Abstract
BACKGROUND
There is a debate regarding the management of ovarian immature teratomas (ITs). In adult women, postoperative chemotherapy is standard except for stage I, grade 1 disease, whereas surgery alone is standard in pediatric patients. To determine the role of chemotherapy, a pooled analysis of pediatric and adult clinical trials was conducted.
METHODS
Data from 7 pediatric trials and 2 adult trials were merged in the Malignant Germ Cell International Collaborative data set. Four trials included patients with newly diagnosed pure ovarian ITs and were selected (Pediatric Oncology Group/Children’s Cancer Group Intergroup Study (INT 0106), Second UKCCSG Germ Cell Tumor Study (GC2), Gynecologic Oncology Group (GOG 0078 and GOG 0090). Adult and pediatric trials were analyzed separately. The primary outcome measures were event-free survival (EFS) and overall survival (OS).
RESULTS
One hundred seventy-nine patients were included (98 pediatric patients and 81 adult patients). Ninety pediatric patients were treated with surgery alone, whereas all adult patients received chemotherapy. The 5-year EFS and OS were 91% and 99%, respectively, for the pediatric cohort and 87% and 93%, respectively, for the adults. There were no relapses in grade 1 patients, regardless of the stage or age. Only 1 adult patient with a grade 2 IT relapsed. Among grade 3 patients, the 5-year EFS was 0.92 (0.72–0.98) for stage I/II and 0.52 (0.22–0.75) for stage III in the pediatric cohort (P = .005) and 0.91 (0.69–0.98) for stage I/II and 0.65 (0.39–0.83) for stage III/IV in the adult cohort (P = .01). Postoperative chemotherapy did not decrease relapses in the pediatric cohort.
CONCLUSIONS
The grade was the most important risk factor for relapse in ovarian ITs. Among grade 3 patients, the stage was significantly associated with relapse. Adjuvant chemotherapy did not decrease relapses in the pediatric cohort; its role in adults remains unresolved.
Keywords: adult, chemotherapy, ovarian immature teratoma, pediatric
INTRODUCTION
Immature teratomas (ITs) of the ovary represent approximately 1% of ovarian tumors. ITs are a specific histological subtype of germ cell tumor (GCT) and are composed of tissues derived from all 3 embryonic layers: the mesoderm, the endoderm, and the ectoderm. They are graded according to the proportion of tissue containing immature neural elements. The grade and stage of these tumors have been shown to be of prognostic significance and are, therefore, used to make therapeutic decisions.1,2
There is no consensus on the management of patients with ovarian ITs. Significant differences exist between pediatric and adult groups about the necessity and utility of chemotherapy for patients with higher grade and stage disease. Therapeutic recommendations for adult women are based on 2 seminal studies. In 1976, Norris et al3 performed a Cancer January 15, 2016 retrospective analysis of 58 patients, and they observed an 18% recurrence rate for grade 2 tumors and a 70% recurrence rate for grade 3 tumors; this resulted in the recommendation to use chemotherapy for grade 2 and 3 tumors. Gershenson et al4 reported outcomes for 41 patients with stage I to IV ovarian ITs and observed recurrences in 94% of the patients treated with surgery alone versus 14% of the patients treated with surgery and chemotherapy. Henceforth, the standard of care for adult women with ovarian ITs has been postoperative chemotherapy for all patients except those with stage I, grade 1 tumors.
Clinical practice in children has been led by evidence provided by a pediatric intergroup trial (Pediatric Oncology Group/Children’s Cancer Group Intergroup Study (INT) 0106) conducted in the United States. Patients with completely resected ovarian ITs were observed closely without postoperative chemotherapy.5,6 Forty-four patients with completely resected ovarian ITs were enrolled; 31 patients had pure ITs with tumor grade 1 (n = 17), 2 (n = 12), or 3 (n = 2). Thirteen patients had microscopic foci of a yolk sac tumor. Gliomatosis peritonei (GP) was present in 27% of the patients, and 3 of the 16 patients with lymph node sampling had nodal gliomatosis. At 4 years, the event-free survival (EFS) rate was 97.7% with an overall survival (OS) rate of 100%. This study concluded that surgery alone is curative for children with completely resected ovarian ITs, regardless of grade or the presence of microscopic foci of a yolk sac tumor.5 A similar study conducted in the United Kingdom followed 124 patients with ITs (54 ovarian ITs) after surgical resection.7 Eleven patients had GP, and 6 patients had nodal gliomatosis. The EFS and OS rates were 85.9% and 95.1%, respectively, and the authors concluded that the treatment of ovarian ITs is primarily surgical.
To highlight these differences and gain clarity into the role of postoperative chemotherapy in the management of a pure IT of the ovary, we conducted a pooled analysis. The Children’s Oncology Group (COG) and the Children’s Cancer and Leukemia Group (CCLG) agreed to merge 25 years of clinical trial data on pediatric GCTs to form the Malignant Germ Cell International Collaborative. Subsequently, data from the Gynecologic Oncology Group (GOG) GCT clinical trials were added. This report presents our analysis of pediatric and adult patients with ovarian ITs. We compare these 2 groups and identify similarities and differences with the goal of establishing a uniform treatment approach across all age groups. Data from some of these trials have been previously published as separate reports.5,7,8
MATERIALS AND METHODS
After the signing of a memorandum of understanding, which specified the variables to be included and how data were to be de-identified, patient data from 7 GCT clinical trials conducted by COG or CCLG between 1983 and 2009 were included in the Malignant Germ Cell International Collaborative data set. Data from 2 GOG clinical trials were added subsequently. Four of the 9 trials included patients with ITs. These were INT 0106 (COG), GC2 (CCLG), GOG 0078, and GOG 0090. Each study had different eligibility criteria for the inclusion of IT patients. The INT 0106 study included patients up to 21 years of age with biopsy-proven ITs (stages I and II and grades 1–3). The presence of GP within the abdomen or pelvis did not result in upstaging in this study. The GC2 study included patients younger than 16 years with biopsy-proven ITs (all stages and grades). The pediatric studies included ITs at all sites in both sexes. The GOG 0078 study included patients with stage I, grade 2/3 ITs and completely resected stage II/III ITs of all grades. The GOG 0090 protocol included patients with incompletely resected stage II to IV, grade 1 to 3 ITs. Each trial had received research ethics board approval from the relevant agencies. This project was approved by the Dana-Farber/Harvard Cancer Center institutional review board.
From the larger data set, we selected only females with a newly diagnosed pure IT of the ovary. Patients with a mixed malignant GCT of the ovary and patients with an extragonadal IT were excluded. Patient characteristics that were included in the data set were the age at diagnosis, histology, tumor markers, stage, grade, administration of adjuvant chemotherapy (yes or no), and clinical outcomes.
Staging
Patients treated with GOG protocols were staged with International Federation of Gynecology and Obstetrics (FIGO) staging,9 whereas pediatric patients were staged with the COG or CCLG staging system.5,7 There are several differences between the COG/CCLG staging system and the FIGO staging system. For instance, positive peritoneal washings are stage III in COG staging but can be stage Ic or IIc in the FIGO system. Similarly, positive lymph nodes are stage III in the FIGO system, but they are stage II in the COG system if they are less than or equal to 2 cm and are stage III if they are greater than 2 cm. Extension to the pelvis is stage IIb in the FIGO system and stage III in the COG system. Because of irreconcilable differences in staging between these systems, adult and pediatric patients were analyzed separately. A few adolescents were treated with GOG protocols. They were analyzed with the adult cohort because they were staged and managed according to the adult treatment algorithm. Within the pediatric staging systems, a major difference between the COG and CCLG systems was that the presence of GP in the COG system did not result in upstaging of stage I patients, whereas in the CCLG system, patients with GP were classified as stage III. To resolve this discrepancy, we retrospectively applied the CCLG staging to COG patients so that all patients with GP were defined as having stage III disease.
Grading
All tumors required central pathology review at the time of enrollment by the respective cooperative group pathologist to confirm the histology, and they were graded according to the criteria of Norris et al3 and Robboy and Scully.10 Grade 1 was defined as immature tissue present in <1 low-power field (4× objective) per slide, grade 2 was defined as immature tissue present in 1 to 3 low-power fields (4× objective) per slide, and grade 3 was defined as immature tissue present in >3 low-power fields (4× objective) per slide.
Tumor Markers
Serum α-fetoprotein (AFP) levels were available for most patients, and they were classified as normal if they were <10 ng/mL and as elevated if they were ≥10 ng/mL. Patients with AFP levels > 1000 ng/mL were excluded because this level of AFP elevation was considered more likely to indicate malignant GCT elements, which would warrant more aggressive treatment.11
Treatment
Complete surgical excision at diagnosis was undertaken when feasible. In the pediatric trials (INT 0106 and GC2), chemotherapy was not recommended after surgery. However, some patients did receive chemotherapy immediately after surgery at the discretion of the treating physician. In the GOG trials (GOG 0078 and GOG 0090), chemotherapy was administered postoperatively for all patients.
Statistical Analysis
The primary outcomes were EFS and OS. EFS was defined as the time interval from the date of diagnosis to relapse or progression, second malignancy, death, or date last seen (whichever occurred first). Patients who experienced a relapse, progression, or second malignancy were considered to have experienced an event; otherwise, the patient was censored at last contact. OS was defined as the time interval from the date of diagnosis to death or date last seen (whichever occurred first). Patients who died, regardless of cause, were considered to have experienced an event; otherwise, the patient was censored at last contact.
The effects of various factors, including the age at diagnosis, stage, grade, tumor marker levels, and treatment received, on the risk for an EFS event or death were estimated with relative risk regression.12 We constructed survival curves with the Kaplan-Meier method.13 Ninety-five percent confidence intervals for the Kaplan-Meier estimates at specified time points were calculated with the complementary log-log transformation.12 The 2-sided log-rank test for comparing EFS across groups defined by the risk factors12 was used to assess the prognostic significance of the characteristics with P values < .05, which were considered significant.
For adult patients, a backwards stepwise procedure was used to identify factors that were significantly associated with the risk for an EFS event. A selection probability of .05 was used to retain factors in the model. Because of issues with the collinearity of predictor variables, only the stage and the grade could be entered into the starting model for the selection process. All analyses were conducted with Stata 13.1 (StataCorp, College Station, Texas).
RESULTS
A total of 193 patients with pure ovarian ITs were identified from the 4 clinical trials. Six pediatric patients were excluded because they had a mixed malignant GCT on central review. Eight adult patients were excluded; 7 patients had recurrent ITs at the time of enrollment, and 1 patient had mixed malignant histology on central review. One hundred seventy-nine patients with pure ITs were included in the final analysis; 98 patients were treated in the pediatric trials, and 81 patients were treated in the adult GOG trials.
Characteristics, Treatment, and Outcomes of Pediatric Patients
The characteristics of the pediatric patients are presented in Table 1. The mean age at presentation was 10 years (range, 0–17 years). Sixty percent of the patients had stage I disease. The mean serum AFP level was 83 ± 182.7 ng/mL. Ninety of the 98 patients were treated with surgery alone, and 8 patients received postoperative chemotherapy. The median follow-up was 6.8 years (range, 1.7–14 years).
TABLE 1.
Characteristics of Pediatric Patients Treated by the Children’s Oncology Group in the United States and by the Children’s Cancer and Leukemia Group in the United Kingdom (n = 98)
| Patient Characteristic | Value |
|---|---|
| Age, mean ± SD (range), y | 10 ± 3 (0–17) |
| Stage, No. (%) | |
| I | 59 (60) |
| II | 12 (12) |
| III | 27 (28) |
| IV | 0 |
| Grade, No. (%) | |
| 1 | 30 (31) |
| 2 | 20 (20) |
| 3 | 38 (39) |
| Missing | 10 (10) |
| AFP at presentation | |
| Mean ± SD, ng/mL | 83 ± 182.7 |
| Normal (<10 ng/mL), No. (%) | 45 (46) |
| High, No. (%) | 44 (45) |
| Missing, No. (%) | 9 (9) |
| Treatment, No. (%) | |
| Surgery and chemotherapy | 8 (8) |
| Surgery | 90 (92) |
Abbreviations: AFP, α-fetoprotein; SD, standard deviation.
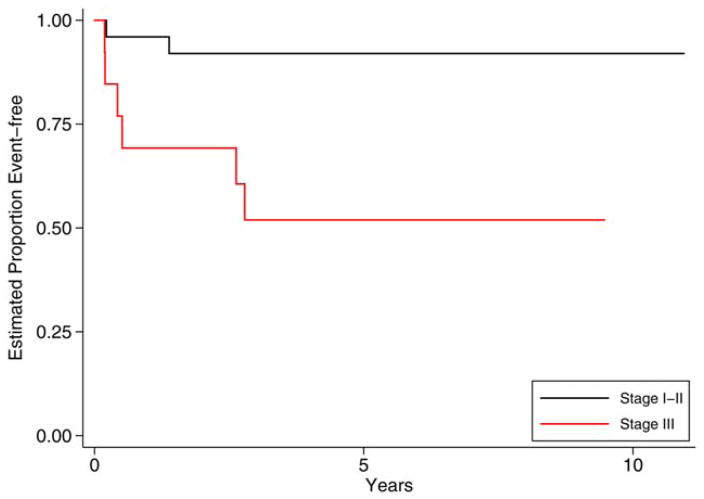
Nine of the 98 patients relapsed with 5-year EFS and OS rates of 0.91 (0.84–0.95) and 0.99 (0.93–1.00), respectively. As shown in Table 2, there were no relapses in patients with grade 1 or grade 2 tumors, regardless of stage. Among patients with grade 3 tumors, 8 of 38 patients (21%) relapsed. Stratifying grade 3 tumors by stage (Fig. 1), we found that the estimated 5-year EFS for patients with grade 3, stage I/II disease was 0.92 (0.72–0.98), whereas it was 0.52 (0.22–0.75) for grade 3, stage III patients (P = .005). The OS for all grade 3 patients, regardless of stage, was 100%. Neither the age at diagnosis nor the AFP level was related significantly to the risk of relapse. The administration of postoperative chemotherapy did not decrease the risk of relapse in the pediatric cohort.
TABLE 2.
Univariate Analysis of Risk Factors for Relapse in Pediatric Patients With Ovarian Immature Teratomas (n = 98)
| Patient Characteristic | Level | No. of Events/No. of Patients | Estimated HR (95% CI) | Pa |
|---|---|---|---|---|
| Age | ≥11 y | 7/49 | 3.76 (0.78–18.10) | .076 |
| <11 y | 2/49 | — | ||
| Stage | III | 7/27 | 10.32 (2.14–49.68) | .0003 |
| I/II | 2/71 | — | ||
| Grade | 3 | 8/38 | —b | .0006 |
| 1/2 | 0/50 | — | ||
| AFP at presentation | High | 5/44 | 1.78 (0.43–7.44) | .42 |
| Normal | 3/45 | — | ||
| Treatment | Chemotherapy | 3/8 | 6.61 (1.65–26.45) | .002 |
| Surgery | 6/90 | — |
Abbreviations: AFP, α-fetoprotein; CI, confidence interval; HR, hazard ratio.
From the log-rank test.
The HR could not be estimated because there was no event for grade 1/2.
Figure 1.
Event-free survival for pediatric patients with ovarian grade 3 immature teratomas by stage. Pediatric patients with grade 3, stage I/II ovarian immature teratomas had a significantly reduced risk for an event-free survival event in comparison with patients with grade 3, stage III disease.
Table 3 outlines the clinical characteristics of the 9 pediatric patients who relapsed. All but 1 of the 9 patients were salvaged (3 with surgery alone).
TABLE 3.
Characteristics of Pediatric Patients Who Relapsed (n = 9)
| Patient | Age, y | Histology | Stage | Grade | AFP, ng/mL | Initial Treatment | Time to Relapse | Second or Subsequent Relapse | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 9 | IT with GP | 3 | 3 | 568 | Surgery, incomplete removal of peritoneal deposits | 2 mo | Yes | Alive |
| 2 | 12 | IT | 3 | 3 | <1 | Surgery | 20 mo | No | Alive |
| 3 | 15 | Bilateral, left MT Right IT with GP | 3 | 3 | —a | Bilateral oophorectomy, JEB (×4) | 5 mo | Yes | Alive |
| 4 | 13 | IT with GP | 3 | —a | 245 | Surgery | 2 mo | Yes | Dead |
| 5 | 14 | IT with GP | 3 | 3 | <1 | Surgery, JEB (×4) | 2 y 7 mo | No | Alive |
| 6 | 4 | IT with nodal gliomatosis | 2 | 3 | 0 | Surgery | 1 y 3 mo | Yes | Alive |
| 7 | 13 | IT | 1 | 3 | 800 | Surgery | 2 mo | Yes | Alive |
| 8 | 15 | Left IT with GP | 3 | 3 | 722 | Surgery, incomplete removal of peritoneal deposits JEB (×3) | 2 y 10 mo | No | Alive |
| 9 | 11 | IT with GP | 3 | 3 | 255 | Surgery | 2 mo | Yes | Alive |
Abbreviations: AFP, α-fetoprotein; GP, gliomatosis peritonei; IT, immature teratoma; JEB, carboplatin, etoposide, and bleomycin; MT, mature teratoma.
The value is missing in the database.
Characteristics, Treatment, and Outcomes of Adult Patients
The characteristics of the 81 adult patients are outlined in Table 4. Fifty-six percent of the patients had grade 3 disease, whereas 39% did in the pediatric cohort. In addition, 7% of the adult patients (but no pediatric patients) had stage IV disease. The mean AFP level was 63.17 ± 155.18 ng/mL. All patients, regardless of stage, were treated with chemotherapy after surgery. The median follow-up was 12 years (range, 0.3–21.9 years).
TABLE 4.
Characteristics of the 81 Patients Treated by the Gynecologic Oncology Group in the United States
| Patient Characteristic | Value |
|---|---|
| Age, mean ± SD (range), y | 26 ± 8.90 (11–49) |
| Stage, No. (%) | |
| I | 43 (53) |
| II | 5 (6) |
| III | 27 (33) |
| IV | 6 (7) |
| Grade, No. (%) | |
| 1 | 7 (9) |
| 2 | 27 (33) |
| 3 | 45 (56) |
| Missing | 2 (2) |
| AFP at presentation | |
| Mean ± SD (ng/mL) | 63.2 ± 155.18 |
| Normal (<10 ng/mL), No. (%) | 27 (33) |
| High, No. (%) | 12 (15) |
| Missing, No. (%) | 42 (52) |
| Treatment, No. (%) | |
| Surgery and chemotherapy | 81 (100) |
| Surgery alone | 0 (0) |
Abbreviations: AFP, α-fetoprotein; SD, standard deviation.
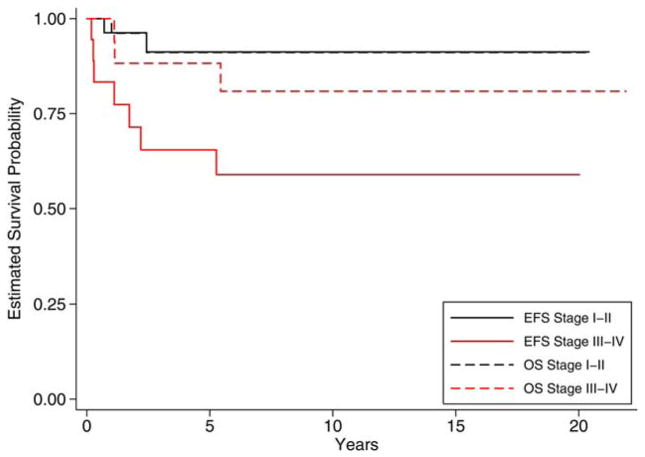
Eleven of the 81 patients relapsed with estimated 5-year EFS and OS rates of 0.87 (0.77–0.93) and 0.93 (0.85–0.97), respectively. There were no relapses in patients with grade 1 tumors, regardless of tumor stage. Among patients with grade 2 tumors, only 1 of the 27 patients (3.7%) relapsed; this patient had stage IIIc disease. Among patients with grade 3 tumors, 9 of 45 patients (20%) experienced a relapse. Stratifying grade 3 disease by stage, we found that the estimated 5-year EFS for patients with grade 3, stage I/II disease was 0.91 (0.69–0.98), whereas it was 0.65 (0.39–0.83) for grade 3, stage III/IV disease (P = .01). The estimated 5-year OS for grade 3, stage I/II disease was 0.91 (0.68–0.98), whereas it was 0.88 (0.61–0.97) for grade 3, stage III/IV disease (P = .41; Fig. 2).
Figure 2.
EFS and OS for adult patients with grade 3 ovarian immature teratomas by stage. Adult patients with grade 3, stage I/II ovarian immature teratomas had a significantly reduced risk for an EFS event in comparison with patients with grade 3, stage III/IV disease. OS was not significantly different between the 2 groups. EFS indicates event-free survival; OS, overall survival.
The extent of resection was not available in the database; however, the eligibility criteria for the adult studies (GOG 0078 and GOG 0090) were based on the stage and extent of resection. Patients with stage III disease were enrolled into GOG 0078 if they had undergone complete resection and into GOG 0090 if they had undergone incomplete resection. Stage IV patients were enrolled only into GOG 0090. Stratifying the grade 3, stage III patients by protocol, we found that the observed 5-year EFS was 0.75 (0.13–0.96) for GOG 0078 and 0.67 (0.28–0.88) for GOG 0090. Because the numbers were so small (9 patients in GOG 0090 and 4 patients in GOG 0078), a log-rank test for this comparison was not performed.
Stepwise selection identified the grade and stage as significantly associated with the risk of relapse (Table 5). The age at diagnosis and the AFP level were not significant risk factors for relapse. Because all the adult patients received postoperative chemotherapy, the effect of surgery versus chemotherapy could not be assessed. Table 6 shows the clinical characteristics of the patients who relapsed. Six of the 11 patients died.
TABLE 5.
Multivariate Analysis of Risk Factors for Relapse in Adult Patients With Ovarian Immature Teratomas (n =79)
| Patient Characteristics | Level | No. of Events/No. of Patients | Estimated HR (95% CI) | Pa |
|---|---|---|---|---|
| Stage | IV | 2/5 | 6.02 (0.84–43.20) | .024 |
| III | 6/27 | 6.76 (1.36–33.67) | ||
| I/II | 2/47 | — | ||
| Grade | 3 | 9/45 | 8.22 (1.01–67.13) | .014 |
| 1/2 | 1/34 | — |
Abbreviations: CI, confidence interval; HR, hazard ratio.
From the relative risk regression.
TABLE 6.
Characteristics of Adult Patients Who Relapsed (n = 11)
| Patient | Age, y | Stage | Grade | AFP, ng/mL | Treatment Protocol | Outcome |
|---|---|---|---|---|---|---|
| 1 | 48 | IVB | 3 | —a | GOG 0090 | Died |
| 2 | 38 | III | 3 | —a | G0G 0078 | Alive |
| 3 | 47 | IV | —a | —a | GOG 0090 | Died |
| 4 | 23 | IIIC | 3 | —a | GOG 0090 | Alive |
| 5 | 41 | IA | 3 | 63 | G0G 0078 | Died |
| 6 | 20 | III | 3 | —a | GOG 0090 | Alive |
| 7 | 21 | IVA | 3 | —a | GOG 0090 | Alive |
| 8 | 37 | IIC | 3 | 4 | G0G 0078 | Died |
| 9 | 13 | III | 3 | —a | GOG 0090 | Died |
| 10 | 21 | IIIC | 3 | —a | GOG 0090 | Died |
| 11 | 45 | IIIC | 2 | —a | GOG 0090 | Alive |
Abbreviation: AFP, α-fetoprotein; GOG, Gynecologic Oncology Group.
The value is missing in the database.
DISCUSSION
This combined analysis was performed in an attempt to simultaneously compare pediatric and adult patients with ovarian ITs to identify common risk factors and compare outcomes for 2 cohorts with different treatment strategies. Our analysis shows several striking similarities in the 2 groups, both in risk factors and in overall outcomes, despite differences in treatment. Risk factor analysis shows that for ovarian ITs, grade is the most important risk factor for relapse across all age groups. In patients with grade 1 tumors, there were no relapses, regardless of stage. Among the 47 pediatric and adult patients with grade 2 tumors, there was only a single relapse. This was an adult patient with stage IIIc disease. The majority of relapses occurred in patients with grade 3 tumors. Twenty percent of the patients (17 of 83) with grade 3 tumors relapsed: 21% in the pediatric cohort and 20% in the adult cohort. For patients with grade 3 tumors, the risk of relapse significantly differed by the stage at presentation. Grade 3 patients with stage I/II disease had excellent EFS in comparison with stage III/IV patients in both the pediatric cohort and the adult cohort. In addition to stage, the completeness of resection influenced EFS. Adult patients were enrolled in 2 protocols, with eligibility based on the stage and extent of resection. Among grade 3, stage III patients, the EFS was improved in patients with complete resection versus those with incomplete resection.
There were important differences in the treatment approach between the pediatric and adult cohorts in our study. Only 8 of the 98 pediatric patients received chemotherapy, whereas all 81 adult patients received postoperative chemotherapy. Despite this, the 5-year EFS and OS were higher in the pediatric cohort versus the adult cohort. This difference in outcomes is likely a reflection of the tumor grade rather than treatment. Tumor grading varied by age, and grade 1 tumors were more frequent in the pediatric cohort versus the adults (31% vs 9%), whereas grade 3 tumors were more common in the adult cohort (56% vs 39%). It is important to note that patients with stage I, grade 1 tumors were not enrolled in the adult GOG studies.
Other smaller studies have corroborated our results. The grade, stage, and completeness of resection have been shown to be important risk factors for relapse.1,3,14 In the study by Norris et al,3 the recurrence rate was 70% for patients with grade 3 tumors and 18% for patients with grade 2 tumors. In a study by Gobel et al,14 which included 116 patients with extracranial ITs, 38 patients underwent incomplete resection. They found that immaturity in incompletely resected teratomas was a risk factor for relapse; there were no relapses in patients with completely resected ITs, even if they were grade 3 tumors.14 It, therefore, appears that in patients with grade 3 tumors, the stage and a lack of complete resection are associated with an increased risk of relapse.
Similarly to our study, other pediatric studies have shown no benefit of adjuvant chemotherapy postoperatively in the management of ovarian ITs. In a nonrandomized study by Gobel et al,14 76 patients were treated by surgery alone, and 40 patients received adjuvant chemotherapy. There was no reduction in the number of subsequent relapses in the IT group receiving chemotherapy. However, risk factors were not balanced between the 2 treatment groups in this study.
In contrast to the pediatric data, chemotherapy was used for all the adult patients with pure ovarian ITs except those with stage I, grade 1 tumors.3,4 Vicus et al15 reported on 34 women with ovarian ITs: 32 were at stage I, 1 was at stage IIB, and 1 was at stage IIIA. Three of the 32 patients with stage I disease experienced recurrence, and all of them had grade 2 or 3 disease. Consequently, the authors recommended surveillance only for stage I, grade 1 tumors and chemotherapy for stage I, grade 2/3 tumors.15 Recently, several studies in adults have questioned the role of chemotherapy for ITs. A multicenter Italian trial (Multicenter Italian Trials in Ovarian Cancer 9) reported on 28 patients with stage I disease.16 Nineteen patients were treated with surgery alone, and 9 patients received adjuvant postoperative chemotherapy. Four of 19 patients treated with surgery alone and 2 of 19 patients treated with adjuvant chemotherapy experienced recurrence. At recurrence, all patients were salvaged. The authors concluded that all patients with stage I ovarian ITs, regardless of grade, could be treated with surgery alone, with chemotherapy reserved for recurrence. A UK study by Patterson et al17 adopted a close surveillance program after surgery for all stage IA ovarian GCTs. Four of 15 patients relapsed, and only 1 of these patients could not be salvaged. They recommended surveillance for all stage IA ITs, regardless of grade. Bonazzi et al18 undertook a prospective trial of patients with pure ovarian ITs. Surgery alone was recommended for patients with stage I/II, grade 1/2 tumors. Twenty-two patients were followed after surgery alone. Two patients relapsed after surgery alone and were salvaged, and this led to the conclusion that such patients could be treated with surgery alone. In our adult cohort, all patients received adjuvant chemotherapy, so we are unable to comment on its efficacy. However, the risk of relapse in the grade 3 patients was not different between the adult and pediatric cohorts (20% vs 21%, respectively), despite major differences in management.
Our study has several limitations. It is a combined database analysis of patients treated in multiple studies; hence, there were missing data, and different staging systems were used. Although a small number of pediatric patients and most adults were treated with chemotherapy, there was no explicit documentation of objective responses to chemotherapy. In addition, clinical details at relapse were missing for adult patients; this included pathologic information and treatment at relapse. Despite these limitations, several important clinical messages can be drawn from our data.
In conclusion, our study shows that for all patients with ovarian ITs, grade is the most important risk factor for relapse. No patients with grade 1 tumors relapsed in the pediatric or adult population. We would thus advocate that for grade 1 tumors, surgery alone should be recommended for all stages across all age groups. In patients with higher grade tumors, the risk of relapse differed by stage, and patients with stage I/II tumors had excellent EFS in comparison with patients with stage III/IV tumors. We currently have a joint COG-NRG clinical trial under review that proposes observation for all stage I tumors, regardless of grade. The results of this analysis further justify this approach. With respect to the approach for grade 2 and 3 tumors at higher stages, in our analyses, we observed only a 2% failure rate for grade 2 tumors. The only group for which the failure rate was a concern was the group with grade 3, stage III/IV tumors. Adjuvant chemotherapy did not decrease the relapse risk in the pediatric cohort. Given our doubts that this subtype of GCT is chemosensitive, even in this higher stage and grade group, we would be in favor of a prospective trial of observation after surgery for patients with grade 2/3, stage II–IV tumors with consideration of second surgery as the first line of treatment in the setting of relapse.
Acknowledgments
FUNDING SUPPORT
This study was supported by the Bridging the Gap Fund, the Dana-Farber Cancer Institute, the Katie Walker Cancer Trust, the Teenage Cancer Trust, and the William and Guy Forbeck Foundation.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
References
- 1.Gobel U, Calaminus G, Blohm M, et al. Extracranial non-testicular teratoma in childhood and adolescence: introduction of a risk score for stratification of therapy. Klin Padiatr. 1997;209:228–234. doi: 10.1055/s-2008-1043955. [DOI] [PubMed] [Google Scholar]
- 2.Heifetz SA, Cushing B, Giller R, et al. Immature teratomas in children: pathologic considerations. Am J Surg Pathol. 1998;22:1115–1124. doi: 10.1097/00000478-199809000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Norris HJ, Zirkin HJ, Benson WL. Immature (malignant) teratoma of the ovary: a clinical and pathologic study of 58 cases. Cancer. 1976;37:2359–2372. doi: 10.1002/1097-0142(197605)37:5<2359::aid-cncr2820370528>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 4.Gershenson DM, del Junco G, Silva EG, Copeland LJ, Wharton JT, Rutledge FN. Immature teratoma of ovary. Obstet Gynecol. 1986;68:624–629. [PubMed] [Google Scholar]
- 5.Cushing B, Giller R, Ablin A, et al. Surgical resection alone is effective treatment for ovarian immature teratoma in children and adolescents: a report of the Pediatric Oncology Group and the Children’s Cancer Group. Am J Obstet Gynecol. 1999;181:353–358. doi: 10.1016/s0002-9378(99)70561-2. [DOI] [PubMed] [Google Scholar]
- 6.Marina N, Cushing B, Giller R, et al. Complete surgical excision is effective treatment for children with immature teratomas with or without malignant elements: a Pediatric Oncology Group/Children’s Cancer Group intergroup study. J Clin Oncol. 1999;17:2137–2143. doi: 10.1200/JCO.1999.17.7.2137. [DOI] [PubMed] [Google Scholar]
- 7.Mann JR, Gray ES, Thornton C, et al. Mature and immature extracranial teratomas in children: the UK Children’s Cancer Study Group experience. J Clin Oncol. 2008;26:3590–3597. doi: 10.1200/JCO.2008.16.0622. [DOI] [PubMed] [Google Scholar]
- 8.Williams S, Blessing JA, Liao SY, Ball Y. Adjuvant therapy of ovarian germ cell tumors with cisplatin, etoposide, and bleomycin: a trial of the Gynecologic Oncology Group. J Clin Oncol. 1994;412:701–706. doi: 10.1200/JCO.1994.12.4.701. [DOI] [PubMed] [Google Scholar]
- 9.Odicino F, Pecorelli S, Zigliani L, Creasman WT. History of the FIGO cancer staging system. Int J Gynaecol Obstet. 2008;101:205–210. doi: 10.1016/j.ijgo.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Robboy SJ, Scully RE. Ovarian teratoma with glial implants on the peritoneum. Hum Pathol. 1970;1:643–653. doi: 10.1016/s0046-8177(70)80062-4. [DOI] [PubMed] [Google Scholar]
- 11.Murray MJ, Nicholson JC. α-Fetoprotein. Arch Dis Child Educ Pract Ed. 2001;96:141–147. doi: 10.1136/adc.2011.213181. [DOI] [PubMed] [Google Scholar]
- 12.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: John Wiley & Sons Inc; 2002. [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 14.Gobel U, Calaminus G, Engert J, et al. Teratomas in infancy and childhood. Med Pediatr Oncol. 1998;31:8–15. doi: 10.1002/(sici)1096-911x(199807)31:1<8::aid-mpo2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 15.Vicus D, Beiner ME, Clarke B, et al. Ovarian immature teratoma: treatment and outcome in a single institutional cohort. Gynecol Oncol. 2011;123:50–53. doi: 10.1016/j.ygyno.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 16.Mangili G, Scarfone G, Gadducci A, et al. Is adjuvant chemotherapy indicated in stage I pure immature ovarian teratoma (IT)? A multicentre Italian trial in ovarian cancer (MITO-9) Gynecol Oncol. 2010;119:48–52. doi: 10.1016/j.ygyno.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 17.Patterson DM, Murugaesu N, Holden L, Seckl MJ, Rustin GJ. A review of the close surveillance policy for stage I female germ cell tumors of the ovary and other sites. Int J Gynecol Cancer. 2008;18:43–50. doi: 10.1111/j.1525-1438.2007.00969.x. [DOI] [PubMed] [Google Scholar]
- 18.Bonazzi C, Peccatori F, Colombo N, Lucchini V, Cantu MG, Mangioni C. Pure ovarian immature teratoma, a unique and curable disease: 10 years’ experience of 32 prospectively treated patients. Obstet Gynecol. 1994;84:598–604. [PubMed] [Google Scholar]