Abstract
Background
Esthesioneuroblastoma (ENB) is a rare cancer of the nasal cavity in children. Radical surgery followed by post-operative radiation is considered the standard of care in adults. A similar approach in children can lead to significant long-term morbidity.
Procedure
A retrospective multi-institutional review of patients <21 years of age diagnosed with ENB between 1990 and 2014 was performed. Clinical features, treatment and outcome were obtained from the medical records.
Results
Twenty-four patients were identified; median age at diagnosis was 14 years (range 0.6 – 20 years). The majority (75%) were female. Headache was the most common presenting symptom, followed by nasal obstruction and epistaxis. Eight patients had Kadish stage B tumors and 16 had Kadish Stage C. Nine patients had metastatic disease. Gross total resection was achieved at diagnosis in eight patients and after neoadjuvant chemotherapy in four patients. Twenty-one patients received radiation therapy (45 Gy – 68.4 Gy). Thirteen patients received neoadjuvant chemotherapy with 84% objective response rate. Seven patients experienced disease progression or relapse; five in central nervous system, one local and one in cervical lymph node. Fifteen patients were alive at last follow-up. The 5-year disease free survival and overall survival were 74% and 73% respectively. Late effects were observed in 78% of long-term survivors. Four patients developed subsequent malignant neoplasms.
Conclusions
Pediatric ENB is a chemosensitive disease. Pre-operative chemotherapy based multimodal approach should be used in patients with advanced stage disease. Radiation therapy is effective for local control but lower doses should be considered in children.
Keywords: Esthesioneuroblastoma, Childhood, Radiation, Chemotherapy, Surgery
INTRODUCTION
Esthesioneuroblastoma (ENB) is a rare cancer arising from the basal progenitor cells of the olfactory epithelium [1]. The majority of patients are diagnosed between 40 and 70 years of age [2]. According to the Surveillance Epidemiology and End Results group data, ENB is the most common malignancy of the nasal cavity in children, accounting for 28% of all cancers in this location [3]. Though some early reports suggested that ENB belonged to the Ewing sarcoma family of tumors, subsequent molecular analysis have not supported this hypothesis [4, 5]. Currently, the World Health Organization groups ENB with Ewing sarcoma under neuroectodermal tumors [3].
ENB is a locally aggressive tumor with a tendency to spread to the cervical lymph nodes. Common presenting symptoms include nasal obstruction, epistaxis, hyposmia, visual symptoms and headache. Rarely, patients may present with paraneoplastic syndromes caused by ectopic secretion of vasopressin or adrenocorticotropic hormone [6]. In addition to cervical lymph nodes, ENB can spread to lung, central nervous system, bone, liver and bone marrow. Due to the rarity of the disease, there is no established standard of care for pediatric ENB. In adults, surgery followed by post-operative irradiation is considered the standard of care [7]. These modalities pose unique challenges in children. Due to smaller size, it may be difficult to perform oncological surgery in the nasal cavities of children without causing significant deformity. There is an added concern of late effects such as craniofacial abnormality, endocrine dysfunction and secondary malignancy due to radiation therapy. Pediatric ENB may be more chemoresponsive than ENB in adults [8, 9]. In order to further the understanding of pediatric ENB, we undertook a multicenter retrospective review of this disease.
METHODS
Patients less than 21 years of age with histologically confirmed ENB diagnosed between 1990 and 2014 at the authors’ institutions were included. Their clinical presentation, disease characteristics, treatment, outcome and complications were retrospectively ascertained by review of medical records. The diagnostic imaging study reports were reviewed to determine the extent of disease and the primary tumor was staged using Kadish staging system [10]; stage A, tumor limited to the nasal cavity, stage B, tumor infiltrating the nasal and paranasal cavities, stage C, tumor extending beyond the nasal and paranasal cavities including orbits, base of skull and intracranium. The response to neoadjuvant chemotherapy was defined as follows: complete response, disappearance of all target lesions; partial response, at least 30% decrease in the size of the lesions; progressive disease, at least 20% increase in the size of the lesions; stable disease, does not meet criteria for partial response or progressive disease. The institutional review board approval was obtained in all participating institutions.
Statistical Analyses
The analysis was descriptive. Disease-free survival (DFS) was defined as the interval between the date of diagnosis and the date of progressive disease or relapse. Overall survival (OS) was defined as the interval between the date of diagnosis and the date of death or last follow-up. Survival analysis was performed using Kaplan-Meier method.
RESULTS
Patient characteristics
Twenty-four patients were treated at the five participating institutions during the study period (Table I). The median age at diagnosis was 14 years (range 0.6 – 20 years). The majority (75%) were female. Headache was the most common presenting symptom, followed by nasal obstruction and epistaxis. Other symptoms included visual disturbance, proptosis, neck swelling, seizure, recurrent sinus infection, facial swelling, dizziness, anosmia and nausea. One patient had a history of global developmental delay and epilepsy. Two patients had a prior history of bilateral retinoblastoma and in another patient ENB was the third malignancy after granulosa theca cell tumor of ovary and malignant cystosarcoma phyllodes of the breast. Eight tumors were Kadish stage B at presentation and 16 were Kadish stage C. Eight patients had radiologic evidence of intracranial extension or infiltration of the cribriform plate. Primary tumor size was available for 15 patients. The median of the largest tumor dimension was 5 cm (range 1.5 – 8 cm). Nine patients had distant metastases at diagnosis; seven had cervical lymph node involvement, one patient had bone marrow involvement, and one patient had both cervical lymph node and bone marrow involvement. All but one patient with metastatic disease had Kadish stage C primary tumor.
Table I.
Patient Characteristics and Outcome
| Patient No. | Age at dx (yr) | Sex | Kadish Stage | Metastatic Sites | Definitive surgery | Neoadjuvant chemotherapy (number of cycles) | Response to chemotherapy | Radiation Dose (Gy) | Vital Status | DFS (mo) | OS (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | M | B | Cervical LN | Upfront biopsy | VDC/IE (12) | CR | NA | Dead (osteosarcoma) | 185 | 185 |
| 2 | 0.6 | M | B | None | Upfront GTR | None | NA | NA | Alive | 57 | 57 |
| 3 | 13 | F | B | None | Upfront GTR | None | NA | 60 | Alive | 82 | 82 |
| 4 | 14 | F | B | None | Upfront GTR | None | NA | 55.8 | Alive | 59 | 59 |
| 5 | 14 | F | B | None | Upfront GTR | None | NA | 50 | Alive | 27 | 27 |
| 6 | 15 | M | B | None | Upfront GTR | None | NA | 55.8 | Alive | 56 | 56 |
| 7 | 19 | F | B | None | Upfront GTR | None | NA | 56 | Dead | 92 | 100 |
| 8 | 5 | F | B | None | Upfront partial resection | None | NA | 36 + boost | Dead (myelodysplastic syndrome) | 53 | 53 |
| 9 | 20 | F | C | Cervical LN | Delayed GTR | CDDPE (2) | PR | 62.5 | Dead | 8 | 11 |
| 10 | 16 | F | C | Cervical LN | Delayed GTR | VDC (5) | PR | 60 | Alive | 46 | 46 |
| 11 | 14 | F | C | None | Delayed GTR | VDC/IE (4) | PR | 50 | Alive | 6 | 6 |
| 12 | 15 | M | C | None | Delayed GTR | VDC/IE (13) | CR | NA | Dead | 12 | 28 |
| 13 | 15 | F | C | None | Delayed partial resection | VDC/IE (4) | PR | 45 | Alive | 10 | 55 |
| 14 | 10 | F | C | None | Delayed partial resection | VDC (3) | SD | 61.2 | Alive | 54 | 54 |
| 15 | 6 | F | C | Cervical LN | Upfront biopsy | CDDPE (4) | CR | 59.4 | Alive with disease | 5 | 8 |
| 16 | 16 | F | C | Cervical LN | Upfront biopsy | CDDPE (5) | CR | 45 | Alive | 263 | 263 |
| 17 | 8 | F | C | None | Upfront biopsy | VDC/IE (8) | PR | 59.4 | Alive | 132 | 132 |
| 18 | 13 | F | C | Bone Marrow | Upfront biopsy | VDC/IE (1) | PD | 68.4 | Dead (anaplastic astrocytoma) | 35 | 35 |
| 19 | 15 | F | C | None | Upfront biopsy | Yes | CR | 54 | Alive | 41 | 46 |
| 20 | 10 | F | C | None | Upfront biopsy | Yes | PR | 60 | Alive | 146 | 146 |
| 21 | 16 | F | C | Cervical LN | Upfront GTR | None | NA | 59.4 | Alive | 10 | 10 |
| 22 | 20 | M | C | Cervical LN | Upfront GTR | None | NA | 63 | Alive with disease | 21 | 25 |
| 23 | 9 | F | C | None | Upfront partial resection | None | NA | 54.6 | Alive | 34 | 34 |
| 24 | 10 | M | C | Bone Marrow, Cervical LN | Upfront partial resection | None | NA | 54 | Dead | 1 | 19 |
M, male; F, female; LN, lymph node; GTR, gross total resection; V, vincristine; D, doxorubicin; C, cyclophosphamide; I, ifosfamide; E, etoposide; CDDP, cisplatin; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; DFS, disease free survival; OS, overall survival; NA, not applicable.
Treatment
The definitive surgical procedure for each patient for ENB is listed in Table I. Gross total resection at diagnosis was achieved in eight patients (six stage B). Three patients underwent partial resection at diagnosis and did not have further surgical resection during treatment. The remaining 13 patients had biopsy only at diagnosis. Following neoadjuvant chemotherapy, four patients underwent gross total resection and two underwent partial resection. Seven patients did not have further surgery after diagnostic biopsy.
Thirteen patients received neoadjuvant chemotherapy. Seven patients had therapy based on a Ewing sarcoma protocol, four patients had cisplatin based therapy and chemotherapy details were not available in two. There were five complete responses, six partial responses, one stable disease and one progressive disease following chemotherapy (Figure 1). Six other patients received adjuvant chemotherapy; three based on a Ewing sarcoma protocol and three with a regimen containing cisplatin. Five patients did not receive chemotherapy; four of whom had gross total resection at diagnosis (Kadish stage B).
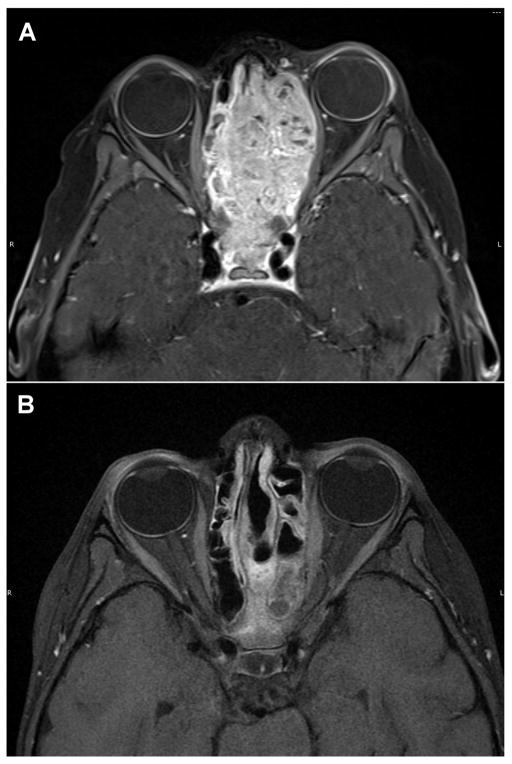
Fig. 1.
T1 axial images from magnetic resonance imaging scan of a patient with esthesioneuroblastoma at diagnosis (A) and after 4 cycles of neo-adjuvant chemotherapy (B).
Twenty-one patients received radiation therapy as part of the initial treatment regimen. The median dose to the primary site was 56 Gy (range 45 Gy – 68.4 Gy). Six of the eight patients with cervical lymph node involvement received neck irradiation with doses ranging from 50 Gy to 57 Gy. Two additional patients received prophylactic irradiation to cervical lymph nodes. Three patients did not receive radiation therapy as part of initial therapy. One patient was 8 months of age at diagnosis and radiation therapy was not given due to young age. Another patient had history of irradiation for bilateral retinoblastoma and was 3 years of age, and the third patient refused radiation therapy.
Outcome
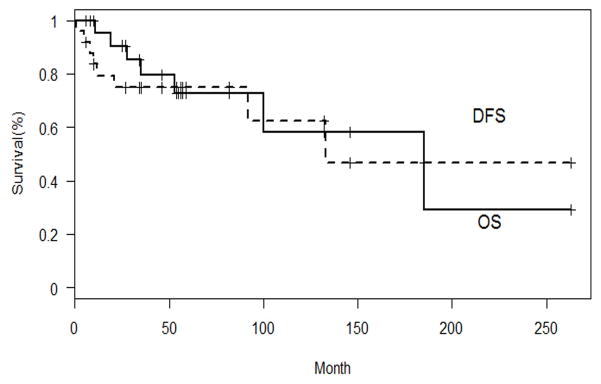
Seven patients experienced disease progression or relapse. Five had central nervous system relapse, one patient had local relapse, and in one patient disease relapsed in the cervical lymph nodes. Only two of the five patients with central nervous system relapse had evidence of intracranial extension or infiltration of the cribriform plate at diagnosis. Disease remission was achieved in the patient with local relapse with chemotherapy. In the patient with cervical lymph node relapse, remission was achieved after partial resection followed by autologous bone marrow transplant with a conditioning regimen containing carboplatin, etoposide and melphalan. All five patients with central nervous system progression had either active disease or were dead at last follow-up in spite of irradiation and chemotherapy. After a median follow-up of 46 months (range 6–263 months), 15 patients were alive without disease, two patients were alive with disease, and seven patients were dead. The cause of death was disease progression in the brain in three patients, development of subsequent malignant neoplasm in three patients and unknown in one patient. All three patients who died due to ENB had Kadish stage C disease at diagnosis; two of them had cervical lymph node metastases. The 5-year DFS and OS were 73.7% (95% CI: 50.5–87.3) and 72.8% (95% CI: 46–87.9) respectively (Figure 2).
Fig. 2.
Disease-free survival (DFS) and overall survival (OS) of patients with esthesioneuroblastoma
Sequelae
Of the 18 patients who had been followed for at least 2 years, 14 had long-term effects documented in their medical records. Recurrent sinus infections, anosmia, craniofacial abnormality, pituitary dysfunction, nasolacrimal duct obstruction and cataract were observed in 2 or more patients. Other sequelae included ptosis, facial pain, retinopathy, Eustachian tube dysfunction, xeropthalmia, epistaxis, hypertension, renal dysfunction, and peripheral neuropathy.
Four patients developed a subsequent malignancy (Table II); in two of them it was the third and fourth malignancy. One each developed myelodysplastic syndrome, malignant fibrous histiocytoma, anaplastic astrocytoma and osteosarcoma of the mandible. In three patients malignancy developed in prior radiation field. The patient who developed myelodysplastic syndrome had received adjuvant chemotherapy with vincristine, doxorubicin, cyclophosphamide, ifosfamide and etoposide for ENB. Three of these 4 patients died due to the subsequent malignancy, accounting for the higher estimation of DFS than OS.
Table II.
Subsequent Malignant Neoplasm
| Patient No | Prior malignancy | Subsequent malignancy | Prior radiation | Time to subsequent malignancy (mo) | Site in prior radiation field |
|---|---|---|---|---|---|
| 1 | Bilateral Retinoblastoma | Osteosarcoma of the mandible | 44.8 Gy (opposing field for retinoblastoma) | 154 | Yes |
| 8 | None | Myelodysplastic syndrome | 36 Gy + boost | 14 | No |
| 13 | None | Malignant fibrous histiocytoma of the right frontal bone | 45 Gy | 49 | Yes |
| 18 | Granulosa Theca cell tumor of ovary Cystosarcoma Phyllodes of breast |
Anaplastic astrocytoma | 68.4 Gy | 29 | Yes |
DISCUSSION
Pediatric ENB is a rare disease that usually presents in the adolescent age group. Some reports mention bimodal distribution of ENB with peaks in 2nd and 6th decades of life [8]. Population based data from the Surveillance Epidemiology and End Results group has shown that this is not the case, and only few cases are diagnosed in the pediatric age group [2]. The eight-month-old patient in this report, to our knowledge, is the youngest patient ever reported with ENB. In adults, ENB is equally distributed between males and females [2]. There were three times as many females as males in our series. In a pediatric series from France, eight of the 11 patients were female, while another series from Italy had more male patients [8, 9]. This variation is likely the result of small numbers. Kadish proposed a staging system (A–C) in 1976 which was later modified by Morita, who added stage D indicating metastatic disease [10, 11]. The Kadish staging system is a significant predictor of survival in adults [2]. Two-third of our patients had Kadish stage C disease, which is in agreement with prior reports demonstrating that children usually present with advanced stage disease [12]. None of the major published reports in children, including the current report, had a patient with Kadish stage A [8, 9, 13].
ENB is a small round blue cell tumor and can be difficult to diagnose [13]. Tumor cells are usually immunopositive for synaptophysin, chromogranin, CD56, neuron-specific enolase, and S-100 and immunonegative for desmin, myogenin, and CD99. One helpful feature is that S-100 would highlight the sustentacular cells by immunohistochemistry. Hyam’s Grading (I–IV) has shown to be of prognostic value in adults, but has not been investigated in children [14].
Combined surgery and radiation therapy has produced the best outcomes in adults [1, 2]. Surgery can be performed either at diagnosis or after chemoradiotherapy. Due to the location, oncologic surgery may involve severe morbidity. Positive margins are common. Some authors have recommended surgery alone for treatment of grossly resected lower stage tumors [13]. This is problematic for two reasons; 1) even with a low stage tumor, it is very difficult to achieve or confirm oncological resection at this location, and 2) multiple studies in adults have shown that local control rates are much higher if surgery is followed by radiation [1]. Tumors unresectable at diagnosis may become resectable after neo-adjuvant chemotherapy or chemoradiotherapy. In a pediatric series published from Germany, complete resection (R0) was reported in 15 out of 17 patients with surgery [13]. Five of these patients received preoperative chemotherapy because the primary was unresectable at diagnosis. The utility of surgery in patients who have radiological remission following neoadjuvant chemotherapy or irradiation is unknown. Several reports of long term survival without surgery have been published [8, 9, 15].
Other reports describe the lack of viable tumor tissue on pathological examination after chemoradiotherapy [15, 16]. On the other hand, while describing the adult experience at the University of Virginia, Polin et al recommended removal of the floor of the anterior fossa even in patients with complete radiological response to reduce the chance of recurrence [17]. In the current series, of the five patients with complete response to chemotherapy, four did not undergo further surgery. None of them had a local relapse of their disease.
Radiation therapy is very effective for local control of ENB, and doses between 50 Gy and 65 Gy have been used in children [8, 9]. Radiation therapy is generally administered in the postoperative setting, but some centers have recommended administration in the neoadjuvant setting [16, 17]. Use of radiation in the neoadjuvant setting with or without chemotherapy in adults resulted in a 50% reduction in tumor volume in half of the patients [17]. Others have attempted concurrent chemoradiotherapy in the preoperative setting, albeit with increased toxicity [16]. Radiation therapy is associated with the development of late effects. In some instances, radiation therapy was not given due to the patient’s age. In a pediatric series by El Kababri et al, a 10 month old was treated with surgery and chemotherapy alone due to age and was a long-term survivor.[8] In another series from Italy, two patients who were treated without radiotherapy due to young age relapsed, but were salvaged with radiotherapy [9]. In the current report, three patients were treated without radiation therapy; in two patients there was no local relapse while the third patient experienced local relapse one year after diagnosis. These data suggest that the omission of radiation therapy from the initial treatment plan should only be considered after a careful discussion of risks and benefits and probably be limited only to a very young patient with either an initial R0 resection or a complete response to chemotherapy. Recently, irradiation using proton beam has been advocated to decrease toxicity [18, 19]. In a small series of 8 children with ENB treated with proton therapy, four developed late toxicity [19]. Due to concern of late effects in children, doses in the range of 54 – 59.4 Gy are recommended [19]. In some adult series, doses as low as 50 Gy have been used without compromising outcomes [20]. Though some authors recommend prophylactic neck irradiation even in patients without cervical node involvement, this is controversial [16, 18]. In general neck imaging should be performed on all patients (especially Kadish stage C) and irradiation should be considered in patients with cervical node involvement. Whether a complete response to initial chemotherapy in the cervical nodes can obviate the need for bilateral cervical radiation therapy is unknown.
ENB is a chemosensitive tumor in children. Commonly used chemotherapy regimens are vincristine/doxorubicin/cyclophosphamide and cisplatin/etoposide. Eighty four percent of patients who received neoadjuvant chemotherapy responded in our series. In pediatric series from France and Italy, the objective response rates to chemotherapy were 66% and 71% respectively [8, 9]. Some groups have adopted neo-adjuvant chemotherapy as the preferred initial approach in children in an attempt to reduce the extent of surgery and surgical complications [9]. Although one patient with relapsed disease in the current report was a long term survivor after high dose chemotherapy and autologous bone marrow transplant, the role of this modality is unclear. There have been isolated case reports of prolonged disease stabilization in adults with refractory disease using newer agents such as sunitinib and temozolomide, though no such data is yet available for the pediatric population [21, 22]. There is no consensus on which chemotherapy regimen to use for ENB in both adults and children. In our series, both Ewing sarcoma based regimens and cisplatin/etoposide regimens produced objective responses. Considering the intensity and length of treatment with Ewing sarcoma based regimens, treatment with 4–6 cycles of cisplatin (100 mg/m2/cycle) and etoposide (300 mg/m2/cycle) may be preferable, but should be weighed against possible ototoxicity and renal toxicity.
In adults, Kadish Stage and Hyam’s histological grade have been shown to predict prognosis [2, 14, 23]. This has not been validated in children. A meta-analysis of published studies on ENB in adults showed a 5-year DFS and OS of 41% and 45%, respectively [1]. Children present with more advanced stage disease than in adults, however, their prognosis is better. In the French series, the 5-year actuarial disease-free survival and overall survival rate was 91% (95% confidence interval, 62%–98%) [8]. In the Italian series, 5-year event free survival was 77.8% (36.6%–93.9%) and overall survival was 88.9% (43.3%–98.4%).[9] In a slightly older series from Germany, 5-year event free survival was 55% and overall survival was 73%. This series reports 5-year DFS of 74% and OS of 73%. These figures, however, must be interpreted with caution given the low sample sizes and resultant wide confidence intervals. Late relapses may occur. One patient in our series relapsed after 11 years.
Treatment and disease related sequelae, including endocrine dysfunctions, anosmia, damage to the dentition, retinopathy and craniofacial abnormalities are common [8, 9, 13]. These were likely under reported since there is no systematic collection of late effects in retrospective studies. Another concerning finding in our study is the development of second malignant neoplasms. In fact, three of our patients died due to a malignancy other than ENB, a rather high and concerning rate given the median follow-up time of under four years. This combined with the fact that at least three patients had a previous history of cancer prior to ENB diagnosis makes us hypothesize that at least some ENB patients may have cancer predisposition. Referral for genetic testing may be indicated for children diagnosed with this rare tumor. Three of our patients developed a subsequent malignancy in the radiation field, and one patient developed myelodysplastic syndrome after receiving chemotherapy. Possible underlying cancer predisposition should be taken into account when planning treatment for patients with ENB.
This study has several limitations. The data was collected retrospectively, and patients were treated over a period of 25 years. The disease staging was performed by review of radiology reports as radiology images were not available in multiple patients. There was no central review of pathology. Definitive recommendation for a chemotherapy regimen could not be derived as patients were treated with different regimens. Many of the patient were treated with older radiation techniques and the late sequelae observed may be less with the use of more conformal techniques.
The optimal treatment of pediatric ENB is not known. Current treatment strategies are based on adult data and often involve morbid surgery and high doses of radiation therapy. Our data and other published reports suggest that pediatric ENB is chemosensitive. Treatment strategies that avoid potentially morbid surgery similar to those used for parameningeal rhabdomyosarcoma and nasopharyngeal carcinoma should be considered in this disease. Conservative doses of irradiation toward the lower end of that described in the literature should be used in children to reduce long-term side effects. Future prospective research with international collaboration is needed to further define the role of chemotherapy in this very rare disease.
Acknowledgments
Funding: None
NIH grant support: T32 CA136432
Footnotes
Conflict of Interest statement
The authors have no conflict of interest to report.
References
- 1.Dulguerov P, Allal AS, Calcaterra TC. Esthesioneuroblastoma: a meta-analysis and review. Lancet Oncol. 2001;2:683–690. doi: 10.1016/S1470-2045(01)00558-7. [DOI] [PubMed] [Google Scholar]
- 2.Jethanamest D, Morris LG, Sikora AG, Kutler DI. Esthesioneuroblastoma: a population-based analysis of survival and prognostic factors. Arch Otolaryngol Head Neck Surg. 2007;133:276–280. doi: 10.1001/archotol.133.3.276. [DOI] [PubMed] [Google Scholar]
- 3.Benoit MM, Bhattacharyya N, Faquin W, Cunningham M. Cancer of the nasal cavity in the pediatric population. Pediatrics. 2008;121:e141–145. doi: 10.1542/peds.2007-1319. [DOI] [PubMed] [Google Scholar]
- 4.Sorensen PH, Wu JK, Berean KW, Lim JF, Donn W, Frierson HF, Reynolds CP, López-Terrada D, Triche TJ. Olfactory neuroblastoma is a peripheral primitive neuroectodermal tumor related to Ewing sarcoma. Proc Natl Acad Sci USA. 1996;93:1038–1043. doi: 10.1073/pnas.93.3.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mezzelani A, Tornielli S, Minoletti F, Pierotti MA, Sozzi G, Pilotti S. Esthesioneuroblastoma is not a member of the primitive peripheral neuroectodermal tumour-Ewing’s group. Br J Cancer. 1999;81:586–591. doi: 10.1038/sj.bjc.6690734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoorn EJ, Monserez DA, Fenton RA, Overdevest I, Apperloo AJ, Zietse R, Hardillo JA. Olfactory Neuroblastoma With Hyponatremia. JCO. 2015;33:e88–e92. doi: 10.1200/JCO.2013.49.1464. [DOI] [PubMed] [Google Scholar]
- 7.Diaz EM, Johnigan RH, Pero C, El-Naggar AK, Roberts DB, Barker JL, DeMonte F. Olfactory neuroblastoma: the 22-year experience at one comprehensive cancer center. Head Neck. 2005;27:138–149. doi: 10.1002/hed.20127. [DOI] [PubMed] [Google Scholar]
- 8.Kababri M, El Habrand JL, Valteau-Couanet D, Gaspar N, Dufour C, Oberlin O. Esthesioneuroblastoma in children and adolescent: experience on 11 cases with literature review. J Pediatr Hematol Oncol. 2014;36:91–95. doi: 10.1097/MPH.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 9.Bisogno G, Soloni P, Conte M, Podda M, Ferrari A, Garaventa A, Luksch R, Cecchetto G. Esthesioneuroblastoma in pediatric and adolescent age. A report from the TREP project in cooperation with the Italian Neuroblastoma and Soft Tissue Sarcoma Committees. BMC Cancer. 2012;12:117. doi: 10.1186/1471-2407-12-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadish S, Goodman M, Wang CC. Olfactory neuroblastoma. A clinical analysis of 17 cases. Cancer. 1976;37:1571–1576. doi: 10.1002/1097-0142(197603)37:3<1571::aid-cncr2820370347>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 11.Morita A, Ebersold MJ, Olsen KD, Foote RL, Lewis JE, Quast LM. Esthesioneuroblastoma: prognosis and management. Neurosurgery. 1993;32:706–714. doi: 10.1227/00006123-199305000-00002. discussion 714–715. [DOI] [PubMed] [Google Scholar]
- 12.Kumar M, Fallon RJ, Hill JS, Davis MM. Esthesioneuroblastoma in children. J Pediatr Hematol Oncol. 2002;24:482–487. doi: 10.1097/00043426-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Eich HT, Müller R-P, Micke O, Kocher M, Berthold F, Hero B. Esthesioneuroblastoma in childhood and adolescence. Better prognosis with multimodal treatment? Strahlenther Onkol. 2005;181:378–384. doi: 10.1007/s00066-005-1362-2. [DOI] [PubMed] [Google Scholar]
- 14.Bell D, Saade R, Roberts D, Ow TJ, Kupferman M, Demonte F, Hanna EY. Prognostic Utility of Hyams Histological Grading and Kadish-Morita Staging Systems for Esthesioneuroblastoma Outcomes. Head Neck Pathol. 2014 doi: 10.1007/s12105-014-0547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharyya N, Thornton AF, Joseph MP, Goodman ML, Amrein PC. Successful treatment of esthesioneuroblastoma and neuroendocrine carcinoma with combined chemotherapy and proton radiation. Results in 9 cases. Arch Otolaryngol Head Neck Surg. 1997;123:34–40. doi: 10.1001/archotol.1997.01900010038005. [DOI] [PubMed] [Google Scholar]
- 16.Sohrabi S, Drabick JJ, Crist H, Goldenberg D, Sheehan JM, Mackley HB. Neoadjuvant Concurrent Chemoradiation for Advanced Esthesioneuroblastoma: A Case Series and Review of the Literature. JCO. 2011;29:e358–e361. doi: 10.1200/JCO.2010.30.9278. [DOI] [PubMed] [Google Scholar]
- 17.Polin RS, Sheehan JP, Chenelle AG, Munoz E, Larner J, Phillips CD, Cantrell RW, Laws ER, Newman SA, Levine PA, Jane JA. The role of preoperative adjuvant treatment in the management of esthesioneuroblastoma: the University of Virginia experience. Neurosurgery. 1998;42:1029–1037. doi: 10.1097/00006123-199805000-00045. [DOI] [PubMed] [Google Scholar]
- 18.Herr MW, Sethi RKV, Meier JC, Chambers KJ, Remenschneider A, Chan A, Curry WT, Barker FG, Deschler DG, Lin DT. Esthesioneuroblastoma: an update on the massachusetts eye and ear infirmary and massachusetts general hospital experience with craniofacial resection, proton beam radiation, and chemotherapy. J Neurol Surg B Skull Base. 2014;75:58–64. doi: 10.1055/s-0033-1356493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucas JT, Ladra MM, MacDonald SM, Busse PM, Friedmann AM, Ebb DH, Marcus KJ, Tarbell NJ, Yock TI. Proton therapy for pediatric and adolescent esthesioneuroblastoma. Pediatr Blood Cancer. 2015 doi: 10.1002/pbc.25494. n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loy AH, Reibel JF, Read PW, Thomas CY, Newman SA, Jane JA, Levine PA. Esthesioneuroblastoma: continued follow-up of a single institution’s experience. Arch Otolaryngol Head Neck Surg. 2006;132:134–138. doi: 10.1001/archotol.132.2.134. [DOI] [PubMed] [Google Scholar]
- 21.Preusser M, Hutterer M, Sohm M, Koperek O, Elandt K, Dieckmann K, Prayer D, Marosi C. Disease stabilization of progressive olfactory neuroblastoma (esthesioneuroblastoma) under treatment with sunitinib mesylate. J Neurooncol. 2010;97:305–308. doi: 10.1007/s11060-009-0027-x. [DOI] [PubMed] [Google Scholar]
- 22.Wick W, Wick A, Küker W, Dichgans J, Weller M. Intracranial metastatic esthesioneuroblastoma responsive to temozolomide. J Neurooncol. 2004;70:73–75. doi: 10.1023/b:neon.0000040826.30636.4a. [DOI] [PubMed] [Google Scholar]
- 23.Van Gompel JJ, Giannini C, Olsen KD, Moore E, Piccirilli M, Foote RL, Buckner JC, Link MJ. Long-term outcome of esthesioneuroblastoma: hyams grade predicts patient survival. J Neurol Surg B Skull Base. 2012;73:331–336. doi: 10.1055/s-0032-1321512. [DOI] [PMC free article] [PubMed] [Google Scholar]