Abstract
In the adult brain, increases in local neural activity are almost always accompanied by increases in local blood flow. However, many functional imaging studies of the newborn and developing human brain have observed patterns of hemodynamic responses that differ from adult responses. Among the proposed mechanisms for the observed variations is that neurovascular coupling itself is still developing in the perinatal brain. Many of the components thought to be involved in actuating and propagating this hemodynamic response are known to still be developing postnatally, including perivascular cells such as astrocytes and pericytes. Both neural and vascular networks expand and are then selectively pruned over the first year of human life. Additionally, the metabolic demands of the newborn brain are still evolving. These changes are highly likely to affect early postnatal neurovascular coupling, and thus may affect functional imaging signals in this age group. This chapter will discuss the literature relating to neurovascular development. Potential effects of normal and aberrant development of neurovascular coupling on the newborn brain will also be explored, as well as ways to effectively utilize imaging techniques that rely on hemodynamic modulation such as fMRI and NIRS in younger populations.
Keywords: Neurovascular coupling, fMRI, Optical imaging, Brain development, Brain metabolism
1 INTRODUCTION
In the adult brain, increases in local neural activity result in increases in local blood flow to the activated region (Attwell and Iadecola, 2002; Heeger and Ress, 2002). This influx of oxygenated, arterial blood far exceeds the consumption of oxygen by the tissue, leading to localized increases in hemoglobin oxygenation. The functional magnetic resonance imaging (fMRI) blood-oxygen-level-dependent (BOLD) signal reports these oxygenation changes, representing decreases in local deoxygenated hemoglobin (HbR) concentration as “positive BOLD”—enabling the noninvasive imaging of a proxy of neural activity (see also chapter “Submillimeter-resolution fMRI: Toward understanding local neural processing” by M. Fukuda et al.).
While this process of neurovascular coupling is relatively consistent in the healthy adult brain, key differences have been observed in functional imaging studies of the early postnatal brain in both humans (Arichi et al., 2010; Born et al., 1998; Meek et al., 1998; Yamada et al., 1997) and rodent models (Colonnese et al., 2008; Kozberg et al., 2013; Zehendner et al., 2013). Common observations include a “negative BOLD” response inverted with respect to the adult response (Born et al., 1998; Yamada et al., 1997), a reduced amplitude BOLD response (Arichi et al., 2012), and a biphasic response that gradually becomes entirely positive with progression to adulthood (Kozberg et al., 2013; Zehendner et al., 2013). These variations in response patterns may represent the progressive maturation of neurovascular coupling itself (Arichi et al., 2012; Hillman and Kozberg, 2013), since many components of neurovascular unit are still developing postnatally (Harris et al., 2011). The presence of seemingly conflicting results may correspond to inter-study differences in stimulation and/or imaging paradigms. These accumulated findings have complicated the interpretation of studies of early brain development and limited the utility of functional imaging as a tool to diagnose and assess abnormal brain development.
The postnatal brain is a highly dynamic system undergoing radical changes in neural connectivity and function that extend into childhood and adolescence. The possibility that neurovascular coupling itself may be developing alongside neuronal development in the postnatal brain suggests that in this developmental period the brain may be experiencing differences in energy supply and demand dynamics compared to the adult brain. Understanding these neurovascular and neurometabolic changes could provide new insights into the medical management of infants and children. Furthermore, substantial vascular development takes place during this neural network expansion, and coordination of development between these two systems may be a critical and potentially vulnerable aspect of brain development. Although developmental differences in early neurovascular coupling may confound conventional interpretation of fMRI BOLD-based studies, neurovascular development could provide a biomarker for assessment of normal or abnormal developmental trajectories that could be directly detectable using functional imaging.
This chapter will review postnatal changes in neurovascular responses, the neural and vascular architecture of the brain, the complete neurovascular unit, and how these components relate to one another in the context of brain function, brain development, disease and functional imaging signals.
2 FUNCTIONAL IMAGING OF THE DEVELOPING BRAIN
2.1 IMAGING TECHNIQUES
The two major imaging technologies used to examine postnatal development in the human brain are fMRI BOLD and near-infrared spectroscopy (NIRS; Graham et al., 2014). As mentioned earlier, fMRI BOLD is a measure of the levels of HbR; decreases in HbR lead to an increase in signal which is termed “positive BOLD” and typically interpreted as an increase in neural activity. Thus it is important to note that positive BOLD does not reflect oxygen consumption by the tissue but rather a robust oversupply of oxygenated blood (functional hyperemia). NIRS takes advantage of the different optical absorption spectra of oxygenated hemoglobin (HbO) and HbR to measure relative changes in each across the cortex using different wavelengths of near-infrared light (see also chapter “Hemodynamic signals in fNIRS” by Y. Hoshi). This enables the assessment of both blood oxygenation and volume changes. NIRS is particularly well suited for imaging in the newborn brain because of the thinness of the newborn skull compared to the adult and ease of acquisition in newborns at the bedside (Ferradal et al., 2015; Liao et al., 2010).
2.2 HUMAN STUDIES OF INFANT HEMODYNAMIC RESPONSES
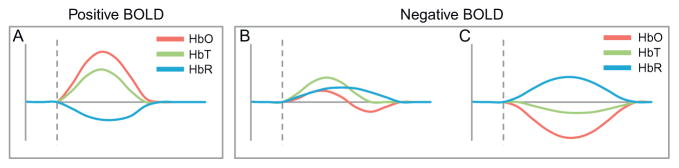
There has been a great deal of variability in the hemodynamic responses reported in the newborn brain (see Table 1). Human studies have broadly demonstrated both negative and positive fMRI BOLD responses to stimulation (Anderson et al., 2001; Arichi et al., 2012; Born et al., 1996; Yamada et al., 1997). Researchers utilizing NIRS have observed adult-like responses (Fig. 1A), as well as two types of “negative BOLD” response (informed by simultaneous imaging of HbO): (1) an increase in HbT, HbR, and HbO, suggesting insufficient hyperemia to meet oxygen consumption demands (Fig. 1B) and (2) A decrease in HbT and HbO with an increase in HbR, suggesting local vasoconstriction exacerbating oxygen consumption needs (Fig. 1C).
Table 1.
Hemodynamic Responses Observed in Human Postnatal Development Using fMRI and NIRS
| Positive BOLD/Decrease in Deoxygenated Hemoglobin
| ||||||
|---|---|---|---|---|---|---|
| Stimulation | Study Details | Age | fMRI/NIRS? | Health Status of Infants | Anesthesia/State | ↑ Or ↓ in [HbT]? |
|
| ||||||
| Visual | Yamada et al. (1997) | <5 weeks | fMRI | Some preterm, all healthy | Pentobarbital sedation | |
| Born et al. (1998, 2000) | <48 weeks | fMRI | Preterm and term, some with visual impairments | Spontaneous sleep or chloral hydrate | ||
| Karen et al. (2008) | 2–10 days | NIRS | All healthy | Spontaneous sleep | Increase | |
| Liao et al. (2010) | 2 days | NIRS | All healthy | Spontaneous sleep | Increase | |
| Auditory | Anderson et al. (2001) | 40–50 weeks | fMRI | Some preterm, all healthy | Awake | |
| Somatosensory | Arichi et al. (2010, 2012) | 0–2 weeks | fMRI | Some preterm, all healthy | Awake | |
|
| ||||||
| Negative BOLD/Increase in Deoxygenated Hemoglobin | ||||||
|
| ||||||
| Stimulation | Study Details | Age | fMRI/NIRS? | Health Status of Infants | Anesthesia/State | ↑ Or ↓ in [HbT]? |
|
| ||||||
| Visual | Yamada et al. (1997) | >5 weeks | fMRI | Some preterm, all healthy | Pentobarbital sedation | |
| Born et al. (1998, 2000) | >56 weeks | fMRI | Preterm and term, some with visual impairments | Spontaneous sleep or chloral hydrate | ||
| Sie et al. (2001) | ~18 months | fMRI | Healthy and with periventricular leukomalacia | Promethazine, pethidine, droperidol, diazepam | ||
| Born et al. (2002) | 4–71 months | fMRI BOLD and perfusion | Three healthy, one with cerebral palsy | Chloral hydrate | Decrease (perfusion MRI) | |
| Meek et al. (1998) | 0–14 weeks | NIRS | All healthy | Awake | Increase | |
| Kusaka et al. (2004) | 4–16 weeks | NIRS | Decrease | |||
| Auditory | Anderson et al. (2001) | >50 weeks | fMRI | Some preterm, all healthy | Awake | |
| Sakatani et al. (1999) | 3 days | NIRS | Some preterm, all healthy | Awake | Increase | |
| Zaramella et al. (2001) | 0–7 weeks | NIRS | Admitted for a variety of diagnoses | Awake and spontaneous sleep | Increase | |
| Somatosensory | Erberich et al. (2006) | 38–46 weeks | fMRI | Some preterm, all healthy | Isoflurane | |
| Heep et al. (2009) | ~39 weeks postconceptional age | fMRI | Preterm | Chloral hydrate | ||
|
| ||||||
| Variable BOLD/Inconsistent Decreases vs Increases in Deoxygenated Hemoglobin | ||||||
|
| ||||||
| Stimulation | Study Details | Age | fMRI/NIRS? | Health Status of Infants | Anesthesia/State | Details |
|
| ||||||
| Visual | Martin et al. (1999) | 1 month to 1 year | fMRI | All healthy | Chloral hydrate, pentobarbital, halothane | <1 month: 30% pos, 30% neg; 1 month to 1 year: 27% pos, 27% neg; 1–6 years: 71% pos, 14% neg |
| Hoshi et al. (2000) | 4–5 days | NIRS | All healthy | Spontaneous sleep | Increase in HbO and HbT, variable HbR responses | |
FIG. 1.
Diagrams of different types of hemodynamic response to stimulus reported in the developing human brain. (A) Classic adult-like hemodynamic response with an increase in oxygenated (HbO) and total hemoglobin (HbT) and a corresponding decrease in deoxygenated hemoglobin (HbR). This response would appear as “positive BOLD” in fMRI. (B and C) Responses that would both be represented by “negative BOLD” in fMRI. (B) Pattern suggests that functional hyperemia is insufficient to meet oxygen demands of the tissue, resulting in an increase in HbT, HbO, and HbR. (C) A decrease in HbT accompanies an increase in HbR suggesting vasoconstriction amplifying increases in HbR.
2.2.1 Observations of negative BOLD responses in the infant brain
Early fMRI studies performed in human newborns identified a negative BOLD response to visual stimulation, hypothesizing that these negative responses were due to oxygen consumption in the presence of minimal functional hyperemia (Born et al., 1996; Joeri et al., 1996). These studies were followed by work from Yamada et al. in 1997, which tracked the progression of fMRI responses to visual stimulation in anesthetized newborns with age (Yamada et al., 1997). They outlined two different responses: in infants under 5 weeks of age, they observed positive BOLD responses, while they observed negative BOLD responses in infants over 5 weeks of age. They attributed this variability to a postnatal increase in cerebral metabolism (see Section 4), hypothesizing that because of low oxygen metabolism rates in younger newborns (Chugani and Phelps, 1986) and a low synaptic density (Huttenlocher et al., 1982), a relatively small increase in blood volume (in comparison to the adult brain) is able to oversupply activated regions in the newborn brain, resulting in a net decrease in the levels of HbR. It was reasoned that older newborns, with a higher cerebral metabolic rate of oxygen (CMRO2) and increased synaptic activity, would use more of the available oxygen, leading to negative BOLD even in the presence of local hyperemia (Muramoto et al., 2002). Subsequently, a number of other groups utilizing fMRI observed negative BOLD responses to visual (Born et al., 1998, 2000; Martin et al., 1999; Sie et al., 2001), auditory (Anderson et al., 2001), and somatosensory stimuli (Erberich et al., 2006) in infants (see Table 1 for details). Studies have also demonstrated negative BOLD responses to somatosensory stimuli in preterm infants (Heep et al., 2009). Since BOLD reports only changes in HbR, negative BOLD responses can be ambiguous and could represent either case shown in Fig. 1B or C. However, a study utilizing perfusion MRI (FAIR) reported concomitant decreases in blood flow in the newborn brain in response to visual stimulation (Born et al., 2002).
Meek et al. published the first hemodynamic study of the awake newborn brain using NIRS (Meek et al., 1998). Their custom NIRS system allowed for infants to sit on examiners’ laps and tolerated small amounts of motion, precluding the need for anesthesia or sleeping. Subjects were presented with blocks of visual stimulation. Resulting increases were observed in both HbO and HbR (Fig. 1B), indicating an increase in oxygen supply insufficient to meet the oxygen demand of the newborn brain. Their subjects ranged in age from 3 to 14 weeks, aligning relatively well with the Yamada et al.’s negative BOLD age group (5 weeks+), but also suggesting that negative BOLD may also be observed at younger ages. Several groups have supported these findings of an increase in both HbO and HbR in newborns using auditory stimulation (Sakatani et al., 1999; Zaramella et al., 2001). Other groups using NIRS have reported fully inverted responses with increases in HbR and decreases in HbO and HbT in response to visual stimulation (Kusaka et al., 2004) suggesting a blood volume decrease in response to stimulation (Fig. 1C).
2.2.2 Observations of positive BOLD in the newborn infant brain
Some groups using both NIRS and fMRI have identified adult-like responses to visual stimulation in early postnatal populations (Anderson et al., 2001; Arichi et al., 2010, 2012; Born et al., 1998, 2000; Hoshi and Tamura, 1993; Karen et al., 2008; Liao et al., 2010; Taga et al., 2003; Watanabe et al., 2008). Notably, however, groups examining these responses via NIRS who reported increases in HbO accompanied by decreases in HbR noted significantly more variability in their HbR responses than HbO responses—and that these responses were occasionally increases (Hoshi et al., 2000; Liao et al., 2010; Taga et al., 2003).
fMRI studies have reported positive BOLD responses, meaning HbR decreases, in response to visual (Born et al., 1998, 2000), auditory (Anderson et al., 2001), and somatosensory stimulation in the immediate postnatal period (Arichi et al., 2010, 2012) as well as in premature infants (Allievi et al., 2016). As discussed earlier, studies that identified both positive and negative responses generally actually observed positive responses in younger infants (Anderson et al., 2001; Born et al., 1998, 2000; Yamada et al., 1997), potentially corresponding to developmental increases in oxygen metabolism (see Table 1 for age group details).
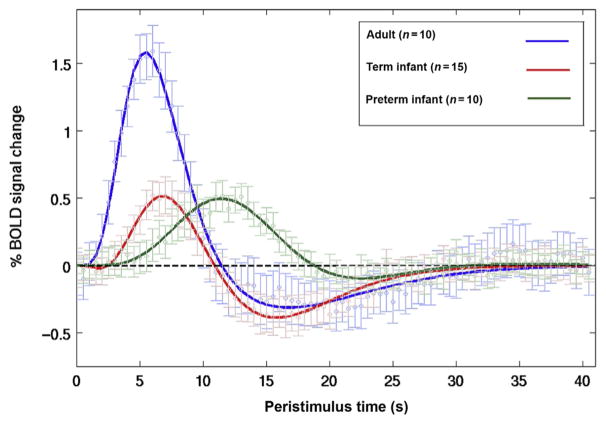
A recent study detailed changes in the temporal shape of the BOLD response to stimulus as a function of developmental age, reporting a delayed positive BOLD response in preterm infants and a biphasic positive–negative BOLD response in term infants (Fig. 2). The same group has suggested that these forms of the hemodynamic response function (HRF) should be used for analysis of perinatal fMRI data, rather than the use of a standard adult HRF (Arichi et al., 2012; Cusack et al., 2015).
FIG. 2.
Human age-dependent hemodynamic response functions to somatosensory stimulation measured using fMRI BOLD.
Figure adapted from Arichi, T., Fagiolo, G., Varela, M., Melendez-calderon, A., Allievi, A., Merchant, N., Tusor, N., Counsell, S.J., Burdet, E., Beckmann, C.F., et al., 2012. Development of BOLD signal hemodynamic responses in the human brain. Neuroimage 63, 663–673.
2.3 RODENT STUDIES OF POSTNATAL NEUROVASCULAR COUPLING
The earlier summaries highlight the wide discrepancies in reports of “normal” hemodynamic responses in the developing human brain. Such studies in human infants pose major challenges, both in terms of image acquisition on neonates that controls for physiological variables, and difficulties in the recruitment of young, healthy subjects for longitudinal studies. Studies thus often include cohorts of premature infants, infants with varied ages or infants with pathologies requiring prolonged postnatal care, making it challenging to establish the underlying cause of conflicting results from different studies.
Studies in rodent models offer the chance to carefully evaluate the underlying cause of changes in responses seen in the human brain. Of course age comparisons between rodents and humans are difficult to validate, particularly in terms of different aspects of brain development, and therefore analysis of developmental trajectory is more meaningful than absolute time points. However, for the work described here, the convention is to assume that the “newborn” human brain is developmentally equivalent to the postnatal day 7 mouse and postnatal day 12 rat, based on the codevelopment of factors such as eye opening and myelination (Quinn, 2005).
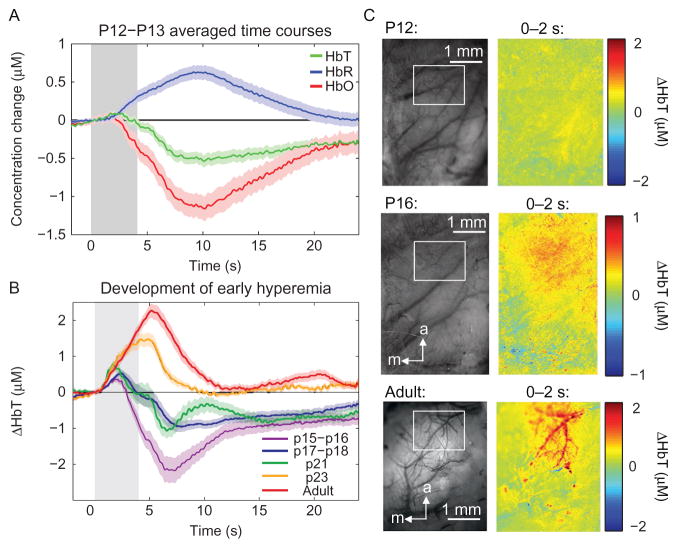
In order to reconcile the above findings of positive and negative BOLD in human infants, in recent work from our laboratory, we charted the hemodynamic response to stimulus as a function of postnatal development in both rats and mice using optical imaging of the exposed cortex at high spatiotemporal resolution (Kozberg et al., 2013). We demonstrated that the time courses of hemodynamic responses to neural activity mature postnatally (Kozberg et al., 2013); application of a somatosensory stimulus to postnatal day 12–13 (P12–13) rats was found to result in no or minimal localized increases in blood volume (functional hyperemia) (Fig. 3). This finding was replicated by Zehendner et al. who used whisker stimulation in P7 mice and demonstrated very minimal increases in local blood flow via laser speckle imaging (Zehendner et al., 2013). We found that when functional hyperemia does emerge several days later in development, it is localized to the brain parenchyma (see Fig. 3), meaning either capillary bed and/or diving arterioles, and does not spread to the larger pial arteries until approximately P23 in rats. This result is summarized in Fig. 3.
FIG. 3.
A functional hyperemia in response to somatosensory stimulation increases significantly with age in early postnatal rats. (A) Observed hemodynamic responses to somatosensory stimulus (gray bar) in P12–P13 rats demonstrating decreases in HbT and corresponding increases in HbR and decreases in HbO. (B) Regional blood volume influxes in response to somatosensory stimulus (gray bar) increase in amplitude with postnatal age. (C) In younger animals, hemodynamic responses are localized to the capillary bed and/or diving arterioles, while in the adult brain larger pial arterioles dilate as well—generating larger amplitude hemodynamic responses.
Figure adapted from Kozberg, M.G., Chen, B.R., Deleo, S.E., Bouchard, M.B., and Hillman, E.M.C., 2013. Resolving the transition from negative to positive blood oxygen level-dependent responses in the developing brain. Proc. Natl. Acad. Sci. 110, 4380–4385.
An important factor in our results was the presence of a delayed, global vasoconstriction in response to stimulus in the developing brain that was present prior to the development of vasodilatory responses (Kozberg et al., 2013; Zehendner et al., 2013). These constrictions were found to remain in place as localized hyperemia develops, resulting in biphasic responses in which there is an increase in total hemoglobin (HbT) followed by a prolonged decrease in HbT as shown in Fig. 3. This global vasoconstriction effect could have a range of causes; a related phenomenon is the “negative surround” commonly interpreted as either “vascular steal” or an indicator of reduced or inhibitory neural activity (Boas et al., 2008; Devor et al., 2008). However, this effect may also be an independent neurovascular coupling mechanism, potentially linked to noradrenergic or cholinergic responses to external stimuli consistent with the hemomodulatory effects of these systems reported in the adult rodent brain (Bekar et al., 2012; Takata et al., 2013). It has been proposed that locus coeruleus-triggered release of norepinephrine (a potent vasoconstrictor) serves to provide counteracting vasoconstriction during stimulation to direct and optimize blood flow to activated regions (Bekar et al., 2012). While this effect requires substantially more investigation, if this vasoconstriction serves to limit the extent of hyperemic responses, its establishment in development prior to hyperemic mechanism maturation could serve to protect fragile perinatal vasculature, which is particularly prone to hemorrhage in premature infants, from uncontrolled vasodilation and systemic blood pressure effects (Volpe, 1997).
These global vasoconstriction effects may be dependent on anesthesia, stimulus type, age, or species; however, as mentioned earlier, some human studies have identified decreases in blood flow (Born et al., 2002) and blood volume (Kusaka et al., 2004) in response to stimulation. A marked arterial vasoconstriction would cause an increase in local HbR (negative BOLD), but observed negative BOLD responses may also be related to underlying oxygen consumption. In the absence of large vasoconstrictions, HbR dynamics would be expected to be a balance of effects relating to oxygen consumption (increasing HbR) and the gradual development of functional hyperemia (decreasing HbR) (eg, the continuum between Fig. 1B and C). This interaction of factors may lead to the increased variability in HbR responses (or BOLD), described earlier.
In our own data, mathematically subtracting this global vasoconstriction effect from acquired data revealed clear evidence of localized oxygen consumption in responding regions of the somatosensory cortex. We observed gradually increasing HbT responses (increasing functional hyperemia as described earlier) with corresponding HbO and HbR dynamics indicating local stimulus-evoked relative hypoxias in early development. As the animals matured, these responses resolved to increases in blood volume balancing consumption effects, until the influx of HbO was sufficient to exceed the oxygen demands of the tissue (Kozberg et al., under review). These results have features consistent with some of the earliest reports of negative BOLD responses in fMRI studies of the human brain (Born et al., 1996).
A study by Colonnese et al. utilized fMRI in a rodent model to explore the postnatal evolution of functional hemodynamics (Colonnese et al., 2008). Although this work reported positive BOLD responses in the postnatal rat brain after P13, responses were prolonged and lower in amplitude compared to the adult brain, and averaged plots do show a marked period of negative BOLD during stimulation and neural activity at P13–15. In rats younger than P11, it is reported that no fMRI BOLD responses (negative or positive) were detected despite the presence of localized neural activity. This condition could indicate that experimental conditions or analysis methods in this study were less susceptible to evoked global vasoconstrictions, such that pre-P11 results confirm an absence of localized functional hyperemia, and minimal (or balanced) effects of oxygen consumption.
2.4 AUTOREGULATION AS A POTENTIAL CONFOUND
In the adult brain, changes in systemic pressure are not directly relayed to the brain due to a process termed autoregulation in which the smooth muscle cells of the brain constrict or relax to counteract increases or decreases in flow, respectively (Iadecola, 2004). However, autoregulation is not fully mature in the postnatal brain (Greisen, 2009). Fluctuations in systemic blood pressure will therefore be reflected in the hemodynamic of the newborn brain (Kozberg et al., 2013) with the potential to profoundly affect the spatiotemporal dynamics and direction of the BOLD signal. Through monitoring systemic blood pressure, we determined that high-amplitude stimuli resulted in large, evoked increases in systemic blood pressure that were closely mirrored by increases in HbT and HbO (and decreases in HbR) in the brain resembling an adult-like “positive BOLD” response. Tuning down stimulus amplitude until no increases in systemic blood pressure were observed yielded a “negative BOLD”-type cortical response that charted the developmental progression shown in Fig. 3. Based on these results, we proposed that a strong startle reflex and immature autoregulation could make the early postnatal brain particularly prone to cortical hyperemia resulting from stimulus-evoked increases in systemic blood pressure. Given the difficulty of monitoring systemic blood pressure in human infants during functional imaging, and difficulties with normalizing stimulus strength in nonverbal subjects who may be anesthetized or asleep, we propose a possible contribution from this blood pressure effect to fMRI and NIRS studies of the immature brain that have reported adult-like fMRI “positive BOLD” responses.
2.5 A POSSIBLE ROLE FOR NEUROVASCULAR DEVELOPMENT
Overall, the studies summarized earlier provide a look at the development of functional hemodynamics in the perinatal brain. Rodent studies have confirmed that the newborn brain exhibits hemodynamic responses that both spatially and temporally differ from those seen in the adult brain. In both Colonnese et al. and recent work from our lab, the neural activity occurring in parallel to these unusual hemodynamic responses has been measured. While the observed neural activity is generally shorter in duration than adult-like responses to the same duration stimuli, this does not fully account for the differences in evoked hemodynamics compared to the adult brain (Colonnese et al., 2008; Kozberg et al., under review). We thus hypothesize that the developmental trajectory of functional hemodynamics in the postnatal brain reflects, at least in part, the gradual development of neurovascular coupling itself. This hypothesis raises the following questions that will be addressed in the remainder of the chapter:
Does early neurovascular immaturity correspond to the physical absence of the mechanisms that link neural activity to blood flow changes? Does neurovascular development represent the establishment of this neurovascular system? Is the primary change made up of structural and functional changes at the level of neurons (or interneurons), vasculature, and/or intermediate cells such as astrocytes and pericytes?
A lack of functional hyperemia during early brain development appears to lead to stimulus-evoked hypoxias local to neural responses to external stimuli. This observation introduces the possibility that the newborn brain exists in a very different metabolic environment to the adult brain, despite being in a period of prolific neural development. What role does this metabolic environment play in normal and abnormal brain development and the effects of therapeutic interventions?
If neurovascular development is a critical component of perinatal brain development, occurring in parallel with neural development, could aberrant neurovascular development be a component in disorders of brain development? Could this developmental process provide new biomarkers or therapeutic targets? Could fMRI provide a unique tool to track and quantify this developmental trajectory?
Immaturity of neurovascular coupling suggests that fMRI data acquired in premature infants, newborns, and possibly children need to be analyzed differently from adult data. Which factors should be considered in the analysis of these data? How do changes in neurovascular coupling affect resting state fMRI methods?
Many changes occur in the newborn brain during early development. It is thus unsurprising that neurovascular coupling might also be assembling and developing in parallel. The following section details what is currently known about the components that might contribute to the establishment of neurovascular coupling.
3 POSTNATAL DEVELOPMENT OF NEUROVASCULAR CELLS AND STRUCTURES
This section explores the known features of development of cellular components that could represent the building blocks of neurovascular development (see also chapter “Mechanisms of cellular plasticity in cerebral perivascular region” by N. Egawa et al.). Although there is considerable debate about the mechanisms of neurovascular signaling itself, we have included discussion of neurons, vasculature, astrocytes, and pericytes, all of which have been proposed to play a role in neurovascular coupling in the adult brain (see chapters “Neurogenic control of parenchymal arterioles in the cerebral cortex” by H. Hotta, “Involvement of astrocytes in neurovascular communication” by M. Nuriya and H. Hirase, and “Vascular potassium channels in NVC” by K. Yamada).
3.1 POSTNATAL DEVELOPMENT OF NEURAL STRUCTURE AND FUNCTION
Neural networks are far from being fully established at birth, both in the human and rodent brain. The elaboration and refinement of these circuits require inputs from the external environment as well as waves of spontaneous activity within the earlier developed circuitry. Neurons initially undergo a robust period of axonal outgrowth and synaptogenesis postnatally (Cahalane et al., 2011; Lewis et al., 2013; Uylings et al., 1990; Wang et al., 2007), followed by synaptic pruning and circuit refinement with synapse elimination (Huttenlocher et al., 1982). These periods of elaboration and refinement vary regionally, but generally continue in the human brain well into adolescence (Harris et al., 2011).
The cortex is formed through multiple rounds of neurogenesis with almost all cortical neurons originating from radial glial progenitor cells. In the rodent brain at birth, layers 2–4 of the cortex exist in a gradient within the cortical plate; however, layers 5 and 6 are already defined, but still expanding. Over the next few days, the laminar organization becomes more defined, and by postnatal day 4 in the rodent brain, there has been clear structural differentiation into each of the layers (McCandlish et al., 1993). Neurons formed in later rounds of neurogenesis migrate to the superficial layers of cortex, past earlier rounds of neurons (Gao et al., 2014), Generally, deeper neurons (early born) are more connected with subcortical structures while superficial neurons (late born) are projection neurons responsible for intracortical connectivity; postnatal elaboration of neural circuitry primarily involves these more superficial cortical layers. Recent studies in the mouse brain have specifically demonstrated that transcortical projections within layer 2/3 are established throughout the first postnatal week (Hand and Polleux, 2011; Wang et al., 2007). While understanding, the establishment of these long-range connections and network firing patterns is a primary goal of many research programs utilizing functional imaging as a proxy for neural activity in development (Graham et al., 2014), there is a possibility that the connectivity of neurons to the vasculature also varies with the elaboration of neural networks.
Postnatal changes to the neural circuitry are in part activity dependent; the proper development of this circuitry requires external inputs for the process of effectively wiring “like” cells together (Katz and Shatz, 1996). Alterations in experiences can have large effects on neural synapse formation particularly in critical periods of neural development. These critical periods vary in timing regionally (Hensch, 2004). One of the paradigms most widely utilized to examine early cortical plasticity is that of monocular deprivation. If a subject is deprived of input from one eye during a “critical period” of visual cortex development, the region of the cortex receiving inputs from the deprived eye will be significantly smaller than that of the contralateral eye which will be proportionally larger, an effect first demonstrated in kittens by Hubel and Wiesel and subsequently in many other model systems (Hensch, 2004; Hubel and Wiesel, 1963). Recent work has also demonstrated that spontaneous activity may also be essential to circuit development (Feller, 1999). For example, prior to exposure of the visual system to external inputs, ie, prior to eye opening, spontaneous waves of retinal activity occur which are transmitted back to the visual cortex, generating neural activity which may aid in early circuit elaboration and refinement (Ackman et al., 2012).
Refinement of neural circuitry is dependent on the development of inhibitory pathways (Crook et al., 1998). GABAergic interneurons develop at different times postnatally corresponding to region-dependent critical periods (Le Magueresse and Monyer, 2013). Notably, the potassium chloride cotransporter KCC2 is upregulated with age, resulting in a developmental switch of the neurotransmitter GABA from depolarizing to hyperpolarizing at approximately postnatal day 7 in mice (Ben-Ari, 2002), changing the excitatory to inhibitory balance of the brain. These changes have the potential to affect the spatiotemporal profile brain hemodynamics, as some researchers hypothesize that interneurons play an essential role in neurovascular coupling (Cauli et al., 2004).
Lastly, an important facet of brain development is the physical expansion of the cortex. In the rodent brain, the cortical surface area more than doubles within the first 20 days of life (Wang et al., 1996). These differences in size of the cortex themselves may affect the energy demands and distribution in the newborn brain as oxygen diffusion limitations emerge.
3.2 POSTNATAL DEVELOPMENT OF THE BRAIN VASCULATURE
The brain’s vasculature itself changes significantly morphologically and functionally during the early postnatal period. The early vascular plexus of the brain is formed embryonically, a disordered plexus of vessels with no true differentiation between arteries, veins, and capillaries. This plexus is used as a scaffold for vascular development with gradual refinement of the vasculature into the different vascular compartments (Herken et al., 1989; Wang et al., 1996); arteries and veins notably only begin to take on their unique characteristics postnatally (Rowan and Maxwell, 1981). Remnants of the early vascular plexus of the brain remain particularly prevalent across the surface of the early postnatal brain, forming a dense meshwork of interconnected vasculature, which is largely pruned postnatally, leaving the larger vessels that become the pial arteries and veins (Wang et al., 1996). While these vessels are pruned, the deeper, intra-cortical network of vasculature elaborates.
3.2.1 Capillaries
Vascular network expansion via angiogenesis occurs primarily in the bed postnatally (Risser et al., 2009) and the pattern of changes in vascular density in many ways echoes the previously noted observations of neural development. For the first few months of human postnatal development and first postnatal month in mice, the capillary bed expands (Harb et al., 2013; Keep and Jones, 1990; Norman, 1986). The process of vascular remodeling occurs through sprout formation followed by either maintenance or pruning of these sprouts (Harb et al., 2013; Rowan and Maxwell, 1981).
Human studies have examined the cerebral blood flow of the cortex, a metric which is deeply entwined with vascular density, demonstrating that cerebral blood flow is significantly lower at early postnatal ages than in the adult brain, increases until the age of 7, and then decreases to adult levels in the late teen years (Chiron et al., 1992; Takahashi et al., 1999), a pattern notably similar to that of neural synapse formation and elimination. Additionally, rodent studies have demonstrated that postnatal brain vascularization occurs in an inside-to-outside pattern in which deeper cortical layers develop their capillary beds earlier (Norman, 1986; Rowan and Maxwell, 1981), a trend similar to the radial development of the neural architecture of the brain described earlier. Ways in which the parallel development of the neural and vascular systems may influence one another will be discussed later.
Microvascular density has a crucial impact on the oxygenation of the brain. Capillaries are known to be the main locations of oxygen exchange; only a small amount of oxygen exchange occurs along the arterioles (Vovenko, 1999). Efficient nutrient delivery to the adult brain depends in part on the density of the capillary bed; recent studies have demonstrated that in the adult brain islands of relative hypoxia exist in regions furthest from the capillaries, particularly in activated tissue (Sakadžić et al., 2014). Some groups hypothesize that the “oversupply” of oxygenated blood following stimulation may account for this variability in oxygenation based on capillary distribution (Devor et al., 2011). Therefore, secondary to relatively low capillary density, the newborn brain may actually have lower baseline levels of oxygenation than the adult brain.
Capillaries may also be essential for the actuation and execution of neurovascular responses. Recent studies have suggested that functional hyperemia is initiated at the capillary bed level (Hall et al., 2014; Hamilton et al., 2010) before spreading up the diving arterioles (Tian et al., 2010) and reaching the larger pial arteries on the surface of the brain (Chen et al., 2011, 2014). Every neuron is within 8–23 μm of a capillary (Lovick et al., 1999), making them a close signaling contact point; recent work has in fact demonstrated their ability to dilate themselves in response to stimulation, an action accomplished through the relaxation of pericytes and/or pericapillary smooth muscle cells (Hall et al., 2014; Hamilton et al., 2010; Hill et al., 2015). These data suggest that the capacity to perform neurovascular coupling may be diminished in an environment with a low density capillary bed, as in the newborn brain.
3.2.2 Arteries
The arterial distribution of major vessels across the surface of the brain is well established at birth (Wang et al., 1996); however, there are crucial differences in their oxygenation and function from those of the adult. Firstly, arteriovenous shunts which do not exist in the adult brain are present throughout the pial vasculature (Wang et al., 1996), and therefore differences in oxygenation between the pial arteries and veins are diminished as compared to the adult brain. Secondly, as mentioned earlier, arteries only begin to take on their unique characteristics postnatally, meaning that their vascular reactivity may differ (Rowan and Maxwell, 1981).
One of the essential mediators of vasodilation is nitric oxide. A recent study in rats demonstrated that while neural nitric oxide synthase decreases in expression with age, endothelial NOS (eNOS) expression is 85% lower in the 1-month-old brain than in the 14-month-old brain (Bustamante et al., 2008). Furthermore, studies of the piglet brain have demonstrated differences in the arterial response to acetylcholine, known in the adult brain to increase NO levels via eNOS (Andresen et al., 2005). Zuckerman et al. found that acetylcholine in the piglet brain in fact generated a NO-independent, prostanoid-mediated arterial constriction when applied to the newborn brain (Zuckerman et al., 1996).
Recent work from our laboratory demonstrated that the vascular endothelium itself is an essential component of neurovascular signaling and the spatiotemporal shape of the hemodynamic response (Chen et al., 2014). As discussed earlier, hemodynamic responses in the adult brain begin at the capillary bed or diving arteriolar level, and this dilation is back propagated along the arterial tree, eventually reaching the larger arteries on the pial surface. We demonstrated that this signaling is dependent on an intact vascular endothelium, hypothesizing the involvement of both gap junction coupling between endothelial cells enabling a long-range hyperpolarization wave (Ujiie et al., 2003) as well as more local nitric oxide-dependent and prostanoid-dependent signaling (Hillman, 2014). Therefore, changes in endothelial properties postnatally, including gap junction and receptor expression, may have large effects on the spatiotemporal profile of observed neurovascular responses.
3.2.3 Resting vascular tone
The aminergic systems which release norepinephrine, dopamine, and serotonin from the locus coeruleus, ventral tegmental area, and raphe nucleus, respectively, also expand postnatally (Goldman-Rakic and Brown, 1982). These transmitters are all vasoconstrictors and are important for the maintenance of resting vascular tone (Iadecola, 2004) and may even be additionally modulated during a stimulus (Bekar et al., 2012). Developmental changes in these networks could lead to relative “predilation” of the early postnatal arterial system and therefore a diminished capacity for active dilation.
3.3 ASTROCYTES
Astrocytes, ideally positioned surrounding both synapses and blood vessels throughout the brain, have been proposed to play an essential role in functional neurovascular coupling (Attwell et al., 2010). Local increases in extracellular glutamate have been demonstrated to drive increases in astrocytic intracellular calcium (Lind et al., 2013), in turn resulting in vascular dilations in the stimulated regions through the release of vasoactive substances stimulating smooth muscle cell relaxation such as prostaglandins and epoxyeicosatrienoic acids (Takano et al., 2006).
Astrocytes are known to be immature in the postnatal rat brain until postnatal day 21 and do not reach adult density levels until postnatal day 50, with changes in individual astrocyte size, branching and connectivity, as well as in number (Seregi et al., 1987; Stichel et al., 1991). Astrocytes gap junction coupling is established only at postnatal day 11 in the rat visual cortex, which could also affect the ability of astrocytes to signal to one another throughout the cortex (Binmoller and Müller, 1992).
3.4 PERICYTES
As mentioned earlier, any given neuron is within close proximity to the capillary bed, making capillaries a widely accessible signaling target for neurovascular coupling. Pericytes are the mural cells of capillaries (as well as other small vessels of the brain). They are similar to smooth muscle cells in many ways including their expression of contractile proteins, enabling them to change the diameter of capillaries. While smooth muscle cells are separated from the endothelium by the internal elastic lamina, pericytes share a basement membrane with the endothelium, potentially enabling enhanced signaling (Armulik et al., 2005). Recent studies have begun to focus on pericytes as a potential mediator of neurovascular coupling, demonstrating that capillaries dilate prior to precapillary arterioles, and that this dilation is particularly seen in regions with pericyte cell bodies, findings that suggest active dilation (Hall et al., 2014).
Pericytes are deeply involved in the structural patterning of the vasculature of the brain, particularly in the stabilization of vessels during angiogenesis. Throughout the process of sprout formation, pericytes are positioned at endothelial sprout tips. These developmental changes present the possibility that pericytes are unable to actively modulate capillary diameter throughout this dynamic period. Additionally, the investment of the pericytes into the endothelial basement membrane may occur postnatally (Fujimoto, 1995), which may affect communication between the cell types.
4 THE NEWBORN BRAIN’S UNIQUE METABOLIC ENVIRONMENT
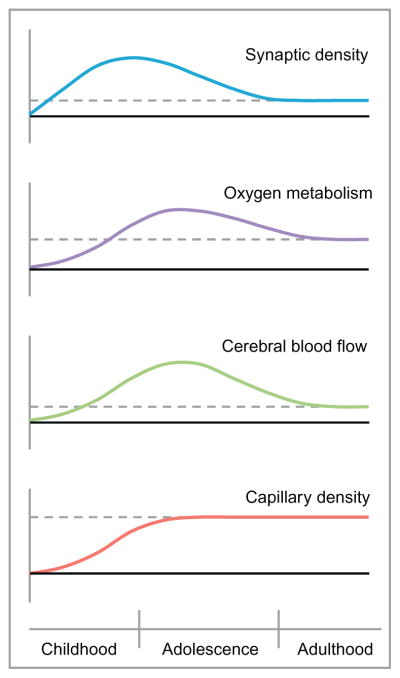
Early positron emission tomography studies of the developing brain indicated key differences in postnatal brain metabolism compared to the adult brain (Altman et al., 1993; Chugani and Phelps, 1986; Takahashi et al., 1999). Specifically, the newborn brain was observed to have a lower baseline CMRO2, which increases to adult rates by age 2 and then continues to rise to above adult levels until age 9. These CMRO2 values then decline to adult values by the late teenage years (Chugani and Phelps, 1986). Additional parameters are depicted in Fig. 4.
FIG. 4.
Developmental changes in neural and vascular density, oxygen metabolism, and cerebral blood flow. Newborn levels are depicted with solid lines and adult levels with dotted lines.
Based on Chiron, C., Raynaud, C., Mazière, B., Zilbovicius, M., Laflamme, L., Masure, M.C., Dulac, O., Bourguignon, M., and Syrota, A., 1992. Changes in regional cerebral blood flow during brain maturation in children and adolescents. J. Nucl. Med. 33, 696–703; Harb, R., Whiteus, C., Freitas, C., and Grutzendler, J., 2013. in vivo imaging of cerebral microvascular plasticity from birth to death. J. Cereb. Blood Flow Metab. 33, 146–156; Huttenlocher, P., de Courten, C., Garey, L., and van der Loos, H., 1982. Synaptogenesis in human visual cortex—evidence for synapse elimination during normal development. Neurosci. Lett. 33, 247–252; Norman, M.G., 1986. The growth and development of microvasculature in human cerebral cortex. J. Neuropathol. Exp. Neurol. 45, 222–232; Risser, L., Plouraboué, F., Cloetens, P., and Fonta, C., 2009. A 3D-investigation shows that angiogenesis in primate cerebral cortex mainly occurs at capillary level. Int. J. Dev. Neurosci. 27, 185–196; and Takahashi, T., Shirane, R., Sato, S., and Yoshimoto, T., 1999. Developmental changes of cerebral blood flow and oxygen metabolism in children. AJNR Am. J. Neuroradiol. 20, 917–922.
These findings suggest that the newborn brain may have less oxygen demand than the adult brain, a concept supported by the high tolerance of the neonatal brain to hypoxia (Singer, 1999) as well as prior studies of enzyme expression which have suggested that the early postnatal brain performs primarily nonoxidative glucose metabolism (Bilger and Nehlig, 1991; Bonavita et al., 1962; Booth et al., 1980). A recent study from our laboratory utilizing flavin adenine dinucleotide fluorescence to image rates of oxidative metabolism in the in vivo mouse brain demonstrated that the newborn brain (postnatal day 7–8) does increase its local rate of oxidative metabolism in response to stimulation, but at seemingly lower levels than in the adult (even in the presence of relatively large neural responses) (Kozberg et al., under review). In addition to the potential of the newborn brain to perform nonoxidative metabolism, demand may also be diminished secondary to the lower levels of spontaneous neural activity in the newborn brain (Kozberg et al., under review). Conversely, the level of oxygen availability in the cortex for oxidative metabolism could actually limit the levels of activity in the brain.
Note that while neurovascular coupling is hypothesized to exist in order to bring oxygen and other metabolites to the metabolically active brain (Heeger and Ress, 2002), the execution of hemodynamic responses to neural activity is not dependent on metabolic demand by the tissue, at least in terms of both oxygen and glucose availability (Lindauer et al., 2010; Wolf et al., 1997). Additionally, as mentioned earlier, the supply of oxygen to the tissue far exceeds the demand by the tissue (Fox et al., 1988). Therefore, while neurovascular coupling appears to be necessary for normal adult brain function (Iadecola, 2004), the relative lack of neurovascular coupling in the newborn brain does not necessarily imply decreased levels of demand or a completely resource limited state.
4.1 FETAL HEMOGLOBIN
Fetal hemoglobin binds to oxygen more strongly than adult hemoglobin, enabling the transfer of oxygen from mother to fetus prenatally. Oxygen exchange within the tissue is thus affected by the strength of the binding between hemoglobin and oxygen. Human infants continue to generate significant amounts of fetal hemoglobin for up to 6 months postnatally (Thein et al., 2009), which impacts (diminishes) oxygen exchange between the vasculature and tissue throughout the body, including in the brain.1 Of note, the absorption spectra of oxygenated and deoxygenated fetal hemoglobin differ only minimally from those of the adult, and therefore changes in expression of fetal hemoglobin are not expected to significantly affect NIRS analysis (Zijlstra et al., 1991).
4.2 NEUROVASCULAR CO-DEVELOPMENT?
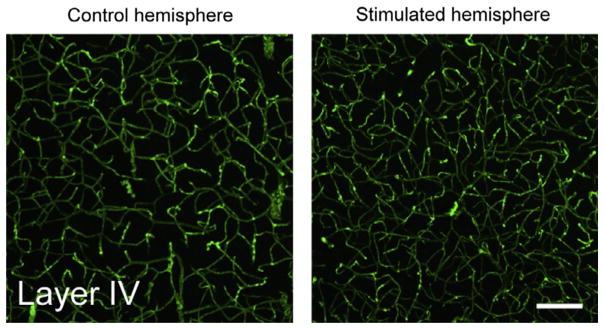
Embryonically, neurons and vasculature may share guidance cues as each is developing (Gelfand et al., 2009). As discussed earlier, neural and vascular networks continue to expand in parallel postnatally and vascular growth appears to be optimized to regions of higher neural activity. Recent studies have focused on determining how modulations in neural activity affect postnatal angiogenesis. Lacoste et al. manipulated inputs into the whisker barrel through both stimulation and selective ablation paradigms and studied the vascularization of the corresponding whisker barrels, finding that regions with fewer neural inputs and activity have decreased vascular density while regions with enhanced activity experience increased levels of postnatal angiogenesis (Lacoste et al., 2014; see Fig. 5). This corresponds well to previous work from Argandoña and Lafuenta who demonstrated decreased vascularization in the visual cortices of dark-reared rats (Argandoña and Lafuente, 1996) as well as work from Black et al. demonstrating increased vascular density in rats raised in complex environments (Black et al., 1987).
FIG. 5.
Whisker stimulation in early postnatal life leading to increased capillary density and branching.
Figure adapted from Lacoste, B., Comin, C.H., Ben-Zvi, A., Kaeser, P.S., Xu, X., Costa, L. da F., and Gu, C., 2014. Sensory-related neural activity regulates the structure of vascular networks in the cerebral cortex. Neuron 83, 1117–1130.
However, a recent study from Whiteus et al. demonstrated that exposing newborn mice to chronic auditory, whisker, or motor stimulation resulted in near arrest of normal postnatal angiogenesis in those respective cortices (Whiteus et al., 2014). Unsurprisingly, this decrease in vascular density made the mice more susceptible to hypoxias in regions furthest from the nearest capillary. Of note, contrary to the results of Lacoste et al. stated earlier, they found that smaller fluctuations in activity driven by whisker trimming or moderate whisker stimulation did not result in alterations in the level of angiogenesis. These data suggest that the type and intensity of stimulation may significantly affect its impact on postnatal vascular growth. There may be states of either “too much” or “too little” activity, each of which could lead to decreased angiogenesis.
Increased regional metabolic demand has also been associated with increased vascular density (Riddle et al., 1993; Weber et al., 2008). Detection of this metabolic demand may be in part through hypoxia signaling, which is well-known to drive vascular elaboration, particularly in the developing brain. Harb et al. demonstrated that young adult mice (<3 months old) placed in hypoxia form significantly more vessels, and that these vessels are not eliminated once the animal is brought back to normoxic conditions (Harb et al., 2013). Hypoxia induces increases in vessel growth through the activation of hypoxia-inducible transcription factors (known as HIFs; see chapter “Development and pathological changes of neurovascular unit regulated by hypoxia response in the retina” by T. Kurihara for details), which in turn upregulate expression of signaling molecules involved in angiogenesis such as VEGF (Lacoste and Gu, 2015; Rey and Semenza, 2010).
If hypoxia is a component of directed/activity-dependent vascular patterning, increased oxygenated blood delivery by neurovascular coupling could actually be detrimental to establishing a complete vascular network responsive to the demands of the tissue. The relative hypoxias that occur in response to neural activity in the absence of high-amplitude neurovascular coupling described earlier could be necessary to drive postnatal angiogenesis (Lacoste and Gu, 2015).
5 CONCLUSIONS
Functional imaging studies using fMRI and NIRS in newborns have identified distinctly different response characteristics in newborn infants, and even children exceeding 36 months, compared to stereotypical adult responses. Mounting evidence suggests that these differences in functional responses correspond to the immaturity of neurovascular coupling, the cellular mechanisms that link neural activity to local increases in blood flow in the brain. The substantial cellular and vascular development known to occur during the postnatal period therefore includes both the expansion of neural and vascular networks and the gradual postnatal development of neurovascular coupling mechanisms.
The resultant lack of coupling in the immature brain (<P8 in mice) raises important questions about developing brain metabolism. The newborn brain supports massive growth, neurogenesis, and learning in a state that appears to be incompletely tuned to the brain’s metabolic demands. Does the newborn brain encounter an environment of low energy availability that limits the ability of neurons to fire? Moreover, is there a key purpose to the development of the brain’s neurovascular system in parallel with the development of neural circuits? From an optimization standpoint, the codevelopment of neural networks and supporting vasculature is logical, as opposed to independent development or initial hardwiring of the vasculature prior to neural expansion and pruning. Some recent results have suggested that relative hypoxic events occur in response to neural activity (in the absence of coupled functional hyperemia), a possible mechanism by which vasculature directs its growth to serve regions of increased neural activity.
5.1 A ROLE IN NORMAL AND ABNORMAL DEVELOPMENT?
The complex (and relatively unexplored) process of neurovascular development continues to occur postnatally in normal development, prompting consideration of the possible role of this developing system in normal and abnormal trajectories of brain development. Since the early postnatal brain appears to operate with a delicate balance of oxygenation, the establishment of functional hyperemia at the right time point in development is likely a key stage of development. Disruptions or delays in the onset of coupled hyperemia could feasibly lead to abnormalities in both neural and vascular development.
The unique metabolic environment of the newborn brain may also have important implications for the care of premature and newborn infants. Several studies have indicated that resuscitation of infants with room air rather than 100% oxygen offers better clinical outcomes and less brain damage, while premature infants may require slight oxygen supplementation (Saugstad, 2010). This oxygen supplementation must be carefully titrated: on the one hand, excess and abnormal vascular development occurs in the eyes of premature infants exposed to high levels of oxygen (retinopathy of prematurity) (Wheatley et al., 2002), while on the other hand ischemia in premature infants is associated with poor neurological outcomes primarily due to cerebral white matter injury (Khwaja and Volpe, 2008). Further studies have raised concerns over the effects of general anesthesia in newborns, indicating that they may have a profound impact on developing neural circuitry (Culley et al., 2012). There is thus an urgent need to understand the optimal metabolic state of the perinatal brain.
5.2 IMPLICATIONS FOR FUTURE FUNCTIONAL IMAGING STUDIES
Both fMRI and NIRS, the primary noninvasive functional imaging modalities used to study human brain development, rely on measurements of hemodynamics as a proxy for neural activity. If indeed, there are periods of early (possibly prenatal) development in which neurovascular coupling is different than in adults, standard analysis and interpretation of fMRI and NIRS data could be misleading. Conversely, with improved knowledge of how to interpret functional imaging signals in these age groups, functional imaging methods could prove invaluable for charting the development of neurovascular coupling.
5.2.1 Functional imaging during stimulation
While observed hemodynamic responses in the newborn brain are varied, there are a few unifying factors. Firstly, most agree that early postnatal hemodynamics differ from the adult in terms of their spatiotemporal dynamics, variations that are likely a result of immature signaling and/or immature vasculature. This necessitates the use of age-specific HRFs in the analysis of functional imaging data (Arichi et al., 2012; Cusack et al., 2015). The question of the age at which human children can be considered to have full “adult-like” hemodynamic responses is still open, with recent studies suggesting that neurovascular responses continue to mature throughout adolescence (Schmithorst et al., 2015) although some studies demonstrate BOLD % signal change equal to the adult in early childhood (Church et al., 2010).
Secondly, unlike in the adult brain in which a large influx in arterial blood in response to stimulation far overcompensates for local oxygen consumption, the balance between local consumption and arterial supply is much more delicate in the developing brain. Because the directionality of the HbR curve (ie, positive vs negative BOLD) is affected by both oxygen metabolism and blood volume changes, interstudy, intersubject, and even intrasubject regional variability may be attributable to slight tips in the balance between these two components.
For example, hyperemia is primarily localized to the capillary bed or diving arterioles of the cortex in the immature brain (Kozberg et al., 2013). This capillary bed is actively expanding in postnatal development following an inside-to-outside maturation pattern suggesting that the deeper layers of the cortex may be best prepared to mount a hemodynamic response. Therefore, in deeper regions of the brain, the balance could be tipped to “positive BOLD” in the early postnatal brain. Notably, this would differentially affect functional imaging modalities as signals from deeper layers of the cortex are less detectable by NIRS, optical intrinsic signal imaging, and speckle than by fMRI. Another important consideration is that the maturation of neurons and vasculature is region dependent, making it likely that there will be stimulus-dependent variability.
Lastly, hemodynamics measurements are often used as a proxy for neural activity, which by nature is an imperfect representation. In the developing brain, it is crucial to remember that while both negative and positive BOLD responses are approximate proxies for neural activity, they are actually reporting two different phenemona, which will have different spatiotemporal distributions.
5.2.2 Resting state functional connectivity mapping
A focus of many recent studies of both the developing and adult brain has been on functional connectivity mapping. This form of imaging takes advantage of spontaneous network activity, neural activity that occurs in the absence of a task. Such activity patterns reveal the underlying architecture of the neural circuitry of the brain with striking accuracy in the healthy adult brain (Fox and Raichle, 2007). These imaging paradigms are much easier to perform in infants than task-based studies because of the speed of imaging and decreased need for subject attention and compliance (Doria et al., 2010; Fransson et al., 2011; Smyser et al., 2010) and have been performed to examine the fetal brain (Ferrazzi et al., 2014). These studies can also be performed using NIRS (Ferradal et al., 2015). However, resting state fMRI data can be more challenging to interpret than stimulus-evoked responses; it remains unknown what precisely drives these slow fluctuations in neural and hemodynamic activity in structurally and functionally connected regions even in the adult brain, and no direct measurement is made to confirm the presence of positive, negative, or absent neurovascular coupling.
Recent work from our laboratory demonstrated diminished amplitude functional hyperemias with accompanying relative local hypoxias in response to spontaneous neural activity in the early postnatal mouse brain, similar to observed hemodynamic responses to stimulus-evoked neural activity (Kozberg et al., under review). This finding indicates that oxygen consumption may also be an important source of signal in functional connectivity mapping of newborns, and potential spatial differences between the localization of oxygen consumption and vascular responses should be considered. Additionally, we identified large, global fluctuations in hemodynamics that were spatiotemporally uncoupled to neural activity. These fluctuations may confound studies reliant on hemodynamic measures of resting state neural activity.
5.3 A LOOK TO THE FUTURE
Neurovascular coupling is an essential and largely underexplored component of early postnatal development that affects both the interpretation of functional imaging studies and the metabolic environment of the developing brain. A more comprehensive understanding of the normal stages of neurovascular coupling maturation could provide important new insights into the etiology of developmental disorders and disorders of prematurity and early childhood and may provide new biomarkers for diagnosis and monitoring and unexplored therapeutic targets for currently intractable conditions.
Acknowledgments
The authors would like to acknowledge the support from National Institutes of Health grants: 1F31NS084538, 1RO1NS063226, and 1RO1NS076628; and National Science Foundation grant: CBET-0954796.
Footnotes
In the rodent, embryonic hemoglobin is largely replaced with adult hemoglobin prior to birth (without an intermediate fetal hemoglobin step as in humans; Iwahara et al., 1996).
References
- Ackman JB, Burbridge TJ, Crair MC. Retinal waves coordinate patterned activity throughout the developing visual system. Nature. 2012;490:219–225. doi: 10.1038/nature11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allievi AG, Arichi T, Tusor N, Kimpton J, Arulkumaran S, Counsell SJ, Edwards AD, Burdet E. Maturation of sensorimotor functional responses in the preterm brain. Cereb Cortex. 2016;26(1):402–413. doi: 10.1093/cercor/bhv203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman DI, Perlman JM, Volpe JJ, Powers WJ. Cerebral oxygen metabolism in newborns. Pediatrics. 1993;92:99–104. [PubMed] [Google Scholar]
- Anderson AW, Marois R, Colson ER, Peterson BS, Duncan CC, Ehrenkranz RA, Schneider KC, Gore JC, Ment LR, et al. Neonatal auditory activation detected by functional magnetic resonance imaging. Magn Reson Imaging. 2001;19:1–5. doi: 10.1016/s0730-725x(00)00231-9. [DOI] [PubMed] [Google Scholar]
- Andresen J, Shafi NI, Bryan RM. Endothelial influences on cerebrovascular tone. J Appl Physiol. 2005;100:318–327. doi: 10.1152/japplphysiol.00937.2005. [DOI] [PubMed] [Google Scholar]
- Argandoña EG, Lafuente JV. Effects of dark-rearing on the vascularization of the developmental rat visual cortex. Brain Res. 1996;732:43–51. doi: 10.1016/0006-8993(96)00485-4. [DOI] [PubMed] [Google Scholar]
- Arichi T, Moraux A, Melendez A, Doria V, Groppo M, Merchant N, Combs S, Burdet E, Larkman DJ, Counsell SJ, et al. Somatosensory cortical activation identified by functional MRI in preterm and term infants. Neuroimage. 2010;49:2063–2071. doi: 10.1016/j.neuroimage.2009.10.038. [DOI] [PubMed] [Google Scholar]
- Arichi T, Fagiolo G, Varela M, Melendez-calderon A, Allievi A, Merchant N, Tusor N, Counsell SJ, Burdet E, Beckmann CF, et al. Development of BOLD signal hemodynamic responses in the human brain. Neuroimage. 2012;63:663–673. doi: 10.1016/j.neuroimage.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekar LK, Wei HS, Nedergaard M. The locus coeruleus-norepinephrine network optimizes coupling of cerebral blood volume with oxygen demand. J Cereb Blood Flow Metab. 2012;32:2135–2145. doi: 10.1038/jcbfm.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Bilger A, Nehlig A. Quantitative histochemical changes in enzymes involved in energy metabolism in the rat brain during postnatal development. I Cytochrome oxidase and lactate dehydrogenase. Int J Dev Neurosci. 1991;9:545–553. doi: 10.1016/0736-5748(91)90015-e. [DOI] [PubMed] [Google Scholar]
- Binmoller F, Müller C. Postnatal development of dye-coupling among astrocytes in the visual cortex. Glia. 1992;6:127–137. doi: 10.1002/glia.440060207. [DOI] [PubMed] [Google Scholar]
- Black J, Sirevaag A, Greenough W. Complex experience promotes capillary formation in young rat visual cortex. Neurosci Lett. 1987;83:351–355. doi: 10.1016/0304-3940(87)90113-3. [DOI] [PubMed] [Google Scholar]
- Boas D, Jones S, Devor A, Huppart T, Dale A. A vascular anatomical network model of the spatio-temporal response to brain activation. Neuroimage. 2008;40:1116–1129. doi: 10.1016/j.neuroimage.2007.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonavita Vi, Ponte F, Amore G. Lactate dehydrogenase isozymes in the developing rat brain. Nature. 1962;196:576–577. doi: 10.1038/196576a0. [DOI] [PubMed] [Google Scholar]
- Booth RF, Patel TB, Clark JB. The development of enzymes of energy metabolism in the brain of a precocial (guinea pig) and non-precocial (rat) species. J Neurochem. 1980;34:17–25. doi: 10.1111/j.1471-4159.1980.tb04616.x. [DOI] [PubMed] [Google Scholar]
- Born P, Rostrup E, Leth H, Peitersen B, Lou H. Change of visually induced cortical activation patterns during development. Lancet. 1996;347:543. doi: 10.1016/s0140-6736(96)91175-7. [DOI] [PubMed] [Google Scholar]
- Born P, Leth H, Miranda MJ, Rostrup E, Stensgaard A, Peitersen B, Larsson HBW, Lou HC. Visual activation in infants and young children studied by functional magnetic resonance imaging. Pediatr Res. 1998;44:578–583. doi: 10.1203/00006450-199810000-00018. [DOI] [PubMed] [Google Scholar]
- Born AP, Miranda MJ, Rostrup E, Toft PB, Peitersen B, Larsson HBW, Lou HC. Functional magnetic resonance imaging of the normal and abnormal visual system in early life. Neuropediatrics. 2000;31:24–32. doi: 10.1055/s-2000-15402. [DOI] [PubMed] [Google Scholar]
- Born AP, Rostrup E, Miranda MJ, Larsson HBW, Lou HC. Visual cortex reactivity in sedated children examined with perfusion MRI (FAIR) Magn Reson Imaging. 2002;20:199–205. doi: 10.1016/s0730-725x(02)00469-1. [DOI] [PubMed] [Google Scholar]
- Bustamante J, Czerniczyniec A, Cymeryng C, Lores-Arnaiz S. Age related changes from youth to adulthood in rat brain cortex: nitric oxide synthase and mitochondrial respiratory function. Neurochem Res. 2008;33:1216–1223. doi: 10.1007/s11064-007-9570-z. [DOI] [PubMed] [Google Scholar]
- Cahalane DJ, Clancy B, Kingsbury MA, Graf E, Sporns O, Finlay BL. Network structure implied by initial axon outgrowth in rodent cortex: empirical measurement and models. PLoS One. 2011;6:e16113. doi: 10.1371/journal.pone.0016113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Tong X, Rancillac A, Serluca N, Lambolez B, Rossier J, Hamel E. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BR, Bouchard MB, Mccaslin AFH, Burgess SA, Hillman EMC. High-speed vascular dynamics of the hemodynamic response. Neuroimage. 2011;54:1021–1030. doi: 10.1016/j.neuroimage.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EMC. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc. 2014;3:e000787. doi: 10.1161/JAHA.114.000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiron C, Raynaud C, Mazière B, Zilbovicius M, Laflamme L, Masure MC, Dulac O, Bourguignon M, Syrota A. Changes in regional cerebral blood flow during brain maturation in children and adolescents. J Nucl Med. 1992;33:696–703. [PubMed] [Google Scholar]
- Chugani HT, Phelps ME. Maturational changes in cerebral function in infants determined by 18FDG positron emission tomography. Science. 1986;231:840–843. doi: 10.1126/science.3945811. [DOI] [PubMed] [Google Scholar]
- Church J, Peterson S, Schlagger B. The “Task B problem” and other considerations in developmental functional neuroimaging. Hum Brain Mapp. 2010;31:852–862. doi: 10.1002/hbm.21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnese MT, Phillips MA, Constantine-paton M, Kaila K, Jasanoff A. Development of hemodynamic responses and functional connectivity in rat somatosensory cortex. Nat Neurosci. 2008;11:72–79. doi: 10.1038/nn2017. [DOI] [PubMed] [Google Scholar]
- Crook JM, Kisvárday ZF, Eysel UT. Evidence for a contribution of lateral inhibition to orientation tuning and direction selectivity in cat visual cortex: reversible inactivation of functionally characterized sites combined with neuroanatomical tracing techniques. Eur J Neurosci. 1998;10:2056–2075. doi: 10.1046/j.1460-9568.1998.00218.x. [DOI] [PubMed] [Google Scholar]
- Culley DJ, Maze M, Crosby G. Reprogramming of the infant brain by surgery with general anesthesia. Mayo Clin Proc. 2012;87:110–113. doi: 10.1016/j.mayocp.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack R, Wild C, Linke AC, Arichi T, Lee DSC, Han VK. Optimizing stimulation and analysis protocols for neonatal fMRI optimizing stimulation and analysis protocols for neonatal fMRI. PLoS One. 2015;10:e0120202. doi: 10.1371/journal.pone.0120202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor A, Hillman EMC, Tian P, Waeber C, Teng IC, Ruvinskaya L, Shalinsky MH, Zhu H, Haslinger RH, Narayanan SN, et al. Stimulus-induced changes in blood flow and 2-deoxyglucose uptake dissociate in ipsilateral somatosensory cortex. J Neurosci. 2008;28:14347–14357. doi: 10.1523/JNEUROSCI.4307-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor A, Sakadz S, Saisan PA, Yaseen MA, Roussakis E, Srinivasan VJ, Vinogradov SA, Rosen BR, Buxton RB, Dale AM, et al. “Overshoot” of O2 is required to maintain baseline tissue oxygenation at locations distal to blood vessels. J Neurosci. 2011;31:13676–13681. doi: 10.1523/JNEUROSCI.1968-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria V, Beckmann CF, Arichi T, Merchant N, Groppo M, Turkheimer FE, Counsell SJ, Murgasova M, Aljabar P, Nunes RG, et al. Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci U S A. 2010;107:20015–20020. doi: 10.1073/pnas.1007921107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erberich SG, Panigrahy A, Friedlich P, Seri I, Nelson MD, Gilles F. Somato-sensory lateralization in the newborn brain. Neuroimage. 2006;29:155–161. doi: 10.1016/j.neuroimage.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Feller MB. Spontaneous correlated activity in developing neural circuits. Neuron. 1999;22:653–656. doi: 10.1016/s0896-6273(00)80724-2. [DOI] [PubMed] [Google Scholar]
- Ferradal SL, Liao SM, Eggebrecht AT, Shimony JS, Inder TE, Culver JP, Smyser CD. Functional imaging of the developing brain at the bedside using diffuse optical tomography. Cereb Cortex. 2015:1–11. doi: 10.1093/cercor/bhu320. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrazzi G, Kuklisova Murgasova M, Arichi T, Malamateniou C, Fox MJ, Makropoulos A, Allsop J, Rutherford M, Malik S, Aljabar P, et al. Resting state fMRI in the moving fetus: a robust framework for motion, bias field and spin history correction. Neuroimage. 2014;101:555–568. doi: 10.1016/j.neuroimage.2014.06.074. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C, Focal D. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Fransson P, Aden U, Blennow M, Lagercrantz H. The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb Cortex. 2011;21:145–154. doi: 10.1093/cercor/bhq071. [DOI] [PubMed] [Google Scholar]
- Fujimoto K. Pericyte-endothelial gap junctions in developing rat cerebral capillaries: a fine structural study. Anat Rec. 1995;242:562–565. doi: 10.1002/ar.1092420412. [DOI] [PubMed] [Google Scholar]
- Gao P, Postiglione MP, Krieger TG, Hernandez L, Wang C, Han Z, Streicher C, Papusheva E, Insolera R, Chugh K, et al. Article deterministic progenitor behavior and unitary production of neurons in the neocortex. Cell. 2014;159:775–788. doi: 10.1016/j.cell.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand M, Hong S, Gu C. Guidance from above: common cues direct distinct signaling outcomes in vascular and neural patterning. Trends Cell Biol. 2009;19:1199–1216. doi: 10.1016/j.tcb.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic P, Brown R. Postnatal development of monoamine content and synthesis in the cerebral cortex of rhesus monkeys. Brain Res. 1982;256:339–349. doi: 10.1016/0165-3806(82)90146-8. [DOI] [PubMed] [Google Scholar]
- Graham AM, Pfeifer JH, Fisher PA, Lin W, Gao W, Fair DA. The potential of infant fMRI research and the study of early life stress as a promising exemplar. Dev Cogn Neurosci. 2014;12:12–39. doi: 10.1016/j.dcn.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greisen G. To autoregulate or not to autoregulate—that is no longer the question. Semin Pediatr Neurol. 2009;16:207–215. doi: 10.1016/j.spen.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D, Hall CN. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics. 2010;2:1–14. doi: 10.3389/fnene.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand R, Polleux F. Neurogenin2 regulates the initial axon guidance of cortical pyramidal neurons projecting medially to the corpus callosum. Neural Dev. 2011;6:30. doi: 10.1186/1749-8104-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harb R, Whiteus C, Freitas C, Grutzendler J. In vivo imaging of cerebral micro-vascular plasticity from birth to death. J Cereb Blood Flow Metab. 2013;33:146–156. doi: 10.1038/jcbfm.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JJ, Reynell C, Attwell D. The physiology of developmental changes in BOLD functional imaging signals. Dev Cogn Neurosci. 2011;1:199–216. doi: 10.1016/j.dcn.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeger DJ, Ress D. What does fMRI tell us about neuronal activity? Nat Rev Neurosci. 2002;3:142–151. doi: 10.1038/nrn730. [DOI] [PubMed] [Google Scholar]
- Heep A, Scheef L, Jankowski J, Born M, Zimmermann N, Sival D, Bos A, Gieseke J, Bartmann P, Schild H, et al. Functional magnetic resonance imaging of the sensorimotor system in preterm infants. Pediatrics. 2009;123:294–300. doi: 10.1542/peds.2007-3475. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Herken R, Gotz W, Wattjes KH. Initial development of capillaries in the neuroepithelium of the mouse. J Anat. 1989;164:85–92. [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron. 2015;87:95–110. doi: 10.1016/j.neuron.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman EMC. Coupling mechanism and significance of the BOLD signal: a status report. Annu Rev Neurosci. 2014;37:161–181. doi: 10.1146/annurev-neuro-071013-014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman EMC, Kozberg MG. What secrets can functional MRI reveal about the developing infant brain? Imaging Med. 2013;5:203–206. [Google Scholar]
- Hoshi Y, Tamura M. Dynamic multichannel near-infrared optical imaging of human brain activity. J Appl Physiol. 1993;75:1842–1846. doi: 10.1152/jappl.1993.75.4.1842. [DOI] [PubMed] [Google Scholar]
- Hoshi Y, Kohri S, Matsumoto Y, Cho K, Matsuda T, Okajima S, Fujimoto S. Hemodynamic responses to photic stimulation in neonates. Pediatr Neurol. 2000;23:323–327. doi: 10.1016/s0887-8994(00)00195-8. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of cells in striate cortex of very young, visually inexperienced kittens. J Neurophysiol. 1963;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P, de Courten C, Garey L, van der Loos H. Synaptogenesis in human visual cortex—evidence for synapse elimination during normal development. Neurosci Lett. 1982;33:247–252. doi: 10.1016/0304-3940(82)90379-2. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Iwahara SI, Abe Y, Okazaki T. Identification of five embryonic hemoglobins of rat and ontogeny of their constituent globins during fetal development. J Biochem. 1996;119:360–366. doi: 10.1093/oxfordjournals.jbchem.a021248. [DOI] [PubMed] [Google Scholar]
- Joeri P, Huisman T, Ekatodramis D, Loenneker T, Rumpel H, Martin E. Functional magnetic resonance imaging (fMRI) of the visual cortex in children. Pediatr Res. 1996;40:535. [Google Scholar]
- Karen T, Morren G, Haensse D, Bauschatz AS, Bucher HU, Wolf M. Hemodynamic response to visual stimulation in newborn infants using functional near-infrared spectroscopy. Hum Brain Mapp. 2008;29:453–460. doi: 10.1002/hbm.20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Keep R, Jones H. Cortical microvessels during brain development: a morphometric study in the rat. Microvasc Res. 1990;40:412–426. doi: 10.1016/0026-2862(90)90036-q. [DOI] [PubMed] [Google Scholar]
- Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93:F153–F161. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozberg MG, Chen BR, Deleo SE, Bouchard MB, Hillman EMC. Resolving the transition from negative to positive blood oxygen level-dependent responses in the developing brain. Proc Natl Acad Sci. 2013;110:4380–4385. doi: 10.1073/pnas.1212785110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozberg MG, Ma Y, Kim SH, Hillman EMC. Rapid postnatal expansion of neural networks occurs in an environment of altered neurovascular and neurometabolic coupling. doi: 10.1523/JNEUROSCI.2363-15.2016. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaka T, Kawada K, Okubo K, Nagano K, Namba M, Okada H, Imai T, Isobe K, Itoh S. Noninvasive optical imaging in the visual cortex in young infants. Hum Brain Mapp. 2004;22(2):122–132. doi: 10.1002/hbm.20020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste B, Gu C. Control of cerebrovascular patterning by neural activity during postnatal development. Mech Dev. 2015;138:43–49. doi: 10.1016/j.mod.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste B, Comin CH, Ben-Zvi A, Kaeser PS, Xu X, da Costa LF, Gu C. Sensory-related neural activity regulates the structure of vascular networks in the cerebral cortex. Neuron. 2014;83:1117–1130. doi: 10.1016/j.neuron.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Magueresse C, Monyer H. GABAergic interneurons shape the functional maturation of the cortex. Neuron. 2013;77:388–405. doi: 10.1016/j.neuron.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Lewis TL, Courchet J, Polleux F. Cell biology in neuroscience: cellular and molecular mechanisms underlying axon formation, growth, and branching. J Cell Biol. 2013;202:837–848. doi: 10.1083/jcb.201305098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao SM, Gregg NM, White BR, Zeff BW, Bjerkaas KA, Inder TE, Culver JP. Neonatal hemodynamic response to visual cortex activity: high-density near-infrared spectroscopy study. J Biomed Opt. 2010;15:1–9. doi: 10.1117/1.3369809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind BL, Brazhe AR, Jessen SJ, Tan FCC, Lauritzen MJ. Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo. Proc Natl Acad Sci. 2013;110:E4678–E4687. doi: 10.1073/pnas.1310065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindauer U, Leithner C, Kaasch H, Rohrer B, Foddis M, Füchtemeier M, Offenhauser N, Steinbrink J, Royl G, Kohl-Bareis M, et al. Neurovascular coupling in rat brain operates independent of hemoglobin deoxygenation. J Cereb Blood Flow Metab. 2010;30:757–768. doi: 10.1038/jcbfm.2009.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovick TA, Brown LA, Key BJ. Neurovascular relationships in hippocampal slices: physiological and anatomical studies of mechanisms underlying flow-metabolism coupling in intraparenchymal microvessels. Neuroscience. 1999;92:47–60. doi: 10.1016/s0306-4522(98)00737-4. [DOI] [PubMed] [Google Scholar]
- Martin E, Joeri P, Loenneker T, Ekatodramis D, Vitacco D, Hennig J, Marcar VL. Visual processing in infants and children studied using functional MRI. Pediatr Res. 1999;46:135–140. doi: 10.1203/00006450-199908000-00001. [DOI] [PubMed] [Google Scholar]
- McCandlish CA, Li CX, Waters RS. Early development of the SI cortical barrel field representation in neonatal rats follows a lateral-to-medial gradient: an electrophysiological study. Exp Brain Res. 1993;92:369–374. doi: 10.1007/BF00229024. [DOI] [PubMed] [Google Scholar]
- Meek JH, Firbank M, Elwell CE, Atkinson J, Braddick O, Wyatt JS. Regional hemodynamic responses to visual stimulation in awake infants. Pediatr Res. 1998;43:840–843. doi: 10.1203/00006450-199806000-00019. [DOI] [PubMed] [Google Scholar]
- Muramoto S, Yamada H, Sadato N, Kimura H, Konishi Y, Kimura K, Tanaka M, Kochiyama T, Yonekura Y, Ito H. Age-dependent change in metabolic response to photic stimulation of the primary visual cortex in infants: functional magnetic resonance imaging study. J Comput Assist Tomogr. 2002;26:894–901. doi: 10.1097/00004728-200211000-00007. [DOI] [PubMed] [Google Scholar]
- Norman MG. The growth and development of microvasculature in human cerebral cortex. J Neuropathol Exp Neurol. 1986;45:222–232. [PubMed] [Google Scholar]
- Quinn R. Comparing rat’s to human’s age: how old is my rat in people years? Nutrition. 2005;21:775–777. doi: 10.1016/j.nut.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Rey S, Semenza GL. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res. 2010;86:236–242. doi: 10.1093/cvr/cvq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle DR, Gutierrez G, Zheng D, White LE, Richards A, Purves D. Differential metabolic and electrical activity in the somatic sensory cortex of juvenile and adult rats. J Neurosci. 1993;13:4193–4213. doi: 10.1523/JNEUROSCI.13-10-04193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser L, Plouraboué F, Cloetens P, Fonta C. A 3D-investigation shows that angiogenesis in primate cerebral cortex mainly occurs at capillary level. Int J Dev Neurosci. 2009;27:185–196. doi: 10.1016/j.ijdevneu.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Rowan RA, Maxwell DS. Patterns of vascular sprouting in the postnatal development of the cerebral cortex of the rat. Am J Anat. 1981;160:247–255. doi: 10.1002/aja.1001600303. [DOI] [PubMed] [Google Scholar]
- Sakadžić S, Mandeville ET, Gagnon L, Musacchia JJ, Yaseen MA, Yucel MA, Lefebvre J, Lesage F, Anders M, Eikermann-haerter K, et al. Large arteriolar component of oxygen delivery implies a safe margin of oxygen supply to cerebral tissue. Nat Commun. 2014;5:5734. doi: 10.1038/ncomms6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakatani K, Chen S, Lichty W, Zuo H, Wang YP. Cerebral blood oxygenation changes induced by auditory stimulation in newborn infants measured by near infrared spectroscopy. Early Hum Dev. 1999;55:229–236. doi: 10.1016/s0378-3782(99)00019-5. [DOI] [PubMed] [Google Scholar]
- Saugstad OD. Resuscitation of newborn infants: from oxygen to room air. Lancet. 2010;376:1970–1971. doi: 10.1016/S0140-6736(10)60543-0. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Vannest J, Lee G, Hernandez-Garcia L, Plante E, Rajagopal A, Holland SK. Evidence that neurovascular coupling underlying the BOLD effect increases with age during childhood. Hum Brain Mapp. 2015;36:1–15. doi: 10.1002/hbm.22608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seregi A, Keller M, Hertting G. Are cerebral prostanoids of astroglial origin? Studies on the prostanoid forming system in developing rat brain and primary cultures of rat astrocytes. Brain Res. 1987;404:113–120. doi: 10.1016/0006-8993(87)91361-8. [DOI] [PubMed] [Google Scholar]
- Sie L, Rombouts S, Valk J, Hart A, Scheltens P, van der Knaap M. Functional MRI of visual cortex in infants with or without periventricular leukomalacia. Dev Med Child Neurol. 2001;43:486–490. doi: 10.1017/s0012162201000895. [DOI] [PubMed] [Google Scholar]
- Singer D. Neonatal tolerance to hypoxia: a comparative-physiological approach. Comp Biochem Physiol A Mol Integr Physiol. 1999;123:221–234. doi: 10.1016/s1095-6433(99)00057-4. [DOI] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 2010;20:2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stichel CC, Müller CM, Zilles K. Distribution of glial fibrillary acidic protein and vimentin immunoreactivity during rat visual cortex development. J Neurocytol. 1991;20:97–108. doi: 10.1007/BF01279614. [DOI] [PubMed] [Google Scholar]
- Taga G, Asakawa K, Maki A, Konishi Y, Koizumi H. Brain imaging in awake infants by near-infrared optical topography. Proc Natl Acad Sci. 2003;100:107222–110727. doi: 10.1073/pnas.1932552100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Shirane R, Sato S, Yoshimoto T. Developmental changes of cerebral blood flow and oxygen metabolism in children. AJNR Am J Neuroradiol. 1999;20:917–922. [PMC free article] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Takata N, Nagai T, Ozawa K, Oe Y, Mikoshiba K, Hirase H. Cerebral blood flow modulation by basal forebrain or whisker stimulation can occur independently of large cytosolic Ca2+ signaling in astrocytes. PLoS One. 2013;8:e66525. doi: 10.1371/journal.pone.0066525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thein SL, Menzel S, Lathrop M, Garner C. Control of fetal hemoglobin: new insights emerging from genomics and clinical implications. Hum Mol Genet. 2009;18:R216–R223. doi: 10.1093/hmg/ddp401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian P, Teng IC, May LD, Kurz R, Lu K, Scadeng M, Hillman EMC, Crespigny AJ, De Arceuil HED, Mandeville JB, et al. Cortical depth-specific microvascular dilation underlies laminar differences in blood oxygenation level-dependent functional MRI signal. Proc Natl Acad Sci U S A. 2010;107:15246–15251. doi: 10.1073/pnas.1006735107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujiie H, Chaytor AT, Bakker LM, Griffith TM. Essential role of Gap junctions in NO- and prostanoid-independent relaxations evoked by acetylcholine in rabbit intracerebral arteries. Stroke. 2003;34:544–550. doi: 10.1161/01.str.0000054158.72610.ec. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Van Eden CG, Parnavelas JG, Kalsbeek A. The prenatal and postnatal development of rat cerebral cortex. In: Kolb B, Tees RC, editors. The Cerebral Cortex of the Rat. MIT Press; Cambridge, MA: 1990. pp. 36–76. [Google Scholar]
- Volpe JJ. Brain injury in the premature infant—from pathogenesis to prevention. Brain Dev. 1997;19:519–534. doi: 10.1016/s0387-7604(97)00078-8. [DOI] [PubMed] [Google Scholar]
- Vovenko E. Distribution of oxygen tension on the surface of arterioles, capillaries and venules of brain cortex and in tissue in normoxia: an experimental study on rats. Pflugers Arch. 1999;437:617–623. doi: 10.1007/s004240050825. [DOI] [PubMed] [Google Scholar]
- Wang DBB, Blocher NC, Spence ME, Rovainen CM, Woolsey TA. Development and remodeling of cerebral blood vessels and their flow in postnatal mice observed with in vivo videomicroscopy. J Cereb Blood Flow Metab. 1996;12:935–946. doi: 10.1038/jcbfm.1992.130. [DOI] [PubMed] [Google Scholar]
- Wang CL, Zhang L, Zhou Y, Zhou J, Yang XJ, Duan S, Xiong ZQ, Ding YQ. Activity-dependent development of callosal projections in the somatosensory cortex. J Neurosci. 2007;27:11334–11342. doi: 10.1523/JNEUROSCI.3380-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Homae F, Nakano T, Taga G. Functional activation in diverse regions of the developing brain of human infants. Neuroimage. 2008;43:346–357. doi: 10.1016/j.neuroimage.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Weber B, Keller AL, Reichold J, Logothetis NK. The microvascular system of the striate and extrastriate visual cortex of the macaque. Cereb Cortex. 2008;18:2318–2330. doi: 10.1093/cercor/bhm259. [DOI] [PubMed] [Google Scholar]
- Wheatley CM, Dickinson JL, Mackey DA, Craig JE, Sale MM. Retinopathy of prematurity: recent advances in our understanding. Arch Dis Child Fetal Neonatal Ed. 2002;87:F78–F82. doi: 10.1136/fn.87.2.F78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteus C, Freitas C, Grutzendler J. Perturbed neural activity disrupts cerebral angiogenesis during a postnatal critical period. Nature. 2014;505:407–411. doi: 10.1038/nature12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf T, Lindauer U, Villringer A, Dirnagl U. Excessive oxygen or glucose supply does not alter the blood flow response to somatosensory stimulation or spreading depression in rats. Brain Res. 1997;761:290–299. doi: 10.1016/s0006-8993(97)00354-5. [DOI] [PubMed] [Google Scholar]
- Yamada H, Sadato N, Konishi Y, Kimura K, Tanaka M, Yonekura Y, Ishii Y. A rapid brain metabolic change in infants detected by fMRI. Neuroreport. 1997;8:3775–3778. doi: 10.1097/00001756-199712010-00024. [DOI] [PubMed] [Google Scholar]
- Zaramella P, Freato F, Amigoni A, Salvadori S, Marangoni P, Suppjei A, Schiavo B, Chiandetti L. Brain auditory activation measured by near-infrared spectroscopy (NIRS) in neonates. Pediatr Res. 2001;49:213–219. doi: 10.1203/00006450-200102000-00014. [DOI] [PubMed] [Google Scholar]
- Zehendner CM, Tsohataridis S, Luhmann HJ, Yang JW. Developmental switch in neurovascular coupling in the immature rodent barrel cortex. PLoS One. 2013;8:e80749. doi: 10.1371/journal.pone.0080749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra WG, Buursma A, Meeuwsen-van der Roest WP. Absorption spectra of human fetal and adult oxyhemoglobin, de-oxyhemoglobin, carboxyhemoglobin, and methemoglobin. Clin Chem. 1991;37:1633–1638. [PubMed] [Google Scholar]
- Zuckerman SL, Armstead W, Hsu P, Shibata M, Leffler C. Age dependence of cerebrovascular response mechanisms in domestic pigs. Am J Physiol Heart Circ Physiol. 1996;271:H535–H540. doi: 10.1152/ajpheart.1996.271.2.H535. [DOI] [PubMed] [Google Scholar]