Abstract
The aim of this study was to determine the relationship between lobar severity of emphysema and lung cancer using automated lobe segmentation and emphysema quantification methods.
This study included 78 patients (74 males and 4 females; mean age of 72 years) with the following conditions: pathologically proven lung cancer, available chest computed tomographic (CT) scans for lobe segmentation, and quantitative scoring of emphysema. The relationship between emphysema and lung cancer was analyzed using quantitative emphysema scoring of each pulmonary lobe.
The most common location of cancer was the left upper lobe (LUL) (n = 28), followed by the right upper lobe (RUL) (n = 27), left lower lobe (LLL) (n = 13), right lower lobe (RLL) (n = 9), and right middle lobe (RML) (n = 1). Emphysema ratio was the highest in LUL, followed by that in RUL, LLL, RML, and RLL. Multivariate logistic regression analysis revealed that upper lobes (odds ratio: 1.77; 95% confidence interval: 1.01–3.11, P = 0.048) and lobes with emphysema ratio ranked the 1st or the 2nd (odds ratio: 2.48; 95% confidence interval: 1.48–4.15, P < 0.001) were significantly and independently associated with lung cancer development.
In emphysema patients, lung cancer has a tendency to develop in lobes with more severe emphysema.
Keywords: computed tomography, CT, lobe, lung cancer, pulmonary emphysema, segmentation
1. Introduction
Lung cancer is the leading cause of cancer death worldwide. While cigarette smoking is a major risk factor for lung cancer, other factors such as family history of lung cancer, prior diagnosis of malignant tumor, occupational exposure to asbestos, and pre-existing lung disease such as chronic obstructive pulmonary disease (COPD) can also increase the risk of lung cancer.[1,2] As emphysema has been considered as a major component of COPD rather than a separate disease entity, the unique effect of emphysema on the development of lung cancer has not drawn much attention. Recently, several studies have reported that visual identification of emphysema and the severity of emphysema on CT assessed by visual scoring method are associated with an increased risk of lung cancer.[3,4] However, studies to determine the relationship between quantitatively assessed emphysema and lung cancer have shown conflicting results[5–8]: Several studies performed on different lung cancer screening groups failed to show correlations between quantitative computed tomographic (CT) measurements of emphysema and lung cancer[5–7]; however, a recent study on a larger population of National Lung Cancer Screening Trial (NLST) showed a correlation between quantitative CT measures of emphysema and lung cancer risk.[8]
In addition, the relationship between lobar severity of emphysema and lung cancer has not been reported previously. Therefore, the objective of the present study was to determine the relationship between the severity of emphysema quantified in each pulmonary lobe and lung cancer on standard chest CT scan.
2. Methods
2.1. Patients
Our Institutional Ethics Committee approved this study. The requirement for informed consent from patients was waived because this was a HIPA A compliant retrospective study. The initial population consisted of 570 consecutive patients who were diagnosed with lung cancer in our hospital between July 2012 and June 2014. The diagnosis of lung cancer was made by histopathologic examinations, clinical evaluations, and findings on follow-up examinations. Of the 570 patients, only those with adequate CT images for automated lobe segmentation and quantitative scoring of emphysema with a single pulmonary nodule/mass on CT images were included in this study. Exclusion criteria were inability to identify the primary lesion, presence of a huge infiltrative mass or multiple masses, a final diagnosis of metastatic lung cancer, presence of interstitial fibrosis, combined obstructive pneumonitis or infection, lung collapse, prior lung resection, and severe deformity of the thorax. Cases with poor-quality chest CT scans and CTs transferred from outside hospitals were also excluded due to technical problems in lobe segmentation and emphysema quantification. As a few isolated pixels were identified as emphysema in the absence of visually discernible emphysema in some patients, patients with emphysema making up <1% of total lung volume were excluded. Finally, a total of 78 patients were included in this study. The patient selection process and reasons for exclusion are summarized in Fig. 1.
Figure 1.
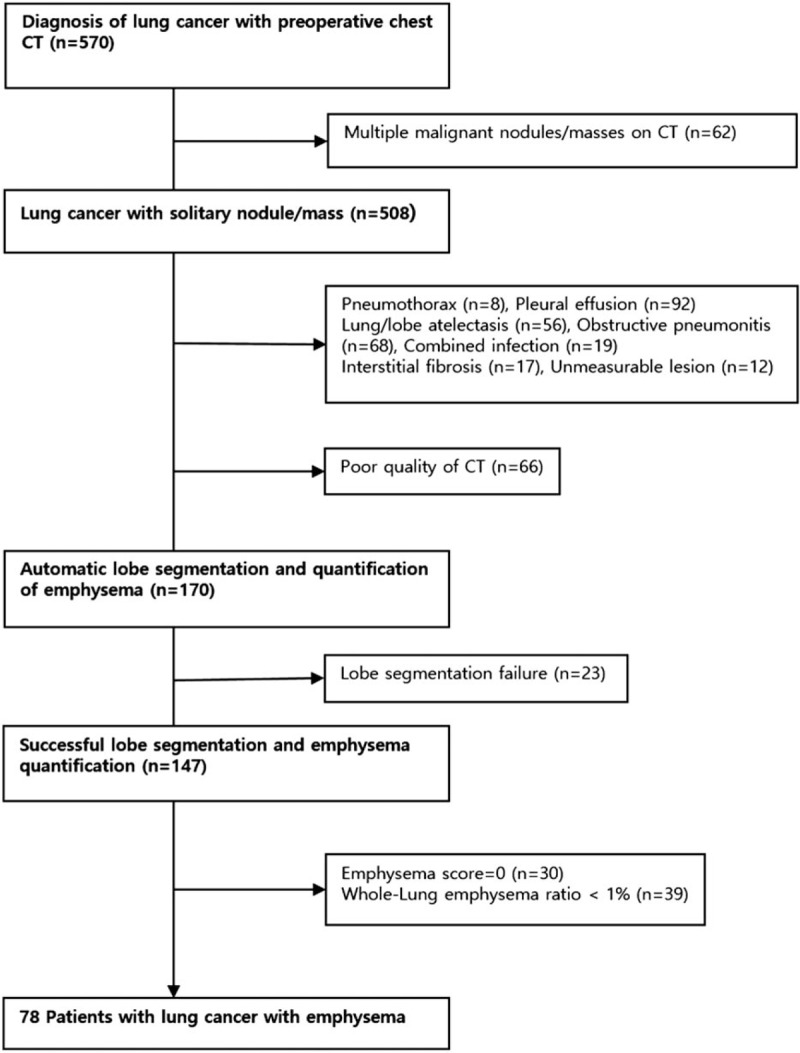
Selection of patients.
2.2. Chest CT examinations
All patients underwent contrast-enhanced chest CT for diagnosis and clinical staging of lung cancer. Chest CT scans were obtained using 64-channel multidetector CT scanners (Brilliance-64; Philips Medical Systems, Best, The Netherlands) during a full-inspiration breath-hold using the following parameters: detector configuration of 64 x 0.625 mm, tube voltage of 120 kVp, tube current of 180 to 200 mAs, pitch of 0.923 or 0.987, and gantry rotation time of 0.5 seconds. Data reconstruction was performed in 2 mm thick axial images with 1 mm of overlapping with a standard reconstruction kernel. The whole lung parenchyma (from the lung apex to the diaphragm) was scanned in craniocaudal direction. The location and size of the nodule or mass were determined by 1 experienced chest radiologist (15 years’ experience) who did not participate in the process of lobe segmentation or quantification of emphysema.
2.3. Emphysema quantification
All chest CT scans were transferred to a workstation (IntelliSpace Portal 7.0; Philips Healthcare, Cleveland, OH). Pulmonary-dedicated software (COPD; Philips Healthcare, Cleveland, OH) was used for segmentation of both lungs into lobes and quantification of emphysema. All image analyses were performed by 1 radiologist who was blinded to the purpose of this study. CT data were loaded first for the quantification procedure. Results were displayed in 3 dimensions (3D) in each step. Borders of each lung, fissures, and central airways were detected automatically. Tracheobronchial tree up to the subsegmental level was identified. Next, both lungs were differentiated from the surrounding chest wall and mediastinal structures. Lobe segmentation was then performed to allow automated delineation for each of the 5 pulmonary lobes using automatic lobe segmentation algorithm (a model-based method). A number of local fissure surface patches were identified by clustering areas with similar gray-value gradients. These fissure surface patches were used to adapt a geometric mesh model for the 5 lobes. Each lobe was then identified on the basis of the adaptation of the mesh model. When lobe segmentation was completed, axial, sagittal, coronal, and volume-rendered images were displayed. A colored mask was superimposed onto the CT images using different color for each lobe. By scrolling through the multiplanar images, it was possible for the operator to evaluate if the automated lobar segmentation was adequate. The operator's interaction with the analysis procedure was minimized as much as possible. However, in a few cases, lobar limits were corrected with a minor adjustment for interlobar boundaries in multiplanar images. For practical purposes, when the correction process took more than 5 minutes, lobe segmentation was abandoned. This was considered as a technical failure. Attenuation of each voxel within segmented lungs was computed automatically. A lower attenuation threshold of -950 HU on high-resolution CT is known to correlate best with morphological emphysema[9]; hence, emphysema volume was calculated as the sum of voxels with attenuation below -950 HU. Lung volume, emphysema volume, and the percent ratio between lung volume and emphysema volume in the lungs as a whole in each separate lung and in individual lobes were displayed in the table (Fig. 2). The mean processing time from lung segmentation to emphysema quantification was 101.9 ± 33.7 seconds (range, 68–260 seconds). The percent ratio between lung volume and emphysema volume in both lungs as a whole and in individual lobes are referred as “whole-lung emphysema ratio” and “lobar emphysema ratio,” respectively. We reviewed patients’ demographics and results of pulmonary function tests [forced expiratory volume in 1 second (FEV1), FEV1/forced vital capacity (FVC)] through medical records.
Figure 2.
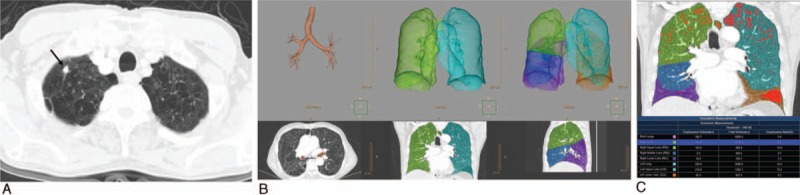
Automatic lobe segmentation process and quantification of emphysema per lobe in a 75-year-old man with lung cancer in the right upper lobe (RUL). (A) An 8 mm sized nodule in RUL (arrow) was confirmed as a squamous cell cancer through wedge resection. (B) Sequential segmentation of airways, lungs, and lobes. (C) Results of volumetric measurement of emphysema in both lungs, each lung, and each lobe (attenuation threshold of voxels, <-950 HU). Emphysematous voxels are shown in red.
2.4. Statistical analysis
Continuous variables (age, pack-years of smoking, emphysema ratio, etc.) were reported as means and standard deviations. Categorical variables (sex, lobar location of lung cancer, etc.) were reported as counts and frequencies. Mann–Whitney U test was used to compare whole-lung emphysema ratio between current smokers and ex-smokers (n = 57) versus never smokers (n = 21). We also used Mann–Whitney U test to compare lobar severity of emphysema between lobes with lung cancer (n = 78) and those without lung cancer (n = 312, 78 x 4).
Logistic regression analysis was used to define significant factors associated with the presence of lung cancer. In this analysis, each individual subject acted as his/her own control because we compared lobes with lung cancer (cases) to lobes without lung cancer (controls). Therefore, there were a total of 78 cases and 312 (78 x 4) controls.
All statistical analyses were performed using SPSS statistical package (SPSS 11.5; SPSS Inc., Chicago, IL). P values ≤0.05 were considered as statistically significant.
3. Results
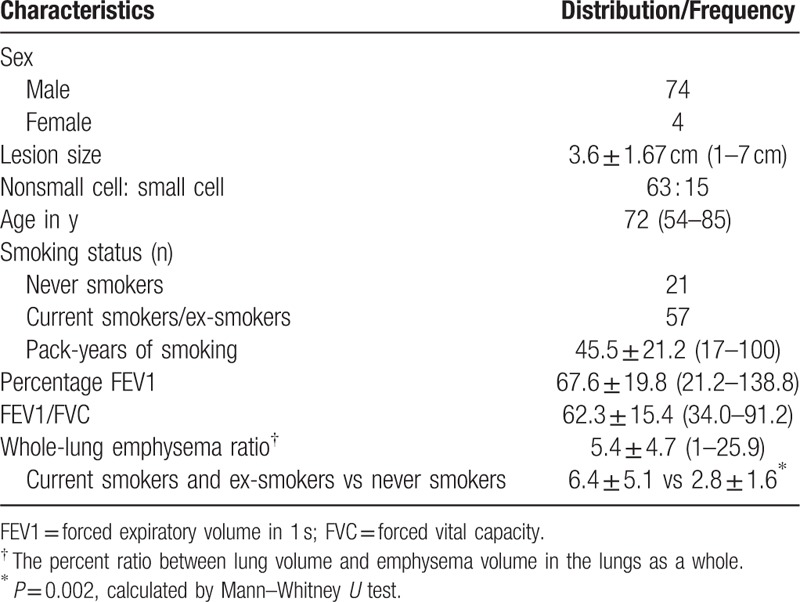
The subjects consisted of 74 males and 4 females with a mean age of 72 years (range, 54–85 years). A total of 21 patients were never smokers and 57 patients were current smokers or ex-smokers. Of the 78 patients, 63 (80.8%) had nonsmall cell lung cancer and 15 (19.2%) had small cell lung cancer. The mean diameter of lung cancer was 3.6 ± 1.7 cm (range, 0.8–7 cm).
The mean whole-lung emphysema ratio was 5.4 ± 4.7% (range, 1–25.9%). The emphysema ratio in smokers and ex-smokers was significantly higher than that in never smokers (6.4 ± 5.1% vs 2.8 ± 1.6%, P = 0.002). The emphysema ratio was negatively correlated with the percentage of FEV1 (percentage FEV1, Pearson coefficient: -0.30, P = 0.01) and the ratio of FEV1 to FVC (FEV1/FVC, Pearson coefficient: -0.36, P = 0.003) (Table 1).
Table 1.
Patient demographics (n = 78).
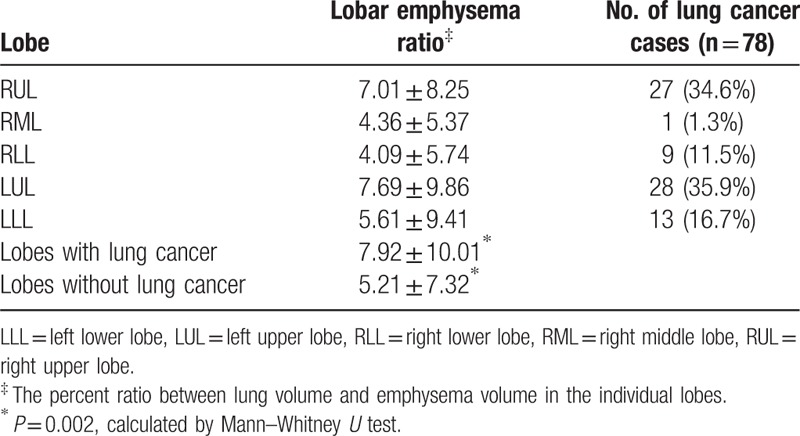
The most frequent location of lung cancer was the left upper lobe (LUL, n = 28, 35.9%), followed by the right upper lobe (RUL, n = 27, 34.6%), left lower lobe (LLL, n = 13, 16.7%), right lower lobe (RLL, n = 9, 11.5%), and right middle lobe (RML, n = 1, 1.3%). Lung cancer developed more frequently in the upper lobes than that in the lower/middle lobes (35.3% vs 9.8%, P < 0.001). The lobar emphysema ratio was the highest in the LUL (7.68%), followed by that in RUL (7.01%), LLL (5.60%), RML (4.36%), and RLL (4.08). Emphysema impacted the upper lobes more severely than it did the lower/middle lobes (7.36 ± 9.01 vs 4.65 ± 7.03, P < 0.001). The mean lobar emphysema ratio of lobes with lung cancer was higher than that of lobes without cancer (7.92 ± 10.01% vs 5.21 ± 7.32%, P = 0.002) (Table 2).
Table 2.
Emphysema ratio in each lobe and incidence of lung cancer.
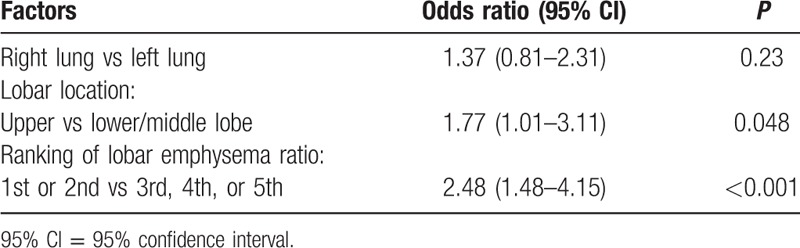
To assess whether lobar emphysema severity was a predictor of the location of lung cancer, the 5 lobes were ranked (1st–5th) according to lobar emphysema ratio in each individual. Of a total of 78 tumors, 26 developed in lobes ranked the first, 20 developed in those ranked the 2nd, 18 developed in those ranked the 3rd, 5 developed in those ranked the 4th, and 9 developed in lobes ranked the 5th. Multivariate logistic regression analysis revealed that the odds of having lung cancer in lobes with the 1st or the 2nd lobar emphysema ratio was 2.48 [95% confidence interval (95% CI): 1.48–4.15, P < 0.001] compared with that in other lobes (3rd, 4th, or 5th). The odds of developing lung cancer in the upper lobes was 1.77 (95% CI: 1.01–3.11, P = 0.048) compared with that in the lower/middle lobes. There was no significant difference in the incidence of cancer in the right lung versus the left lung (Table 3).
Table 3.
Results of multivariate logistic regression analyses of factors that may affect the presence of lung cancer.
4. Discussion
In the era of lung cancer screening with CT, many indeterminate nodules are being detected on chest CT.[10,11] Besides the identification of potentially malignant lung nodules, detection of emphysema is also increasing because CT allows direct visualization of lung destruction area.[12] Many studies have been conducted to reveal the effect of emphysema on the development of lung cancer and their biological relationship.[13–15] Because of the common pathogenic process of the 2 disease entities and heterogeneous distribution of emphysema in the lungs, it has been postulated that regional severity of emphysema may determine the location of lung cancer. A few studies have shown that regional magnitude of emphysema is related to the location of lung cancer.[16,17] However, in those studies, the severity of emphysema was measured in a semi-quantitative manner through visual assessment. Although emphysema scoring was done by experienced thoracic radiologists, visual assessment is subjective. It can be biased by the presence of lung cancer in CT images.[18] On the contrary, automated quantification of emphysema using CT densitometry is objective. It correlates better with pathologic severity than visual scoring.[19]
In the present study, to assess the association between regional emphysema severity and the location of lung cancer, we quantitatively measured emphysema in each lobe. In prior studies with similar study subjects, lungs were divided by nonanatomical transverse lines according to lung height.[8,16,17] However, lobar distribution of emphysema is significantly different from the distribution between the upper and lower halves of the lungs divided by a transverse plane. Especially when patients have mild to moderate emphysema as in our patients, the superior segment of the lower lobe found at the level of the upper lobes can dilute the predominance of upper-lobe emphysema. Therefore, lobe segmentation provides more accurate information than nonanatomic approach in ascertaining the effect of regional emphysema on lung cancer.[20] Recently, Gierada et al[8] have demonstrated a difference in quantitative emphysema measurement between patients with lung cancer and those without lung cancer in a National Lung Screening Trial (NLST). The association between lung cancer and quantitatively measured emphysema in the upper lungs is found to be the strongest. To the best of our knowledge, that study was the first one to show a significant relationship between quantitatively measured emphysema and lung cancer. However, they did not identify any practical value of quantifying emphysema in screening because the relationship they found was too weak. In their study, the population was skewed toward lower percentage values of emphysema. Their use of nonanatomic lung segmentation might have weakened the relationship between regional emphysema quantity and lung cancer.
In our study, more severe emphysema was seen in the upper lobes. A higher frequency of lung cancer was also seen in the upper lobes. Our results are in agreement with previous studies.[21–23] In addition, there was a greater amount of emphysema in lobes with lung cancer than that in lobes without lung cancer. We conducted multivariate logistic regression analysis to see if the prevalence of emphysema in lobes with lung cancer could be accounted for the fact that both lung cancers and emphysema were prevalent in the upper lobes. The odds of lung cancer in the upper lobes was 1.77 compared with the lower/middle lobes. The odds of lung cancer in the lobes with more severe emphysema (lobar emphysema ratio ranked 1st or 2nd) was 2.48 than the other lobes with less severe emphysema (lobar emphysema ratio ranked 3rd, 4th, or 5th), which was higher than the odds of lung cancer according to lobar location. Our study showed a significant relationship between quantitatively measured emphysema and lung cancer using lobe segmentation. Automated volume measurement and quantification of emphysema in each lobe have not been widely used in clinical studies due to technical difficulties in lobe segmentation.[24,25] Because of recent advances in lobe segmentation software, we were able to automatically measure the quantity of emphysema in each pulmonary lobe in just a few minutes. Quantitative measurements of lung volume and the amount of emphysema per lobe would also be useful for estimating postoperative lung function in patients with lung cancer.
This study had several limitations. First, the study population was relatively small and predominantly male. Results might be different in a larger population. Second, most patients with severe emphysema were not included in the study group because most of them had conditions (such as pneumonia, pleural effusion, and lung atelectasis) that might affect measurement of lung volume or emphysema. This might affect the statistical analysis. In a previous study, no dose–response effect of emphysema on the risk of lung cancer was found in patients with severe emphysema.[4] Thus, including patients with severe emphysema could weaken the relationship between lobar emphysema quantity and lung cancer. Third, we excluded patients with whole-lung emphysema ratios less than 1%. In addition, some patients were excluded following lobe segmentation failure due to technical reasons. These factors could act as a selection bias. Fourth, we did not analyze subjects according to specific types of lung cancer. It is possible that some types of lung cancer are more likely to be affected by emphysema than other types.[26]
In conclusion, using automated lobe segmentation and quantification methods, we found that lung cancer was most likely to occur in pulmonary lobes with more severe emphysema. To validate the relationship between the quantitative severity of regional emphysema and the likelihood of lung cancer, further study is needed with a larger population.
Footnotes
Abbreviations: COPD = chronic obstructive pulmonary disease, CT = computed tomography, FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity, LLL = left lower lobe, LUL = left upper lobe, RLL = right lower lobe, RML = right middle lobe, RUL = right upper lobe.
The authors have no funding and conflicts of interests to disclose.
References
- [1].Marcus MW, Raji OY, Field JK. Lung cancer screening: identifying the high risk cohort. J Thorac Dis 2015;7suppl 2:S156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Turner MC, Chen Y, Krewski D, et al. Chronic obstructive pulmonary disease is associated with lung cancer mortality in a prospective study of never smokers. Am J Respir Crit Care Med 2007;176:285–90. [DOI] [PubMed] [Google Scholar]
- [3].de Torres JP, Bastarrika G, Wisnivesky JP, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest 2007;132:1932–8. [DOI] [PubMed] [Google Scholar]
- [4].Wilson DO, Weissfeld JL, Balkan A, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med 2008;178:738–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kishi K, Gurney JW, Schroeder DR, et al. The correlation of emphysema or airway obstruction with the risk of lung cancer: a matched case-controlled study. Eur Respir J 2002;19:1093–8. [DOI] [PubMed] [Google Scholar]
- [6].Maldonado F, Bartholmai BJ, Swensen SJ, et al. Are airflow obstruction and radiographic evidence of emphysema risk factors for lung cancer? A nested case-control study using quantitative emphysema analysis. Chest 2010;138:1295–302. [DOI] [PubMed] [Google Scholar]
- [7].Wilson DO, Leader JK, Fuhrman CR, et al. Quantitative computed tomography analysis, airflow obstruction, and lung cancer in the Pittsburgh lung screening study. J Thorac Oncol 2011;6:1200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gierada DS, Guniganti P, Newman BJ, et al. Quantitative CT assessment of emphysema and airways in relation to lung cancer risk. Radiology 2011;261:950–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gevenois PA, De Vuyst P, de Maertelaer V, et al. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 1996;154:187–92. [DOI] [PubMed] [Google Scholar]
- [10].Yip R, Henschke CI, Yankelevitz DF, et al. CT screening for lung cancer: alternative definitions of positive test result based on the national lung screening trial and international early lung cancer action program databases. Radiology 2014;273:591–6. [DOI] [PubMed] [Google Scholar]
- [11].Aberle DR, Adams AM, Berg CD, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Omori H, Nakashima R, Otsuka N, et al. Emphysema detected by lung cancer screening with low-dose spiral CT: prevalence, and correlation with smoking habits and pulmonary function in Japanese male subjects. Respirology 2006;11:205–10. [DOI] [PubMed] [Google Scholar]
- [13].Mikita K, Saito H, Sakuma Y, et al. Growth rate of lung cancer recognized as small solid nodule on initial CT findings. Eur J Radiol 2012;81:e548–53. [DOI] [PubMed] [Google Scholar]
- [14].Henschke CI, Yip R, Boffetta P, et al. CT screening for lung cancer: importance of emphysema for never smokers and smokers. Lung Cancer 2015;88:42–7. [DOI] [PubMed] [Google Scholar]
- [15].Kinsey CM, San Jose Estepar R, Wei Y, et al. Regional emphysema of a non-small cell tumor is associated with larger tumors and decreased survival rates. Ann Am Thorac Soc 2015;12:1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hohberger LA, Schroeder DR, Bartholmai BJ, et al. Correlation of regional emphysema and lung cancer: a lung tissue research consortium-based study. J Thorac Oncol 2014;9:639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bishawi M, Moore W, Bilfinger T. Severity of emphysema predicts location of lung cancer and 5-y survival of patients with stage I non-small cell lung cancer. J Surg Res 2013;184:1–5. [DOI] [PubMed] [Google Scholar]
- [18].Cavigli E, Camiciottoli G, Diciotti S, et al. Whole-lung densitometry versus visual assessment of emphysema. Eur Radiol 2009;19:1686–92. [DOI] [PubMed] [Google Scholar]
- [19].Bankier AA, De Maertelaer V, Keyzer C, et al. Pulmonary emphysema: subjective visual grading versus objective quantification with macroscopic morphometry and thin-section CT densitometry. Radiology 1999;211:851–8. [DOI] [PubMed] [Google Scholar]
- [20].Owsijewitsch M, Ley-Zaporozhan J, Kuhnigk JM, et al. Quantitative emphysema distribution in anatomic and non-anatomic lung regions. COPD 2015;12:257–66. [DOI] [PubMed] [Google Scholar]
- [21].Byers TE, Vena JE, Rzepka TF. Predilection of lung cancer for the upper lobes: an epidemiologic inquiry. J Natl Cancer Inst 1984;72:1271–5. [PubMed] [Google Scholar]
- [22].Kinsey CM, Estepar RS, Zhao Y, et al. Invasive adenocarcinoma of the lung is associated with the upper lung regions. Lung Cancer 2014;84:145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Thurlbeck WM, Muller NL. Emphysema: definition, imaging, and quantification. AJR Am J Roentgenol 1994;163:1017–25. [DOI] [PubMed] [Google Scholar]
- [24].Revel MP, Faivre JB, Remy-Jardin M, et al. Automated lobar quantification of emphysema in patients with severe COPD. Eur Radiol 2008;18:2723–30. [DOI] [PubMed] [Google Scholar]
- [25].Qi S, van Triest HJ, Yue Y, et al. Automatic pulmonary fissure detection and lobe segmentation in CT chest images. Biomed Eng Online 2014;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Smith BM, Schwartzman K, Kovacina B, et al. Lung cancer histologies associated with emphysema on computed tomography. Lung Cancer 2012;76:61–6. [DOI] [PubMed] [Google Scholar]


