Abstract
Purpose
We aimed to compare the efficacy and safety of midazolam plus ketamine versus fentanyl plus propofol combination administered to children undergoing upper gastrointestinal endoscopy (UGE) and to determine the most appropriate sedation protocol.
Materials and methods
This prospective, randomized, single-blind study included patients between the ages of 4 and 17 years who underwent UGE for diagnostic purposes. Patients were divided randomly into groups A (midazolam–ketamine combination, n=119) and B (fentanyl plus propofol combination, n=119). The effectiveness of the sedation and complications during the procedure and recovery period were recorded.
Results
The processes started without an additional dose of the drug for 118 patients (99.1%) in group A and for 101 patients (84.8%) in group B (P=0.001). The average dose of ketamine administered to the patients in group A was 1.03±0.15 mg/kg and the average dose of propofol administered to the patients in group B was 1.46±0.55 mg/kg. None of the patients stopped the endoscopic procedure in group A, but one patient (0.8%) had to discontinue the endoscopic procedure in group B. 27 patients in group A (22.7%) and 41 patients (34.5%) in group B developed complications during the procedure (P=0.044). The rate of complications during the recovery of group A (110 patients, 92.4%) was significantly higher than that in group B (48 patients, 40.3%) (P=0.001).
Conclusion
In children, UGE procedures can be quite comfortable when using the midazolam–ketamine combination. However, adverse effects related to ketamine were observed during recovery.
Keywords: children, endoscopy, sedation
Introduction
The use of upper gastrointestinal endoscopy (UGE) for diagnosis and treatment is becoming increasingly more frequent in children. Unlike adults, children require deep sedation 1. It is difficult to adjust the depth of anaesthesia in invasive procedures outside the operating room. Although target-controlled infusion (TCI) or total intravenous anaesthesia (TIVA) infusion pumps enable rapid adjustments in sedation level, optimal conditions may not be available everywhere or every time. Although superficial anaesthesia in children prevents successful performance, very deep anaesthesia or sedation may cause respiratory depression and other related side effects. A large number of sedatives and anaesthetic drugs are used in the UGE process in children. The most commonly used drugs are ketamine, propofol, midazolam and fentanyl.
The effects of ketamine, one of the most commonly used agents in UGE of children, are rapid, but short; ketamine offers quick recovery and has wide confidence intervals 2,3. When used for sedation and anaesthesia, ketamine causes dissociative anaesthesia characterized by amnesia and analgesia 4,5. However, side effects such as aspiration, stridor, laryngospasm and after-sedation nausea have been reported 6,7. In addition, there may be some effects called emergence reactions such as nightmares, delirium, excitation and physical aggression 8. Ketamine is used in combination with benzodiazepines (especially midazolam) to reduce the frequency of these side effects 2,3,9–11. Midazolam is a very short-acting benzodiazepine. It has sedative, hypnotic, anxiolytic and anticonvulsant properties and causes anterograde amnesia 12,13.
Another agent used frequently in the UGE of children is propofol. It provides rapid and reliable unconsciousness and consciousness with its sedative, hypnotic and amnestic effects. However, its analgesic effect is weak 14. Despite its use by anaesthesiologists in many centres, it can cause hypotension and respiratory depression at high doses 15,16. The use of propofol in combination with other sedatives provides a synergistic effect and enables the use of lower doses 17,18. Fentanyl is used in combination with propofol as an adjuvant to allow for effective use of propofol at lower doses 14.
The best sedation procedure with minimal side effects providing the best comfort both for the patient and for the endoscopist during the UGE of children is still under discussion. The primary aim of this study was to investigate the efficiency and safety of midazolam plus ketamine versus fentanyl plus propofol administered to children undergoing UGE and to determine the most appropriate sedation protocol. As a secondary aim, we compared the groups with respect to comfort and complications.
Materials and methods
This prospective, randomized, single-blind study was carried out in the department of Pediatric Gastroenterology, Hepatology and Nutrition at the Kanuni Training and Research Hospital. The study was carried out after receiving approval from the local ethics committee (Registry Url: 2015/18 Identifier: Trabzon Kanuni Training and Research Hospital Clinical Research Ethics Committee) and informed parental consent in accordance with the Declaration of Helsinki. The study was planned for 250 patients who underwent UGE for diagnostic purposes. The clinical trial was registered before patient enrollment and patient consent was written. All the patients were American Society of Anesthesiologists (ASA) physical status I or II 19. Patients with respiratory tract infections, glaucoma, psychosis, porphyria, hypertension, metabolic or neurologic diseases, increased intracranial pressure and intracranial mass and patients known to be allergic to the drugs used were excluded from the study. Patients undergoing therapeutic or urgent endoscopy were not included in this study. This study was registered on 7 April 2016 at https//:clinicaltrials.gov as NCT02732132.
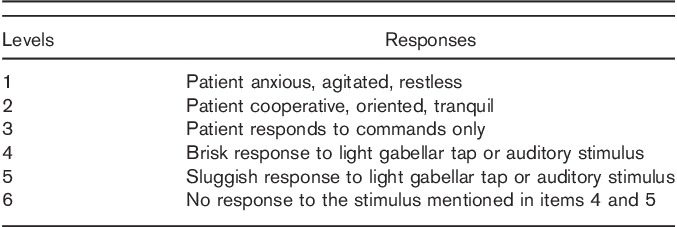
The procedures were applied in the endoscopy unit by a paediatric endoscopist and an endoscopy nurse. All sedation procedures were performed by trained and experienced anaesthesiologists. Patients’ follow-up was performed by another nurse in the recovery room after the procedure. All procedures were performed using the Olympus GIF-H180 device (Olympus Corporation, Tokyo, Japan) in the left lateral position. After 6-h fasting 20, blinded patients were randomized 1 : 1 to receive midazolam plus ketamine combination in group A or fentanyl plus propofol combination in group B for sedation. The effectiveness of sedation during the procedure was evaluated according to a modified Ramsay sedation score (RSS) (Table 1). A score from 1–6 was assigned according to the response of the patient to the stimuli. A score of 5 or above indicates adequate sedation 21. RSS of 5 or above was aimed at for a comfortable procedure in our study.
Table 1.
Ramsay scale for the assessment of the level of sedation
The patients were administered a spray of lidocaine 10% (Xylocaine spray; AstraZeneca, Silk Rod, UK) as a topical pharyngeal anaesthesia before sedation. An intravenous midazolam (Dormicum, 1 mg/ml, 5 ml; Deva Holding, Istanbul, Turkey) bolus dose 0.1 mg/kg (maximum 4 mg) was administered to group A. Two minutes later, ketamine (Ketalar, 50 mg/ml, 10 ml; Pfizer, Sandwich, UK) bolus dose 1 mg/kg was administered intravenously. Patient responses to verbal and tactile stimuli were evaluated 2 min after the administration of ketamine. Ketamine 0.5 mg/kg (maximum of 2 mg/kg) was added at 2-min intervals if adequate sedation was not achieved initially. The endoscopy process was initiated by the endoscopist if the child was well sedated. If the patients were agitated after the start of the procedure, a single dose of ketamine 0.5 mg/kg was administered. Intravenous fentanyl (Fentanyl, 0.05 mg/ml, 10 ml; Johnson & Jonhson, Turnhout, Belgium) bolus dose 1 µg/kg was administered to group B. Two minutes later, propofol (Propofol-Lipuro, 10 mg/ml, 20 ml; B. Braun, Melsungen, Germany) bolus dose 1 mg/kg was administered intravenously. Patient responses to verbal and tactile stimuli were evaluated 2 min after the administration of propofol. Propofol 0.5 mg/kg was added at 2-min intervals if adequate sedation was not achieved. The endoscopy process was initiated by the endoscopist if there was no response. If the patients were agitated after the procedure started, a single dose of propofol 0.5 mg/kg was administered. None of the patients were administered an antidote after the process during recovery.
All patients were monitored for peripheral oxygen saturation, heart rate (HR), respiratory rate and RSS during the procedure. Oxygen (2 l/min) by a nasal cannula was administered to all patients during the procedure. Cardiac arrest, apnoea and laryngospasm were assessed as major complications, whereas hypoxia (peripheral oxygen saturation <90% during 60 s), tachycardia (defined as 30% more than the average HR by age), bradycardia (30% less than the average HR by age), increase in oral secretions (copious oral secretions requiring suctioning), flushing, coughing and vomiting were assessed as minor complications. All complications were recorded. The start and end of the process were recorded after providing appropriate sedation. The completion of the procedure without any major complications indicated the success rate of sedations.
The recovery of the patients was assessed using a modified Aldrete score. The modified Aldrete score was determined by scoring from 0 to 10 according to the patient’s activity, oxygen saturation, consciousness, respiration and circulation 22. Patients with an Aldrete score of 9 or greater were discharged from the endoscopy unit. The time between the end of the process and departure from the endoscopy unit (the recovery time) was recorded. Complications during the recovery time such as double vision, dizziness, nausea and vomiting were recorded. Hallucinations, agitation and emergence reactions were evaluated and recorded. Emergence reactions included severe agitations such as nightmares, hallucinations and delirium observed during the recovery time 11.
Mean±SD were used as descriptive statistics; an independent two-sample t-test was used for normal distribution in the comparison of two groups and the Mann–Whitney U-test was used to identify non-normally distributed variables. A χ2-test was used to compare categorical variables. P less than 0.05 was considered statistically significant. The data were evaluated using the SPSS, version 13.0 (SPSS Inc., Chicago, Illinois, USA) statistical package program.
Results
Five parents did not agree to participate in the study and seven patients were excluded from the study because of missing data. Therefore, 238 patients were evaluated (119 patients in group A and 119 patients in group B). The median age of the patients was 12 years (range: 4–17 years; mean SD: 12.21±3.38 years) and the median weight was 40 kg (range: 17–95 kg; mean±SD: 41.92±15.24 kg). There were no differences in age, sex and weight between the groups. The most frequent indication for endoscopy was chronic abdominal pain (60.5%). Other indications were chronic dyspepsia (19.3%), reflux-related symptoms (14.2), suspected coeliac disease (2.1%) and chronic diarrhoea (1.4%).
The endoscopy process started without an additional dose of the drug for 118 patients (99.1%) in group A and for 101 patients (84.8%) in group B (P=0.001). Six patients in group A were agitated during the endoscopic procedure and were administered additional doses of ketamine (5.0%) and 45 patients in group B (36.1%) were administered additional propofol (P=0.001). The average dose of ketamine administered to the patients in group A was 1.03±0.15 mg/kg and the average dose of propofol administered to the patients in group B was 1.46±0.55 mg/kg (Table 2). The endoscopic procedure was not discontinued for any of the patients in group A, but for one patient in group B (0.8%), the endoscopic procedure had to be discontinued as the patient was agitated during the procedure.
Table 2.
Evaluation of patients during and after endoscopy
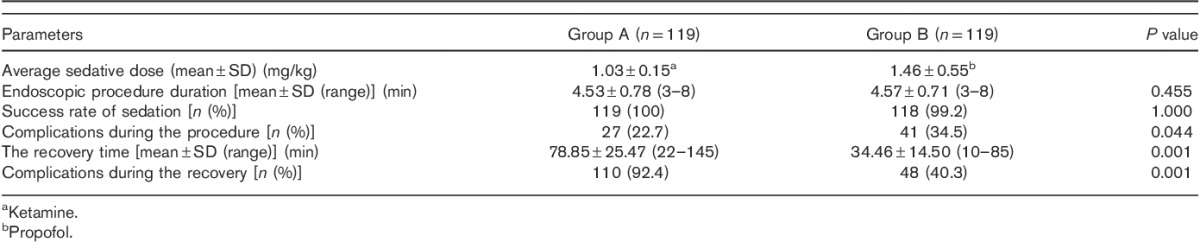
The average endoscopic procedure duration was about 5 min (range: 3–8 min, mean±SD: 4.55±0.75 min). No difference was recorded in the endoscopic procedure times between the two groups (Table 2). RSS measured during the intervention (after the endoscope passed the oesophagus and until the end of the procedure) was significantly higher in group A than in group B (mean±SD: 4.94±1.17 vs. 2.98±1.88, P<0.01). There were 41 patients with an RSS score of 6 in group A (34.5%) and five patients in group B with an RSS score of 6 (4.2%). There were only six patients with an RSS score of one in group A (5.0%), but 52 patients with an RSS score of one in group B (43.7%) (Fig. 1). A post-hoc power analysis showed that from the data obtained from our study, power was calculated to be 99%, indicating a significant difference in RSS between the groups.
Fig. 1.
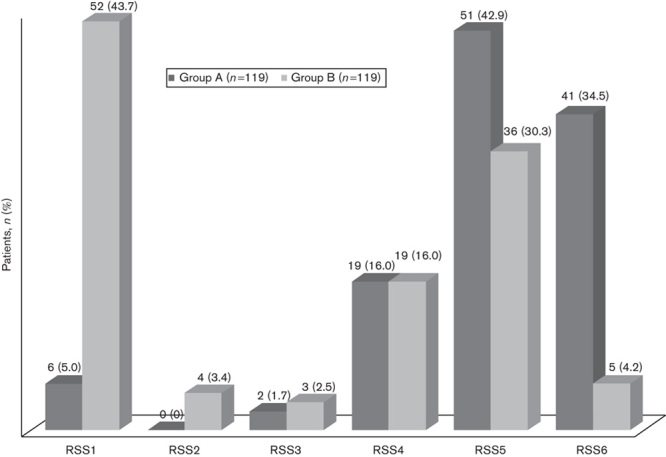
Ramsay sedation score (RSS) of the patients.
No patients developed major complications such as cardiac arrest, apnoea or laryngospasm during the procedure. A total of 68 patients (28.6%) developed minor complications; 27 patients (22.7%) in group A and 41 patients (34.5%) in group B developed complications [P=0.044, odds ratio (OR): 0.55, 95% confidence interval (CI): 0.31–0.98] (Fig. 2). Vomiting, cough, tachycardia and increased oral secretions were observed more often in group A, but flushing, hypoxia and bradycardia were observed more frequently in group B. There was a statistically significant difference only in the occurrence of bradycardia among these complications (one patient in group A and 11 patients in group B) (P=0.003, OR: 0.08, 95% CI: 0.01–0.65) (Fig. 2). Oxygen saturation of six patients developing hypoxia improved in a short time with additional oxygen (4–6 l/min) and the positioning of the airway. The endoscopic procedures were completed successfully with the improvement in oxygen saturation and the endoscopic procedures were not stopped in any patient. Although an increase in secretion is a risk in aspiration and laryngospasm, these complications were observed in none of our patients. Symptoms suggestive of aspiration pneumonia were not observed in the recovery room after the procedure or during outpatient follow-up of these patients. Bradycardia did not cause any haemodynamic disorders such as hypotension in any of the patients. Normal pulse rate was obtained after applying 0.02 mg/kg atropine (Atropine Sulfate Biofarma, 0.25 mg/ml, 1 ml; Biofarma, Istanbul, Turkey) and the endoscopic procedures were completed successfully. All endoscopic procedures were completed successfully in group A. An endoscopic procedure had to be terminated in group B as the patient was agitated during the process. No correlation was discovered between weight and sex of the patients and complications during the endoscopic procedures.
Fig. 2.
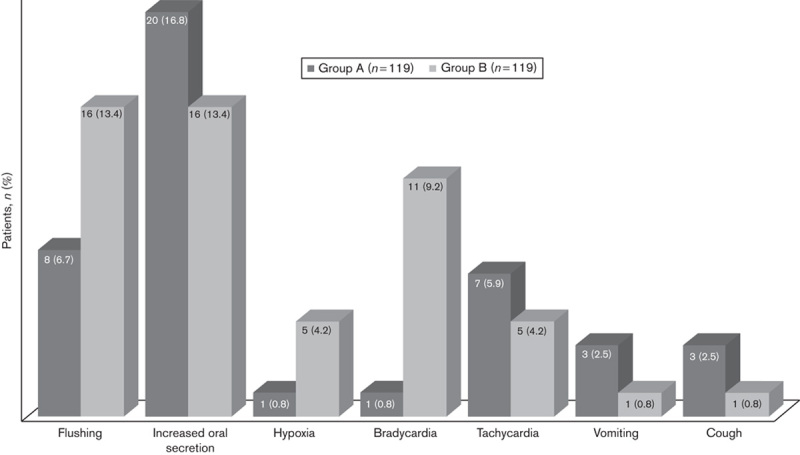
Frequency of complications during the procedure.
The average recovery time was 55 min (range: 10–145 min, mean±SD: 56.65±30.37 min). The recovery time of group A was significantly higher than that of group B (78.85±25.47 vs. 34.46±14.50 min, P<0.01).
A total of 158 patients (66.4%) developed complications during the recovery. Dizziness was the most frequent complication (142 patients, 59.7%). Complication rates of group A (110 patients, 92.4%) were significantly higher than those of group B (48 patients, 40.3%) (P=0.001, OR: 18.07, 95% CI: 8.35–39.12) (Table 2). Headache, dizziness, hallucinations, double vision, anxiety, nausea and vomiting were significantly more prevalent in group A than group B (Fig. 3). Emergence reactions were observed in six patients (5.0%) in group A, but none in group B. No correlation was found between sex and age of the patients and complications during the recovery.
Fig. 3.
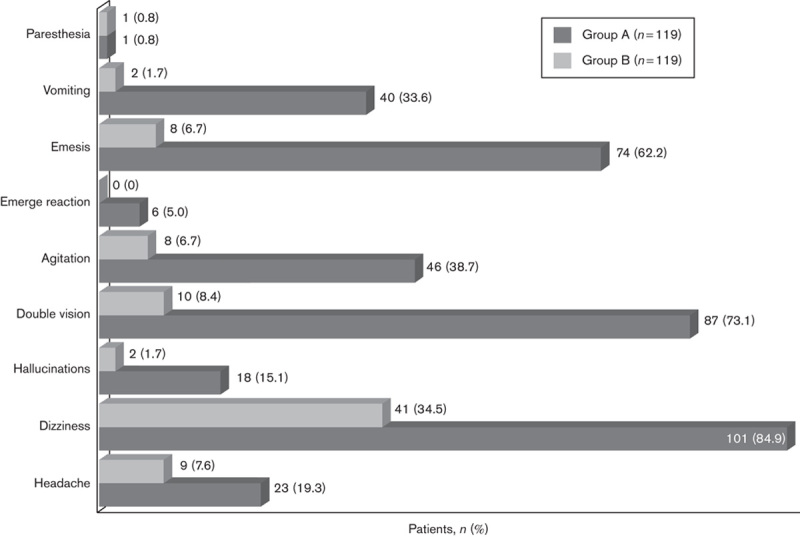
Frequency of complications during the recovery.
Discussion
In this study, effective sedation was provided with midazolam plus ketamine (midazolam–ketamine) and fentanyl plus propofol (fentanyl–propofol) when performing an upper gastrointestinal UGE on children. The procedures performed with the midazolam–ketamine combination were more comfortable than that in the fentanyl–propofol group during the procedure. However, the recovery time was longer in the midazolam–ketamine group. However, the fentanyl–propofol group was more comfortable in the recovery period in terms of complications.
The UGE is performed in specific units out of the operating theatre. It may not always be suitable for general anaesthesia everywhere because of equipment and physical conditions. Sedation is generally used for this endoscopic procedure. Therefore, we preferred sedation. Mature teenagers can tolerate the UGE under mild sedation, but children, especially those younger than 10 years of age, may not be cooperative and may not tolerate this procedure. Therefore, we preferred deep sedation (RSS>5) to perform the UGE. However, the need for deeper sedation during procedures and anatomical differences contribute towards the development of complications. Higher rates of complications associated with sedation are encountered in children compared with adults 23. The most appropriate sedative drugs and experienced personnel are needed to reduce the risks associated with sedation in children.
Numerous studies have investigated the use of ketamine when performing UGE on children. These studies showed that ketamine plus midazolam is more effective and has fewer side effects than ketamine alone 2,3,11. In our previous study, we used 0.1 mg/kg midazolam and a 0.5 mg/kg low dose of ketamine in UGE for children; the success rate was 97.42% 24. Brecelj et al. 11 reported that the initial dose of ketamine should be 1–1.5 mg/kg and its efficacy is not sufficient at lower doses. In the present study, we used 1.03 mg/kg ketamine, on average, and achieved 100% success, which supports their view.
Sedation with propofol is effective. The ratio of unsuccessful sedation reported in previous studies ranged between 0 and 0.4% 25–27. However, 14.2% of the children undergoing UGE experienced extreme discomfort when propofol was used alone 28. In contrast, a high dose of propofol causes respiratory depression 27. It has been stated that propofol has no analgesic properties and it may be combined with analgesics for all painful procedures 29. Propofol in combination with midazolam or fentanyl was shown to provide greater comfort during the procedure and to lead to fewer side effects 1,16. A combination of these drugs enables the use of lower doses of propofol 15. In the study carried out by Tosun et al. 1, 1 µg/kg fentanyl and 1.2 mg/kg propofol were administered initially, and 50% of the patients had to be administered additional doses of propofol. In that study, the average amount of propofol administered to the patients was 2.48 mg/kg. In our study, 52.9% of the patients who were administered 1 mg/kg propofol at the beginning of sedation had to be administered an additional dose of propofol (1.46 mg/kg, on average). Despite the high success rate of the procedure in these patients, their comfort during the process was significantly lower than that for the patients in the midazolam plus ketamine group (the RSS of 43.7% of the patients was 1). We believed that the dose of propofol and fentanyl was lower because of the absence of TCI for TIVA in our hospital conditions; thus, the comfort of this group could not reach the level of the other group.
Ketamine increases airway secretions and can cause laryngospasm 30. In a meta-analysis, 3.9% of the serious airway or respiratory side effects were associated with ketamine sedation in children 31. In particular, administration of high doses of ketamine (initial dose of >2.5 mg/kg) and accompanied use of benzodiazepine increase the risk of complications for children under the age of 2 years. Although mild respiratory side effects, such as a temporary decrease in saturation, have been recorded in sedation with propofol, major respiratory complications, such as severe hypoxia, apnoea and total airway obstruction, have rarely been reported 32. In the study by Larsen and colleagues, minor respiratory complications occurred in 35% of the children under the age of 1 year and in 12.5% of the children under the age of 10 years 27. In their study involving 811 children, Disma et al. 16 reported that 14 patients (1.7%) who developed laryngospasm were younger than 4 years of age. In our study, hypoxia was more frequent in the fentanyl–propofol group. We believed that respiratory depression because of propofol caused hypoxia. Because positioning the airway led to stimuli of respiration, desaturation of oxygen was restored. However, the finding of hypoxia was not statistically significant, and it soon improved in both groups after positioning the airway and providing additional oxygen. In our study, no major side effects such as laryngospasm developed. The emerging minor complication rate was low and the procedures were completed successfully.
Fentanyl has very little effect on the cardiovascular system. However, it can cause a moderate sinus bradycardia when used with other sedative drugs. It was reported that the combination of fentanyl with those other drugs may cause bradycardia, especially in children younger than 2 years of age 14. The reason why bradycardia is associated with the use of fentanyl is not known exactly, but it is considered that the drug suppresses the vagal afferent neurons coming from the nucleus to the heart through the µ-opioid receptors, thereby causing bradycardia 33. In our study, significantly higher bradycardia was observed in the fentanyl–propofol group (11 patients, 9.2%) compared with the midazolam–ketamine group (one patient, 0.8%). Patients who developed bradycardia had to be administered atropine, but no circulatory complications resulting in the need to stop the procedure occurred.
In our study, the rate of complications during the recovery was significantly higher in the midazolam–ketamine group. Agitation and emergence reactions are significant side effects observed during recovery in ketamine-based sedation. In the meta-analysis carried out, although side effects occurred in 30% of the adults, they only occurred in 1.4% of the children 31. The incidence of emergence reactions increases, especially when ketamine is used at high doses, when a fast injection (<1 minute) is administered and when excessive visual or verbal stimuli exist during the recovery 2,4. In our previous study, we used 0.5 mg/kg ketamine and the emergence reaction was 1.2%. However, in the present study, we used 1.03 mg/kg ketamine and the emergence reaction was 5% 24. Several studies in the literature have reported that fentanyl and propofol reduce the incidence of emergence reactions 34–36. In our study, similarly, none of the patients administered the fentanyl–propofol combination developed emergence reactions.
Ketamine-associated vomiting differs with the procedure and it occurs in 3.5 to 28.4% of all cases 37. After endoscopy, nausea and vomiting during the recovery period cause unrest, especially in parents. Vomiting occurs more often in gastrointestinal tract procedures than in other processes. Motamed et al. 4 reported that vomiting occurred in 17.6% of the children who underwent UGE procedures. Some studies have reported that administration of atropine along with ketamine sedation reduces vomiting, but there is no consensus on this issue 37–39. In our study without prophylactic atropine, nausea was found in 62.2% of the patients in the midazolam–ketamine group and vomiting was found in 33.6% of these patients, which is higher than the findings of previous studies. Propofol has been shown to have antiemetic effects in different surgical procedures 40–43. In our study, significantly less nausea and vomiting was also observed in the fentanyl–propofol group.
Our study has some limitations, despite adequate sedation in both drug combinations. First, this study included only diagnostic UGE procedures. These doses of drugs may be inadequate in interventional procedures. In addition, patients younger than 4 years of age were excluded from the study. Therefore, probable complications in a younger age group, especially in children under the age of 1 year, could not be considered. The exclusion of younger children and of patients with ASA status more than II are a significant limitation of the study as young and sicker children have a higher need for sedation. As an example, ‘neurological’ children (most of whom are ASA III) represent a significant proportion of patients undergoing digestive endoscopy and we need more data on safe sedation in these diseases. The administration of propofol by a TCI device and the evaluation of sedation level by a bispectral index device are more reliable than the conventional method for the regulation of sedation and evaluation of comfort. If we had used TCI and bispectral index devices, we would have obtained more objective results.
Conclusion
Our study showed that midazolam–ketamine and fentanyl–propofol combinations provide effective sedation of children during UGE. In children, UGE procedures can be quite comfortable when using the midazolam–ketamine combination. The wide margin of safety of the ketamine dose and the absence of cardiopulmonary suppressive property as well as the anxiolytic and analgesic effects in children make this combination ideal. However, adverse effects related to ketamine were observed during the recovery, which causes concern in parents. In our study, with no antidote, more side effects were observed in comparison with previous studies. Our study was prospective, and all the side effects were recorded; this might have led to a higher rate of side effects compared with the findings from previous studies. Although the UGE procedure is less comfortable with the fentanyl–propofol combination in comparison with the midazolam–ketamine combination, the recovery time is less with fentanyl–propofol and there are fewer side effects during the recovery period.
Acknowledgements
This study was funded by the Kanuni Training and Research Hospital.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Tosun Z, Aksu R, Guler G, Esmaoglu A, Akin A, Aslan D, et al. Propofol–ketamine vs propofol–fentanyl for sedation during pediatric upper gastrointestinal endoscopy. Paediatr Anaesth 2007; 17:983–988. [DOI] [PubMed] [Google Scholar]
- 2.Gilger MA, Spearman RS, Dietrich CL, Spearman G, Wilsey MJ, Jr, Zayat MN. Safety and effectiveness of ketamine as a sedative agent for pediatric GI endoscopy. Gastrointest Endosc 2004; 59:659–663. [DOI] [PubMed] [Google Scholar]
- 3.Kirberg A, Sagredo R, Montalva G, Flores E. Ketamine for pediatric endoscopic procedures and as a sedation complement for adult patients. Gastrointest Endosc 2005; 61:501–502. [DOI] [PubMed] [Google Scholar]
- 4.Motamed F, Aminpour Y, Hashemian H, Soltani AE, Najafi M, Farahmand F. Midazolam–ketamine combination for moderate sedation in upper GI endoscopy. J Pediatr Gastroenterol Nutr 2012; 54:422–426. [DOI] [PubMed] [Google Scholar]
- 5.White PF, Way WC, Trevor AJ. Ketamine – its pharmacology and therapeuticuses. Anesthesiology 1982; 56:119–136. [DOI] [PubMed] [Google Scholar]
- 6.Reich D, Silvay G. Ketamine: an update on the first twenty five years of clinical experience. Can J Anaesth 1989; 36:186–197. [DOI] [PubMed] [Google Scholar]
- 7.Green SM, Johnson NE. Ketamine sedation for pediatric procedures: part 2, review and implications. Ann Emerg Med 1990; 19:1033–1046. [DOI] [PubMed] [Google Scholar]
- 8.Green SM, Roback MG, Kennedy RM, Krauss B. Clinical practice guideline for emergency department ketamine dissociative sedation: 2011 update. Ann Emerg Med 2011; 57:449–461. [DOI] [PubMed] [Google Scholar]
- 9.Reves JG, Fragen RJ, Vinik R, Greenblatt DJ. Midazolam: pharmacology and uses. Anesthesiology 1985; 62:310–324. [PubMed] [Google Scholar]
- 10.Sener S, Eken C, Schultz CH, Serinken M, Ozsarac M. Ketamine with and without midazolam for emergency department sedation in adults: a randomized controlled trial. Ann Emerg Med 2011; 57:109–114. [DOI] [PubMed] [Google Scholar]
- 11.Brecelj J, Trop TH, Orel R. Ketamine with and without midazolam for gastrointestinal endoscopies in children. J Pediatr Gastroenterol Nutr 2012; 54:748–752. [DOI] [PubMed] [Google Scholar]
- 12.Malamed SF. Malamed SF. Sedation: a guide to patient management. Pharmacology. St Louis, MO: Mosby Company; 1989. 330–379. [Google Scholar]
- 13.Reves JG, Glass PSA, Lubarsky DA. Miller RD. Non barbiturate intravenous Anesthetics. Anesthesia. Philadelphia, PA: Churchill Livingstone; 2000. 228–272. [Google Scholar]
- 14.Bedirli N, Egritas O, Cosarcan K, Bozkirli F. A comparison of fentanyl with tramadol during propofol-based deep sedation for pediatric upper endoscopy. Paediatr Anaesth 2012; 22:150–155. [DOI] [PubMed] [Google Scholar]
- 15.Elitsur Y, Blankenship P, Lawrence Z. Propofol sedation for endoscopic procedures in children. Endoscopy 2000; 32:788–791. [DOI] [PubMed] [Google Scholar]
- 16.Disma N, Astuto M, Rizzo G, Rosano G, Naso P, Aprile G, et al. Propofol sedation with fentanyl or midazolam during oesophagogastroduodenoscopy in children. Eur J Anaesthesiol 2005; 22:848–852. [DOI] [PubMed] [Google Scholar]
- 17.Cohen LB, Hightower CD, Wood DA, Miller KM, Aisenberg J. Moderate levels sedation during endoscopy: a prospective study using low-dose propofol, meperidine/fentanyl, and midazolam. Gastrointest Endosc 2004; 59:795–803. [DOI] [PubMed] [Google Scholar]
- 18.Van Natta ME, Rex DK. Propofol alone titrated to deep sedation versus propofol in with opiods and/or benzodiazepines and titrated to moderate sedation for colonoscopy. Am J Gastroenterol 2006; 101:2209–2217. [DOI] [PubMed] [Google Scholar]
- 19.Lowrie L, Weiss AH, Lacombe C. The pediatric sedation unit: a mechanism for pediatric sedation. Pediatrics 1998; 102:30. [DOI] [PubMed] [Google Scholar]
- 20.De Silva AP, Amarasiri L, Liyanage MN, Kottachchi D, Dassanayake AS, de Silva HJ. One-hour fast for water and six-hour fast for solids prior to endoscopy provides good endoscopic vision and results in minimum patient discomfort. J Gastroenterol Hepatol 2009; 24:1095–1097. [DOI] [PubMed] [Google Scholar]
- 21.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxolone. Br Med J 1974; 22:656–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aldrete JA. Modifications to the postanesthesia score for use in ambulatory surgery. J Perianesth Nurs 1998; 3:148–155. [DOI] [PubMed] [Google Scholar]
- 23.Chiaretti A, Benini F, Pierri F, Vecchiato K, Ronfani L, Agosto C, et al. Safety and efficacy of propofol administered by paediatricians during procedural sedation in children. Acta Paediatr 2014; 103:182–187. [DOI] [PubMed] [Google Scholar]
- 24.Akbulut UE, Cakir M. Efficacy and safety of low dose ketamine and midazolam combination for diagnostic upper gastrointestinal endoscopy in children. Pediatr Gastroenterol Hepatol Nutr 2015; 18:160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbi E, Petaros P, Badina L, Pahor T, Giuseppin I, Biasotto E, et al. Deep sedation with propofol for upper gastrointestinal endoscopy in children, administered by specially trained pediatricians: a prospective case series with emphasis on side effects. Endoscopy 2006; 38:368–375. [DOI] [PubMed] [Google Scholar]
- 26.Khoshoo V, Thoppil D, Landry L, Brown S, Ross G. Propofol versus midazolam plus meperidine for sedation during ambulatory esophagogastroduodenoscopy. J Pediatr Gastroenterol Nutr 2003; 37:146–149. [DOI] [PubMed] [Google Scholar]
- 27.Larsen R, Galloway D, Wadera S, Kjar D, Hardy D, Mirkes C, et al. Safety of propofol sedation for pediatric outpatient procedures. Clin Pediatr 2009; 48:819–823. [DOI] [PubMed] [Google Scholar]
- 28.Paspatis GA, Charoniti I, Manolaraki M, Vardas E, Papanikolaou N, Anastasiadou A, et al. Synergistic sedation with oral midazolam as a premedication and intravenous propofol versus intravenous propofol alone in upper gastrointestinal endoscopies in children: a prospective, randomized study. J Pediatr Gastroenterol Nutr 2006; 43:195–199. [DOI] [PubMed] [Google Scholar]
- 29.Strauss JM, Giest J. Total intravenous anesthesia. On the way to standard practice in pediatrics. Anaesthesist 2003; 52:763–777. [DOI] [PubMed] [Google Scholar]
- 30.Fredette ME, Lightdale JR. Endoscopic sedation in pediatric practice. Gastrointest Endosc Clin N Am 2008; 18:739–751. [DOI] [PubMed] [Google Scholar]
- 31.Green SM, Roback MG, Krauss B, Brown L, McGlone RG, Agrawal D, et al. Predictors of emesis and recovery agitation with emergency department ketamine sedation: an individual-patient data meta-analysis of 8282 children. Ann Emerg Med 2009; 54:171–180. [DOI] [PubMed] [Google Scholar]
- 32.Van Beek EJ, Leroy PL. Safe and effective procedural sedation for gastrointestinal endoscopy in children. J Pediatr Gastroenterol Nutr 2012; 54:171–185. [DOI] [PubMed] [Google Scholar]
- 33.Griffioen K, Venkatesan P, Huang ZG, Wang X, Bouairi E, Evans C, et al. Fentanyl inhibits GABAergic neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Brain Res 2004; 1007:109–115. [DOI] [PubMed] [Google Scholar]
- 34.Kim MS, Moon BE, Kim H, Lee JR. Comparison of propofol and fentanyl administered at the end of anaesthesia for prevention of emergence agitation after sevoflurane anaesthesia in children. Br J Anaesth 2013; 110:274–280. [DOI] [PubMed] [Google Scholar]
- 35.Van Hoff SL, O’Neill ES, Cohen LC, Collins BA. Does a prophylactic dose of propofol reduce emergence agitation in children receiving anesthesia? A systematic review and meta-analysis. Paediatr Anaesth 2015; 25:668–676. [DOI] [PubMed] [Google Scholar]
- 36.Bakhamees HS, Mercan A, El-Halafawy YM. Combination effect of low dose fentanyl and propofol on emergence agitation in children following sevoflurane anesthesia. Saudi Med J 2009; 30:500–503. [PubMed] [Google Scholar]
- 37.Lee JS, Jeon WC, Park EJ, Min YG, Jung YS, Kim GW, et al. Adjunctive atropine versus metoclopramide: can we reduce ketamine-associated vomiting in young children? A prospective, randomized, open, controlled study. Acad Emerg Med 2012; 19:1128–1133. [DOI] [PubMed] [Google Scholar]
- 38.Heinz P, Geelhoed GC, Wee C, Pascoe EM. Is atropine needed with ketamine sedation? A prospective, randomised, double blind study. Emerg Med J 2006; 23:206–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green SM, Roback MG, Krauss B. Anticholinergics and ketamine sedation in children: a secondary analysis of atropine versus glycopyrrolate. Acad Emerg Med 2010; 17:157–162. [DOI] [PubMed] [Google Scholar]
- 40.Lee DW, Lee HG, Jeong CY, Jeong SW, Lee SH. Postoperative nausea and vomiting after mastoidectomy with tympanoplasty: a comparison between TIVA with propofol–remifentanil and balanced anesthesia with sevoflurane–remifentanil. Korean J Anesthesiol 2011; 61:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Won YJ, Yoo JY, Chae YJ, Kim DH, Park SK, Cho HB, et al. The incidence of postoperative nausea and vomiting after thyroidectomy using three anaesthetic techniques. J Int Med Res 2011; 39:1834–1842. [DOI] [PubMed] [Google Scholar]
- 42.Park SK, Cho EJ. A randomized controlled trial of two different interventions for the prevention of postoperative nausea and vomiting: total intravenous anaesthesia using propofol and remifentanil versus prophylactic palonosetron with inhalational anaesthesia using sevoflurane–nitrous oxide. J Int Med Res 2011; 39:1808–1815. [DOI] [PubMed] [Google Scholar]
- 43.Erdem AF, Yoruk O, Silbir F, Alici HA, Cesur M, Dogan N, et al. Tropisetron plus subhypnotic propofol infusion is more effective than tropisetron alone for the prevention of vomiting in children after tonsillectomy. Anaesth Intensive Care 2009; 37:54–59. [DOI] [PubMed] [Google Scholar]


