Abstract
Aim
To evaluate whether the presence of apical periodontitis (AP), root canal treatment (RCT) and endodontic burden (EB) – as the sum of AP and RCT sites – were associated with long-term risk of incident cardiovascular events (CVE), including cardiovascular-related mortality, using data on participants in the Baltimore Longitudinal Study of Aging (BLSA).
Methodology
This retrospective cohort included 278 dentate participants in the BLSA with complete medical and dental examinations. Periodontal disease (PD) and missing teeth were recorded. Total number of AP and RCT sites was determined from panoramic radiographs. EB was calculated as the sum of AP and RCT sites. Oral inflammatory burden (OIB) was calculated combining PD and EB. The main outcome was, incident CVE including angina, myocardial infarction and cardiovascular-related death. Participants were monitored for up to 44 years (mean=17.4±11.1 years) following dental examination. Relative Risks (RR) were calculated through Poisson regression models, estimating the relationship between AP, RCT, EB, PD, OIB and incident CVE.
Results
Mean age at baseline was 55.0±16.8 years and 51.4% were men. Sixty two participants (22.3%) developed CVE. Bivariate analysis showed that PD, EB, number of teeth and OIB were associated with incident CVE. Multivariate models, adjusted for socio-demographic and medical variables, showed that age≥60 years (RR=3.07, 95%CI=1.68-5.62), hypertension (RR=2.0, 95%CI=1.16-3.46) and EB≥3 (RR=1.77, 95%CI=1.04-3.02) were independently associated with incident CVE. The association between OIB and incident CVE was reduced to non-significance after adjustments (RR=1.97, 95%CI=0.83-4.70).
Conclusions
EB in mid-life was an independent predictor of CVE among community-dwelling participants in the BLSA. Prospective studies are required to evaluate cardiovascular risk reduction with the treatment of AP.
Keywords: atherosclerosis, cardiovascular diseases, inflammation, oral-systemic diseases, risk factors
Introduction
Cardiovascular disease (CVD) is the primary noncommunicable cause of global mortality. Ischemic heart disease and stroke accounted for 12.9 million deaths in 2010, reflecting nearly 25% of all deaths worldwide (Lozano et al. 2012). CVD is a result of the interaction of genetic predisposition and a constellation of environmental risk factors. However, recognized risk factors for CVD, such as gender, smoking, dyslipidemia, diabetes, hypertension, serological markers of inflammation and obesity, do not fully explain all the clinical and epidemiological features of the disease. Over the past two decades, oral infections and resulting oral inflammatory diseases have been investigated as additional risk factors for CVD and mortality (Mattila et al. 1989, DeStefano et al. 1993).
Observational studies have provided consistent evidence suggesting that periodontal disease (PD) is independently associated with atherosclerosis and CVD (Bahekar et al. 2007, Lockhart et al. 2012, Tonetti et al. 2013). Tooth loss, which serves as a surrogate measure of history of oral disease, was also found independently associated with atherosclerosis and early mortality (Padilha et al. 2008, Gomes et al. 2012a). Detailed reviews have addressed the biologic plausibility and potential pathogenic mechanisms linking oral infections, atherogenesis and CVD (Kebschull et al. 2010, Cotti et al. 2011b, Janket et al. 2015).
Apical periodontitis (AP) is a common late consequence of dental caries and endodontic infection. AP shares important similarities with the microbial spectrum and the inflammatory response involved in PD (Rupf et al. 2000). AP is associated with increased levels of C-reactive protein (CRP), IL-1, IL-2, IL-6, ADMA, IgA, IgG, and IgM in humans (Gomes et al. 2013), consistent with a systemic immune response to endodontic infection. Moreover, chronic AP may go undiagnosed for years, potentially leading to increased systemic inflammation. Serum levels of inflammatory markers are known to predict CVD risk (Blake et al. 2002).
Thus, AP may also represent a risk factor for CVD; however, few epidemiological studies have examined this potential association (Frisk et al. 2003, Caplan et al. 2006, Joshipura et al. 2006, Cotti et al. 2011a, Pasqualini et al. 2012, Willershausen et al. 2014). One longitudinal investigation found a relationship between incident AP and time to coronary heart disease diagnosis, especially among young men (Caplan et al. 2006). However, no longitudinal clinical studies have addressed the association between AP, RCT and endodontic burden (EB) – using AP and RCT sites combined – and cardiovascular events (CVE) including cardiovascular-related deaths.
The Baltimore Longitudinal Study of Aging (BLSA) is the longest-running scientific study of human aging in the United States (starting in 1958), in which several detailed systemic conditions were assessed concurrently with oral clinical examinations, oral radiographies and oral-health questionnaires (Padilha et al. 2008, Gomes et al. 2012c). The purpose of this study was to evaluate whether the presence of AP, RCT and EB was associated with long-term risk of incident (CVE) using data from participants in the BLSA, testing the hypothesis that endodontic variables are independently associated with CVE.
Methods
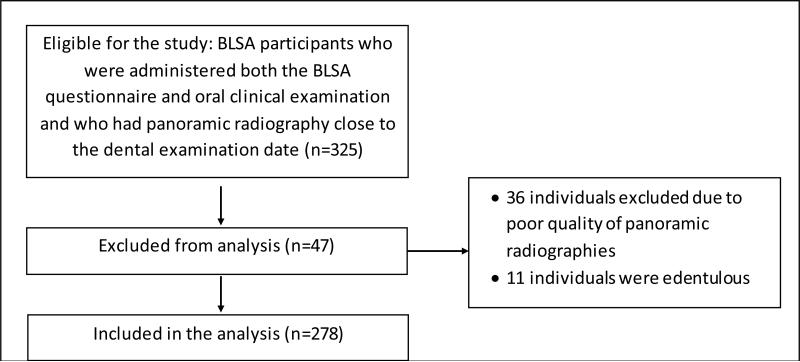
The BLSA has continuing approval from the Institutional Review Board (IRB) of the National Institute of Environmental Health Sciences. Written informed consent was obtained from all participants and all data were de-identified for analysis. Present observational study conforms to the STROBE guidelines. The initial sample of this retrospective cohort comprised 325 subjects who were administered both the BLSA questionnaire and oral clinical examination and who had a panoramic radiography performed within 12 months of the examination. Detailed information on dental status was collected between 1962 and 1995. Thirty-six individuals were excluded due to poor quality of panoramic radiography. Of the 289 remaining subjects, 11 were edentulous and excluded from the analysis, leaving a total of 278 subjects. Figure 1 shows the flowchart of the study sample.
Figure 1.
Flowchart of the study sample.
Sociodemographic data included age (dichotomised as <60 or ≥60 years), sex and years of formal education (dichotomised as ≤12 or >12 years). Smoking was dichotomised as current or former smoker versus never smoker. Complete medical examinations, laboratory exams and clinical oral assessment were performed according to previously described procedures and criteria (Padilha et al. 2008, Nesbitt et al. 2010).
Blood values used in the analysis included fasting glucose, serum glucose assessed 2 hours post-glucose challenge (75 g of glucose in 300 mL of solution), LDL cholesterol, HDL cholesterol and triglycerides. Laboratory assays were performed at the time of the participant's visit to the clinical laboratories at the National Institute on Aging.
Dyslipidemia was defined as total cholesterol >200 and/or LDL>130 and/or triglycerides >200. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared, and was dichotomised as ≤25 (eutrophic) or >25 (overweight/obese). Hypertension was defined as blood pressure ≥140 mmHg systolic and/or ≥90 mmHg diastolic and/or antihypertensive drug therapy. Myocardial infarction (MI) was defined by history of medical diagnosis and/or typical Q waves on a resting electrocardiogram. Angina pectoris was identified by medical history and current use of anti-ischemic agents or positive ischemic treadmill stress testing and clinical symptoms (Rose criteria). Clinical diabetes was defined by medical diagnosis with current use of insulin or hypoglycemic agents or two consecutive positive oral glucose tolerance tests (200 mg/dL or more at 120 min after 75 g glucose beverage intake). Cancer was defined as non-skin cancer documented by medical history and hospitalization records. A history of coronary artery disease (CAD) or stroke was confirmed by medical history and hospitalization records.
Mortality of inactive participants was ascertained by telephone follow-up, correspondence from relatives and annual searches of the National Death Index. Cause of death was determined by physician consensus cause of death, CID9 and CID10 codes.
The main exposures of total number of AP and RCT sites were determined from panoramic radiographies, according to pre-established criteria (De Moor et al. 2000). The radiographic analysis was conducted by an experienced endodontist examiner (MSG) trained in the analysis of panoramic radiographs and blinded to the medical data. As described in a previous study (Gomes et al. 2012c), prior to conducting the radiographic analysis the examiner underwent a training period using 34 images independent of the BLSA radiographies. Calibration training was performed by two examiners, both experienced specialists in Endodontics, who independently evaluated the panoramic radiographies twice, with a period of 45 days between the first and the second evaluation. Inter-examiner agreement levels after the second examination yielded a Kappa of 0.912 for RCT and a Kappa of 0.801 for AP. The intra-examiner agreement (MSG) demonstrated a Kappa of 0.983 for RCT and a Kappa of 0.959 for AP.
For analytical purposes, both AP and RCT variables were dichotomised as absent or ≥1 AP or RCT. EB was calculated as the sum of the total number of teeth with AP and/or RCT for each individual. EB was categorised as zero, 1-2, or ≥3 teeth, based on the stratification of a classic study (Mattila et al. 1989). PD, decayed/missing/filled teeth index (DMFT) and missing teeth were recorded through oral clinical examination. PD was also evaluated radiographically by measuring alveolar bone loss, and was dichotomised as none/slight or moderate/severe (Nesbitt et al. 2010). DMFT and number of teeth were dichotomised as <20 or ≥20 teeth (Padilha et al. 2008). Oral inflammatory burden (OIB) was calculated combining PD and EB and was defined according to four categories: PD none/slight & EB < 3 teeth; PD moderate/severe & EB < 3 teeth; PD none/slight & EB ≥3 teeth; PD moderate/severe & EB ≥3 teeth. All oral variables (AP, RCT, EB, PD, DMFT, OIB) were obtained only at the baseline clinical and radiographic examinations, since no dental longitudinal data was available in the BLSA.
The main outcome, long-term risk of incident CVE, comprised incident angina, incident myocardial infarction (MI) and/or cardiovascular-related death (which included stroke, coronary artery disease (CAD) and other cardiac causes). Participants were monitored for up to 44 years (mean of 17.4±11.1 years, minimum of 1 year, maximum of 44 years) for CVE and mortality from time of dental examination visit through 2011.
Bivariate and multivariate models using Poisson regression with robust variance estimated the relationship (Relative Risk) between AP, RCT, EB, PD, OIB and long-term risk of incident CVE. All variables associated with CVE in the bivariate analysis with a p-value <0.25 were considered potential confounders (known risk factors for CVD) and were included in the multivariate models predicting CVE, where the value for rejection of the null hypothesis was set at p ≤ 0.05. Wald Chi-Square test estimated the strength of the association.
Results
Mean age at baseline dental examination was 55.0±16.8 years (22 to 89 years), with 143 (51.4%) males. In total, 62 participants (22.3%) developed CVE, which included: angina (N=16, 5.8%), MI (N=17, 6.1%) and cardiovascular-related death (N=36, 39.6%). Seven participants presented more than one incident CVE. Among participants who died due to cardiovascular reasons, 20 (55.5%) had CAD, 10 (27.8%) had stroke, and 6 (16.7%) died due to other cardiac reasons. Fifty-five (60.4%) participants died for reasons other than cardiovascular-related deaths, including 24 (43.6%) due to cancer.
Table 1 shows the distribution of socio-demographic, medical and dental characteristics of participants according to incident CVE, as well as the unadjusted p-values. Age (RR=4.6, 95%CI=2.6-8.1), BMI (RR=1.6, 95%CI=1.1-2.6), hypertension (RR=3.3, 95%CI=2.0-5.3), dyslipidemia (RR=2.1, 95%CI=1.1-3.7), diabetes (RR=2.1, 95%CI=1.2-3.6), EB ≥ 3 (RR=1.9, 95%CI=1.1-3.3), PD (RR=1.8, 95%CI=1.2-2.8), number of teeth (RR=1.8, 95%CI=1.1-2.9) and OIB (RR=2.7, 95%CI=1.4-5.3) were significantly associated with incident CVE in the bivariate analysis. When individually analysed, the presence of AP (RR=1.46, 95%CI=0.91-2.33) and RCT (RR=1.25, 95%CI=0.80-1.94) were not associated with incident CVE. Among the 37 individuals with EB ≥ 3, 13 (35.1%) developed CVE; among these, 6 had incident angina, 4 had incident MI, 1 had both incident angina and MI, and 4 participants died due to CVD.
Table 1.
Socio-demographic, medical and dental characteristics of participants (N, %), by incident Cardiovascular Event (CVE). Poisson Regression with robust variance.
| Variables (N) | no incident CVE N (%) |
incident CVE N (%) |
p-value* |
|---|---|---|---|
| Socio-demographic | |||
| Age (278) | |||
| ≥60 years | 76 (35.2) | 49 (79.0) | <0.01 |
| Sex (278) | |||
| Male | 105 (48.6) | 38 (61.3) | 0.08 |
| Education (271) | |||
| >12 years | 184 (86.4) | 48 (82.8) | 0.48 |
| Medical | |||
| BMI (277) | |||
| overweight / obese (>25) | 93 (43.1) | 36 (59.0) | 0.03 |
| Smoking Status (278) | |||
| smoker / ex-smoker | 121 (56.0) | 40 (64.5) | 0.24 |
| Hypertension (278) | |||
| Yes | 71 (32.9) | 43 (69.4) | <0.01 |
| Dyslipidemia (257) | |||
| Yes | 121 (60.5) | 45 (78.9) | 0.02 |
| Diabetes (278) | |||
| Yes | 12 (5.6) | 9 (14.5) | 0.01 |
| Dental | |||
| Apical Periodontitis (278) | |||
| ≥1 AP | 43 (19.9) | 18 (29.0) | 0.12 |
| Root Canal Treatment (278) | |||
| ≥1 RCT | 76 (35.2) | 26 (41.9) | 0.33 |
| Endodontic Burden (278) | |||
| no AP and/or RCT | 132 (61.1) | 30 (48.4) | |
| 1 to 2 AP and/or RCT | 60 (27.8) | 19 (30.6) | 0.31 |
| ≥3 AP and/or RCT | 24 (11.1) | 13 (21.0) | 0.02 |
| Periodontal Disease (270) | |||
| moderate/severe | 43 (20.5) | 22 (36.7) | 0.01 |
| DMFT (262) | |||
| ≥20 | 91 (44.0) | 26 (47.3) | 0.67 |
| Number of Teeth (278) | |||
| <20 | 23 (10.6) | 13 (21.0) | 0.02 |
| Oral inflammatory burden (270) | |||
| PD none/slight & EB <3 | 150 (71.4) | 31 (51.7) | |
| PD moderate/severe & EB <3 | 36 (17.1) | 16 (26.7) | 0.03 |
| PD none/slight & EB ≥3 | 17 (8.1) | 7 (11.7) | 0.14 |
| PD moderate/severe & EB ≥3 | 7 (3.3) | 6 (10.0) | <0.01 |
| Total (278) | 216 (77.7) | 62 (22.3) | - |
p-value for bivariate analysis, Poisson regression;
BMI=body mass index;
AP=apical periodontitis;
RTC=root canal treatment;
PD = periodontal disease;
EB = endodontic burden;
DMFT=“decayed, missing, filled teeth” index
Table 2 shows the results of the multivariate analysis for the association between EB and incident CVE. Model A is adjusted for sociodemographic variables, model B is adjusted for sociodemographic and medical variables, and model C is adjusted for sociodemographic, medical and dental covariates. Model C demonstrates that EB≥3 (p=0.035), age (p<0.001) and hypertension (p=0.013) were significantly associated with incident CVE after adjustment for covariates. Wald chi-square tests revealed that age (13.2), hypertension (6.14) and EB≥3 (4.46) presented the highest strengths of association after adjustment.
Table 2.
Multivariate analysis: adjusted models for the association between endodontic burden (EB) and incident cardiovascular events (CVE). Boldface type indicates statistical significance (P value <0.05), Relative Risk (RR) and Wald Chi-Square, Poisson Regression with robust variance.
| Variables | A | B | C | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI |
95% CI |
95% CI |
|||||||||||||
| Wald | RR | Lower | Upper | p* | Wald | RR | Lower | Upper | p* | Wald | RR | Lower | Upper | p* | |
| EB (zero) | |||||||||||||||
| EB (1-2) | 0.02 | 0.97 | 0.60 | 1.56 | 0.89 | 0.06 | 0.94 | 0.57 | 1.52 | 0.81 | 0.01 | 0.99 | 0.61 | 1.60 | 0.97 |
| EB (≥3) | 4.14 | 1.70 | 1.02 | 2.84 | 0.04 | 4.36 | 1.76 | 1.04 | 2.99 | 0.04 | 4.46 | 1.77 | 1.04 | 3.02 | 0.03 |
| Age (≥60y) | 28.9 | 4.61 | 2.64 | 8.04 | <0.01 | 13.2 | 3.00 | 1.66 | 5.43 | <0.01 | 13.2 | 3.07 | 1.68 | 5.62 | <0.01 |
| Sex (male) | 2.13 | 1.38 | 0.90 | 2.12 | 0.14 | 1.29 | 1.32 | 0.82 | 2.14 | 0.26 | 1.80 | 1.40 | 0.86 | 2.27 | 0.18 |
| BMI (>25) | 2.56 | 1.44 | 0.92 | 2.24 | 0.11 | 1.64 | 1.33 | 0.86 | 2.06 | 0.20 | |||||
| Smoking | 0.26 | 1.14 | 0.69 | 1.88 | 0.61 | 0.15 | 1.10 | 0.67 | 1.82 | 0.70 | |||||
| Hypertension | 4.83 | 1.87 | 1.07 | 3.22 | 0.03 | 6.14 | 2.00 | 1.16 | 3.46 | 0.01 | |||||
| Dyslipidemia | 2.74 | 1.61 | 0.92 | 2.81 | 0.10 | 3.00 | 1.70 | 0.93 | 3.10 | 0.08 | |||||
| Diabetes | 0.46 | 1.25 | 0.66 | 2.38 | 0.50 | 0.13 | 1.13 | 0.59 | 2.20 | 0.71 | |||||
| PD | 0.04 | 0.96 | 0.59 | 1.54 | 0.85 | ||||||||||
| Number Teeth (<20) | 1.08 | 0.75 | 0.44 | 1.28 | 0.30 | ||||||||||
p-value for multivariate analysis, Poisson regression; BMI=body mass index; PD = periodontal disease.
Table 3 shows the results of the multivariate analysis for the association between OIB and incident CVE. Model A is adjusted for sociodemographic variables and model B is adjusted for sociodemographic and medical covariates. In model A, OIB was independently associated with incident CVE (RR=2.01, 95%CI=1.19-3.62). However, model B reveals that the association between OIB and incident CVE was weakened after the inclusion of medical covariates (RR=1.98, 95%CI=0.83-4.71).
Table 3.
Multivariate analysis: adjusted models for the association between oral inflammatory burden (OIB) and incident cardiovascular events (CVE). Boldface type indicates statistical significance (P value <0.05), Relative Risk (RR) and Wald Chi-Square, Poisson Regression with robust variance.
| Variables | A | B | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI |
95% CI |
|||||||||
| Wald | RR | Lower | Upper | p* | Wald | RR | Lower | Upper | p* | |
| Oral Inflammatory Burden | ||||||||||
| PD none/slight & EB <3 | ||||||||||
| PD moderate/severe & EB <3 | 0.02 | 1.04 | 0.61 | 1.76 | 0.89 | 0.01 | 1.02 | 0.62 | 1.68 | 0.94 |
| PD none/slight & EB ≥3 | 1.49 | 1.54 | 0.78 | 3.10 | 0.22 | 2.54 | 1.70 | 0.89 | 3.25 | 0.11 |
| PD moderate/severe & EB ≥3 | 6.63 | 2.01 | 1.19 | 3.62 | 0.01 | 2.36 | 1.98 | 0.83 | 4.71 | 0.12 |
| Age (≥60y) | 26.4 | 4.85 | 2.65 | 8.86 | <0.01 | 12.7 | 3.19 | 1.69 | 6.04 | <0.01 |
| Sex (male) | 1.70 | 1.34 | 0.86 | 2.01 | 0.19 | 1.56 | 1.36 | 0.84 | 2.20 | 0.21 |
| BMI (>25) | 1.65 | 1.34 | 0.86 | 2.09 | 0.20 | |||||
| Smoking | 0.20 | 1.12 | 0.67 | 1.89 | 0.66 | |||||
| Hypertension | 5.57 | 1.97 | 1.12 | 3.44 | 0.02 | |||||
| Dyslipidemia | 2.74 | 1.65 | 0.91 | 2.99 | 0.10 | |||||
| Diabetes | 0.31 | 1.20 | 0.63 | 2.28 | 0.58 | |||||
p-value for multivariate analysis, Poisson regression;
BMI=body mass index; PD = periodontal disease; EB = endodontic burden.
Sample power calculation was based on N=278, RR=1.77 for EB≥3, 11.1% of individuals exposed to EB≥3 among non-CVE participants, α=5%, and resulted in a power of 0.76.
Discussion
The results of this study indicate that EB in mid-life independently predicted CVE among community-resident participants in the BLSA. This study is novel in providing up to 44 years of longitudinal data on the relationship between incident CVE including cardiovascular-related mortality and baseline radiographic findings of both RCT and AP.
Potential mechanisms linking oral infections - including AP - to atherogenesis were extensively described (Kebschull et al. 2010, Cotti et al. 2011b, Janket et al. 2015) and are based on the role of oral pathogens and their products in the development of endothelial dysfunction, formation of fatty streaks and maturation of atherosclerotic plaques, increasing their predisposition to rupture and resultant vascular thrombosis. Moreover, AP may contribute to CVD through metastatic pathways, such as the spread of endodontic infection through transient bacteremia, a metastatic injury by circulating endodontic microbial toxins, or systemic inflammation arising from an immune response to endodontic pathogens (Cotti et al. 2011b; Gomes et al. 2013).
The present results are in accordance with previous findings from epidemiological studies (Caplan et al. 2006, Joshipura et al. 2006, Cotti et al. 2011a, Pasqualini et al. 2012, Willershausen et al. 2014) which, taken together, suggest an independent relationship between the overall burden of endodontic disease and CVD. One cross-sectional study did not confirm this association (Frisk et al. 2003), possibly because the sample included women exclusively who have low CVD risk relative to men, had a wide age variation (38-84 years) with no young individuals presenting CVD (the association between oral infections and CVD risk is more pronounced in persons aged 50 years and younger (Mattila et al. 2000, Caplan et al. 2006)), and had a low prevalence of AP.
Oral diseases are primarily associated with noncommunicable chronic diseases through shared common risk factors such as age, lifestyle, diet, smoking, and low socioeconomic status. Accordingly, there is evidence that after adjusting for these risk factors, the relationship between oral diseases and CVD may be weakened (Tuominen et al. 2003). Nevertheless, results from our study indicate that baseline EB was an independent predictor of incident CVE, even after adjustments for several sociodemographic, medical and oral variables (Table 2, model C). Additionally, the association of EB≥3 with CVD was strong (RR=1.77 and Wald=4.46), approaching that for hypertension (RR=2.00 and Wald=6.14), one of the strongest risk factors for CVD. Furthermore, the RR for EB≥3 in this BLSA sample was comparable to overall population tobacco smoking risk, which is known to increase mortality from CAD and cerebrovascular disease 2–3 fold (Mackay et al. 2004). Thus, based on present findings, it is possible to suppose that EB may add to the overall inflammatory burden, contributing to increase cardiovascular risk of susceptible individuals.
The present results suggested a gradient effect on the association between endodontic variables and CVE, since not only EB but also AP and RCT showed a higher incidence of CVE in individuals with a greater number of teeth with AP or RCT, even though the difference for the last two variables failed to reach statistical significance. For illustration purposes, when the AP and RCT variables were categorised the same way as EB (i.e., none / 1-2 / ≥3), AP≥3 (p=0.081) and RCT≥3 (p=0.055) approached significance in the regression analysis. Nevertheless, for analytical purposes, we decided to dichotomise AP and RCT in order to isolate the association between each exposure and the outcome. In contrast, EB was categorised to analyse the association between endodontic disease load and the incidence of CVE.
In the preliminary results from the present study (Gomes et al. 2012b), with a reduced sample size, Cox regression analysis was used to estimate the relationship among baseline endodontic variables and time to CVE. In the present report, the RR was calculated using Poisson regression analysis with robust variance, considering the low number of deaths and also the fact that the proportional hazard model probably violates the assumption of proportionality. Notably, the independent association between EB and incident CVE was statistically confirmed regardless of the analytical approach used.
Interestingly, the multivariate analysis showed that PD, tooth loss and OIB were not associated with incident CVE after adjustment for sociodemographic, medical and dental covariates. Thus, among BLSA participants, EB was found to be the only oral health parameter predictive of CVD. Additionally, the diagnostic criteria of PD in this BLSA population, based on panoramic radiographies and dichotomised into none-slight or moderate-severe PD (Nesbitt et al. 2010), may have represented a “rough measure” of PD, possibly resulting in an underestimate of PD, attenuating its association with CVE after adjustment for endodontic variables. As previously described (Manau et al. 2008), PD definition may determine the association between periodontitis and other systemic outcomes. Noteworthy, most epidemiological studies reporting an independent association between PD and CVD (Bahekar et al. 2007, Lockhart et al. 2012) fail to omit endodontic variables, particularly AP, as potential oral confounders in the multivariate analysis, which should be revisited in future studies in this field.
Some methodological features warrant consideration. Edentulous participants were excluded in the analysis, since no determination of RCT or AP history prior to extractions was possible. Although AP had a p-value <0.25 in the bivariate analysis, it was not included in the multivariate models since the inclusion of both AP and EB variables would produce collinearity issues. As extensively discussed in a previous study (Gomes et al. 2012c), another aspect is related to the radiographic analysis and its inherent limitations, particularly for the detection of AP. Although several epidemiological studies have used panoramic radiographies (De Moor et al. 2000, Gulsahi et al. 2008), a complete intra-oral radiographic series or cone-beam computed tomography (CBCT) yields greater accuracy for the diagnosis of AP (Ridao-Sacie et al. 2007, Estrela et al. 2008). Comparisons of panoramic and periapical films for diagnosis of AP suggest that there is some, though not dramatic, reduction in the detectability of periapical lesions (Ridao-Sacie et al. 2007). In addition, periapical radiographs and CBCT are more accurate only for the diagnosis of incipient and small lesions, but not for medium- or large-sized AP (Estrela et al. 2008). Moreover, CBCT was shown to be a very sensible method but it may lack specificity in the early detection of AP lesions (Pope et al. 2014). Nevertheless, considering the low radiation doses, costs, and field of interest, the panoramic radiograph provides a valuable survey film for this type of investigation. Furthermore, it must be considered that BLSA's dental data were collected between 1962 and 1995 when CBCT was not available.
In the present study AP was assessed based on panoramic radiographies using pre-established criteria (De Moor et al. 2000; Gomes et al. 2012c) instead of using the PAI score system (Ørstavik et al. 1986). The PAI score system was originally designed for the analysis of periapical radiographs, and it was decided not to use it to minimize over-scoring of disease. Additionally, detection of medium- and large-size AP through panoramic radiographs is usually accurate. Most importantly, it is plausible to infer that the larger AP lesions are possibly those with the greatest potential to influence general health, since they tend to develop over longer periods and may present a more complex and virulent microbiota.
Possibly, the main limitation of the present study is the lack of access to incident oral diseases; unfortunately, dental data in the BLSA was restricted to the baseline clinical and radiographic examinations. Hence, unlike a previous investigation (Caplan et al. 2006), this study could not relate incident AP and RCT to incident CVE. This insurmountable limitation implies that patients free of AP and/or RCT at baseline may have developed endodontic diseases during the follow-up period. However, this information is unknown and, thus, a possible source of assessment bias.
Some limitations must be considered regarding the external validity of the present results. The BLSA population is a convenience sample representing largely well-educated north-American white adults, with access to dental treatment. Since stroke and cardiovascular-related death is higher among those of African-American, Hispanic or Asian ethnicity, relative to Caucasian (Mackay et al. 2004), extrapolation of these findings to populations with different patterns of ethnicity, education, culture, and access to dental care should be done with caution. Moreover, this study evaluated whether EB was associated with long-term risk of incident CVE in consecutive participants in the BLSA, however it is unclear whether EB would predict incident CVE among younger adults.
Extensive research is under way to identify novel risk factors that can improve the ability to accurately predict CVD risk and improve current prognostic algorithms (Madjid et al. 2011). In the last decade, studies revealed a heritable component to the CVD (Nabel 2003). A recent report could not find a significant correlation between CD14 polymorphism, AP and coronary heart disease (Pasqualini et al. 2012). However, similarly to the present study, results from that research suggest that AP may be an unconventional risk factor for the development of CVD.
The major established risk factors for CVD meet three criteria (Mackay et al. 2004): a high prevalence in many populations; a significant independent impact on the risk of coronary heart disease or stroke; and treatment and control reduce risk. Hence, based on available evidence, AP seems to fill the first two criteria. However, to establish AP as a novel risk factor for CVD (which would include a causal relationship rather than an association), further interventional controlled prospective studies are definitively required to substantiate the present observational findings and evaluate a hypothetical CVD risk reduction with the treatment of AP.
Endodontics plays an important role in maintaining good oral health by controlling pain, reducing infection and ultimately preserving the natural dentition. The possible association of endodontic diseases with the risk of developing CVD has a major impact on the rationale and case selection for RCT, primarily highlighting the preventive efforts to avoid pulpal infection and all deleterious consequences.
Conclusion
The results suggest that the number of teeth with AP and/or RCT (EB) in mid-life was an independent predictor of CVE among community-dwelling participants in the BLSA. Prospective studies are required to evaluate CVD risk reduction with the treatment of AP.
Acknowledgements
The authors thank Dr. Earl Jeffrey Metter and Dr. Angelo Bós for their invaluable help in providing and organizing the BLSA database. This study was supported by: the Intramural Research Program of the NIH/NIA, National Institute on Aging; the CAPES Foundation, Ministry of Education of Brazil, doctorate scholarship number 1433/11-3; the Military Police, State Government of Rio Grande do Sul, Brazil.
Footnotes
The authors deny any conflicts of interest related to this study.
References
- Bahekar AA, Singh S, Saha S, Molnar J, Arora R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: a meta-analysis. American Heart Journal. 2007;154:830–7. doi: 10.1016/j.ahj.2007.06.037. [DOI] [PubMed] [Google Scholar]
- Blake GJ, Ridker PM. Inflammatory bio-markers and cardiovascular risk prediction. Journal of Internal Medicine. 2002;252:283–94. doi: 10.1046/j.1365-2796.2002.01019.x. [DOI] [PubMed] [Google Scholar]
- Caplan DJ, Chasen JB, Krall EA, et al. Lesions of endodontic origin and risk of coronary heart disease. Journal of Dental Research. 2006;85:996–1000. doi: 10.1177/154405910608501104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotti E, Dessi C, Piras A, et al. Association of endodontic infection with detection of an initial lesion to the cardiovascular system. Journal of Endodontics. 2011a;37:1624–9. doi: 10.1016/j.joen.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Cotti E, Dessi C, Piras A, Mercuro G. Can a chronic dental infection be considered a cause of cardiovascular disease? A review of the literature. International Journal of Cardiology. 2011b;148:4–10. doi: 10.1016/j.ijcard.2010.08.011. [DOI] [PubMed] [Google Scholar]
- De Moor RJ, Hommez GM, De Boever JG, Delme KI, Martens GE. Periapical health related to the quality of root canal treatment in a Belgian population. International Endodontic Journal. 2000;33:113–20. doi: 10.1046/j.1365-2591.2000.00295.x. [DOI] [PubMed] [Google Scholar]
- DeStefano F, Anda RF, Kahn HS, Williamson DF, Russell CM. Dental disease and risk of coronary heart disease and mortality. British Medical Journal. 1993;306:688–91. doi: 10.1136/bmj.306.6879.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrela C, Bueno MR, Leles CR, Azevedo B, Azevedo JR. Accuracy of cone beam computed tomography and panoramic and periapical radiography for detection of apical periodontitis. Journal of Endodontics. 2008;34:273–9. doi: 10.1016/j.joen.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Frisk F, Hakeberg M, Ahlqwist M, Bengtsson C. Endodontic variables and coronary heart disease. Acta Odontologica Scandinavica. 2003;61:257–62. doi: 10.1080/00016350310005510. [DOI] [PubMed] [Google Scholar]
- Gomes MS, Blattner TC, Sant'Ana Filho M, et al. Can apical periodontitis modify systemic levels of inflammatory markers? A systematic review and meta-analysis. Journal of Endodontics. 2013;39:1205–17. doi: 10.1016/j.joen.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Gomes MS, Chagas P, Padilha DM, et al. Association between self-reported oral health, tooth loss and atherosclerotic burden. Brazilian Oral Research. 2012a;26:436–42. doi: 10.1590/s1806-83242012005000019. [DOI] [PubMed] [Google Scholar]
- Gomes MS, Hugo FN, Hilgert JB, et al. Apical periodontitis and cardiovascular events in the BLSA: preliminary results. IADR Annual Meeting, Journal of Dental Research. 2012b;91 (Spec Iss A) [Google Scholar]
- Gomes MS, Hugo FN, Hilgert JB, et al. Validity of Self-reported History of Endodontic Treatment in the Baltimore Longitudinal Study of Aging. Journal of Endodontics. 2012c;38:589–93. doi: 10.1016/j.joen.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulsahi K, Gulsahi A, Ungor M, Genc Y. Frequency of root-filled teeth and prevalence of apical periodontitis in an adult Turkish population. International Endodontic Journal. 2008;41:78–85. doi: 10.1111/j.1365-2591.2007.01324.x. [DOI] [PubMed] [Google Scholar]
- Janket SJ, Javaheri H, Ackerson LK, Ayilavarapu S, Meurman JH. Oral Infections, Metabolic Inflammation, Genetics, and Cardiometabolic Diseases. Journal of Dental Research. 2015 doi: 10.1177/0022034515580795. (in press) [DOI] [PubMed] [Google Scholar]
- Joshipura KJ, Pitiphat W, Hung HC, Willett WC, Colditz GA, Douglass CW. Pulpal inflammation and incidence of coronary heart disease. Journal of Endodontics. 2006;32:99–103. doi: 10.1016/j.joen.2005.10.039. [DOI] [PubMed] [Google Scholar]
- Kebschull M, Demmer RT, Papapanou PN. “Gum bug, leave my heart alone!”--epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. Journal of Dental Research. 2010;89:879–902. doi: 10.1177/0022034510375281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart PB, Bolger AF, Papapanou PN, et al. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association?: a scientific statement from the American Heart Association. Circulation. 2012;125:2520–44. doi: 10.1161/CIR.0b013e31825719f3. [DOI] [PubMed] [Google Scholar]
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay J, Mensah G, Mendis S, Greenlund K, World Health Organization. Dept. of Management of Noncommunicable Diseases . The atlas of heart disease and stroke. World Health Organization; Geneva: 2004. [Google Scholar]
- Madjid M, Willerson JT. Inflammatory markers in coronary heart disease. British Medical Bulletin. 2011;100:23–38. doi: 10.1093/bmb/ldr043. [DOI] [PubMed] [Google Scholar]
- Manau C, Echeverria A, Agueda A, Guerrero A, Echeverria JJ. Periodontal disease definition may determine the association between periodontitis and pregnancy outcomes. Journal of Clinical Periodontology. 2008;35:385–97. doi: 10.1111/j.1600-051X.2008.01222.x. [DOI] [PubMed] [Google Scholar]
- Mattila KJ, Asikainen S, Wolf J, Jousimies-Somer H, Valtonen V, Nieminen M. Age, dental infections, and coronary heart disease. Journal of Dental Research. 2000;79:756–60. doi: 10.1177/00220345000790020901. [DOI] [PubMed] [Google Scholar]
- Mattila KJ, Nieminen MS, Valtonen VV, et al. Association between dental health and acute myocardial infarction. British Medical Journal. 1989;298:779–81. doi: 10.1136/bmj.298.6676.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel EG. Cardiovascular disease. New England Journal of Medicine. 2003;349:60–72. doi: 10.1056/NEJMra035098. [DOI] [PubMed] [Google Scholar]
- Nesbitt MJ, Reynolds MA, Shiau H, Choe K, Simonsick EM, Ferrucci L. Association of periodontitis and metabolic syndrome in the Baltimore Longitudinal Study of Aging. Aging Clinical and Experimental Research. 2010;22:238–42. doi: 10.1007/bf03324802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørstavik D, Kerekes K, Eriksen HM. The periapical index: a scoring system for radiographyic assessment of apical periodontitis. Endodontics & Dental Traumatology. 1986;2:20–34. doi: 10.1111/j.1600-9657.1986.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Padilha DM, Hilgert JB, Hugo FN, Bos AJ, Ferrucci L. Number of teeth and mortality risk in the Baltimore Longitudinal Study of Aging. The Journals of Gerontology. Series A: Biological Sciences and Medical Sciences. 2008;63:739–44. doi: 10.1093/gerona/63.7.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualini D, Bergandi L, Palumbo L, et al. Association among oral health, apical periodontitis, CD14 polymorphisms, and coronary heart disease in middle-aged adults. Journal of Endodontics. 2012;38:1570–7. doi: 10.1016/j.joen.2012.08.013. [DOI] [PubMed] [Google Scholar]
- Pope O, Sathorn C, Parashos P. A comparative investigation of cone-beam computed tomography and periapical radiography in the diagnosis of a healthy periapex. Journal of Endodontics. 2014;40:360–5. doi: 10.1016/j.joen.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Ridao-Sacie C, Segura-Egea JJ, Fernandez-Palacin A, Bullon-Fernandez P, Rios-Santos JV. Radiological assessment of periapical status using the periapical index: comparison of periapical radiography and digital panoramic radiography. International Endodontic Journal. 2007;40:433–40. doi: 10.1111/j.1365-2591.2007.01233.x. [DOI] [PubMed] [Google Scholar]
- Rupf S, Kannengiesser S, Merte K, Pfister W, Sigusch B, Eschrich K. Comparison of profiles of key periodontal pathogens in periodontium and endodontium. Endodontics & Dental Traumatology. 2000;16:269–75. doi: 10.1034/j.1600-9657.2000.016006269.x. [DOI] [PubMed] [Google Scholar]
- Tonetti MS, Van Dyke TE. Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. Journal of Clinical Periodontology. 2013;40(Suppl 14):S24–9. doi: 10.1111/jcpe.12089. [DOI] [PubMed] [Google Scholar]
- Tuominen R, Reunanen A, Paunio M, Paunio I, Aromaa A. Oral health indicators poorly predict coronary heart disease deaths. Journal of Dental Research. 2003;82:713–8. doi: 10.1177/154405910308200911. [DOI] [PubMed] [Google Scholar]
- Willershausen I, Weyer V, Peter M, Weichert C, Kasaj A, Münzel T, Willershausen B. Association between chronic periodontal and apical inflammation and acute myocardial infarction. Odontology. 2014;102:297–302. doi: 10.1007/s10266-013-0112-7. [DOI] [PubMed] [Google Scholar]