Supplemental Digital Content is available in the text
Keywords: adverse events, canagliflozin, HbA1c, meta-analysis, type 2 diabetes
Abstract
Background:
Canagliflozin is a new SGLT2 inhibitor which has been approved as an adjunct to diet and exercise for the treatment of adults with type 2 diabetes (T2D) mellitus in more than 30 countries. To evaluate the efficacy and safety of canagliflozin in patients with T2D, we carried out a meta-analysis of phase III clinical trials to offer an additional evidence of the efficacy and safety of canagliflozin for evidence-based clinical practice, strictly restricting the treatment durations to 26 weeks (core period) and 52 weeks (extension period).
Methods:
Randomized controlled trials (RCTs) published in English were searched in PubMed, Embase, and the Cochrane Library database (before April 2016). The studies reporting the efficacy and safety of canagliflozin in patients with T2DM were considered. Two authors separately performed data extraction. The differences were discussed and resolved. Pooled weighted mean differences (WMDs) or relative risks and 95% confidence intervals (CIs) were computed by using either fixed- or random-effects models.
Results:
At the end of the selection process, 7 RCTs were collected and included in the present analysis. Placebo-subtracted WMDs (%) of glycosylated hemoglobin (HbA1c) were −0.63 (95% CI: −0.77, −0.49) and −0.80 (95% CI: −0.98, −0.62) for canagliflozin 100 and 300 mg, respectively, from baseline to week 26. At week 26, canagliflozin 100 and 300 mg significantly reduced the body weight from baseline when compared with that of placebo, with a WMD of −2.23 and −3.00 in percent changes (P < 0.001 for both). The fasting and postmeal glucose, blood pressure (BP), and triglycerides were also reduced. These reductions were sustained over 52 weeks but had no significant differences between the 100 and 300 mg doses. The overall safety of canagliflozin was good, with the exception of high incidence of genital mycotic infections and osmotic diuresis-related adverse events.
Conclusion:
Canagliflozin was found to reduce HbA1c, fasting and postmeal glucose, body weight, BP, and triglycerides, and it was generally well tolerated in patients with T2DM.
Key Points
Canagliflozin can reduce HbA1c, fasting, and postmeal glucose.
Canagliflozin can reduce body weight, blood pressure, and triglycerides.
Canagliflozin was generally well tolerated in patients with T2DM.
1. Introduction
Diabetes mellitus is a major global health problem, with an estimated prevalence of 8.3% (387 million) in 2014, which is expected to rise to 592 million by 2035; 46.3% (179 million) people with diabetes are undiagnosed. In 2014, diabetes led to at least 612 billion USD in health expenditure and 4.9 million deaths. Worldwide, a person dies from diabetes every 7 seconds.[1,2] In adults, type 2 diabetes (T2D), which is a major cause of heart disease, stroke, and kidney failure, accounts for approximately 90% to 95% of all diagnosed cases of diabetes.[3]
People with T2D can often initially manage their disease condition through exercise and diet. When lifestyle intervention is inadequate, metformin is recommended. Insulin or other oral antihyperglycemic drugs, such as sulfonylureas and thiazolidinediones, can be considered as combination drugs.[4] However, side effects like hypoglycemia, weight gain,[5] fluid retention, and bone loss[6–8] make it difficult to give full play to their long-term effects in maintaining glucose control. There might be unavoidable publication bias as their efficacies for preventing the clinical complications of T2D (macro- and microvascular complications) are not demonstrated with a high level of evidence. In this circumstance, many new drugs have been developed to break new ground in improving hypoglycemic effects and reducing side effects. Sodium-glucose cotransporter 2 (SGLT2) inhibitors offer an antidiabetic effect by increasing the urinary glucose excretion and subsequently lowering the plasma glucose without inducing excessive insulin secretion.[9] Both animal studies and clinical trials have revealed the efficacy and safety of SGLT2 inhibition for T2D.[10–14] Canagliflozin is a new SGLT2 inhibitor. Its adverse events (AEs) are increased urinary and genital infections as well as salt and volume depletion.[12,15,16] This agent has been approved as an adjunct to diet and exercise for the treatment of adults with type 2 diabetes (T2D) mellitus in more than 30 countries, including the United States and the European Union.
Three previous meta-analyses have reviewed the efficacy and safety of canagliflozin. However, 2 of them included both phase II and phase III clinical trials with different treatment durations.[17,18] Among their pooled articles, the treatment durations were vastly different (12, 24, 26, and 52 weeks). Yang et al[17] evaluated the efficacy and safety of canagliflozin 300 mg versus those of the control groups. Another pooled analysis of phase III studies only included 4 randomized controlled trials (RCTs).[19] Clinical trials with different designs, conditions, dosages, and follow-up time might provide different results and make it intricate in guiding clinical medication. We carried out a meta-analysis of phase III clinical trials to offer an additional evidence of the efficacy and safety of canagliflozin for evidence-based clinical practice, strictly restricting the treatment durations to 26 weeks (core period) and 52 weeks (extension period). Placebo-subtracted efficacy and safety as well as the differences between the different treatment groups were also evaluated.
2. Methods
2.1. Ethical consideration
The current study was reviewed and approved by the ethical committee of the Central Hospital of Wuhan.
2.2. Literature search
We performed this review according to a protocol predefining the selection criteria, all efficacy and safety outcomes, and main analyses. We have presented the methods and results of our meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses recommendations.[20]
A comprehensive, computerized literature search was performed in the PubMed, Embase, and the Cochrane Library database (before April 2016) for studies reporting the efficacy and safety of canagliflozin in patients with T2DM. The search was conducted using the terms “canagliflozin” or “INVOKANA” or “TA-7284” or “JNJ-28431754.” Only RCTs published in English were considered. To identify completed but unpublished trials, we also searched the websites of relevant pharmaceutical companies and public registers of clinical trials (www.clinicaltrials.gov/).
2.3. Selection criteria
The studies were eligible for inclusion in the meta-analysis if they met the following criteria:
-
(1)
The participants were nonpregnant adults (aged over 18 years) with T2D. The patients in the study had a glycosylated hemoglobin (HbA1c) between 7% and 10.5% and a fasting plasma glucose (FPG) < 270 mg/dL (15 mmol/L) from baseline. The mean duration of T2DM was more than 1 year.
-
(2)
The treatment intervention was canagliflozin, and the duration of the intervention was at least 26 weeks.
-
(3)
The study design was randomized, double-blind, placebo-controlled, or active-controlled, parallel-group study.
-
(4)
The primary efficacy endpoint was the change in percentage of HbA1c from the baseline. For multiple publications in the same RCT, only the article with the most comprehensive data was included.
Articles were excluded if they were letters, editorials, conference abstracts, reviews, and commentaries.
2.4. Quality assessment of the trials
The quality of RCTs was assessed with the Cochrane risk of bias tool, which is the recommended approach for assessing the risk of bias in studies included in Cochrane reviews. This tool assesses the risk of bias in 2 parts, addressing the following specific domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other issues.[21]
2.5. Data extraction
A data extraction form was predesigned to extract the following information from each study: first author's surname, year of publication, study name, national clinical trial number, study design, patients’ profile, control groups, dose of canagliflozin, background therapy, rescue therapy, total number enrolled, mean age at baseline, sex, mean HbA1c in baseline, study duration, completion time of primary outcomes, number analyzed in each group, percent change in HbA1c, changes in other glycemic efficacy endpoints, such as FPG and 2-hour postprandial glucose (2h-PPG), changes in body weight, blood pressure (BP) and lipids, change in homeostasis model assessment of β-cell function (HOMA-β), and AEs observed in each group. To get a more detailed data of AEs, we searched the websites of public registers of clinical trials. Two authors separately performed data extraction. The differences were discussed and resolved.
2.6. Data synthesis and statistical analysis
To synthesize the efficacy outcomes, Review Manager 5 (The Cochrane Collaboration, Oxford, UK) was used to calculate the estimates and 95% confidence intervals (CIs) of the weighted mean differences (WMDs) between the intervention group (canagliflozin 100 or 300 mg daily) and the control group for quantitative variables and relative risks (RRs) for categorical variables, using either fixed or random effects models with an inverse variance method. If a study did not report a standard deviation, it was calculated from the sample size and the standard error or the 95% CI. In this section, we focused on the efficacy changes from the baseline to week 26 (core period).
Some studies designed a 26-week, active-controlled extension period in which the placebo-treated patients were switched to sitagliptin 100 mg; hence, there were insufficient number of articles to explore the 52-week efficacy discrepancies between the intervention and control groups. Considering the different canagliflozin doses, we turned our attention to the comparison between the 2 intervention groups. The same software and method were used as previously mentioned to pool the WMDs and RRs between the 300- and 100-mg groups from baseline to week 26 and from baseline to week 52, respectively.
P values less than 0.05 were considered statistically significant for the pooled results. Heterogeneity among the trials was assessed using the χ2 test defined as a P value less than 0.10 and was further quantified through the I2 statistics. The presence of heterogeneity was defined using I2 values over 40%, making it suitable for random effects models. Publication bias was assessed using Egger linear regression test and Begg rank correlation test, conducted using STATA version 12.0 (StataCorp LP, College Station, TX). P values more than 0.05 were considered as no evidence of publication bias.
The number of AEs was extracted from different articles and combined together. RRs were used to compare the differences between the groups.
To explore the sources of heterogeneity among the studies and test the robustness of the changes, we conducted several sensitivity analyses by excluding a trial carried out on patients having T2DM with reduced kidney function. Based on this consideration, we suggested that patients with reduced kidney function might have different reactions to the drug. For the primary efficacy endpoint HbA1c, we also conducted random-effects meta-regression to explore the possible causes of heterogeneity. Canagliflozin doses, percentage of males, mean age, HbA1c at baseline, and duration of diabetes mellitus were assessed as possible cofactors for the potential changes. WMDs were dependent variables of meta-regression. Thus, we could assess whether changes seen in HbA1c were associated with these covariables.
In addition, we conducted a Bayesian meta-analysis, which has become prominent in the recent years. The Bayesian analysis itself combines the prior distribution with the data, turning it into a posterior probability distribution. A 95% Bayesian credible interval is a summary of the posterior distribution, such that the probability that the true quantity is within the interval is equal to 95%.[22] We used WinBUGS (Medical Research Council's Biostatistics Unit, Cambridge, UK) for the Bayesian meta-analysis.
3. Results
3.1. Literature search and characteristics of the included studies
The search strategy initially identified 57 articles. After selection, 7 randomized, double-blind, placebo-controlled or active-controlled, parallel-group trials met the selection criteria, with a total enrollment of 5215 patients with T2DM. In Fig. 1, the selection steps and results have been outlined. The characteristics of the 7 included studies are shown in Supplementary table 1. The risk of summary and risk of graph are shown in Supplementary figures 1 and 2, respectively. All articles had good quality.
Figure 1.
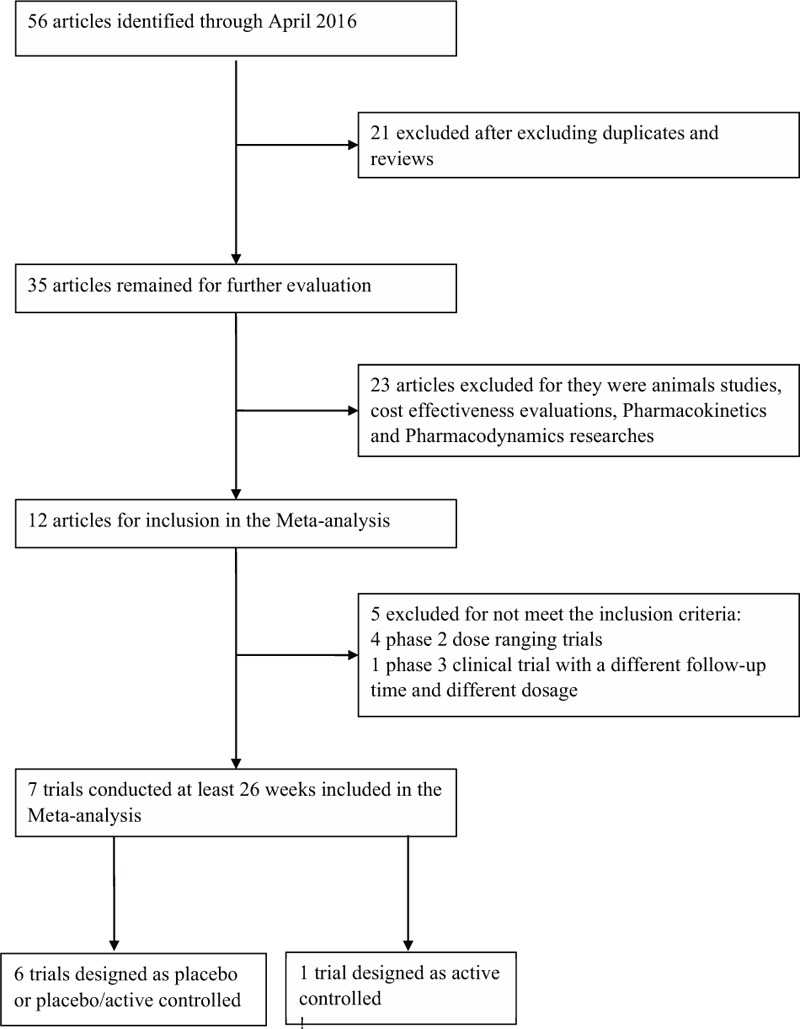
Selection of studies that examined the efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus.
3.2. Efficacy
The efficacy of canagliflozin was stated based on the following aspects: glycemic efficacy endpoints (changes in HbA1c, percentage of patients with HbA1c < 7%, change in FPG, change in 2h-PPG; body weight, BP, and lipids (percent change in body weight, change in systolic BP (SBP) and diastolic BP (DBP), percent change in triglycerides, high-density lipoprotein–cholesterol (HDL-C), low-density lipoprotein–cholesterol (LDL-C), non-HDL-C, and LDL-C/HDL-C ratio; and β-cell function (change in HOMA-β).
3.3. Glycemic efficacy endpoints
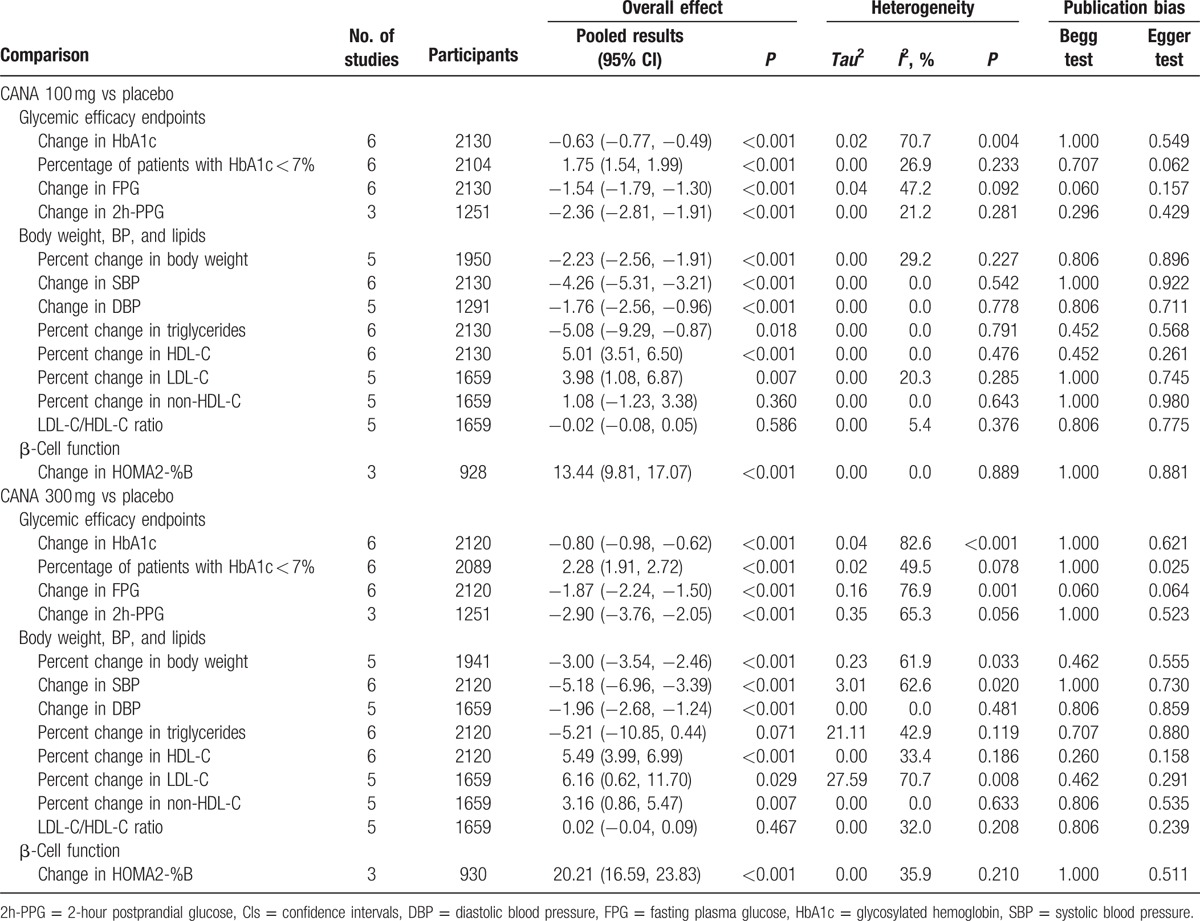
When comparing intervention groups to the placebo group, the placebo-subtracted WMDs (%) of HbA1c for canagliflozin 100 and 300 mg were −0.63 (95% CI: −0.77, −0.49) and −0.80 (95% CI: −0.98, −0.62), respectively, from the baseline to week 26 (Table 1), P < 0.001 for both. The pooled RRs of the percentage of patients with HbA1c < 7% for canagliflozin 100 and 300 mg were 1.75 (95% CI: 1.54, 1.99) and 2.28 (95% CI: 1.91, 2.72), respectively. FPG and 2h-PPG were both significantly reduced from the baseline.
Table 1.
Summary of pooled results of efficacy changes from baseline to week 26.
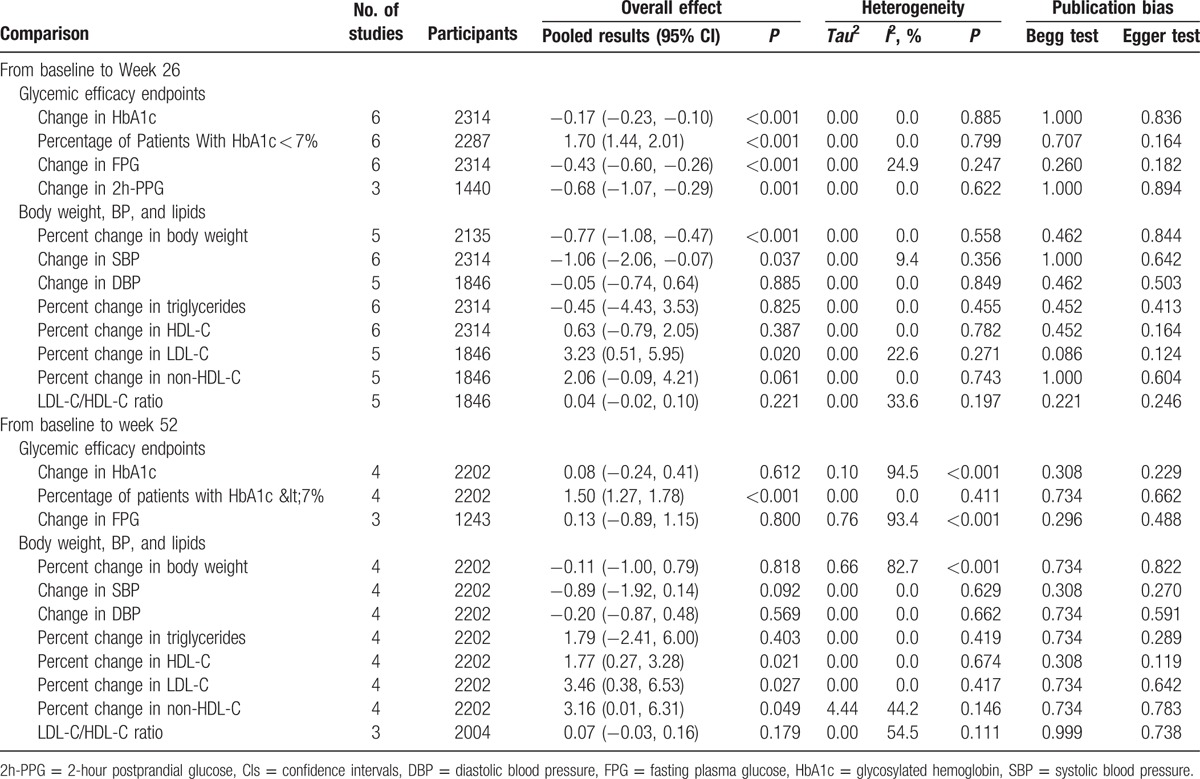
Compared to the 100 mg dosage group, 300 mg dosage group had a WMD of −0.17 (95% CI: −0.23,−0.10; P < 0.001) change in HbA1c from baseline to week 26 and 0.08 (95% CI: −0.24, 0.41; P = 0.612) to week 52 (Table 2). The pooled RRs of the percentage of patients with HbA1c < 7% were 1.70 (95% CI: 1.44, 2.01) and 1.50 (95% CI: 1.27, 1.78), respectively, P < 0.001 for both. The changes in FPG from baseline was 0.43% lower in week 26, but 0.13% higher in week 52 for 300 mg dosage group versus 100 mg dosage group, P < 0.001 for the former and P = 0.800 for the latter.
Table 2.
Summary of pooled results of efficacy changes comparing CANA 300 mg with CANA 100 mg.
3.4. Body weight, BP, and lipids
At week 26, canagliflozin 100 and 300 mg significantly reduced the body weight from baseline compared with the placebo, with a WMD of −2.23 and −3.00 in percent changes (P < 0.001 for both). With regard to the 300 mg dosage group versus 100 mg dosage group, the percent change from baseline was significant with a −0.77 mean difference (95% CI: −1.08, −0.47; P < 0.001) in week 26. However, it turned out to be not statistically different for a −0.11 mean difference (95% CI: −1.00, 0.79; P = 0.818) in week 52.
WMDs of change from baseline in SBP were all significant for canagliflozin 100 mg versus placebo (WMD: −4.26 [95% CI: −5.31, −3.21] mm Hg; P < 0.001), canagliflozin 300 mg versus placebo (WMD: −5.18 [95% CI: −6.96, −3.39] mm Hg; P < 0.001). At the same time, for the same 2 comparisons, the changes in DBP were also significant, with detailed results shown in Table 1. When comparing the 300-mg dosage group with the 100-mg dosage group, only the change in SBP from baseline to week 26 was significant, with a WMD of −1.06 mm Hg (95% CI: −2.06, −0.07; P = 0.037). However, there were no statistical differences in the remaining 3 comparisons in BP (Table 2).
Reductions from baseline to week 26 in triglycerides were seen across the treatment groups relative to the placebo, with numerically greater and statistically significant decrease of percent change for canagliflozin 100 and 300 mg (WMD: –5.08 and –5.21, respectively; Table 1). Canagliflozin 100 and 300 mg showed numerical increase from baseline to week 26 for percent changes in cholesterol (HDL-C, LDL-C, and non-HDL-C; Table 2). Canagliflozin 100 and 300 mg showed slight increase in LDL-C/HDL-C ratio relative to the placebo at week 26, but these differences did not reach a statistical significance (Table 1).
When comparing 2 intervention groups, the percent changes from baseline in lipid indices were not significant at week 26, except for LDL-C. The percent changes in LDL-C increased significantly (WMD: 3.23 [95% CI: 0.51, 5.95]; P = 0.020). After long-term drug administration (52 weeks), canagliflozin 300 mg were associated with WMDs of percent changes in HDL-C, LDL-C, and non-HDL-C versus 100 mg of 1.77 (95% CI: 0.27, 3.28), 3.46 (95% CI: 0.38, 6.53), and 3.16 (95% CI: 0.01, 6.31), respectively (Table 2). Numerical but not statistically significant increase in triglyceride levels was also seen with canagliflozin 300 mg relative to 100 mg at week 52. No significant change in LDL-C/HDL-C ratio was observed for canagliflozin 300 and 100 mg.
3.5. β-cell function
Numerical higher mean changes in HOMA-β were observed for canagliflozin 100 and 300 mg compared with placebo at week 26. The WMDs were 13.44 (95% CI: 9.81, 17.07; P < 0.001) and 20.21 (95% CI: 16.59, 23.83; P < 0.001). Owing to the limited number of articles offering change in HOMA-β from baseline to week 52, we did not get a pooled result.
3.6. Heterogeneity and publication bias
The detailed information, namely Tau2, I2, and P value, of heterogeneity is presented in Tables 1 and 2. An obvious evidence of ridiculous heterogeneity among the studies was not found for body weight, BP, and lipids. Substantial heterogeneity was significant for several glycemic efficacy endpoints. Meta-regression was carried out, which will be examined later in this article.
The evidence of publication bias for every meta-analysis assessing different efficacy outcomes is noted in Tables 1 and 2, by using Begg rank correlation test and Egger linear regression test for each kind of comparison. Almost all the results indicated the absence of publication bias, with the exception of a few P values less than 0.05.
3.7. Safety and tolerability
Summary of the overall safety and selected AEs from baseline to week 26 and from baseline to week 52 is shown in Table 3. The RRs of comparisons between different groups are shown in detail in Supplementary table 2.
Table 3.
Summary of overall safety and selected AEs from baseline to week 26 and from baseline to week 52.
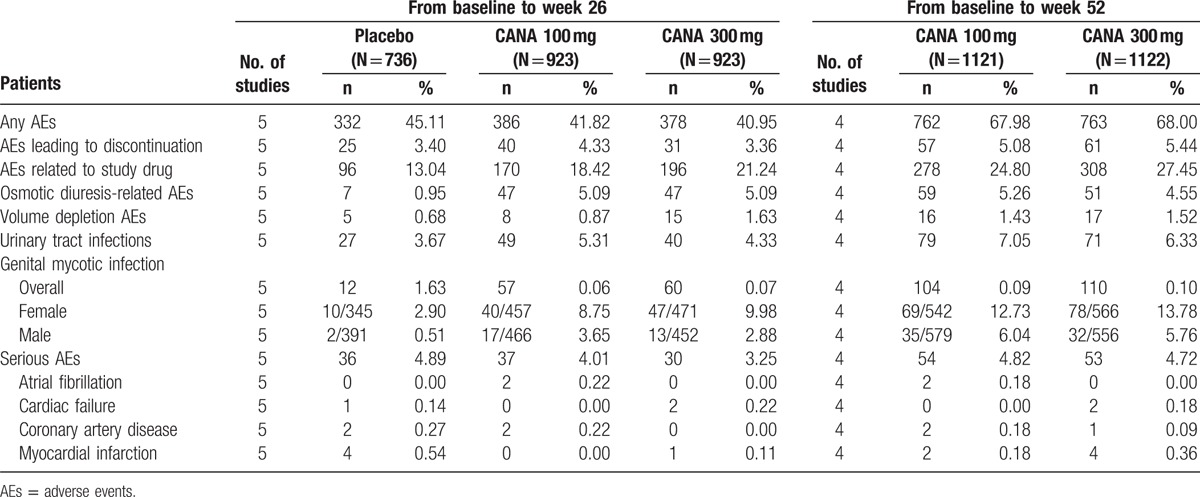
The overall incidence of AEs was similar over the 26-week treatment period, with 45.11%, 41.82%, and 40.95% for placebo, canagliflozin 100 and 300 mg, respectively. Regarding the comparisons between treatment groups and placebo groups, the differences were not significant with an RR of 0.93 (95% CI: 0.83, 1.04) for canagliflozin 100 mg and 0.91 (95% CI: 0.81, 1.01) for canagliflozin 300 mg relative to the placebo. The incidence of AEs leading to study discontinuation was generally comparable across the group over 26 weeks, with slightly higher incidences in the canagliflozin 100 mg group. The incidence of AEs related to the study drug was higher in groups treated with canagliflozin (1.41 [95% CI: 1.12, 1.78] for 100 mg and 1.63 [95% CI: 1.30, 2.04] for 300 mg) compared with that of the placebo. Serious AE rates were higher for the placebo group when compared to those for both doses of canagliflozin, although not significantly different. Several specific AEs occurred frequently than others, such as osmotic diuresis-related AEs, volume depletion AEs, urinary tract infections, and genital mycotic infection. Of the 4 selected AEs, the incidence of osmotic diuresis-related AEs and genital mycotic infections was numerically higher for canagliflozin (both 100 and 300 mg) compared with that for the placebo. The treatment groups both had over 4-fold higher incidence of osmotic diuresis-related AEs than that of the placebo group. Canagliflozin was associated with approximately 2-fold higher overall incidence of genital mycotic infections, both in men and women. The incidence of the remaining 2 selected AEs was slightly higher, but not significant in the canagliflozin groups relative to that of the placebo group.
The incidence was comparable between canagliflozin 300 and 100 mg for all AEs mentioned above, irrespective of the duration (over 26 or 52 weeks) from baseline.
3.8. Sensitivity analyses
After excluding a trial carried out on patients with T2DM having reduced kidney function, we conducted several sensitivity analyses on the glycemic efficacy endpoints from baseline to week 26. The results are shown in Supplementary table 3. The pooled estimate had little substantial change in glycemic efficacy endpoints from baseline to week 26.
3.9. Bayesian meta-analysis
The pooled results of changes in HbA1c from baseline to week 26 using the classical and Bayesian meta-analysis are detailed in Supplementary table 4. Considering the prior distribution, the mean differences of HbA1c were still statistically significant, with −0.64 (95% CI: −0.81, −0.46) for canagliflozin 100 mg versus placebo, −0.80 (95% CI: −1.03, −0.56) for canagliflozin versus placebo, and −0.17 (95% CI: −0.23, −0.10) for canagliflozin 300 mg versus canagliflozin 100 mg from baseline to week 26. Monte Carlo errors were negligibly small, and the Gibbs trace figure indicated well convergent iteration.
3.10. Meta-regression
Various possible factors affected the hypoglycemic effect such as dose, proportion of male patients, age, baseline HbA1c, and duration of T2DM. These factors were taken into the random-effects meta-regression models as covariables. However, we still could not find out the source of heterogeneity on HbA1c. All models had an extremely poor fitting degree with low adjusted R square.
The random-effects meta-regression analysis showed no significant association between the baseline HbA1c and HbA1c reductions with canagliflozin (P = 0.811). Analyses were also performed to assess the association between the drug dose and HbA1c changes. No significant association was observed (P = 0.705). Similar negative results were obtained for the proportion of male patients, age, baseline HbA1c, and duration of T2DM. The results of several models are presented in Supplementary table 8.
4. Discussion
The main findings of this meta-analysis indicated a better hypoglycemic effect for the 26-week treatment of T2DM than that of the placebo, as reflected by the proportion of patients who achieved HbA1c < 7%, FPG, and 2h-PPG. A 24-week, placebo-controlled, phase III study of canagliflozin monotherapy in Japanese patients with T2DM, which was inadequately controlled with diet and exercise, was excluded owing to its different drug administration time and dosage. In this study, canagliflozin was also found to significantly improve glycemic control, in accordance with our pooled results.[23] In addition, an active control group was set up in 2 of the included studies. In one of them, it was shown that canagliflozin provides greater HbA1c reduction than glimepiride does.[16] In the other study, canagliflozin 100 mg showed noninferiority and canagliflozin 300 mg showed statistical superiority to sitagliptin 100 mg in HbA1c-lowering effect at week 52.[24] Mechanism research showed that canagliflozin lowers PPG and insulin levels by delaying the intestinal glucose absorption in addition to increasing the urinary glucose excretion.[25] Owing to the fact that a higher dose provides more sustained maximal decrease in renal threshold for glucose than a lower dose,[26,27] canagliflozin 300 mg showed higher decrease in HbA1c, FPG, and 2h-PPG levels, which resulted in more patients achieving HbA1c < 7%, compared to that of canagliflozin 100 mg from baseline to week 26. At week 52, however, there were no significant differences observed in the hypoglycemic effect. One possible reason is the declining initial stimulus and growing drug tolerance. In a previous study, urinary tract infections were reported without dose dependency in 3% to 9% of canagliflozin and 6% of placebo.[28] This showed that our findings were reasonable. Furthermore, the results from a review of the efficacy and safety of canagliflozin in elderly patients indicated that elderly patients with T2DM have lesser HbA1c reduction compared with that in younger patients,[18] which needs further researches.
Similar with the hypoglycemic effect, canagliflozin also had a remarkable placebo-subtracted effect on reducing body weight and BP especially SBP. The reductions in the 300 mg group were a little higher than the 100 mg group at week 26, but undifferentiated at week 52. Notable reduction in triglyceride levels and increase in HDL-C and LDL-C levels were seen in patients treated with canagliflozin compared with those in the placebo. There were no obvious differences observed between different doses, irrespective of duration (week 26 or 52). Previous studies that assessed the changes in the body composition have suggested that approximately 2/3 of the body weight loss with canagliflozin was owing to the loss of fat mass.[16,29] In clinical studies conducted among obese patients, modest reductions in body weight have been associated with favorable improvements in cardiovascular risk factors, including lipids and BP.[30,31]
In addition to this, at week 26, canagliflozin was found to be better than placebo for enhancing β-cell function, with great increase in HOMA-β. It is clear that β-cell dysfunction exists in individuals with T2DM.[32,33] SGLT2 transporters did not directly act on β-cells, so this improvement is unlikely a direct mechanism. Researches have suggested that the improvements in β-cell function might reflect the reversal of glucotoxicity.[34] As urinary glucose excretion increases, blood glucose decreases, and the load of β-cell was alleviated, resulting in improvements in β-cell function.
The overall safety of canagliflozin was comparable to placebo at week 26. Canagliflozin treatment was associated with an increased risk of genital mycotic infections. One possible explanation for this is that the enhanced glucose content in diabetic urine enhances bacterial growth.[35] Osmotic diuresis-related AEs including dry mouth, thirst, micturition urgency, pollakiuria, polyuria, and nocturia were observed significantly more in patients treated with canagliflozin. Several previous trials focused on urinary tract infections.[3,28,36] However, a discrepant risk was not found between the treatment and placebo groups in our results. These AEs generally occurred during the first 26 weeks of the treatment. They were considered mild or moderate in intensity and infrequently related to discontinuation. Serious AEs were similar and very few serious cardiovascular events were observed both in the treatment and placebo groups. It might partly attribute to its reduction effect on body weight, BP, and triglycerides, which are known as risk factors for cardiovascular events. The safety and tolerability were almost the same on comparing the 2 doses, whether patients were treated for 26 or 52 weeks.
In conclusion, canagliflozin offered an antidiabetic effect and reduced the body weight, BP, and triglycerides. During a short-term administration, high-dose canagliflozin acted better than low dose, while both doses showed almost the same effect during a long-term administration. Considering the load on metabolism and possible safety issues, we suggest low-dose, long-term, oral administration of canagliflozin monotherapy once daily or add-on treatment.
4.1. Strengths and limitations of the review
The present meta-analysis was based on several randomized, double-blind, placebo-controlled or active-controlled, parallel-group studies. All patients included in this review had T2DM, and the number of overall participants was large enough. Our results were consistent with a previous meta-analysis, which did not control the treatment time,[17] and an integrated analysis of data pooled from 4 placebo-controlled, phase III studies.[19] Compared to these studies, the advantage of our study is that the drug administration time and dosage were strictly predetermined in the inclusion criteria and more evidences can be obtained. In addition, we performed quality assessment through the Cochrane risk of bias tool to guarantee the quality of the included articles. We also performed several sensitivity analyses, meta regression, and Bayesian meta-analysis to make the pooled results more credible. Therefore, our evidences were strong enough. Notably, in this meta-analysis, only phase III studies were included, which allowed the analyses of clinical outcomes rather than biological outcomes.
It is well known that meta-analysis has certain unavoidable limitations. Although we had limited this review to well designed RCTs and performed quality assessment to reduce the possible selective bias, the present meta-analysis still had several potential limitations.
First, the present meta-analysis compared canagliflozin with placebo rather than other active antidiabetic drugs. The active-controlled trials were so few to conduct a meta-analysis. Second, a major limitation of clinical trials was that most of the trials were funded by the industry. Industry funding has been found to be strongly associated with favorable outcomes.[37] It may create bias, which can threaten the facticity of the results. Third, there was unavoidable heterogeneity between the studies. Notably, the heterogeneity did not exceedingly limit our results, judging by the results of sensitivity analyses and meta regression. Furthermore, few articles reported the incidence of specific serious AEs, especially cardiovascular events and mortality, which are very important when evaluating a new drug. Although we collected data from public registers of clinical trials, we were still restricted by the presentation and quality of results on the web pages. Moreover, limiting the search to publications in English language might be a little restrictive, but it would not change our conclusion.
5. Conclusion
In summary, canagliflozin was found to reduce HbA1c, fasting and postmeal glucose, body weight, BP, and triglycerides. These reductions were sustained over 52 weeks but had no apparent differences between the 100 and 300 mg doses. Canagliflozin was generally well tolerated in patients with T2DM. Physicians need to pay more attention to genital mycotic infections and osmotic diuresis-related AEs during the drug administration process. These findings can provide further support for the clinical utility of canagliflozin in patients with T2DM. On the basis of our results, we suggest low-dose, long-term, oral administration, once daily.
Supplementary Material
Footnotes
Abbreviations: 2h-PPG = 2-hour postprandial glucose, CIs = confidence intervals, DBP = diastolic blood pressure, FPG = fasting plasma glucose, RRs = relative risks, SBP = systolic blood pressure, T2DM = type 2 diabetes mellitus, WMDs = weighted mean differences.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Key Findings 2014. 2014; http://www.idf.org/diabetesatlas/update-2014 [Accessed April 24, 2015]. [Google Scholar]
- [2].Diabetes: Facts and Figures. 2014; http://www.idf.org/worlddiabetesday/toolkit/gp/facts-figures [Accessed April 24, 2015]. [Google Scholar]
- [3].Nicolle LE, Capuano G, Fung A, et al. Urinary tract infection in randomized phase III studies of canagliflozin, a sodium glucose co-transporter 2 inhibitor. Postgrad Med 2014;126:7–17. [DOI] [PubMed] [Google Scholar]
- [4].About Diabetes. 2014; http://www.idf.org/about-diabetes [Accessed April 24, 2015]. [Google Scholar]
- [5].Piya MK, Tahrani AA, Barnett AH. Emerging treatment options for type 2 diabetes. Br J Clin Pharmacol 2010;70:631–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Graham DJ, Drinkard CR, Shatin D. Incidence of idiopathic acute liver failure and hospitalized liver injury in patients treated with troglitazone. Am J Gastroenterol 2003;98:175–9. [DOI] [PubMed] [Google Scholar]
- [7].Mancini T, Mazziotti G, Doga M, et al. Vertebral fractures in males with type 2 diabetes treated with rosiglitazone. Bone 2009;45:784–8. [DOI] [PubMed] [Google Scholar]
- [8].Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457–71. [DOI] [PubMed] [Google Scholar]
- [9].Katsuno K, Fujimori Y, Ishikawa-Takemura Y, et al. Long-term treatment with sergliflozin etabonate improves disturbed glucose metabolism in KK-A y mice. Eur J Pharmacol 2009;618:98–104. [DOI] [PubMed] [Google Scholar]
- [10].Han S, Hagan DL, Taylor JR, et al. Dapagliflozin, a selective SGLT2 inhibitor, improves glucose homeostasis in normal and diabetic rats. Diabetes 2008;57:1723–9. [DOI] [PubMed] [Google Scholar]
- [11].Shah NK, Deeb WE, Choksi R, et al. Dapagliflozin: a novel sodium-glucose cotransporter type 2 inhibitor for the treatment of type 2 diabetes mellitus. Pharmacotherapy 2012;32:80–94. [DOI] [PubMed] [Google Scholar]
- [12].Inagaki N, Kondo K, Yoshinari T, et al. Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, 12-week study. Diabetes Obes Metab 2013;15:1136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nicolle LE, Capuano G, Ways K, et al. Effect of canagliflozin, a sodium glucose co-transporter 2 (SGLT2) inhibitor, on bacteriuria and urinary tract infection in subjects with type 2 diabetes enrolled in a 12-week, phase 2 study. Curr Med Res Opin 2012;28:1167–71. [DOI] [PubMed] [Google Scholar]
- [14].Zhang M, Zhang L, Wu B, et al. Dapagliflozin treatment for type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Res Rev 2014;30:204–21. [DOI] [PubMed] [Google Scholar]
- [15].Nyirjesy P, Zhao Y, Ways K, et al. Evaluation of vulvovaginal symptoms and Candida colonization in women with type 2 diabetes mellitus treated with canagliflozin, a sodium glucose co-transporter 2 inhibitor. Curr Med Res Opin 2012;28:1173–8. [DOI] [PubMed] [Google Scholar]
- [16].Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 2013;382:941–50. [DOI] [PubMed] [Google Scholar]
- [17].Yang XP, Lai D, Zhong XY, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes: systematic review and meta-analysis. Eur J Clin Pharmacol 2014;70:1149–58. [DOI] [PubMed] [Google Scholar]
- [18].Elmore LK, Baggett S, Kyle JA, et al. A review of the efficacy and safety of canagliflozin in elderly patients with type 2 diabetes. Consult Pharm 2014;29:335–46. [DOI] [PubMed] [Google Scholar]
- [19].Usiskin K, Kline I, Fung A, et al. Safety and tolerability of canagliflozin in patients with type 2 diabetes mellitus: pooled analysis of phase 3 study results. Postgrad Med 2014;126:16–34. [DOI] [PubMed] [Google Scholar]
- [20].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. [DOI] [PubMed] [Google Scholar]
- [21].Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2014. Available from www.cochrane-handbook.org. [Google Scholar]
- [22].Jones HE. Introduction to meta-analysis. Paediatric and Perinatal Epidemiology 2010;24: [DOI] [PubMed] [Google Scholar]
- [23].Inagaki N, Kondo K, Yoshinari T, et al. Efficacy and safety of canagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled with diet and exercise: a 24-week, randomized, double-blind, placebo-controlled, Phase III study. Expert Opin Pharmacother 2014;15:1501–15. [DOI] [PubMed] [Google Scholar]
- [24].Lavalle-Gonzalez FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia 2013;56:2582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Polidori D, Sha S, Mudaliar S, et al. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo-controlled study. Diabetes Care 2013;36:2154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sha S, Devineni D, Ghosh A, et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab 2011;13:669–72. [DOI] [PubMed] [Google Scholar]
- [27].Polidori D, Sha S, Ghosh A, et al. Validation of a novel method for determining the renal threshold for glucose excretion in untreated and canagliflozin-treated subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab 2013;98:E867–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rosenstock J, Aggarwal N, Polidori D, et al. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care 2012;35:1232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Toubro S, Cefalu W, Xie J, et al. Canagliflozin, a sodium glucose co-transporter 2 inhibitor, reduces body weight mainly through loss of fat mass in subjects with type 2 diabetes. Presented at 48th EASD Annual Meeting; October 1–5, 2012; Berlin, Germany. [Google Scholar]
- [30].Davidson MH, Hauptman J, DiGirolamo M, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA 1999;281:235–42. [DOI] [PubMed] [Google Scholar]
- [31].Neter JE, Stam BE, Kok FJ, et al. Influence of weight reduction on blood pressure a meta-analysis of randomized controlled trials. Hypertension 2003;42:878–84. [DOI] [PubMed] [Google Scholar]
- [32].Kahn SE. The importance of β-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab 2001;86:4047–58. [DOI] [PubMed] [Google Scholar]
- [33].Mathis D, Vence L, Benoist C. β-Cell death during progression to diabetes. Nature 2001;414:792–8. [DOI] [PubMed] [Google Scholar]
- [34].Wajchenberg BL. Clinical approaches to preserve β-cell function in diabetes. Advances in Experimental Medicine & Biology 2010;654:515–35. [DOI] [PubMed] [Google Scholar]
- [35].Geerlings SE, Brouwer EC, Gaastra W, et al. Effect of glucose and pH on uropathogenic and non-uropathogenic Escherichia coli: studies with urine from diabetic and non-diabetic individuals. J Med Microbiol 1999;48:535–9. [DOI] [PubMed] [Google Scholar]
- [36].Nicolle L, Capuano G, Ways K, et al. Effect of canagliflozin, a sodium glucose co-transporter 2 (SGLT2) inhibitor, on bacteriuria and urinary tract infection in subjects with type 2 diabetes enrolled in a 12-week, phase 2 study. Curr Med Res Opin 2012;28:1167–71. [DOI] [PubMed] [Google Scholar]
- [37].Khan SN, Mermer MJ, Myers E, et al. The roles of funding source, clinical trial outcome, and quality of reporting in orthopedic surgery literature. Am J Orthop (Belle Mead NJ) 2008;37:E205–12. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


