Abstract
Although it has been thought that female mammals develop all the eggs they will ever have by the time they are born, new research suggesting otherwise has now sparked a debate.
Although long held as true, the notion that females are born with a finite number of nonreplenishable oocytes is somewhat remarkable. How do eggs, made during embryonic life, ‘last’ so long? This traditional narrative of oogenesis was recently challenged; indeed, the paper by Johnson et al.1 highlighted here suggests the radical notion that the adult ovary must and does have the capacity to create new oocytes. After it was published, the reproductive science community collectively asked, “How did we miss this?,” and thus new studies were launched to validate these findings. To date, however, the overall evidence still favors the established dogma. Nonetheless, the field has been energized by this vigorous debate.
The purpose of germ cells, from pollen grains to mouse oocytes, is to produce future progeny and recreate the ‘self’, not necessarily for the individual but for the species as a whole. Unraveling the mechanisms that drive germ cell development and persistence in mammals is fascinating and challenging. The prevailing dogma of mammalian reproduction states that females lose the capacity to produce germ cells during fetal development and are born with a finite number of follicle-enclosed oocytes, only a small number of which will be ovulated after puberty (Fig. 1). Several recent studies from the laboratory of Jonathan Tilly at Harvard led to the hypothesis that adult mice are capable of regenerating oocytes, suggesting the existence of adult germline stem cells1,3,4. These papers created a schism within the reproductive science community that is now coming to a resolution.
Figure 1.
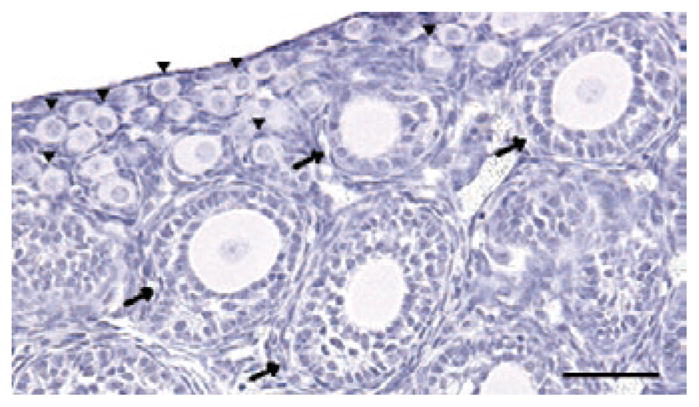
Follicles are the functional unit of the ovary. Most of these follicles are present as nongrowing primordial follicles (arrowheads). Once they are activated, the centrally located oocyte and the surrounding granulosa cells begin to grow (arrows) in a coordinated way leading toward ovulation. Scale bar, 50 μm. Figure courtesy of C. Tingen, Northwestern University.
In humans, fewer than 300,000 of the original pool of about one million healthy oocytes present at birth survive to puberty5,6. This number continues to decline throughout adulthood to the point of extinction around age 50, thus driving menopause. In a series of high-profile papers1,3,4, it was postulated that, at least in mice, the postnatal follicle cohort was dying so quickly that normal adult fertility could not be maintained if it depended solely on the follicle pool present at birth. Indeed, on the basis of this observation, a profoundly provocative statement was made that neo-oogenesis occurs in the adult mammal1. Moreover, it was posited that the source of the germline stem cells is tissue-based cells in the ovarian epithelium or, in a change from the first paper, from the bone marrow1,3. More recently, the group has contended that the ovary contains germ cell niches (resident follicles) that can accept these cells and help them transform into oocytes4. On the basis of these studies, the authors suggest that the adult oocyte population is renewable and that this process is necessary to supplant an insufficient number of follicles formed during embryonic development.
The reproductive research community began to think critically about the experiments and their interpretation and to design studies to examine the provocative new ideas7–14. Alternative interpretations of the data presented in the original papers and the absence of corroborating evidence of appreciable numbers of stem cells within the adult ovary were the first two hints that the original theories from last century may still stand. Indeed, the preponderance of experimental data collected over the past four years supports the nonrenewable germ cell pool theory. These findings show that circulating adult stem cells (provided through parabiosis) do not contribute to the ovulated follicle pool10, empty germ cell niches are not repopulated by newly formed oocytes11 and markers of stem cells are absent in the postnatal ovary but do appear in the ovarian vasculature where stem cells are known to exist12. Additionally, mathematical models evaluating the kinetics of follicle transitions indicate that the canonical nonreplenishable follicle pool model describes empirical observations of follicle numbers better than models incorporating neo-oogenesis13,14. Taken together, these papers teach against the idea that germline stem cells initiate neo-oogenesis in the adult animal and support the concept that female germ cells formed before birth are all that will be available from puberty to menopause. Thus, nature has given the female ovary a limited number of nonreplenishable eggs.
However, what if an occasional wandering stem cell, or a local one, was to transform into a germline stem cell in the adult? If such an event could be documented, that cell would face a high set of hurdles on its way to becoming a functional oocyte, including progressing from a proliferative cell to a meiotically arrested, follicle-enclosed cell. The hurdles heighten if this oocyte must eventually mature to an egg that can be fertilized and sustain embryogenesis. We know there is something unique about the embryonic gonad that supports oogonia proliferation. And we know that there is something equally singular about the adult ovary, which maintains the majority of oocytes in a quiescent pool. Some of the key factors involved in embryonic neo-oogenesis have been identified15,16, and the field is looking forward to the next revelation of genes and mechanisms. There is equal interest in recreating oocytes from embryonic stem (ES) cells. Indeed, oocyte-like structures have been formed from ES cells in vitro, again creating a rich vein of investigation17,18. These are the areas where reproductive biology is breaking new ground and creating new paradigms.
The publication of the initial adult oogenesis paper created a splash in the journal and in the media, suggesting that young women facing life-preserving but sometimes fertility-threatening cancer treatments might have an option for restoring their fertility. However, the ability to repopulate the ovary with a bone marrow transplant or stem cell transfusion now seems unachievable. Despite this reality, a number of existing and emerging reproductive interventions offer new options to young patients with cancer19. These include conventional hormone stimulation with embryo or egg cryobanking and ovarian tissue cryopreservation, with promising tissue transplantation and follicle maturation technologies also on the horizon (refs. 20–23, and see the related News & Views on this topic on pages 1182–1184). Such strategies are welcome and exciting reproductive interventions that can be offered to those women who will have irretrievable damage to the resident ovarian follicles as a result of the treatment for their cancer.
It is hard to look back on the past four years and not appreciate the number of key discoveries that emerged as a consequence of this discourse. The field has moved forward by affirming what it knew—that making an egg is a biological puzzle that has yet to be entirely solved. New concepts about the essential characteristics of the oocyte, which include its ability to persist over long spans of time, provide the next generation of questions that can be solved by multiple groups bringing varied perspectives and experimental methods to the problem. Reproductive science and medicine have been strengthened by virtue of the discussion and are now poised to move to the next frontier.
References
- 1.Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Nature. 2004;428:145–150. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- 2.Zuckerman S. Recent Prog Horm Res. 1951;6:63–108. [Google Scholar]
- 3.Johnson J, et al. Cell. 2005;122:303–315. doi: 10.1016/j.cell.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 4.Lee HJ, et al. J Clin Oncol. 2007;25:3198–3204. doi: 10.1200/JCO.2006.10.3028. [DOI] [PubMed] [Google Scholar]
- 5.Baker TG. Proc R Soc Lond B. 1963;158:417–433. doi: 10.1098/rspb.1963.0055. [DOI] [PubMed] [Google Scholar]
- 6.Gougeon A, Chainy GB. J Reprod Fertil. 1987;81:433–442. doi: 10.1530/jrf.0.0810433. [DOI] [PubMed] [Google Scholar]
- 7.Byskov AG, Faddy MJ, Lemmen JG, Andersen CY. Differentiation. 2005;73:438–446. doi: 10.1111/j.1432-0436.2005.00045.x. [DOI] [PubMed] [Google Scholar]
- 8.Telfer EE, et al. Cell. 2005;122:821–822. doi: 10.1016/j.cell.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Johnson J, et al. Cell Cycle. 2005;4:1471–1477. doi: 10.4161/cc.4.11.2186. [DOI] [PubMed] [Google Scholar]
- 10.Eggan K, Jurga S, Gosden R, Min IM, Wagers AJ. Nature. 2006;441:1109–1114. doi: 10.1038/nature04929. [DOI] [PubMed] [Google Scholar]
- 11.Begum S, Papaioannou VE, Gosden RG. Hum Reprod. 2008;23:2326–2330. doi: 10.1093/humrep/den249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bristol-Gould SK, et al. Dev Biol. 2006;298:132–148. doi: 10.1016/j.ydbio.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 13.Bristol-Gould SK, et al. Dev Biol. 2006;298:149–154. doi: 10.1016/j.ydbio.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 14.Faddy M, Gosden R. Cell Cycle. 2007;6:1951–1952. doi: 10.4161/cc.6.15.4517. [DOI] [PubMed] [Google Scholar]
- 15.Daggag H, et al. Biol Reprod. 2008;79:468–474. doi: 10.1095/biolreprod.107.067348. [DOI] [PubMed] [Google Scholar]
- 16.Anderson EL, et al. Proc Natl Acad Sci USA. 2008;105:14976–14980. doi: 10.1073/pnas.0807297105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tilgner K, et al. Stem Cells. 2008 Sep 18; doi: 10.1634/stemcells.2008-0289. published online. [DOI] [Google Scholar]
- 18.Hubner K, et al. Science. 2003;300:1251–1256. doi: 10.1126/science.1083452. [DOI] [PubMed] [Google Scholar]
- 19.Woodruff TK. Cancer Treat Res. 2007;138:3–11. doi: 10.1007/978-0-387-72293-1_1. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal SK, Chang RJ. Cancer Treat Res. 2007;138:15–27. doi: 10.1007/978-0-387-72293-1_2. [DOI] [PubMed] [Google Scholar]
- 21.Gosden RG. Reprod Biomed Online. 2002;4(Suppl 1):64–67. doi: 10.1016/s1472-6483(12)60014-5. [DOI] [PubMed] [Google Scholar]
- 22.Silber SJ, et al. Hum Reprod. 2008;23:1531–1537. doi: 10.1093/humrep/den032. [DOI] [PubMed] [Google Scholar]
- 23.Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue Eng. 2006;12:2739–2746. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]


