Abstract
Peritoneal dissemination (PD) of cancer is difficult to diagnose. Previous reports have shown that carbohydrate antigen 125 (CA125) is a sensitive marker of PD of gastric cancer. However, CA125 has not been evaluated as a marker of colorectal cancer (CRC), and its accuracy in men is controversial. The aim of this study was to compare the ability of CA125 and carcinoembryonic antigen (CEA) to predict PD of CRC in men and women.
Preoperative CA125 and CEA concentrations were measured in 853 people (510 men, 343 women) over 10 years. PD was confirmed intraoperatively in 57 patients. The predictive ability was compared between CA125 and CEA.
Compared with CEA, CA125 concentration had a lower sensitivity, higher specificity, and diagnostic accuracy, and significantly greater area under the curve. Further analysis of CA125's sensitivity and specificity among CEA-negative group (n = 514) showed acceptable sensitivity (57.1%) and good specificity (92.0%). In men and women, CA125 concentration did not increase with stage from I to IV unless PD was present (P < 0.001). CEA concentration was increased in women with metastasis with PD (P < 0.001) or without PD (P < 0.001), but was increased only in men with metastasis without PD (P < 0.01). CA125 concentration correlated with PD grade for men and women, but CEA concentration correlated with grade only in women.
When analyzed according to the primary tumor site, CA125 concentration in men did not differ between patients with the primary site in the right or left colon, or the rectum, regardless of PD status. By contrast, CA125 concentration differed between PD-positive and PD-negative patients with cancer in the right (P < 0.001) or left (P < 0.001) colon but not in the rectum. CEA concentration in men did not differ according to the primary site or PD status. In women, CA125 and CEA concentrations differed significantly between the PD-positive and PD-negative groups in patients with the primary tumor in the right (P < 0.001) or left (P < 0.001) colon; tumor sites did not differ between the PD-positive and PD-negative groups.
These findings suggest that CA125 is a better tumor marker than CEA for predicting PD of CRC in both men and women.
Keywords: CA125, CEA, colorectal cancer, peritoneal dissemination
1. Introduction
Peritoneal dissemination (PD) is one of the most troublesome metastases of colorectal cancer (CRC) and has a significant impact on the prognosis.[1] Imaging procedures, mainly computed tomography (CT), have insufficient sensitivity to help in the diagnosis of small PD lesions,[2] and PD is usually not diagnosed without an operation.
First reported by Bast et al in 1981, carbohydrate antigen 125 (CA125) is a sensitive tumor marker for ovarian cancer.[3] CA125 is found in mesothelial cells of the peritoneum, pleura, pericardium, epithelium of the fallopian tubes, endometrium, and endocervix.[4] Irritation of these foci in certain reactive or neoplastic lesions causes the serum CA125 concentration to increase. CA125 can also be detected in the tissue of gastrointestinal cancers, including stomach and colon cancer.[5] Previous reports have shown that CA125 is a sensitive marker of PD of gastric cancer.[6,7] We hypothesized that the mechanism underlying the linkage between CA125 and PD of gastric cancer may be similar to that of CRC.
It was recently reported that a high serum CA125 concentration is not a good predictor of gastrointestinal malignancy in men.[8] The patient's sex may be an issue in the interpretation of CA125 concentration, which is rarely evaluated in men. It is possible that the markers of PD may have different patterns in men and women because of the involvement of the genital organs, especially the ovary. Ovarian metastasis is specifically included in PD staging in the Japanese Classification of Colorectal Cancer (JCCRC).[9] The ovary produces abundant growth factors, whose levels may correlate with many markers.
In this study, we investigated whether CA125 concentration is a better predictor of PD of CRC than carcinoembryonic antigen (CEA) concentration and, if so, whether the predictive ability differs in men and women. We performed a retrospective analysis of a prospectively collected database of a large population. Because the primary tumor site can affect tumor behavior and prognosis,[10–12] we also compared CA125 concentration in patients with different primary tumor sites.
2. Methods
2.1. Patients
Between June 2000 and December 2010, 1077 consecutive patients underwent elective resection for CRC (adenocarcinoma or mucinous adenocarcinoma) by 2 surgeons (S-HY and J-KJ) at Taipei Veterans General Hospital. The Institutional Review Board of the Taipei Veterans General Hospital approved the study. After excluding patients with synchronous cancers besides CRC (n = 9), rectal cancer receiving neoadjuvant radiotherapy (n = 108), or with insufficient data (n = 107), the data for 853 patients were included. All patients had received standard preoperative evaluations, including CT of the chest and abdomen, colonoscopy, and biochemical analyses. A positron emission tomography (PET) scan was not included as a routine. The presence of PD was defined as any grossly visible peritoneal tumor seeding found during operative exploration. All PD cases were confirmed by pathology. Omental metastasis was also recognized as PD. In the analysis of primary tumor location, right-sided tumors were defined as those proximal to the splenic flexure and left-sided tumors as those distal to the splenic flexure. Rectal cancer was defined as a tumor within 15 cm of the anal verge. The PD grade was defined according to the JCCRC criteria as follows: P0, no peritoneal metastasis; P1, metastases to the adjacent peritoneum but not distant peritoneum; P2, a few metastases to the distant peritoneum; and P3, numerous metastases to the distant peritoneum.[9] Ovarian metastasis was classified as P2. The TNM staging of American Joint Committee on Cancer (AJCC) (7th ed.) was used for staging.[13] The sensitivity for diagnosing PD was compared between CT and CA125.
2.2. Assays
Peripheral blood samples obtained preoperatively for measurement of CEA and CA125 concentrations were sent within 30 days before surgery to the Department of Nuclear Medicine of this hospital. The concentrations of both tumor markers were measured by radioimmunoassay: CEA using a CEA-RIACT Kit (CIS bio international, Gif-sur-Yvette, France) and CA125 using a 125I IRMA Kit (DiaSorin, Stillwater, MN). A CEA concentration ≥5 ng/mL and CA125 concentration ≥35 U/mL were defined as abnormal.
2.3. Statistical analyses
An independent t test was used to compare 2 independent groups, and 1-way analysis of variance was performed for multiple comparisons with the post hoc Bonferroni test. Pearson chi-squared test was used to compare categorical variables. The area under the receiver-operating characteristic curve (AUC) was analyzed using the method of Hanley and McNeil (Intercooled Stata 9.2 for Windows, StataCorp LP, College Station, TX).[14] A P value < 0.05 was considered to be significant.
3. Results
The distributions of clinicopathological factors in men and women are shown in Table 1. This study included data for 510 men and 343 women, whose median age was 66.6 years (range 26.5–94.4). The median CA125 concentration was 12.1 U/mL (range 0.1–1503) and CEA concentration was 3.5 ng/mL (0.1–3539). The tumor locations were as follows: 286 in the right side of the colon, 363 in the left side of the colon, and 204 in the rectum. The AJCC staging was 169 with Stage I disease, 258 with Stage II disease, 245 with Stage III disease, and 181 with Stage IV disease. There were 57 patients with PD (27 men and 30 women) in the Stage IV group. The locations of the metastases were as follows: 117 in the liver, 37 in the lung, 43 in distant nodes, and 7 in other sites. The distributions of all of these factors did not differ between men and women. In the PD group, there were 27 patients with pure PD and 30 with extra-PD metastases. Table 2 shows that the presence of PD correlated significantly with all types of extra-PD metastases.
Table 1.
Demographic distribution according to genders.
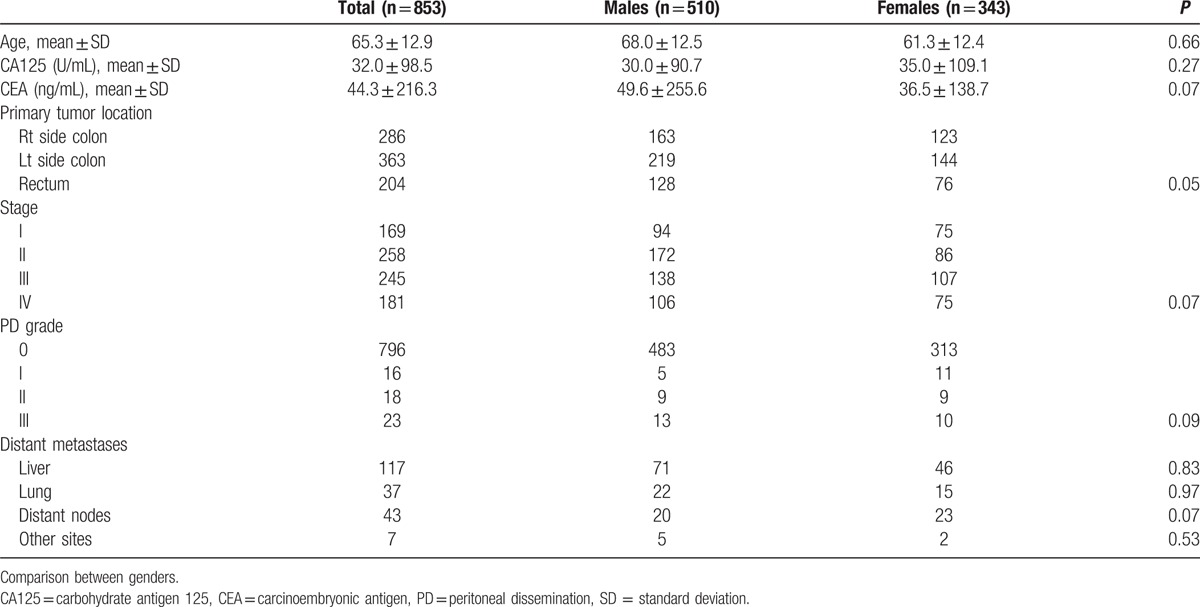
Table 2.
Metastasis site distribution according to peritoneal dissemination.
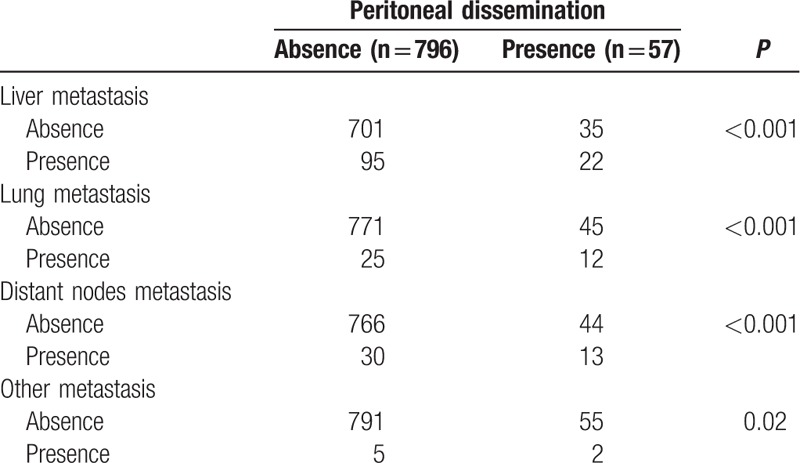
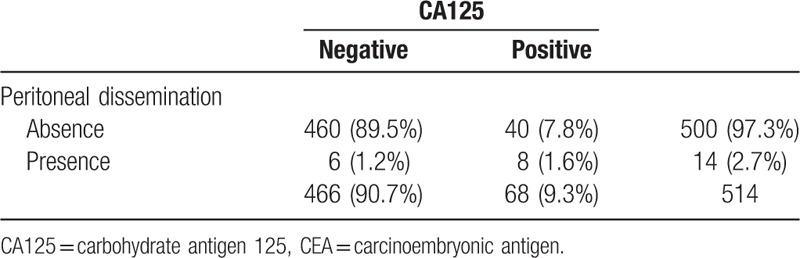
Table 3 shows the comparisons of CA125 and CEA concentrations for predicting PD. CA125 concentration had a lower sensitivity, higher specificity, and higher diagnostic accuracy rate. Comparison of the AUCs showed that CA125 concentration had better coverage than CEA concentration (P < 0.01), which suggested that CA125 concentration is a better predictor of PD than is CEA concentration. Further analysis of CA125's sensitivity and specificity among CEA-negative group (n = 514) was shown in Table 4. The sensitivity was 57.1%, specificity 92.0%.
Table 3.
Diagnostic ability of CA125 and CEA for peritoneal dissemination.
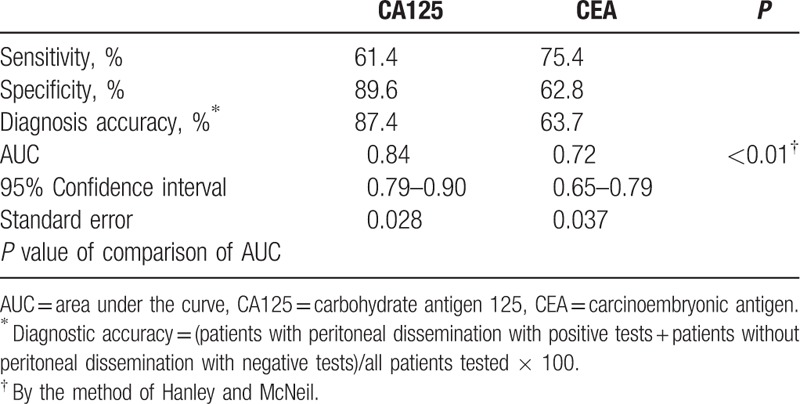
Table 4.
Diagnostic ability of CA125 among CEA-negative group (n = 514) for peritoneal dissemination.
The distributions of CA125 and CEA concentrations according to tumor stages for both men and women are shown in Fig. 1. In men (Fig. 1A), CA125 concentration did not increase significantly from Stage I to IV (without PD) unless PD was present (P < 0.001) CEA concentration was increased significantly only in patients with Stage IV without PD (P < 0.01). CEA concentration was not significantly higher in patients with PD compared with patients without metastases. In women (Fig. 1B), CA125 concentration was significantly increased in those with PD (P < 0.001), as observed for men. In patients with Stage IV disease, both patients with PD (P < 0.001) and without PD (P < 0.001) had a significantly higher CEA concentration compared with patients without metastases. CEA concentration did not differ between patients with and without PD.
Figure 1.
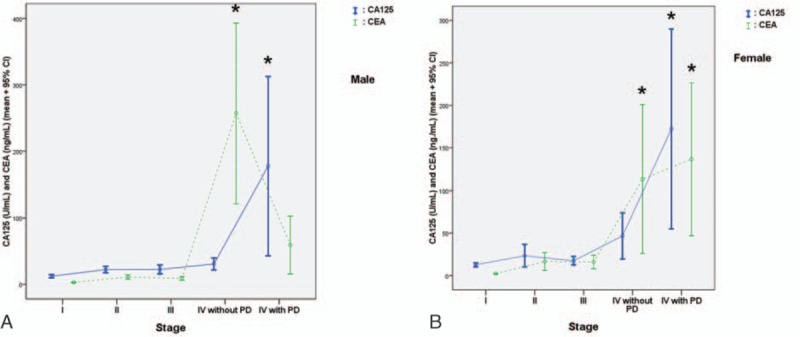
CA125 and CEA concentrations grouped according to tumor stage in men (A) and women (B). The asterisk indicates a significant difference compared with the nonmetastases group. CA125 = carbohydrate antigen 125, CEA = carcinoembryonic antigen, PD = peritoneal dissemination.
For the analysis of the association between CA125 and CEA concentrations and PD grade, because of the small number of cases, we combined grades P1 and P2 (P1 + P2). Figure 2A shows that, in men, CA125 concentration increased with increasing grade and that the difference was significant between each grade. By contrast, CEA concentration did not differ according to grade. Figure 2B shows a similar pattern for CA125 concentration in women as in men. CEA concentration was higher in patients with P3 and the combined P1 + P2 group compared with those with P0 but did not differ significantly between the P3 and P1 + P2 groups.
Figure 2.
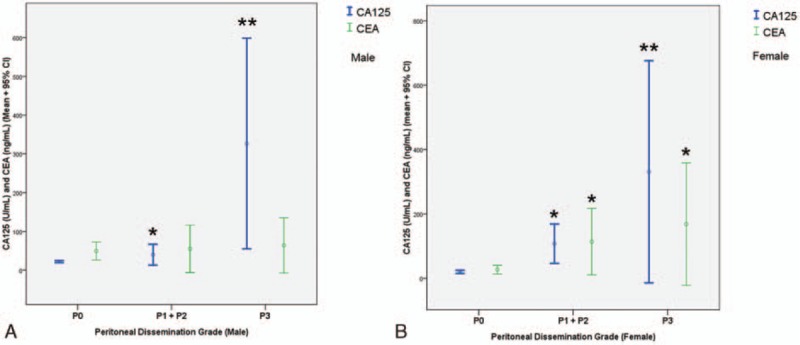
CA125 and CEA concentrations grouped according to grade of peritoneal dissemination. The asterisk indicates a significant difference compared with the P0 group (P < 0.05); the double asterisk indicates a significant difference compared with P0 and the combined P1 + P2 group (P < 0.05). CA125 = carbohydrate antigen 125, CEA = carcinoembryonic antigen.
CA125 and CEA concentrations were analyzed further according to the primary tumor site for men and women. Figure 3A shows that, in men, CA125 concentration did not differ according to tumor location (right side of the colon, left side of the colon, and rectum) in patients with or without PD. By contrast, CA125 concentration differed between the PD-positive and PD-negative groups in the patients with the primary tumor located in the right (P < 0.001) or left (P < 0.001) colon, but not in the rectum. CEA concentration did not differ according to tumor site in patients with or without PD, and did not differ between the PD-positive and PD-negative groups at any tumor site (Fig. 3B).
Figure 3.
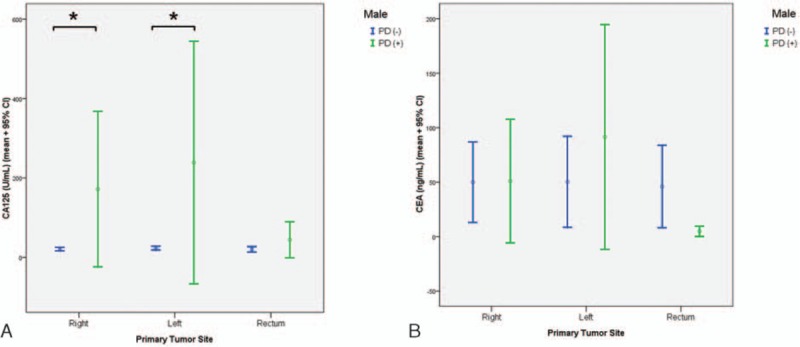
CA125 (A) and CEA (B) concentrations in PD-negative and PD-positive patients grouped according to the 3 primary tumor sites in men. The asterisk indicates a significant difference between the PD-positive and PD-negative groups at a given tumor site. CA125 = carbohydrate antigen 125, CEA = carcinoembryonic antigen, PD = peritoneal dissemination.
CA125 concentration in women showed a similar pattern as in men: significant difference between the PD-positive and PD-negative groups in patients with the primary tumor in the right (P < 0.001) or left colon (P < 0.001). The tumor sites did not differ between the PD-positive and PD-negative groups (Fig. 4A). The analysis was not conducted for the rectum because there was only 1 patient with a PD-positive tumor in the rectum. CEA concentration showed a similar pattern as for CA125 concentration in women: a significant difference between PC-positive and PD-negative in patients with the primary tumor in the right (P < 0.01) or left (P < 0.01) colon (Fig. 4B).
Figure 4.
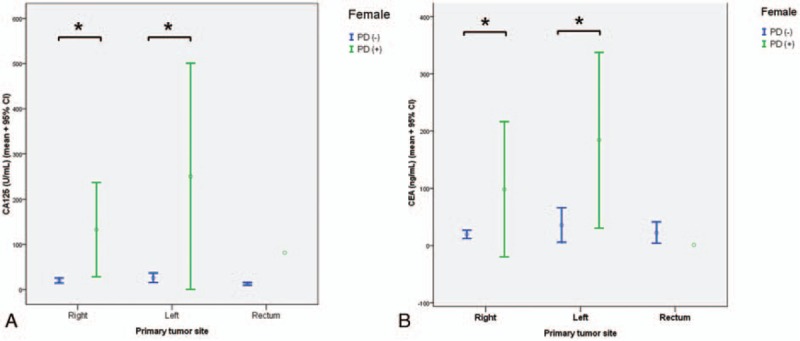
CA125 (A) and CEA (B) concentrations in PD-negative and PD-positive patients grouped according to the 3 primary tumor sites in women. The asterisk indicates a significant difference between the PD-positive and PD-negative groups at a given tumor site. CA125 = carbohydrate antigen 125, CEA = carcinoembryonic antigen, PD = peritoneal dissemination.
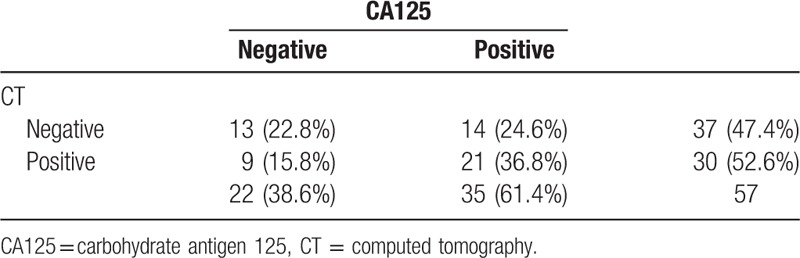
The sensitivity of CT and CA125 concentration for diagnosing PD was compared (Table 5). The sensitivity of CA125 concentration (61.4%) was slightly higher than that of CT (52.6%).
Table 5.
Diagnostic sensitivity of CA125 and CT for peritoneal dissemination.
4. Discussion
In this study, CA125 concentration correlated with the severity of PD and was a better predictive marker of PD in both men and women. The primary tumor site had no effect on the predictive ability of CA125 concentration. To our knowledge, this is the first study to show that CA125 concentration may be useful for predicting PD of CRC and to compare this in men and women. The ability to predict PD is consistent with findings of previous studies of gastric cancer. Our data suggest that CA125 concentration should be included in the pretreatment evaluation to detect PD, especially when a deep T stage is suspected clinically.
These findings provide clinicians a reference marker for predicting and managing the possibility of PD in patients with CRC. The sensitivity of CT (52.6%) for detecting PD in this series was less than that of CA125 (61.4%). The main difference was because of the inferior ability of CT to detect early PD in grade I lesions (data not shown). Both the Peritoneal Surface Disease Severity Score study by American Society of Peritoneal Surface Malignancies,[15] and Peritoneal Cancer Index study by Faron et al showed that PD severity correlated strongly with the prognosis in patients receiving hyperthermic intraperitoneal chemotherapy.[16] Aggressive treatments for early PD cases would obtain better result. Based on the performance of CA125 concentration in our study, we think measurement of this marker may help in the early diagnosis and planning for optimum treatment.
Some studies have reported on an association between CA125 concentration and liver metastasis of CRC.[17,18] In our series, patients with liver metastasis without PD did not have a higher CA125 concentration compared with other groups of patients (data not shown). It is believed that CA125 concentration may be related to PD in a few very specific cases of liver metastasis, but not in the analysis of the whole group. In our study, the higher diagnostic accuracy of CA125 concentration related mainly to its high specificity, even though its sensitivity was lower than that of CEA concentration. Among CEA-negative group, CA125 still has acceptable sensitivity (57.1%) and good specificity (92.0%). The higher specificity in the whole group leads us to believe that CEA concentration should remain the principle marker for evaluating CRC.
We need to explain the Stage IV cases in this series. Of the 181 patients with Stage IV disease who received resections, 57 had PD and 124 did not. Among the PD-negative group, 14 patients had received a diversion stoma because of prior acute obstruction, 53 received simultaneous metastasectomy and achieved R0 resection, and 57 had subacute bleeding or obstruction complications, or staged resection under the concern of potentially convertibly resectable metastasis. Among the PD-positive group, 17 patients had received a diversion stoma because of prior acute obstruction, 11 had pure PD metastasis that was not diagnosed before the operation, 8 received simultaneous metastasectomy including all PD lesions, and 21 had subacute bleeding or obstruction complications. Most of the primary tumor resections were performed on the basis of total tumor removal with metastasectomy or because of the presence of tumor complications. Primary tumor resection has been reported to have survival benefit even with unresectable metastasis[19,20] or PD.[21] In our series, the use of a multidisciplinary team, which planned CRC treatment through weekly regular group meetings, was begun in October 2007. Aggressive and high-quality treatments involving intensive cooperation has conferred significant survival benefit for patients with Stage IV disease.[22]
The primary tumor site does not appear to change the predictive ability of CA125 concentration. Its good performance was seen in patients with the primary tumor in the right or left side of the colon. Because we excluded patients who received irradiation for rectal cancer in this series, there were too few cases of rectal cancer to include in our analyses, and the performance of CA125 concentration in predicting PD of rectal cancer remains inconclusive.
CA125 concentration has been studied rarely in males. This study shows that its concentration may also increase in men with PD. It is intriguing that the tumor markers differed slightly between men and women. The main difference was that the CEA concentration in the PD-positive men did not increase correspondingly with CA125 concentration, as observed in the PD-positive women. We thought that men with PD should also have a higher CEA concentration than the nonmetastasis group because there was significant proportion of synchronous extra-PD metastases in the men. A possible explanation is the number of patients in the PD-positive group, which is an important shortcoming of this study.
The shortcomings of this study are the smaller PD group, its use of data collected at a single institution, and the retrospective design covering a period of 10 years. Many cases of rectal cancer were excluded because of neoadjuvant radiotherapy, which would cause collection bias. CA125 concentration was measured with preoperative CT but not with PET scans, which may have improved the sensitivity and merits further study.
In conclusion, CA125 concentration seems to be a better tumor marker than CEA concentration for predicting PD in CRC in both men and women. This finding suggests that CA125 concentration should be measured as part of the pretreatment evaluation. Early detection that leads to a well-prepared therapy with curative intent might improve the prognosis of CRC patients with PD.
Acknowledgments
The authors thank Ms Hui-Chen Lee of Division of Experimental Surgery, Taipei-Veterans General Hospital for her valuable assistance in statistical analysis.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, AUC = area under the curve, CA125 = carbohydrate antigen 125, CEA = carcinoembryonic antigen, CRC = colorectal cancer, CT = computed tomography, JCCRC= Japanese Classification of Colorectal Cancer, PD = peritoneal dissemination, PET = positron emission tomography, ROC = received operating characteristic.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Hotokezaka M, Jimi S, Hidaka H, et al. Factors influencing outcome after surgery for stage IV colorectal cancer. Surg Today 2008;38:784–9. [DOI] [PubMed] [Google Scholar]
- [2].Archer AG, Sugarbaker PH, Jelinek JS. Radiology of peritoneal carcinomatosis. Cancer Treat Res 1996;82:263–88. [DOI] [PubMed] [Google Scholar]
- [3].Bast RC, Jr, Feeney M, Lazarus H, et al. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest 1981;68:1331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kabawat SE, Bast RC, Jr, Bhan AK, et al. Tissue distribution of a coelomic-epithelium-related antigen recognized by the monoclonal antibody OC125. Int J Gynecol Pathol 1983;2:275–85. [DOI] [PubMed] [Google Scholar]
- [5].Streppel MM, Vincent A, Mukherjee R, et al. Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum Pathol 2012;43:1755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nakata B, Hirakawa YSCK, Kato Y, et al. Serum CA 125 level as a predictor of peritoneal dissemination in patients with gastric carcinoma. Cancer 1998;83:2488–92. [DOI] [PubMed] [Google Scholar]
- [7].Haga Y, Sakamoto K, Egami H, et al. Clinical significance of serum CA125 values in patients with cancers of the digestive system. Am J Med Sci 1986;292:30–4. [DOI] [PubMed] [Google Scholar]
- [8].Zhu X, Zhou W, Chen Y, et al. High serum carbohydrate antigen 125 concentration can predict serous effusion but not gastrointestinal malignancy in male patients. Tumour Biol 2014;35:5129–35. [DOI] [PubMed] [Google Scholar]
- [9].Japanese Society for Cancer of Colon and Rectum. Japanese Classification of Colorectal Carcinoma. 2nd English ed. Tokyo: Kanehara & Co. Ltd; 2009. [Google Scholar]
- [10].Bauer KM, Hummon AB, Buechler S. Right-side and left-side colon cancer follow different pathways to relapse. Mol Carcinog 2012;51:411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lu HJ, Lin JK, Chen WS, et al. Primary tumor location is an important predictive factor for wild-type KRAS metastatic colon cancer treated with cetuximab as front-line bio-therapy. Asia Pac J Clin Oncol 2016;12:207–15. [DOI] [PubMed] [Google Scholar]
- [12].Price TJ, Beeke C, Ullah S, et al. Does the primary site of colorectal cancer impact outcomes for patients with metastatic disease? Cancer 2015;121:830–5. [DOI] [PubMed] [Google Scholar]
- [13].Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. American Joint Committee on Cancer—AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- [14].Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982;143:29–36. [DOI] [PubMed] [Google Scholar]
- [15].Esquivel J, Piso P, Verwaal V, et al. American Society of Peritoneal Surface Malignancies opinion statement on defining expectations from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with colorectal cancer. J Surg Oncol 2014;110:777–8. [DOI] [PubMed] [Google Scholar]
- [16].Faron M, Macovei R, Goere D, et al. Linear relationship of peritoneal cancer index and survival in patients with peritoneal metastases from colorectal cancer. Ann Surg Oncol 2016;23:114–9. [DOI] [PubMed] [Google Scholar]
- [17].Mavligit GM, Estrov Z. CA 125: a clinically useful tumor marker in the management of colorectal carcinoma metastatic to the liver in patients with normal carcinoembryonic antigen. Am J Clin Oncol 2000;23:213–5. [DOI] [PubMed] [Google Scholar]
- [18].Zhang D, Yu M, Xu T, et al. Predictive value of serum CEA, CA19-9 and CA125 in diagnosis of colorectal liver metastasis in Chinese population. Hepatogastroenterology 2013;60:1297–301. [DOI] [PubMed] [Google Scholar]
- [19].Ishihara S, Hayama T, Yamada H, et al. Prognostic impact of primary tumor resection and lymph node dissection in stage IV colorectal cancer with unresectable metastasis: a propensity score analysis in a multicenter retrospective study. Ann Surg Oncol 2014;21:2949–55. [DOI] [PubMed] [Google Scholar]
- [20].Kim MS, Chung M, Ahn JB, et al. Clinical significance of primary tumor resection in colorectal cancer patients with synchronous unresectable metastasis. J Surg Oncol 2014;110:214–21. [DOI] [PubMed] [Google Scholar]
- [21].Furuhata T, Okita K, Nishidate T, et al. Oncological benefit of primary tumor resection with high tie lymph node dissection in unresectable colorectal cancer with synchronous peritoneal metastasis: a propensity score analysis of data from a multi-institute database. Int J Clin Oncol 2015;20:922–7. [DOI] [PubMed] [Google Scholar]
- [22].Lan YT, Jiang JK, Chang SC, et al. Improved outcomes of colorectal cancer patients with liver metastases in the era of the multidisciplinary teams. Int J Colorectal Dis 2016;31:403–11. [DOI] [PubMed] [Google Scholar]


