Abstract
Psychosocial stress influences cognitive abilities such as long-term memory retrieval. However, less is known about the effects of stress on cognitive flexibility, which is mediated by different neurobiological circuits and could thus be regulated by different neuroendocrine pathways. In this study we randomly assigned healthy adults to an acute stress induction or control condition and subsequently assessed participants’ cognitive flexibility using an open-source version of the Wisconsin Card Sort task. Drawing on work in rodents, we hypothesized that stress would have stronger impairing effects on cognitive flexibility in men than women. As predicted, we found that stress impaired cognitive flexibility in men but did not significantly affect women. Our results thus indicate that stress exerts sex-specific effects on cognitive flexibility in humans and add to the growing body of research highlighting the need to consider sex differences in effects of stress.
Keywords: Stress, Cognitive Flexibility, Sex Differences, Wisconsin Card Sorting Test, Orbitofrontal Cortex, Men, Women
Psychosocial stress has important effects on many cognitive processes (Shields, Lam, et al., 2016; Allen et al., 2014). Indeed, stress typically impairs in long-term memory retrieval and working memory (Shields, Sazma, et al., 2016; Schoofs et al., 2013) but enhances other cognitive abilities such as decision-making competence (Shields, Lam, et al., 2016; though see Starcke & Brand, 2012) and response inhibition (Schwabe et al., 2013). Understanding how stress influences higher cognitive abilities has important implications for solving problems that arise in daily life (Diamond 2013). Despite the well-known effects of stress on many cognitive processes and the importance of these effects, it is still unclear how stress might impact many important cognitive processes, such as cognitive flexibility.
Prior research with both humans (Alexander et al., 2007; Plessow et al., 2011) and rodents (Laredo et al., 2015) has suggested that stress may impair cognitive flexibility—that is, the ability to flexibly switch between thoughts or rules in a goal-directed manner. However, when studying effects of stress, it is important to examine potential sex differences, given the dramatically different effects stress can have on males and females (Trainor et al., 2013); stress should not be presumed to influence males and females similarly (Cahill, 2012; Felmingham et al., 2012; Schoofs et al., 2013; Zoladz et al., 2013). Indeed, work with rodents has suggested that the impairing effects of stress on cognitive flexibility may be stronger in males than females (Laredo et al., 2015), but sex differences have not been examined in human studies of stress and cognitive flexibility. Based on these findings, we tested whether sex moderates the effects of stress on cognitive flexibility in human subjects.
To this end, we randomly assigned healthy adult men and women to either an acute stress induction or control task and subsequently assessed participants’ cognitive flexibility using an open-source version of the Wisconsin Card Sorting Task. To confirm a stress induction, we assessed both negative affect and salivary cortisol at both baseline and 15min post-manipulation. Consistent with prior research (Alexander et al., 2007), we hypothesized that stress would impair cognitive flexibility and—based upon work with rodents (Laredo et al., 2015)—that this effect would be stronger in men than women.
Method
Participants
Participants were 113 healthy young adults attending the University of California, Davis. We did not invite participants who had a current illness, diabetes, history of stroke, neurological disorders, current or former diagnosis of posttraumatic stress disorder, hospitalization for a psychiatric disorder within the past year, current injury or illness within the past week, major sleep disturbances within the past six weeks, or consumption of more than eight caffeinated beverages a day. Similarly, individuals who were pregnant, nursing, on any form of medication (including hormonal birth control or asthma medication) or illegal drugs, had taken any mood-altering medications within the past two months, or had taken corticosteroids within the past three months were not invited to participate. Participants were instructed not to eat, drink, use tobacco, brush their teeth or floss, or engage in exercise for two hours prior to the start of the study. Compliance with these instructions and inclusion criteria was assessed using a questionnaire at the beginning of the study; women also reported the date of the first day of their last menstrual period using that questionnaire. Menstrual cycle phase was approximated by days since preceding cycle had begun (i.e., 5 or less days: menstrual period; 6–13 days: follicular phase; 14+ days: luteal phase).
Fifty-six individuals (36 women, 20 men) were randomly assigned to the stress induction condition and 57 individuals (39 women, 18 men) were randomized to the non-stressful control condition. Participants ranged in age from 18 to 54 years-old (M=20.20, SD=3.8). 16.7% of women were tested during their menstrual period, 28.3% during the follicular phase, and 55% during the luteal phase of their menstrual cycle.
Materials and Procedure
Participants came to the laboratory at either 12pm or 3pm for three- or four-participant group sessions. Upon arrival, an experimenter immediately greeted each participant and brought the participant into a cubicle in order to prevent the participants from interacting with each other. Once in the cubicle, each participant provided informed consent and completed miscellaneous measures, including a measure of baseline affect, for approximately 10min to allow acclimation to the testing environment. Prior to learning of the stressor task and immediately after the task, participants used an unmarked scale, ranging from 1(Not at All) to 7(Very Much), to indicate the extent they currently felt a variety of negative affective states, which were then averaged to create a negative affect composite. Negative affect was assessed at baseline (α=.90) and after the stress manipulation (α=.92). Participants’ computers then reached a password-protected screen that instructed them to wait for instructions from the experimenters. Participants waited until all other participants for the session completed the initial measures, upon which time the first (baseline) saliva sample was taken.
Next, participants completed the laboratory-based stressor or control task, depending upon their time slot’s assigned condition. An experience of acute stress was induced using the Trier Social Stress Test for Groups (TSST-G; von Dawans et al., 2011). This task includes two conditions: a stress induction condition and a non-stressful control condition. In brief, participants in the stress induction condition were conspicuously recorded while they spoke on their real qualifications for the job they would like to have in front of a live panel of two trained, stern evaluators, and afterwards were evaluated as they completed a difficult math task wherein each participant counted backwards in steps of 16 starting from a large four-digit number (e.g., 3,329) that differed for each participant in the session. During the math task the participant was periodically instructed to count faster, and if the participant made a mistake, the participant was told his/her answer was incorrect and s/he had to restart. In contrast, participants in the control condition quietly read aloud a scientific article and subsequently completed a simple forward counting task without any social evaluation.
The TSST-G lasted approximately 30 minutes (including anticipation), after which time participants returned to their computers and waited for the experimenters to enter a password to allow the participant to continue. This transition from the stressor to the participants’ computers took approximately 5min. Participants then completed the post-stressor affect measure, which took approximately 40 seconds. Participants then completed the cognitive flexibility task. We measured cognitive flexibility using the Berg Card Sorting Test, which is an open-source version of the Wisconsin Card Sorting Test (Mueller and Piper, 2014). This task is a well-validated task requiring cognitive flexibility (Miyake et al., 2000). The primary outcome in this task is the number of perseverative errors a person makes, which indicates a continued application of a card-sorting rule that is no longer appropriate instead of shifting to the use of a new rule (i.e., cognitive inflexibility); higher scores thus indicate worse performance.
The card-sorting test took on average approximately 6min to complete. Participants then waited quietly until 15min total had elapsed since the offset of the stress or control manipulation and then provided a second saliva sample. Finally, participants completed the demographics questionnaire before being debriefed, thanked, and dismissed.
Cortisol
Participants provided two saliva samples (baseline and post-manipulation) using a passive drool method. Immediately after collection, the saliva vials were placed in a −20°C freezer until assayed in duplicate using high-sensitivity Salivary Cortisol ELISA Kits (Salimetrics LLC, State College, PA) according to manufacturer instructions. The inter-assay CV was 7.45% and the average intra-assay CV was 2.82%. Sensitivity for these assays was 0.012 μg/dL. All controls were in the expected ranges. Cortisol concentrations were converted from μg/dL to nmol/L for consistency with most human stress literature.
Data Reduction and Analysis
All variables were inspected for conformity to a normal distribution and the natural logarithm transformation was applied when variables evidenced significant skew (i.e., cortisol, both baseline and post-manipulation, and perseverative errors). Because the acute stress manipulation necessitated randomization of participant sessions to conditions (i.e., rather than participants), analyses required a multilevel model to account for shared variability within sessions. Thus, all analyses were linear mixed models with participants nested within Session. We used a mixed model ANOVA nesting measurement occasions within participants and further nesting participants within Session to assess changes in cortisol from baseline to post-manipulation. All analyses were conducted in R, version 3.2.1. Mixed models were fit using the lmerTest package. Least-squares means and their corresponding standard errors were derived using the lsmeans package.
Results
Preliminary Analyses
Participants in the stress induction versus control conditions did not differ with respect to menstrual cycle phase, age, race, or sex (ps>.15, uncorrected). Thus, our random assignment was successful.
We examined changes in negative affect from baseline to post-manipulation to confirm the success of both our stress manipulation and our control condition. As expected, the Time × Condition interaction was significant, F(1,111.0)=11.22, p=.001. Participants in the stress induction group significantly increased in negative affect from pre- to post-manipulation, t(111.0)=−2.66, p=.009, whereas participants in the control group significantly decreased in negative affect from pre- to post-manipulation, t(111.0)=−2.08, p=.040. We did not find evidence for a Sex × Time × Condition interaction, F(1,109.0)=2.15, p=.146.
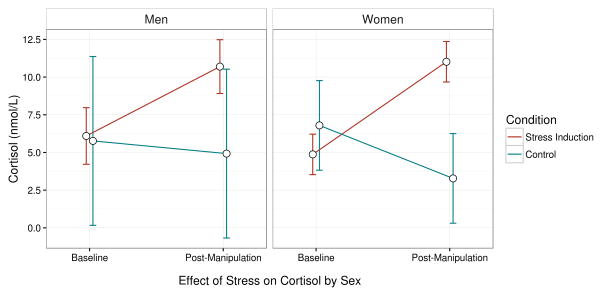
We next examined cortisol reactivity over time for the stress induction group and a randomly-selected 10 participants in the control group. As expected, the Time × Condition interaction was significant, F(1,58.6)=12.66, p<.001 (Figure 1). Participants in the stress induction group significantly increased from pre- to post-manipulation, t(61.0)=4.89, p<.001, whereas participants in the control group tended to decrease from pre- to post-manipulation, t(60.1)=−1.84, p=.071. In addition, post-manipulation cortisol was significantly greater in the stress-induction group than the control group, t(47.1)=2.86, p=.006. We did not find evidence for a Sex × Time × Condition interaction, p=.411, but because we only had 10 participants with cortisol assayed in the control group, we had very little power to determine a sex by condition effect.
Figure 1.
Effect of the stress manipulation on cortisol. Values are presented in nmol/L for comparison with other studies, but analyses were conducted using log-transformed cortisol values to correct for skew. As expected, cortisol increased from pre- to post-manipulation in the stress induction group, p<.001, whereas cortisol tended to decrease in the randomly-selected 10 participants in control group we assayed, p=.071. There was no evidence of sex differences in reactivity to the stressor.
Primary Analyses
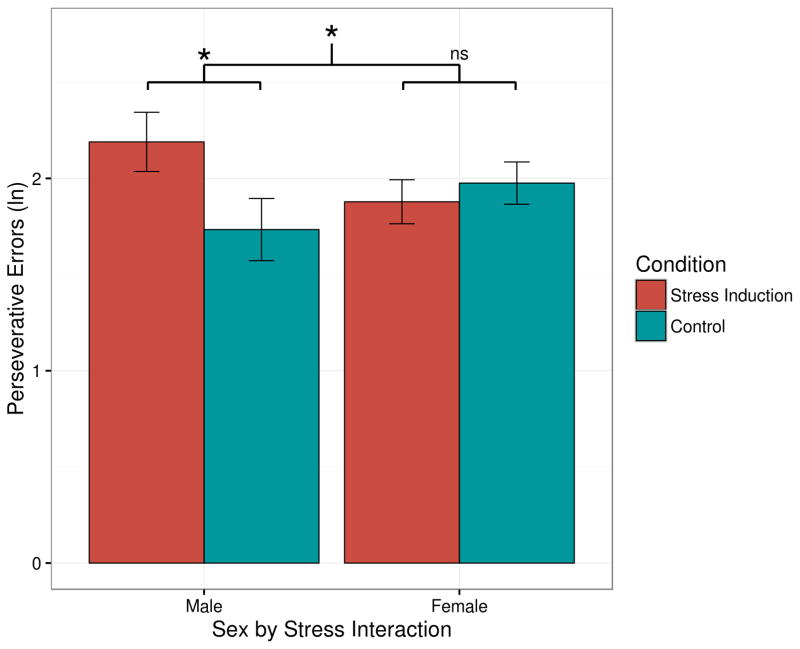
We next examined what effect, if any, acute stress had on cognitive flexibility using a mixed-model ANOVA with Sex and Condition as between-subjects factors. There was no main effect of Sex, indicating that men and women committed the same amount of perseverative errors overall F(1,113.0)=0.69, p=.794. Similarly, although there was a descriptive tendency for participants in the stress induction group (M=2.03, SE=0.10) to commit more perseverative errors than participants in the control group (M=1.85, SE=0.10), the effect of Condition was not significant, F(1,113.0)=1.82, p=.180. However, as hypothesized, the Sex × Condition interaction was significant, F(1,113.0)=4.32, p=.040, η2partial=.037, indicating stress differentially influenced cognitive flexibility by sex. As shown in Figure 2, men in the stress condition (M=2.19, SE=0.15) committed significantly more perseverative errors than men in the control condition (M=1.73, SE=0.16), t(95.5)=2.04, p=.044, d=0.68; however, women in the stress condition (M=1.89, SE=0.11) did not commit more perseverative errors than women in the control condition (M=1.98, SE=0.11), t(51.9)=−0.61, p=.543, d=−0.15. An additional analysis determined that there was no significant moderating effect of menstrual cycle phase on cognitive flexibility, F(2,60)=1.85, p=.167. Across all participants, changes in cortisol (post-manipulation minus baseline) did not predict perseverative errors, p=.781; changes in cortisol did not predict perseverative errors when restricting analyses to only males, p=.205 or only females, p=.844. Thus, acute stress impaired cognitive flexibility in men, but not women.
Figure 2.
Sex-specific effects of acute stress on cognitive flexibility. Men in the stress induction group committed significantly more perseverative errors (log transformed) than men in the control group, p=.044, indicating that stress impaired cognitive flexibility in men. In contrast, women in the stress induction condition did not commit a different number of perseverative errors than women in the control group, p=.543.
Discussion
Although stress is known to influence cognitive processes such as memory, the influence of stress on cognitive flexibility has received comparatively little attention. In addition, prior research in humans has not examined potential sex differences in stress effects on cognitive flexibility. Consistent with work in rodents, we found that acute stress impaired cognitive flexibility in men but not women. Thus, sex appears to be an important factor for understanding stress effects on cognitive flexibility in humans.
Prior studies examining stress effects on cognitive flexibility have found that stress impaired cognitive flexibility in a number of ways—such as solving fewer anagrams, engaging in less creative thinking, and reducing trial-by-trial modulation of behavior in a Simon task—in samples composed of 50% men and 50% women (Alexander et al., 2007; Plessow et al., 2011). Our results suggest that these previous studies of stress and cognitive flexibility may have found larger effects if their analyses were restricted to males.
The biological mechanism(s) behind our observed effects are currently unclear. Although differentially influenced by stress in men and women (Shields, Sazma, et al., 2016), cortisol does not appear to be playing a role, as itwas not related to cognitive flexibility in our data or in a recent meta-analysis (Shields et al., 2015). Work in mice has identified the μ-opioid receptor as an important mechanism mediating stress’s sex-specific effects on cognitive flexibility (Laredo et al., 2015), indicating that the μ-opioid system may contribute to our observed effects. Alternatively, sex differences have been observed in noradrenergic activity following stress (Bangasser & Valentino, 2014), and noradrenergic activity is necessary for producing stress effects on cognitive flexibility (Alexander et al., 2007). Similarly, sex differences in dopaminergic activity exist, and dopamine is important for executive functioning (Shansky & Lipps, 2013). Thus, future work should attempt to elucidate the biological mechanism(s) underpinning sex-specific stress effects on cognitive flexibility in humans.
This study has several limitations worth noting. First, because we used a sample of undergraduate students, it is unknown if our results would generalize to a nonstudent population. Second, the cognitive flexibility task we used is accuracy-based, and it is unknown whether our results generalize to other tasks requiring cognitive flexibility, such as tasks with shift costs as an outcome. Third, assaying cortisol for only ten participants in the control group weakened our neuroendocrine conclusions, as it limited our ability to detect a stress by sex by time effect on cortisol. Fourth, our panels of evaluators were not always mixed-sex panels, and the sex of the evaluators may have influenced stress responses; however, our use of a multi-level model that nested participants within sessions partially controls for this limitation, as this model accounts for similarities between participants in sessions (e.g., being evaluated by the same panel). Fifth, we did not obtain cardiovascular markers or other biological indices of a stress response. Sixth, sampling cortisol 15min after our stressor finished (which lasted 30min) may have missed the cortisol peak, which typically occurs 20–30min after stressor onset. Finally, our stress manipulation was an acute stressor, and it is unknown whether chronic stress would produce the same sex-specific effects on cognitive flexibility as we observed in response to acute stress.
Conclusion
In conclusion, consistent with prior work with rodents, we found that stress impaired cognitive flexibility in men but not women. These results add to a growing literature that cognitive flexibility is more sensitive to stress or other insults in men compared to women. Our research thus adds to the growing body of work highlighting the need to investigate sex differences in effects of stress. In addition, our results suggest that women may be more suited to high-stress environments requiring cognitive flexibility than men. Future research should be careful to consider sex as an important factor in stress effects on cognitive flexibility or any potential effect of stress.
Acknowledgments
The authors wish to thank numerous research assistants for assistance with data collection.
Footnotes
Declaration of Interest
This research was supported by a University of California, Davis Psychology Department Summer Grant in Aid of Research to Grant S. Shields, NIH MH103322 to Brian C. Trainor, a University of California, Davis Provost’s Undergraduate Fellowship to Jovian C. W. Lam, and NIH MH059352 and EY025999 to Andrew Yonelinas. These organizations had no role in designing the study; in collecting, analyzing, or interpreting the data; in writing this report; or in deciding to submit this report for publication.
The authors declare no conflict of interest with respect to this work.
References
- Alexander JK, Hillier A, Smith RM, Tivarus ME, Beversdorf DQ. Beta-adrenergic modulation of cognitive flexibility during stress. J Cogn Neurosci. 2007;19:468–78. doi: 10.1162/jocn.2007.19.3.468. [DOI] [PubMed] [Google Scholar]
- Allen AP, Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Biological and psychological markers of stress in humans: Focus on the Trier Social Stress Test. Neurosci Biobehav Rev. 2014;38:94–124. doi: 10.1016/j.neubiorev.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: Neurobiological perspectives. Front Neuroendocrinol. 2014;35:303–19. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L. A half-truth is a whole lie: On the necessity of investigating sex influences on the brain. Endocrinology. 2012;153:2541–3. doi: 10.1210/en.2011-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annu Rev Psychol. 2013;64:135–68. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham KL, Tran TP, Fong WC, Bryant RA. Sex differences in emotional memory consolidation: The effect of stress-induced salivary alpha-amylase and cortisol. Biol Psychol. 2012;89:539–44. doi: 10.1016/j.biopsycho.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Laredo SA, Steinman MQ, Robles CF, Ferrer E, Ragen BJ, Trainor BC. Effects of defeat stress on behavioral flexibility in males and females: Modulation by the mu-opioid receptor. Eur J Neurosci. 2015;41:434–41. doi: 10.1111/ejn.12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Mueller ST, Piper BJ. The Psychology Experiment Building Language (PEBL) and PEBL test battery. J Neurosci Methods. 2014;222:250–9. doi: 10.1016/j.jneumeth.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessow F, Fischer R, Kirschbaum C, Goschke T. Inflexibly focused under stress: Acute psychosocial stress increases shielding of action goals at the expense of reduced cognitive flexibility with increasing time lag to the stressor. J Cogn Neurosci. 2011;23:3218–27. doi: 10.1162/jocn_a_00024. [DOI] [PubMed] [Google Scholar]
- Schoofs D, Pabst S, Brand M, Wolf OT. Working memory is differentially affected by stress in men and women. Behav Brain Res. 2013;241:144–53. doi: 10.1016/j.bbr.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Höffken O, Tegenthoff M, Wolf OT. Stress-induced enhancement of response inhibition depends on mineralocorticoid receptor activation. Psychoneuroendocrinology. 2013;38:2319–26. doi: 10.1016/j.psyneuen.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Lipps J. Stress-induced cognitive dysfunction: Hormone-neurotransmitter interactions in the prefrontal cortex. Front Hum Neurosci. 2013;7:123. doi: 10.3389/fnhum.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Bonner JC, Moons WG. Does cortisol influence core executive functions? A meta-analysis of acute cortisol administration effects on working memory, inhibition, and set-shifting. Psychoneuroendocrinology. 2015;58:91–103. doi: 10.1016/j.psyneuen.2015.04.017. [DOI] [PubMed] [Google Scholar]
- Shields GS, Lam JCW, Trainor BC, Yonelinas AP. Exposure to acute stress enhances decision-making competence: Evidence for the role of DHEA. Psychoneuroendocrinology. 2016;67:51–60. doi: 10.1016/j.psyneuen.2016.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Sazma MA, McCullough AM, Yonelinas AP. The effects of acute stress on memory: A meta-analysis and integrative review. 2016 doi: 10.1037/bul0000100. Manuscript in Preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcke K, Brand M. Decision making under stress: A selective review. Neurosci Biobehav Rev. 2012;36:1228–48. doi: 10.1016/j.neubiorev.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Takahashi EY, Campi KL, Florez SA, Greenberg GD, Laman-Maharg A, et al. Sex differences in stress-induced social withdrawal: Independence from adult gonadal hormones and inhibition of female phenotype by corncob bedding. Horm Behav. 2013;63:543–50. doi: 10.1016/j.yhbeh.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Dawans B, Kirschbaum C, Heinrichs M. The Trier Social Stress Test for Groups (TSST-G): A new research tool for controlled simultaneous social stress exposure in a group format. Psychoneuroendocrinology. 2011;36:514–22. doi: 10.1016/j.psyneuen.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Warnecke AJ, Woelke SA, Burke HM, Frigo RM, Pisansky JM, Lyle SM, Talbot JN. Pre-learning stress that is temporally removed from acquisition exerts sex-specific effects on long-term memory. Neurobiol Learn Mem. 2013;100:77–87. doi: 10.1016/j.nlm.2012.12.012. [DOI] [PubMed] [Google Scholar]