Abstract
Background and Purpose
The advent of intra-arterial neurothrombectomy (IAT) for acute ischemic stroke opens a potentially transformative opportunity to improve neuroprotection studies. Combining a putative neuroprotectant with recanalization could produce more powerful trials but could introduce heterogeneity and adverse event possibilities. We sought to demonstrate feasibility of IAT in neuroprotectant trials by defining IAT selection criteria for an ongoing neuroprotectant clinical trial.
Methods
The study drug, 3K3A-APC, is a pleiotropic cytoprotectant and may reduce thrombolysis associated hemorrhage. The NeuroNEXT trial NN104 (RHAPSODY) is designed to establish a maximally tolerated dose of 3K3A-APC. Each trial site provided their IAT selection criteria. An expert panel reviewed site criteria and published evidence. Finally, the trial leadership designed IAT selection criteria.
Results
Derived selection criteria reflected consistency among the sites and comparability to published IAT trials. A protocol amendment allowing IAT (and relaxed age, NIHSS, and time limits) in the RHAPSODY trial was implemented on June 15, 2015. Recruitment before and after the amendment improved from 8 enrolled patients (601 screened, 1.3%) to 51 patients (821 screened, 6.2%), OR [95%CL] of 4.9 [2.3,10.4], p<0.001). Gross recruitment was 0.11 patients/site/month vs. 0.43 patients/site/month, respectively, before and after the amendment.
Conclusions
It is feasible to include IAT in a neuroprotectant trial for acute ischemic stroke. Criteria are presented for including such patients in a manner that is consistent with published evidence for IAT while still preserving the ability to test the role of the putative neuroprotectant.
Clinical Trial Registration
Clinical Trial Registration-URL: http://www.clinicaltrials.gov. Unique identifier: NCT02222714.
Clinical trials of neuroprotection for acute ischemic stroke, including rapid pre-hospital treatment, continue to fail. Possible contributors to this failure include the unsuitability of pre-clinical animal models1, inadequate clinical trial design2, 3, and inappropriate patient selection4. One of the most obvious and potentially powerful differences between animal and human neuroprotection studies is the influential role of recanalization/reperfusion. Recanalization refers to the re-opening of the principal artery supplying a vascular territory; reperfusion implies successful restoration of flow in the microvasculature. Recanalization without reperfusion fails to salvage brain after larger artery occlusion5. Pre-clinical studies repeatedly document that putative neuroprotectants provide greater benefit when combined with recanalization6. Many neuroprotectants show benefit in animal stroke models, but they all work best if given in combination with recanalization7. In contrast, documentation of arterial recanalization has not been required during human neuroprotection trials, and this could be one of the many reasons for clinical trial failures. Even after the advent of intravenous thrombolysis as an effective stroke therapy8, the typical clinical neuroprotectant stroke trial did not require documentation of successful recanalization as an enrollment criterion. If recanalization enhances the chance of successful neuroprotection, then the failure of so many stroke neuroprotection trials appears less surprising.
The advent of intra-arterial neurothrombectomy (IAT) for acute ischemic stroke opens a potentially transformative opportunity for improved study of neuroprotection. Combining a putative neuroprotectant with IAT could result in more powerful clinical trials, since all enrolled patients would have documented recanalization. On the other hand, allowing IAT in a clinical trial brings additional heterogeneity into the response outcome variable; introduces additional adverse event possibilities; and may limit generalizability of the trial results to centers that offer such therapy. As well, the published trials of IAT used a wide variety of inclusion/exclusion criteria, possibly making it difficult to design a clinical trial protocol that is consistent with published data9, 10. Health organizations and professional societies have published guidelines for clinical implementation of IAT that seek to allow a wide range of options for physicians11, 12. Wider latitude of treatment options allows individual physicians to personalize their approach to each patient with acute ischemic stroke13, but practice variation among clinical trial study sites could introduce variation in the primary outcome sufficient to obscure treatment benefit or adverse effects of the study therapy14. The different, competing priorities of a clinical trial compared to clinical practice are summarized in Table One. Thus, the clinical trial design and protocol must specify a range of IAT treatment options that is sufficient to allow sites to use the therapy in a wide variety of patients without introducing excessive variability into the primary efficacy and safety outcomes.
Table One. Selection Priorities in Standard Care vs. a Clinical Trial.
Patient selection priorities in the standard care of patients seek to provide effective treatment safely to the widest possible range of patients. Such criteria should emphasize physician judgment in applying published data to single patients13. In contrast, clinical trial protocol selection criteria seek to focus the treatment on patients most likely to respond, thus demonstrating a beneficial treatment effect with the smallest number of patients14. In a clinical trial, adherence to the protocol assures optimal homogeneity of the final study sample.
| Standard Care | Clinical Trial Protocol |
|---|---|
| Widest possible selection based on evidence | Narrower selection to prioritize consistency |
| Physician judgment, personalized care | Patient safety |
| Optimize chances of patient benefit | |
| Avoid “off-label” procedures that could interfere with trial assessments |
|
| Professional Society Guidelines | Trial specific guidelines |
| Presumption of ELVO based on NIHSS, NCCT, CTA/MRA or DSA |
Confirmed ELVO on baseline CTA/MRA |
| Wide range of upper age limits | Age limit based on neuroprotectant to be studied |
We sought to define IAT selection criteria for an ongoing clinical trial of a putative neuroprotectant. The study drug takes advantage of the neuroprotective and vasculoprotective effects of activated protein C (APC), which is naturally active at the protease activated receptor-1 (PAR1)15. To avoid anticoagulation without changing the cytoprotective effects, the native APC structure was mutated in a targeted manner, such that 3 lysines were replaced by alanines removing >90% of the anticoagulant activity with preserved cytoprotection16. The resultant molecule, 3K3A-APC, exhibits considerable cytoprotective effects in vitro and in animal stroke models. 3K3A-APC protects neurons, astrocytes and endothelial cells directly, via action on the transmembrane G-coupled protein PAR1, but also shows vasculoprotective effects that could be interpreted as protection from ‘reperfusion injury’17. The drug shows greatest effect when combined with recanalization, and is effective after treatment delay of up to 12 hours after stroke onset18. 3K3A-APC has satisfied the Stroke Treatment Academic Industry Roundtable (STAIR) suggestions for pre-clinical drug assessment and has an established safety and pharmacokinetic profile in human volunteers19, 20.The NeuroNEXT trial NN104 (RHAPSODY) is a dose-escalation trial designed to establish a maximally tolerated dose of 3K3A-APC (Protocol synopsis in Supplement). Although 3K3A-APC exhibits beneficial effect after permanent occlusion, the effect is greater in pre-clinical models that include recanalization18, 19, 21. Thus, the RHAPSODY trial provides an opportunity to test whether allowing IAT (and thereby documenting recanalization) contributes favorably or negatively to clinical trial recruitment. We convened a panel of experts in both clinical trial design and IAT to review and critique proposed inclusion/exclusion criteria. Shortly after the publication of the key IAT studies, we surveyed our clinical trial sites to assess their local implementation of IAT selection criteria. Based on the summarized published primary results and published society guidelines, expert reviews, and local implementation at our sites, we defined IAT selection criteria for RHAPSODY. We adopted a clinical trial protocol amendment to allow IAT based on these selection criteria, and carefully monitored the implementation of the amended protocol in the study sites. Here we report on the results of the review process and the impact of the amendment on study recruitment.
METHODS
The RHAPSODY trial (A multi-center, Phase 2 study using a continual reassessment method to determine the safety and tolerability of 3K3A-APC, a Recombinant Variant of Human Activated Protein C (APC), in combination with tissue plasminogen activator (tPA), mechanical thrombectomy or both in moderate to severe acute ischemic stroke, NCT02222714) is a multicenter, prospective, randomized, controlled, double-blinded Phase 2 study intended to evaluate the safety, pharmacokinetics and preliminary efficacy of 3K3A-APC following tPA or mechanical thrombectomy or both in participants with moderate to severe acute ischemic stroke. Approximately 100 participants will be randomized, which includes 88 participants in groups of four randomized to either 3K3A-APC or placebo (3:1 ratio) and additional placebo participants enrolled during safety review pauses. This study utilizes a modified version of the continual reassessment method (CRM) to establish a maximum tolerated dose (MTD)22. For the purposes of this study, we assumed an established background symptomatic intracerebral hemorrhage (sICH) rate of 3–6%23–28. Correspondingly, the MTD is defined as the highest dose with a dose limiting toxicity (DLT) rate of 10% or less. Participants are enrolled at the dose estimated from the assumed dose-response model and prior data to be closest to the MTD. Dose-limiting toxicities are assessed from the first dose to 48 hours following the last dose of study treatment. An elevation of PTT, sICH, systemic bleeding requiring transfusion, or hepatic toxicity that appears “related” to study treatment is considered a DLT. Abnormal laboratory values and any event that prompts cessation of study drug are evaluated as a possible DLT as well.
3K3A-APC is administered as a 100 mL IV infusion over 15 minutes every 12 hours (± 1 hour) for 5 doses. Four dose levels of 3K3A-APC will be considered: 120, 240, 360, and 540 µg/kg. Matching placebo, visually indistinguishable from the drug, is administered in the same manner as the active product. The drug may not begin until 30 minutes after the end of the rt-PA infusion, for safety reasons, and no later than 120 minutes following completion of rt-PA infusion or initiation of mechanical thrombectomy (skin puncture), whichever is sooner, although investigators are encouraged to being study drug infusion as soon as possible. Patients will be examined on Days 7, 14, 30 and 90 for safety and outcome. Participants are not considered part of the intent-to-treat (ITT) cohort until they receive any amount of 3K3A-APC or placebo. Thus, subjects who become ineligible (e.g. rapid responder whose NIHSS drops to <5) between randomization and initiation of study drug will be removed from the study and replaced. Detailed brain imaging is required prior to enrollment, at 24 hours, 7d, 30d, and 90d after enrollment, and will be analyzed for ischemic stroke size and hemorrhagic transformation.
Following the publication of studies reporting efficacy of IAT, we sought to assess the readiness of our study sites to include IAT in the treatment of enrolled patients. Each site provided any written guidance they used for specifying the patients they would consider eligible for IAT therapy. The results were summarized and sites that provided insufficient responses were contacted and asked to consider re-submitting. Next we assembled a panel of experts including principal investigators of some of the main IAT publications. The IAT selection document from each site was then reviewed and approved, rejected, or queried. Finally, using the input from the expert panel and the sites, the trial leadership designed study selection criteria for allowing IAT treated patients. Clinical trial recruitment was tracked before and after adoption of the protocol amendment. Additional changes implemented as part of the same protocol amendment were: extended the upper time window for administration of investigational drug from 90 to 120 minutes; extended the upper age range from 80 to 90; broadened the NIHSS range to ≥ 5 (from 7–20 previously). Criteria specific to IAT were also added: onset time to arterial puncture time < 6 hours and a baseline CT revealing large core occlusions as defined by local protocol, ASPECTS or CT perfusion.
RESULTS
The key variables that influence efficacy and safety outcomes included in the published IAT trials are summarized in Table Two. Despite very different trial designs, the 5 trials actually enrolled samples that showed relatively consistent age, severity, and times to important milestones. The unexpected demographic consistency among these trials allows some specificity in designing clinical trial selection criteria.
Table Two. Intra-arterial Trials’ Study Factors Relevant to Neuroprotection.
For the 5 major trials of IAT for acute stroke that showed benefit, the key factors are summarized. “Median time to IAT” is the median of the time from symptom onset (or last known well, LKW) to the initiation of the procedure, in minutes. “Max Time to IAT” is the maximum protocol-allowed time to initiate recanalization therapy after stroke symptom onset. Mean age, median NIHSS, and mean (or median in ESCAPE and MR CLEAN) time to bridging therapy with IV rt-PA were comparable in the groups compared in all studies.
| Trial | Sample Size (n) |
Median Time to IAT (min) |
Max Time to IAT (hours) |
Mean Age IA vs. Control (years) |
Median NIHSS IA vs. Control |
Median Time to IVT IA vs. Control (min) |
|---|---|---|---|---|---|---|
| ESCAPE | 316 | 200 | 12 | 71/70 | 16/17 | 110/125 |
| EXTEND-IA | 70 | 210 | 6 | 69/70 | 17/13 | 127 /145 |
| MR CLEAN | 500 | 260 | 6 | 66/66 | 17/18 | 85/87 |
| REVASCAT | 206 | 269 | 8 | 66/67 | 17/17 | 117/105 |
| SWIFT PRIME | 196 | 224 | 6 | 65/66 | 17 /17 | 110/117 |
The most critical factor influencing outcome after IAT is the incidence of successful recanalization. The main trials’ recanalization rates are summarized in Table Three. Due to a wide variation in the method for determining recanalization, the rates summarized in Table Three are not uniformly comparable among the trials. All trials except ESCAPE used the mTICI score to assess recanalization in the intervention arm; ESCAPE used the TICI score. ESCAPE used the mAOL score to assess recanalization on repeat CTA (within 2–8 hrs of tPA) in the control arm; some other trials assessed recanalization/reperfusion in the control arm (not in all patients) after 24 hrs29, 30. The critical safety outcomes include symptomatic intracerebral hemorrhage (sICH) and mortality. As shown in Table Three, the rate of sICH in the published IAT trials is comparable, or perhaps lower, than that seen in prior trials of IV thrombolysis. Mortality is similarly comparable to published trials of intravenous thrombolytic therapy.
Table Three. Intra-arterial Trial Results Relevant to Neuroprotection.
Recanalization is predicted to improve the odds of success in a neuroprotectant clinical stroke trial. The outcomes of most relevance in designing selection criteria for a neuroprotection trial are rates of successful reperfusion, eventual disability (mRS), symptomatic ICH, and mortality. Although trials reported successful recanalization at different times, we looked for “late” recanalization meaning sometime after the immediate treatment period, typically 24 hours following IAT.
| Trial | Late Recanalization or Reperfusion (%) IA vs. Control |
mRS 0–2 IA vs. Control |
sICH (%) IA vs. Control |
Mortality IA vs. Control |
|---|---|---|---|---|
| ESCAPE | 72.4§\31.2¶ | 53\29.3 | 3.6\2.7 | 10.4\19.0 |
| EXTEND-IA | 89♯\34♯ (86*\not reported) |
71\40 | 0\6 | 9\20 |
| MR CLEAN | 84¶\57.5¶ (58.7*\Not Reported) |
32.6\19.1 | 7.7\6.4 | 21\22 |
| REVASCAT | 66*\Not Reported | 43.7\28.2 | 1.9\1.9 | 18.4\15.5 |
| SWIFT PRIME | 83§§\40§§ | 60\35 | 0\3 | 9\12 |
After reviewing the assembled protocols from our study sites, as well as the results from published IAT trials, we derived a set of selection criteria that reflect consistency among the sites and comparability to the published IAT trials (Table Four). The protocol amendment allowing IAT (as well as relaxed age, NIHSS and time limits) in the RHAPSODY trial was adopted on May 1, 2015 and fully implemented at all sites by June 18, 2015. For the purposes of this analysis, we examined recruitment as of February 22, 2016. Recruitment before and after the amendment is shown in the Figure and in Table Five, which document considerable improvement in trial recruitment. Prior to the amendment, 8 patients were enrolled from 601 screened patients (1.3%) while after the amendment, 51 (2 IAT only, 25 IV rt-PA with IAT, and 24 IV rt-PA only) patients were enrolled from 821 screened patients (6.2%) with no change in the number of sites that were recruiting. Clinical trial recruitment depends on the number of active enrolling sites as well as the inclusion/exclusion criteria: the gross recruitment rate prior to the amendment was 0.11 patients/site/month, and after the amendment 0.43 patients/site/month. The final version of the RHAPSODY patient selection criteria is listed in the Supplement.
Table Four. Selection Guidelines in a Neuroprotectant Trial.
Based on our review of the published trials, as well as the user-survey from our 15 clinical trial sites, we were able to identify an allowed range of selection criteria. The “RHAPSODY Target” criteria are selected from the allowed range for our use in the RHAPSODY trial, but future clinical trialists could consider any value within the allowed range, based on their own considerations of the stroke population they wish to target. For example, an ASPECTS score above 7 was required by the RHAPSODY steering committee to elevate the presumed safety of this early-development, Phase 2 trial, even though some IAT trials admitted patients with more severe “core size” measurements.
| Variable | Allowed Range | Target |
|---|---|---|
| Age (years) | < 80–90 | <90 |
| Time: door to Puncture (min) | 90 to 120 | 90 |
| Time: LKNW to revascularization (hours) | 5 to 8 | 6 |
| Devices | Any 2nd or 3rd generation device | Stent retrievers |
| NIHSS | 5 to 32 | >=5 |
| Imaging Modality | CT or MRI | NCCT |
| Vascular imaging | ELVO on any modality | ELVO on CTA/MRA |
| Perfusion imaging | Mismatch | Salvageable penumbra |
| Core size | ASPECTS >6, CT hypo > 1/3, DWI > 70cc |
ASPECTS > 7 |
Abbreviations: LKNW: last known normal or well. NCCT: non-contrast cranial computed tomography. ELVO: eligible large vessel occlusion. ASPECTS: Alberta Stroke Program Early CT Score. CT hypo: early hypodensity visible on NCCT. CTA: computed tomographic angiogram. DWI: diffusion weighted imaging hyperintensity.
Figure. Recruitment, actual compared against expected.
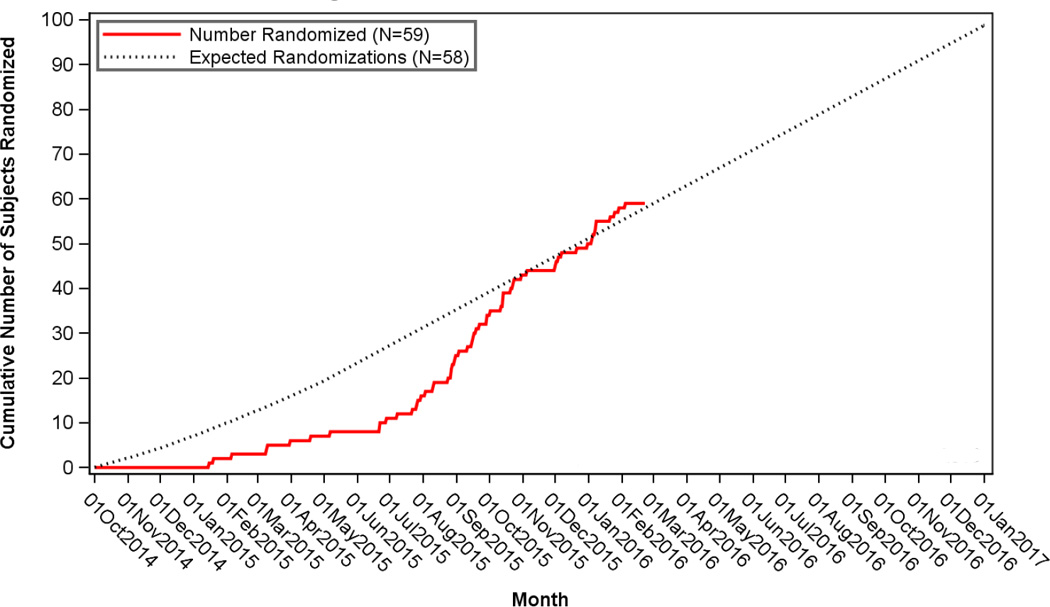
The cumulative recruitment into the RHAPSODY trial as of February 22nd, 2016 is shown from the beginning of the trial. The protocol amendment described here was approved in May 2015 (arrow), and took effect in June 2015. As sites finalized adoption of the new protocol, there was a dramatic increase in recruitment beginning in July 2015.
Table Five. Exclusions due to age, NIHSS, time, and use of IAT before and after the protocol amendment.
The criteria for both inclusion and exclusion changed with the protocol amendment, so the selection of patients for screening and for enrollment changed. There were dramatic decreases in the proportion of patients excluded for age, NIHSS, and time to presentation, which reflect the amended selection criteria. In addition, prior to the amendment 78 patients were excluded from the trial because they underwent IAT, a criterion that did not exist after the amendment. Enrollment increased dramatically from 1.3 to 6.2% of screened patients after the amendment. Due to the asymmetric sample sizes, and the fact that both screening and enrollment criteria changed, a statistical test of significance is not appropriate, but for illustration purposes only, the Fisher’s Exact Test Odds Ratio [95%CL] for increased enrollment is 4.9 [2.3,10.4], with p<0.001.
| Selection Variable | Patients Excluded Before Protocol Amendment (% of Total Screened) |
Patients Excluded After Protocol Amendment (% of Total Screened) |
|---|---|---|
| Age | 130 (21.9) | 29 (3.8) |
| NIHSS | 270 (45.5) | 162 (21.3) |
| Late (3 hours before; 6 hours after) | 75 (12.6) | 27 (3.6) |
| No. excluded for IAT | 78 (13.1) | N/A |
| Total Enrolled and Treated | 8 (1.3) | 51 (6.2) |
| Total Screened | 601 | 821 |
DISCUSSION
We sought to incorporate IAT into a clinical trial of neuroprotection for acute ischemic stroke. To our knowledge, there is no previous demonstration of the feasibility of this approach. Based on a summary of the published IAT trials and the selection criteria in use at 15 clinical trial study sites, we identified a range of acceptable selection criteria that could be used in similar trials (Table Four). After implementing these criteria, as well as relaxed age, NIHSS and timing limits, trial recruitment accelerated dramatically (Figure). The data suggest that it is feasible and likely quite desirable to allow IAT in the context of a neuroprotectant stroke trial.
A plethora of putative neuroprotectants has emerged from pre-clinical testing, and many have shown early promise in small Phase 1 or Phase 2 trials. After testing in large, adequately powered, properly blinded and randomized pivotal trials, however, no neuroprotectant strategy has proven successful31. Pre-clinical studies typically include recanalization (although permanent vessel occlusion can be modeled) and combining a putative neuroprotectant with recanalization may provide the greatest chance for success. Some putative neuroprotectants successfully reduced infarct volumes in animals only if administered prior to recanalization/reperfusion. Such drugs might not be practical for clinical use in which recanalization with iv rt-PA or IAT must occur as quickly as possible after hospital arrival. Clinical trialists designing protocols for such agents may therefore wish to modify the selection criteria offered here.
Other differences between human and pre-clinical studies also matter: such experimental models typically involve young animals free of co-morbid conditions (e.g. diabetes, hypertension) that impact outcome after stroke, although the putative compound in the RHAPSODY trial, 3K3A-APC, has shown effectiveness in aged and in hypertensive animals18. Another critical factor in demonstrating neuroprotection is timing: drugs that show benefit in experimental models when given within minutes or an hour of recanalization have rarely been so tested in clinical trials. In RHAPSODY we require study drug infusion as soon as possible after consent, but no later than 120 minutes after skin puncture (or rt-PA completion). In this way, we suggest the protocol is optimized to show benefit since the drug will be combined with proven recanalization as verified during IAT. An alternative approach would be to require recanalization prior to study-drug treatment, thus limiting generalizability to patients who receive IAT alone or rt-PA plus IAT. This approach could be the ideal one, especially for a treatment in which pre-clinical testing suggests recanalization is absolutely necessary for drug efficacy. While we chose in RHAPSODY to allow patients treated with only IV rt-PA to allow broader generalization of the results, we suggest that other trialists may choose differently under different circumstances.
The selection criteria listed in Table Four represent a compromise between the need for ‘looser’ criteria to allow more rapid enrollment versus ‘tighter’ criteria to focus the study on patients most likely to respond. An upper limit on age is arbitrary, but needed for regulatory approval of the clinical trial protocol32. The limit on times (door to puncture, LKNW to revascularization) are the most important, because no variable impacts the likelihood of favorable outcome more than time33–35. The lower limit on NIHSS serves to select a population more likely to show large vessel occlusion, a requirement for IAT36. The requirement for demonstration of salvageable penumbra or limited volume of irretrievable core, or both, is controversial: while there is no sense—and some potential risk—in reperfusing dead tissue, it is unclear whether current imaging methods unequivocally identify brain beyond salvage37. Nevertheless, for acceptance at both regulatory and site-investigator levels, some limit on infarct volume seemed important.
Our findings should be interpreted with some limitations in mind. Most importantly, the 15 study sites in RHAPSODY are carefully selected and currently participate in the NINDS sponsored NeuroNEXT clinical trial network. The sites were selected through a competitive process, and have demonstrated commitment to clinical trial quality, regulatory compliance and recruitment/retention. They all joined the study simultaneously, and participation in competing clinical trials was discouraged. On the other hand, when NeuroNEXT was being organized there was no premium allotted to selecting study sites with demonstrated capability as comprehensive stroke centers. Thus, the trial includes some centers with world-class recognition for their neuro-interventional programs as well as other sites with more typical capabilities in delivering IAT. Therefore, our results would likely predict the experience to be seen in other clinical trials with a heterogeneous group of stroke centers. Another limitation is that the study material, 3K3A-APC, is relatively easy to administer: a simple 15 min infusion every 12 hours for 5 doses. More complex protective strategies, such as constant infusions that must be titrated, may be more difficult to implement. Another limitation is that IAT as a standard care treatment is only in its infancy. Likely the broad criteria we present here (Table Four) will be refined over time.
These findings raise a number of questions that must be addressed in future investigations. Site selection criteria will require refinement to optimize clinical trial execution without limiting the number of qualifying sites too severely: what is the impact of metrics such as door-to-needle time, door-to-puncture time, and recanalization success rates? The effect of transportation (drip-and-ship) on eligibility, consent, safety, and outcome is unknown. The timing of recanalization relative to study drug treatment may impact efficacy if, for example, the study drug can produce benefit only if administered before or only after recanalization. The effect of peri-interventional management may alter safety and outcome measures significantly: study is needed to assess the effect of local vs. general anesthesia; co-administration of anticoagulants, platelet inhibitors, and glycoprotein IIb/IIIa inhibitors; and use of proximal carotid stenting when required. Finally, the most significant opportunity offered by IAT—besides documented recanalization—is the capability to administer neuroprotection locally38. While intriguing, the idea of administering an immediate, local dose of a putative neuroprotectant will require considerable development in terms of safety and possible efficacy.
Our data do not address whether recanalization is achieved with tPA alone, but some estimates suggest the rate could be as high as 50%39. Post-rt-PA recanalization is rarely documented using imaging. The variability in definitions of measuring late recanalization begs for some standardization in how studies are measuring reperfusion, given the importance that has been placed on it. In future trials, some means of documenting recanalization in rt-PA-only patients will be needed.
In conclusion, it is feasible to include IAT in a neuroprotectant trial for acute ischemic stroke. Criteria are presented for including such patients in a manner that is consistent with evidence based support for IAT while still preserving the ability to test the role of the putative neuroprotectant.
Supplementary Material
Acknowledgments
The Partners Human Research Affairs for coordination and implementation of the single IRB model.
Funding Sources. This study was funded by the National Institutes of Neurological Disorders and Stroke (U01NS088312). The NeuroNEXT Network is supported by the National Institutes of Neurological Disorders and Stroke (Clinical Coordinating Center: U01NS077179, Data Coordinating Center: U01NS077352).
PL is the recipient of U01 NS088312, and KP the recipient of X01 NS088312, both titled “A multi-center, Phase 2 study using a CRM to determine the safety and tolerability of 3K3A-APC in combination with tPA in moderately severe acute hemispheric ischemic stroke.” SW and KP are employees of, and HL is consultant to ZZ Biotech, LLC.
This report does not represent the official view of the National Institute of Neurological Disorders and Stroke (NINDS), the National Institutes of Health (NIH), or any part of the US Federal Government. No official support or endorsement of this article by the NINDS or NIH is intended or should be inferred.
Footnotes
Disclosures
The remainder of the authors have no disclosures.
References
- 1.Jonas A, Aiyagari V, Vieira D, Figueroa M. The failure of neuronal protective agents versus the success of thromolysis for the treatment of ischemic stroke. The predictive value of animal models. Ann NY Acad Sci. 2001;939:257. doi: 10.1111/j.1749-6632.2001.tb03633.x. [DOI] [PubMed] [Google Scholar]
- 2.Sena ES, Currie GL, McCann SK, Macleod MR, Howells DW. Systematic reviews and meta-analysis of preclinical studies: why perform them and how to appraise them critically. J Cereb Blood Flow Metab. 2014;34:737–742. doi: 10.1038/jcbfm.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bath PM, Lees KR, Schellinger PD, Altman H, Bland M, Hogg C, et al. Statistical analysis of the primary outcome in acute stroke trials. Stroke. 2012;43:1171–1178. doi: 10.1161/STROKEAHA.111.641456. [DOI] [PubMed] [Google Scholar]
- 4.Wahlgren NG, Ahmed N. Neuroprotection in cerebral ischaemia: facts and fancies--the need for new approaches. Cerebrovasc Dis. 2004;17(Suppl 1):153–166. doi: 10.1159/000074808. [DOI] [PubMed] [Google Scholar]
- 5.Tsai JP, Albers GW. Reperfusion versus recanalization: the winner is. Stroke. 2015;46:1433–1434. doi: 10.1161/STROKEAHA.115.009268. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A, Aakriti, Gupta V. A review on animal models of stroke: An update. Brain Res Bull. 2016;122:35–44. doi: 10.1016/j.brainresbull.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Aronowski J, Strong R, Grotta JC. Reperfusion injury: demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab. 1997;17:1048–1056. doi: 10.1097/00004647-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 8.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 9.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 10.Mokin M, Rojas H, Levy EI. Randomized trials of endovascular therapy for stroke - impact on stroke care. Nat Rev Neurol. 2016;12:86–94. doi: 10.1038/nrneurol.2015.240. [DOI] [PubMed] [Google Scholar]
- 11.Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46:3020–3035. doi: 10.1161/STR.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 12.Sacks D, Black CM, Cognard C, Connors JJ, 3rd, Frei D, Gupta R, et al. Multisociety Consensus Quality Improvement Guidelines for Intraarterial Catheter-directed Treatment of Acute Ischemic Stroke, from the American Society of Neuroradiology, Canadian Interventional Radiology Association, Cardiovascular and Interventional Radiological Society of Europe, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, European Society of Minimally Invasive Neurological Therapy, and Society of Vascular and Interventional Neurology. AJNR Am J Neuroradiol. 2013;34:E0. [PMC free article] [PubMed] [Google Scholar]
- 13.Caplan LR. How well does "evidence-based" medicine help neurologists care for individual patients? Rev Neurol Dis. 2007;4:75–84. [PubMed] [Google Scholar]
- 14.Lyden PD, Meyer BC, Hemmen TM, Rapp KS. An ethical hierarchy for decision making during medical emergencies. Ann Neurol. 2010;67:434–440. doi: 10.1002/ana.21997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin JH, Zlokovic BV, Mosnier LO. Protein C anticoagulant and cytoprotective pathways. Int J Hematol. 2012;95:333–345. doi: 10.1007/s12185-012-1059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosnier LO, Yang XV, Griffin JH. Activated protein C mutant with minimal anticoagulant activity, normal cytoprotective activity, and preservation of thrombin activable fibrinolysis inhibitor-dependent cytoprotective functions. J Biol Chem. 2007;282:33022–33033. doi: 10.1074/jbc.M705824200. [DOI] [PubMed] [Google Scholar]
- 17.Bai J, Lyden PD. Revisiting cerebral postischemic reperfusion injury: new insights in understanding reperfusion failure, hemorrhage, and edema. Int J Stroke. 2015;10:143–152. doi: 10.1111/ijs.12434. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Zhao Z, Chow N, Rajput PS, Griffin JH, Lyden PD, et al. Activated protein C analog protects from ischemic stroke and extends the therapeutic window of tissue-type plasminogen activator in aged female mice and hypertensive rats. Stroke. 2013;44:3529–3536. doi: 10.1161/STROKEAHA.113.003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams PD, Zlokovic BV, Griffin JH, Pryor KE, Davis TP. Preclinical safety and pharmacokinetic profile of 3K3A-APC, a novel, modified activated protein C for ischemic stroke. Curr Pharm Des. 2012;18:4215–4222. doi: 10.2174/138161212802430413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyden P, Levy H, Weymer S, Pryor K, Kramer W, Griffin JH, et al. Phase 1 safety, tolerability and pharmacokinetics of 3K3A-APC in healthy adult volunteers. Curr Pharm Des. 2013;19:7479–7485. doi: 10.2174/1381612819666131230131454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Zhang Z, Chow N, Davis TP, Griffin JH, Chopp M, et al. An activated protein C analog with reduced anticoagulant activity extends the therapeutic window of tissue plasminogen activator for ischemic stroke in rodents. Stroke. 2012;43:2444–2449. doi: 10.1161/STROKEAHA.112.658997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodman SN, Zahurak ML, Piantadosi S. Some practical improvements in the continual reassessment method for phase I studies. Stat Med. 1995;14:1149–1161. doi: 10.1002/sim.4780141102. [DOI] [PubMed] [Google Scholar]
- 23.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 24.Wahlgren N, Ahmed N, Davalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369:275–282. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 25.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 26.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 27.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 28.Saver JL, Goyal M, Diener HC Investigators SP. Stent-Retriever Thrombectomy for Stroke. N Engl J Med. 2015;373:1077. doi: 10.1056/NEJMc1508744. [DOI] [PubMed] [Google Scholar]
- 29.Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44:2650–2663. doi: 10.1161/STROKEAHA.113.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khatri P, Neff J, Broderick JP, Khoury JC, Carrozzella J, Tomsick T. Revascularization end points in stroke interventional trials: recanalization versus reperfusion in IMS-I. Stroke. 2005;36:2400–2403. doi: 10.1161/01.STR.0000185698.45720.58. [DOI] [PubMed] [Google Scholar]
- 31.O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 32.Sandercock P, Wardlaw JM, Lindley RI, Dennis M, Cohen G, Murray G, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet. 2012;379:2352–2363. doi: 10.1016/S0140-6736(12)60768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marler JR, Tilley BC, Lu M, Brott TG, Lyden PC, Grotta JC, et al. Early stroke treatment associated with better outcome: the NINDS rt-PA stroke study. Neurology. 2000;55:1649–1655. doi: 10.1212/wnl.55.11.1649. [DOI] [PubMed] [Google Scholar]
- 34.Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–1935. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khatri P, Yeatts SD, Mazighi M, Broderick JP, Liebeskind DS, Demchuk AM, et al. Time to angiographic reperfusion and clinical outcome after acute ischaemic stroke: an analysis of data from the Interventional Management of Stroke (IMS III) phase 3 trial. Lancet Neurol. 2014;13:567–574. doi: 10.1016/S1474-4422(14)70066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heldner MR, Zubler C, Mattle HP, Schroth G, Weck A, Mono ML, et al. National Institutes of Health stroke scale score and vessel occlusion in 2152 patients with acute ischemic stroke. Stroke. 2013;44:1153–1157. doi: 10.1161/STROKEAHA.111.000604. [DOI] [PubMed] [Google Scholar]
- 37.Albers GW, Goyal M, Jahan R, Bonafe A, Diener HC, Levy EI, et al. Ischemic core and hypoperfusion volumes predict infarct size in SWIFT PRIME. Ann Neurol. 2016;79:76–89. doi: 10.1002/ana.24543. [DOI] [PubMed] [Google Scholar]
- 38.Mattingly TK, Denning LM, Siroen KL, Lehrbass B, Lopez-Ojeda P, Stitt L, et al. Catheter based selective hypothermia reduces stroke volume during focal cerebral ischemia in swine. J Neurointerv Surg. 2016;8:418–422. doi: 10.1136/neurintsurg-2014-011562. [DOI] [PubMed] [Google Scholar]
- 39.Dorado L, Millan M, Perez de la Ossa N, Guerrero C, Gomis M, Aleu A, et al. Time to recanalization and risk of symptomatic intracerebral haemorrhage in patients treated with intravenous thrombolysis. Eur J Neurol. 2012;19:1251–1255. doi: 10.1111/j.1468-1331.2012.03743.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


