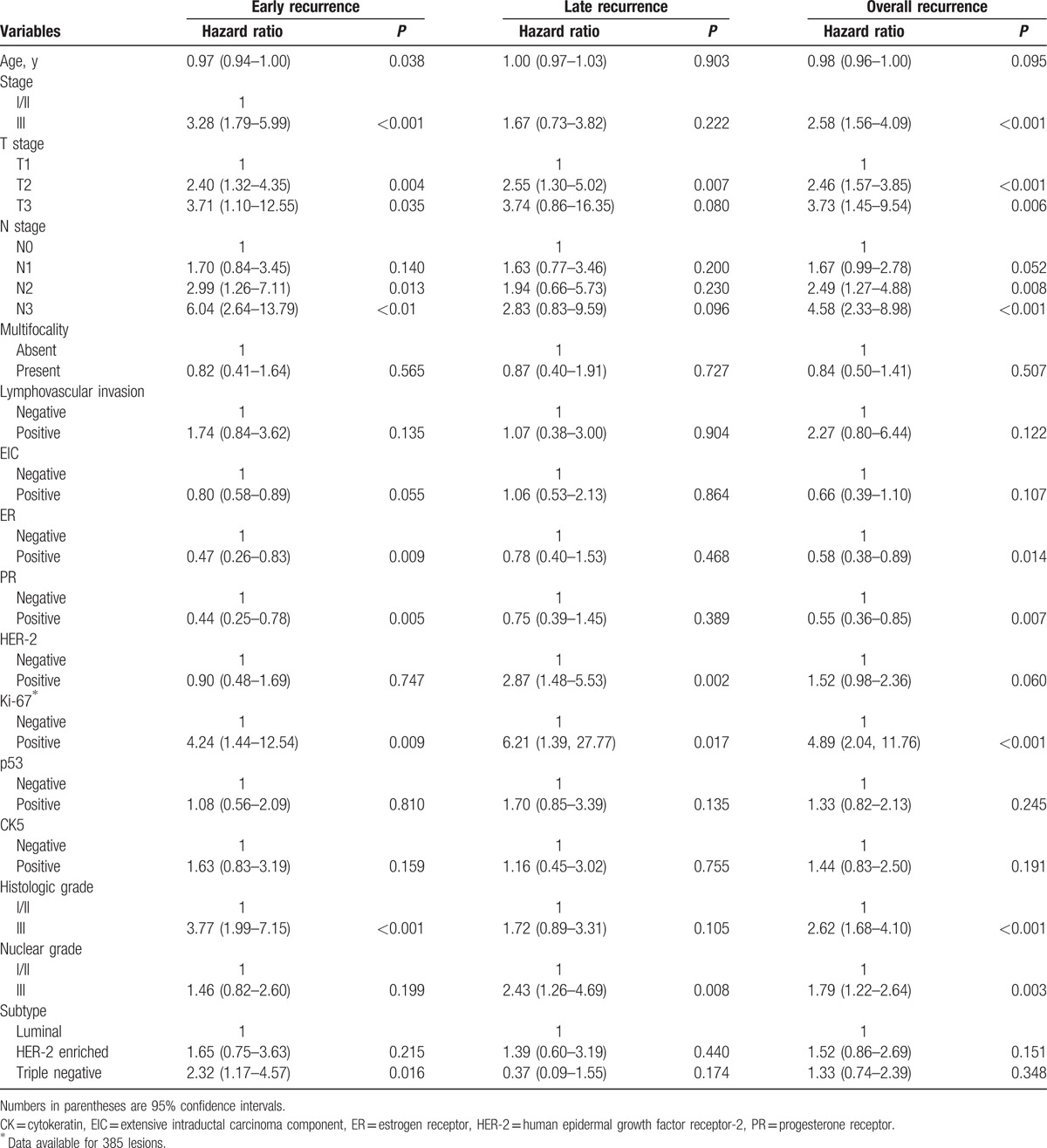
Table 4.
Univariate Cox proportional hazard analysis of DCE-MRI variables.
3.3. Recurrence-free survival analysis: multivariate
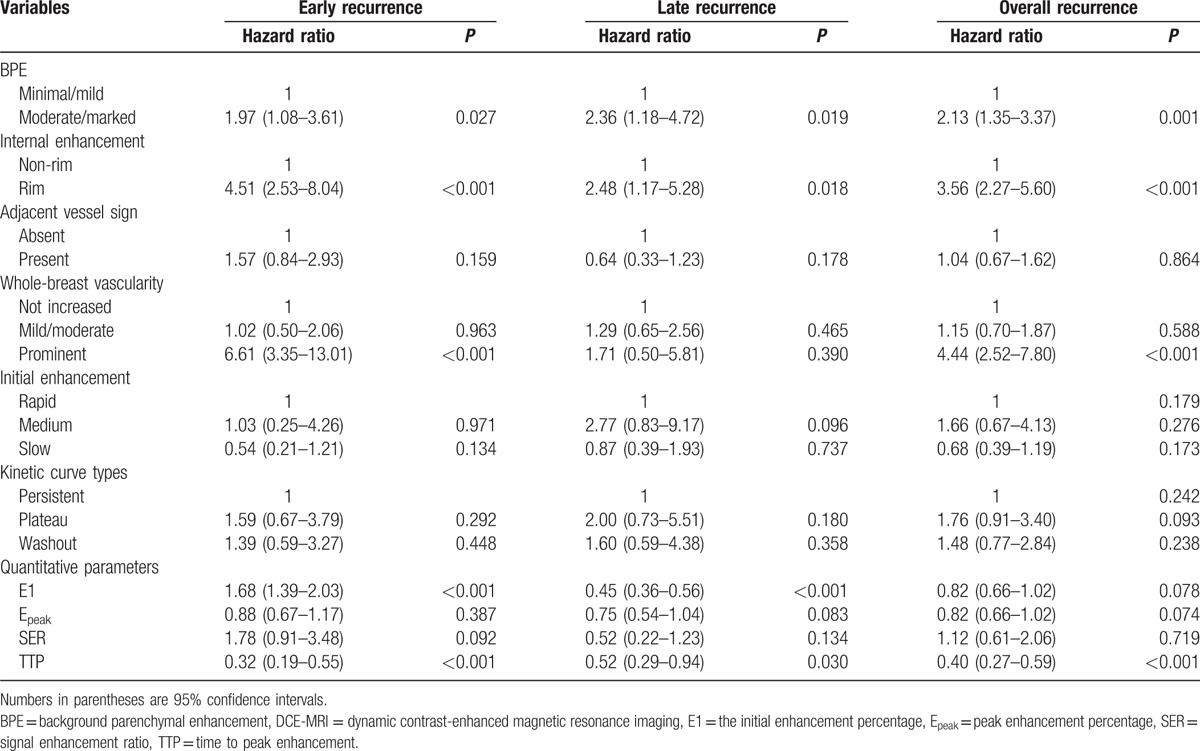
Among the clinico-pathologic variables tested (Table 5), stage III, histologic grade III, and nuclear grade III were significantly associated with overall recurrence. For early recurrence, ER negativity had independent predictive power. For late recurrence, T2 stage and nuclear grade III had independent predictive power. Among the tested DCE-MRI features (Table 5), prominent increased ipsilateral whole-breast vascularity showed the highest risk of overall (HR, 4.75; 95% CI, 2.54–8.91; P < 0.001) and early recurrence (HR, 2.86; 95% CI, 1.39–5.88; P = 0.004) (Fig. 3). BPE and rim enhancement were significantly associated with overall and late recurrence (P < 0.05) (Fig. 4). Shorter TTP was significantly associated with overall recurrence (P = 0.029)
Table 5.
Multivariate Cox proportional hazard analysis of clinico-pathologic and DCE-MRI variables.
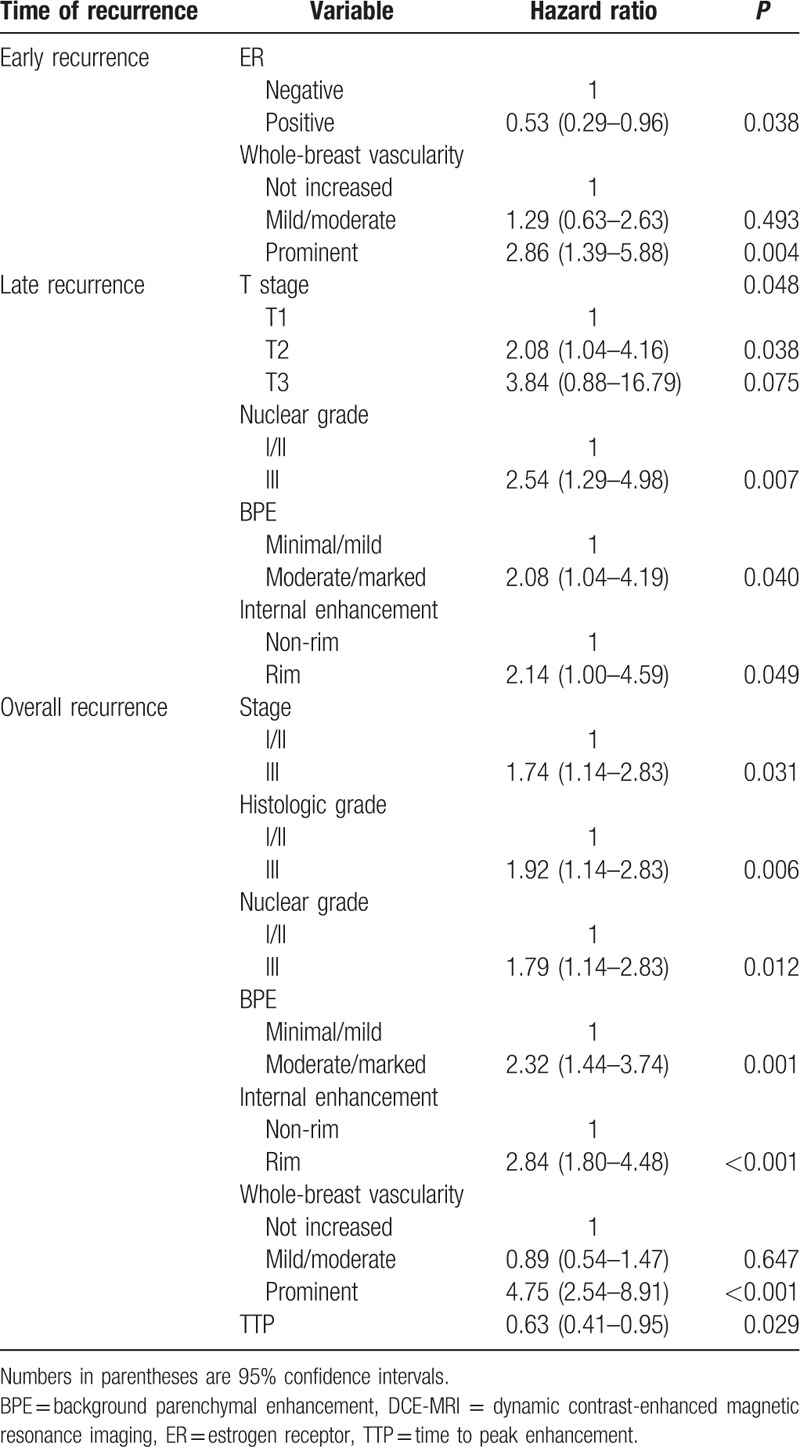
Figure 3.
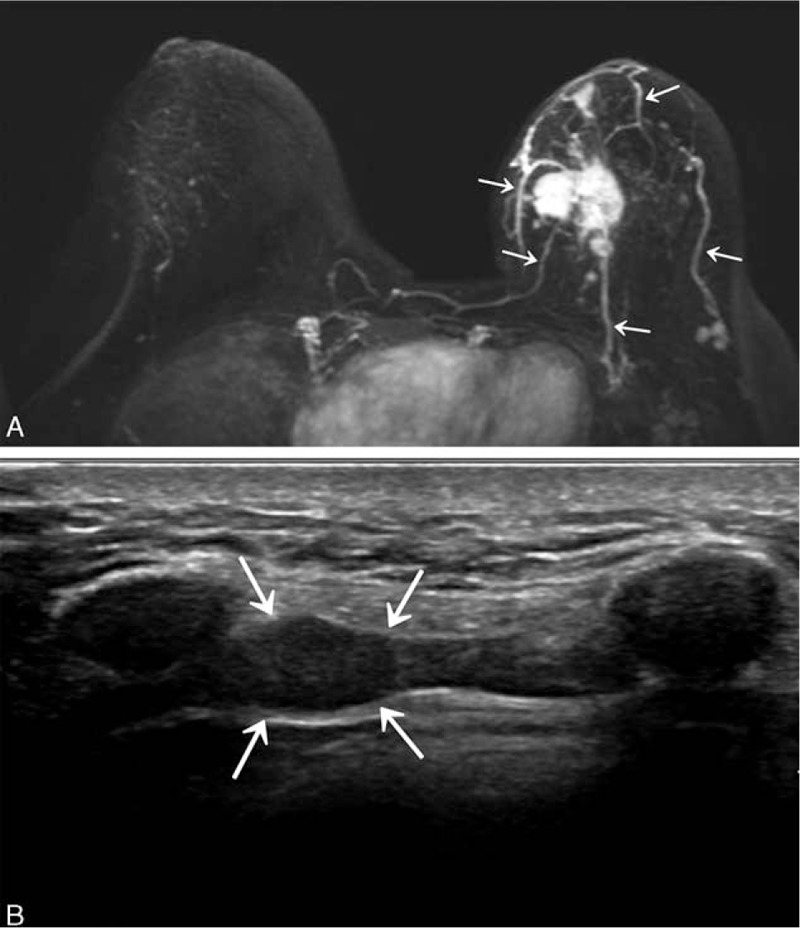
(A) Maximum-intensity-projection image from a 39-year-old woman shows 5 vessels (arrows) in the left that which were interpreted as prominent increased ipsilateral whole-breast vascularity compared with contralateral breast. After surgery, the cancer was confirmed to be stage IIB (T2N1M0), ER-negative, PR-negative, HER-2-positive invasive ductal carcinoma with nuclear grade 2, and histologic grade 3. (B) Follow-up breast ultrasonography obtained after 20.3 months shows a 1-cm-sized internal mammary lymph node (arrows) confirmed to be lymph node metastasis of breast cancer by fine-needle aspiration. ER = estrogen receptor, PR = progesterone receptor, HER-2 = human epidermal growth factor receptor 2.
Figure 4.
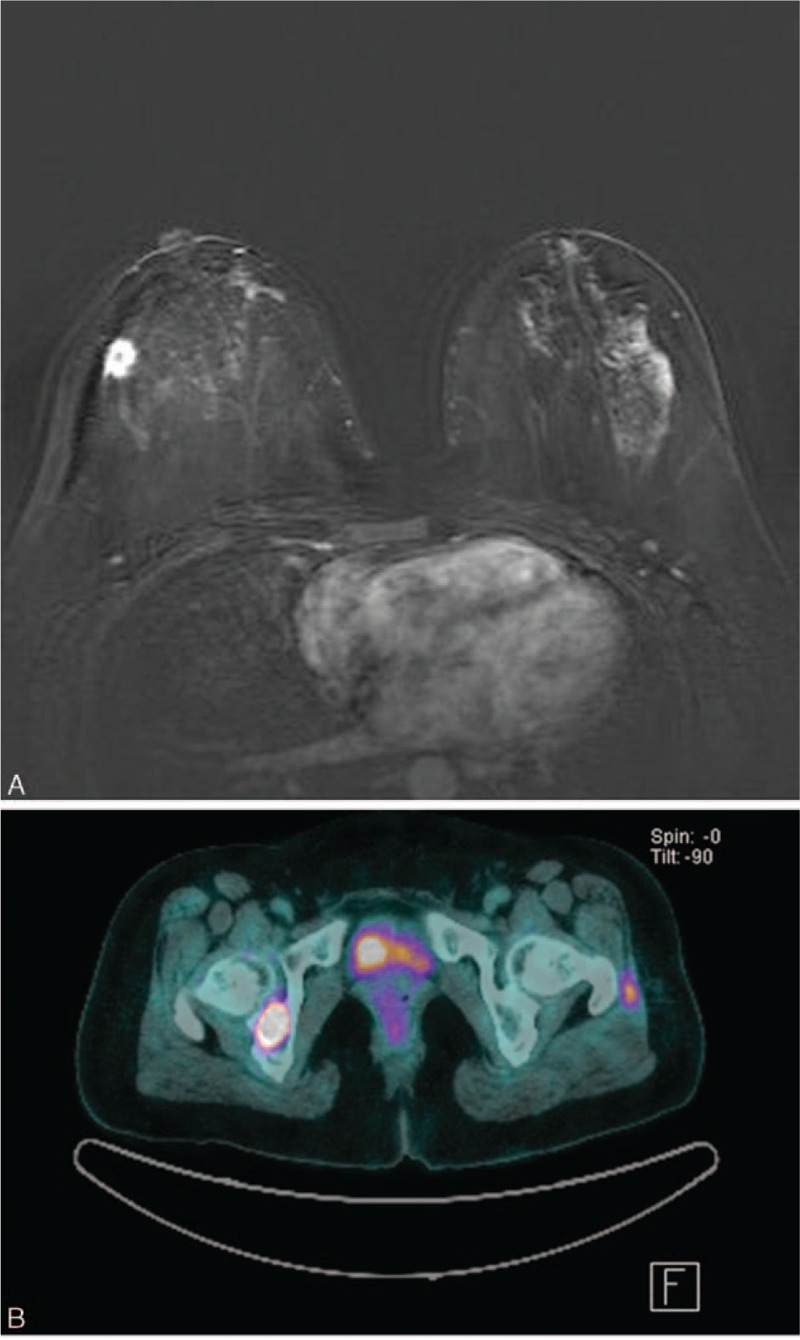
(A) Subtraction image on T1-weighted contrast-enhanced breast MR imaging from a 43-year-old woman shows an irregular mass with rim enhancement and moderate degree of background parenchymal enhancement. After surgery, the cancer was confirmed to be stage IIA (T1N1M0), ER-positive, PR-positive, HER-2-positive invasive ductal carcinoma with nuclear grade 3, and histologic grade 1. (B) Follow-up PET/CT obtained after 37.5 months shows focal areas of increased 18F-FDG uptake in right acetabulum, corresponding to bone metastasis. ER = estrogen receptor, PR = progesterone receptor, HER-2 = human epidermal growth factor receptor 2, PET/CT = positron emission tomography/computed tomography.
In breast cancer recurrence, the AUC of DCE-MRI features were higher for early recurrence and overall recurrence and lower for late recurrence than that of clinico-pathologic variables. However, no significant differences were found (Table 6).
Table 6.
Area under the receiver operating characteristic curve based on selected DCE-MRI and clinico-pathologic variables.

4. Discussion
In this study, we found that prominent increased ipsilateral whole-breast vascularity was associated with early breast cancer recurrence and that moderate or marked BPE and rim enhancement were associated with late recurrence in DCE-MRI. In predicting early, late, or overall recurrence, preoperative DCE-MRI features showed similar performance to clinico-pathologic variables.
In general, vascular blood flow provides nutrients for tumor growth as well as a mechanism for hematogenous spread of malignant cells. It is unsurprising that tumor aggressiveness and breast cancer recurrence are critically dependent on tumor angiogenesis.[23] The prevalence of increased whole-breast vascularity associated with cancer in DCE-MRI may be due to reduced flow resistance in the tumor vessels, higher tumor metabolism, angiogenic stimulation of the whole breast, or a combination of these factors. In addition, a previous study reported that increased whole-breast vascularity was frequently present in patients with multifocal disease and large tumor size and in patients with metastatic disease in the axillary nodes, indicating poor prognosis.[24] Therefore, it can be hypothesized that increased vascular blood flow within a cancer-bearing breast may be a reflection of the growth and metastatic potential of a primary breast tumor, which can seed tumor cells into the peripheral blood, eventually increasing the probability of early recurrence.
Moderate or marked BPE was found to be a significant DCE-MRI feature predictive of late and overall recurrence in this study. BPE is due to increased T1 relaxation of tissue after gadolinium administration and the degree of BPE is directly associated with vascular supply and permeability. Additionally, parenchymal enhancement around a tumor on preoperative DCE-MRI may reflect the status of the microenvironment that is associated with cancer recurrence following breast-conserving treatment.[25] A relatively higher BPE around the breast cancer in the recurrence group indicates increased endothelial permeability that allows the contrast agent to move to and from vessels surrounding the tumor.[25] In other words, increased endothelial permeability results in a higher probability of direct interaction of breast cancer cells and vasculature, thereby facilitating tumor growth and metastasis. Although prominent increased whole-breast vascularity and BPE have similar mechanisms for breast cancer recurrence, BPE was associated with late recurrence, unlike prominent increased whole-breast vascularity, which was associated with early recurrence. Despite a recent surge in investigation of tumor angiogenesis, the effects of large-vessel angiogenesis, including whole-breast vascularity, remain largely unknown. One possibility is that angiogenic stimulation by breast cancer involves not only microvessels, but also larger vessels. More large-vessel angiogenesis might facilitate tumor growth and development of early breast cancer recurrence more effectively, with lower flow resistance than increased endothelial permeability of small vessels.
Rim enhancement on DCE-MRI was found to predict late and overall recurrence in this study. This MR feature is caused by a lower microvessel density within the tumor rather than an elevated microvessel density in the periphery of tumor. In addition, rim enhancement is correlated with tumor fibrosis and necrosis.[6] A central fibrotic focus in breast cancer that is correlated with rim enhancement is a marker of intratumoral hypoxia and its size is correlated with lower microvessel density within the tumor and higher histologic grade.[26–28] However, the relationship between microvessel density, intratumoral hypoxia, and late recurrence of breast cancer remains poorly understood. Several studies have shown a correlation between rim enhancement and rapid tumor growth, decreased tumor differentiation, and other poor prognostic features such as a higher histological grade, ER negativity, higher expression of Ki-67, and higher lymph node stage.[6,7,14,19] Among these poor prognostic features, lymph node status is associated with late recurrence, as is rim enhancement on DCE-MRI.[8]
Among the clinico-pathologic variables investigated in this study, ER-negativity was associated with early breast cancer recurrence, which is similar to what was found in a previous study reporting that ER-positivity conferred a significantly lower recurrence risk than ER-negativity in the 2-year follow-up period.[29] For late recurrence, we observed an independent association with T2 stage and high nuclear grade. In general, tumor size is a well-known important prognostic factor for recurrence.[10] Unlike T2 stage, however, the T3 stage was not independently associated with late recurrence in this study. This might be attributed to a small number of patients with T3 cancer. A previous study reported that high nuclear grade was associated with early metastasis, which is not consistent with our results.[30] However, the cut-off between early and late recurrence was 3.7 years in the previous study, which is different from the 2.5 years used in our study. If we consider this difference in cut-off time, our results are consistent with this previous study.
In terms of the performance in predicting breast cancer recurrence of DCE-MRI features and clinico-pathologic variables, there were no significant differences in the AUC between DCE-MRI features and clinico-pathologic variables for early, late, and overall recurrence (Table 6). In practice, the clinico-pathologic variables associated with recurrence can only be obtained after surgery and are thus not useful for preoperative prediction of breast cancer recurrence. Although some clinico-pathologic variables such as histologic grade or nuclear grade can be available even after core needle biopsy, there is still the issue of discordant results between core needle biopsy and surgical excision. However, DCE-MRI is a noninvasive imaging modality for preoperative evaluation of tumor characteristics and staging through tumor vascularity. Therefore, our results suggest that DCE-MRI features could be useful for preoperative prediction of early, late, and overall recurrence.
This study has some limitations. First, this study included a small number of patients with breast cancer recurrence from a single institution. Therefore, our patients might not be representative of the general population. Second, this study was retrospective and the follow-up period was not long enough (median follow-up, 62.3 months), considering that the luminal subtype of breast cancer may recur even after 10 years. Third, 13% of the eligible 694 patients were lost to follow-up, which might have introduced bias. However, no significant difference was found between the group of loss to follow-up and the enrolled group in clinico-pathologic features except patient age. The age difference between 2 groups would be unlikely to affect the study results. Fourth, we did not evaluate the status of adjuvant hormonal therapy or adjuvant chemotherapy, which could have affected the recurrence period. Lastly, MR imaging was not scheduled according to the women's menstrual cycles, which could have affected the BPE. Although scheduling of screening MR imaging in the second week of a woman's menstrual cycle is routinely recommended to minimize the effect of BPE, diagnostic MR imaging for staging of breast cancer is usually performed regardless of the menstrual cycle.
In conclusion, prominent increased whole-breast vascularity for early recurrence and moderate or marked BPE and rim enhancement for late recurrence on DCE-MRI could be useful for preoperative prediction of early and late recurrences and to establish an appropriate follow-up protocol.
Footnotes
Abbreviations: AUC = area under the receiver operating characteristic curve, BI-RADS = Breast Imaging Reporting and Data System, BPE = background parenchymal enhancement, CI = confidence interval, DCE-MRI = dynamic contrast-enhanced magnetic resonance imaging, E1 = initial enhancement percentage, Epeak = peak enhancement percentage, ER = estrogen receptor, HER-2 = human epidermal growth factor receptor 2, HR = hazard ratio, PR = progesterone receptor, ROC = receiver-operating characteristic, ROI = region of interest, SER = signal enhancement ratio, TTP = time to peak enhancement.
EJC and HMC authors contributed equally to this study.
Funding: This study was supported by Research Fund of Chonbuk National University in 2014.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Bio 2007;608:1–22. [DOI] [PubMed] [Google Scholar]
- [3].Lowery AJ, Kell MR, Glynn RW, et al. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat 2012;133:831–41. [DOI] [PubMed] [Google Scholar]
- [4].Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 1991;324:1–8. [DOI] [PubMed] [Google Scholar]
- [5].Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat 1995;36:169–80. [DOI] [PubMed] [Google Scholar]
- [6].Teifke A, Behr O, Schmidt M, et al. Dynamic MR imaging of breast lesions: correlation with microvessel distribution pattern and histologic characteristics of prognosis. Radiology 2006;239:351–60. [DOI] [PubMed] [Google Scholar]
- [7].Lee SH, Cho N, Kim SJ, et al. Correlation between high resolution dynamic MR features and prognostic factors in breast cancer. Korean J Radiol 2008;9:10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Song WJ, Kim KI, Park SH, et al. The risk factors influencing between the early and late recurrence in systemic recurrent breast cancer. J Breast Cancer 2012;15:218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nishimura R, Osako T, Nishiyama Y, et al. Evaluation of factors related to late recurrence—later than 10 years after the initial treatment—in primary breast cancer. Oncology 2013;85:100–10. [DOI] [PubMed] [Google Scholar]
- [10].Kennecke H, McArthur H, Olivotto IA, et al. Risk of early recurrence among postmenopausal women with estrogen receptor-positive early breast cancer treated with adjuvant tamoxifen. Cancer 2008;112:1437–44. [DOI] [PubMed] [Google Scholar]
- [11].Jonat W, Gnant M, Boccardo F, et al. Effectiveness of switching from adjuvant tamoxifen to anastrozole in postmenopausal women with hormone-sensitive early-stage breast cancer: a meta-analysis. Lancet Oncol 2006;7:991–6. [DOI] [PubMed] [Google Scholar]
- [12].Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26suppl 5:v8–30. [DOI] [PubMed] [Google Scholar]
- [13].Joensuu K, Leidenius M, Kero M, et al. ER, PR, HER2, Ki-67 and CK5 in early and late relapsing breast cancer-reduced CK5 expression in metastases. Breast Cancer (Auckland) 2013;7:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ahn SG, Lee HM, Cho SH, et al. The difference in prognostic factors between early recurrence and late recurrence in estrogen receptor-positive breast cancer: nodal stage differently impacts early and late recurrence. PloS One 2013;8:e63510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Morris EA, Comstock CE, Lee CH, et al. ACR BI-RADS® Magnetic Resonance Imaging. In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- [16].Dietzel M, Baltzer PA, Vag T, et al. The adjacent vessel sign on breast MRI: new data and a subgroup analysis for 1,084 histologically verified cases. Korean J Radiol 2010;11:178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sardanelli F, Iozzelli A, Fausto A, et al. Gadobenate dimeglumine-enhanced MR imaging breast vascular maps: association between invasive cancer and ipsilateral increased vascularity. Radiology 2005;235:791–7. [DOI] [PubMed] [Google Scholar]
- [18].Kang DK, Kim EJ, Kim HS, et al. Correlation of whole-breast vascularity with ipsilateral breast cancers using contrast-enhanced MDCT. AJR Am J Roentgenol 2008;190:496–504. [DOI] [PubMed] [Google Scholar]
- [19].Szabo BK, Aspelin P, Wiberg MK, et al. Dynamic MR imaging of the breast. Analysis of kinetic and morphologic diagnostic criteria. Acta Radiologica 2003;44:379–86. [DOI] [PubMed] [Google Scholar]
- [20].Esserman L, Hylton N, George T, et al. Contrast-enhanced magnetic resonance imaging to assess tumor histopathology and angiogenesis in breast carcinoma. Breast J 1999;5:13–21. [DOI] [PubMed] [Google Scholar]
- [21].Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol 1996;14:2738–46. [DOI] [PubMed] [Google Scholar]
- [22].Mansell J, Monypenny IJ, Skene AI, et al. Patterns and predictors of early recurrence in postmenopausal women with estrogen receptor-positive early breast cancer. Breast Cancer Res Treat 2009;117:91–8. [DOI] [PubMed] [Google Scholar]
- [23].Heimann R, Ferguson D, Gray S, et al. Assessment of intratumoral vascularization (angiogenesis) in breast cancer prognosis. Breast Cancer Res Treat 1998;52:147–58. [DOI] [PubMed] [Google Scholar]
- [24].Han M, Kim TH, Kang DK, et al. Prognostic role of MRI enhancement features in patients with breast cancer: value of adjacent vessel sign and increased ipsilateral whole-breast vascularity. AJR Am J Roentgenol 2012;199:921–8. [DOI] [PubMed] [Google Scholar]
- [25].Kim MY, Cho N, Koo HR, et al. Predicting local recurrence following breast-conserving treatment: parenchymal signal enhancement ratio (SER) around the tumor on preoperative MRI. Acta Radiologica 2013;54:731–8. [DOI] [PubMed] [Google Scholar]
- [26].Narisada H, Aoki T, Sasaguri T, et al. Correlation between numeric gadolinium-enhanced dynamic MRI ratios and prognostic factors and histologic type of breast carcinoma. AJR Am J Roentgenol 2006;187:297–306. [DOI] [PubMed] [Google Scholar]
- [27].Colpaert C, Vermeulen P, van Beest P, et al. Intratumoral hypoxia resulting in the presence of a fibrotic focus is an independent predictor of early distant relapse in lymph node-negative breast cancer patients. Histopathology 2001;39:416–25. [DOI] [PubMed] [Google Scholar]
- [28].Colpaert C, Vermeulen P, Jeuris W, et al. Early distant relapse in “node-negative” breast cancer patients is not predicted by occult axillary lymph node metastases, but by the features of the primary tumour. J Pathol 2001;193:442–9. [DOI] [PubMed] [Google Scholar]
- [29].Gray RJ. Flexible methods for analyzing survival data using splines, with applications to breast cancer prognosis. J Am Stat Assoc 1992;87:942–51. [Google Scholar]
- [30].Westenend PJ, Meurs CJ, Damhuis RA. Tumour size and vascular invasion predict distant metastasis in stage I breast cancer. Grade distinguishes early and late metastasis. J Pathol 2005;58:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]


