Abstract
Background:
Direct antiviral agent (DAA) has been the standard of care for patients with hepatitis C virus (HCV) infection. Twelve weeks of paritaprevir/ritonavir/ombitasvir plus dasabuvir (PROD) with or without ribavirin has shown to have a sustained virological response at post-treatment 12 weeks (SVR12) rate of >90% in HCV genotype 1 (HCV-1) patients.
Methods:
We report a HCV-1b patient who received only 25 days of PROD treatment.
Results:
The patient early terminated treatment due to dengue fever but eventually achieved SVR12. It may attribute to low baseline viral loads and extraordinarily rapid suppression of HCV after treatment day1.
Conclusions:
The finding may shed light for possible response-guided-therapy for so-called ultra-super-responders in the DAA era. Whether the dengue virus, the Flaviviridae family as with HCV, enhanced the HCV clearance remains unclear and needs further exploration.
Keywords: DAA, dengue fever, HCV
1. Introduction
Direct antiviral agent (DAA) has been the standard of care for patients with hepatitis C virus (HCV) infection. The current strategy has moved to interferon-free regimens. The mainstream treatment duration are 12 to 24 weeks depending on regimens and patient characteristics.[1] Paritaprevir/ritonavir/ombitasvir plus dasabuvir (PROD) with or without ribavirin has shown to have a sustained virological response at post-treatment 12 weeks (SVR12) rate of >90% in HCV genotype 1 (HCV-1) patients. In addition to the development of pangenotypic, high potency DAAs, efforts now are made trying to abbreviate treatment duration to 4 to 8 weeks without compromising efficacy. We herein report an HCV-1b patient who received only 25 days of PROD treatment. The patient early terminated treatment due to dengue fever but eventually achieved SVR12.
2. Patient information, therapeutic Intervention, follow-up, and outcome
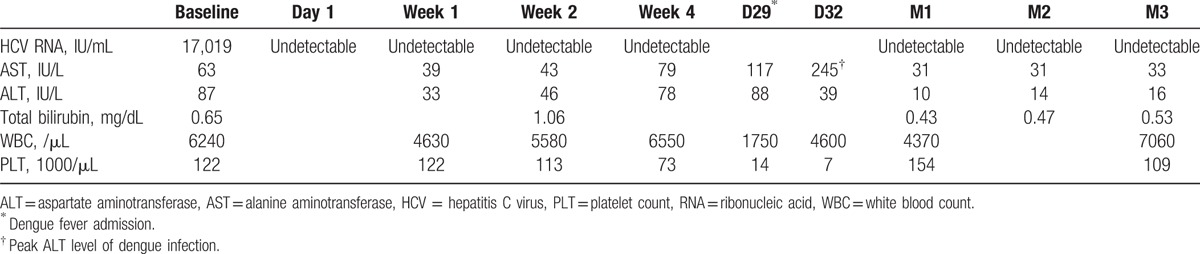
The 81-year-old female had the history of chronic HCV infection for >30 years and co-morbidity of depressive disorder and dementia under regular treatment with Quetiapine 50 mg/day. Due to fear of adverse events and underlying psychiatric disorder, she was ineligible to pegylated interferon/ribavirin, the current reimbursed regimen in Taiwan. The genotype and pretreatment viral loads was HCV-1b and 17,019 IU/mL (real-time HCV; Abbott Molecular, Des Plaines IL; detection limit: 12 IU/mL). The sonography revealed heterogeneous liver parenchyma without splenomegaly and visible collateral vessels. The result of the acoustic radiation force impulse (ARFI) was 1.3 m/s, which corresponded to fibrosis stage 2.[2] Daily fixed-dose combination of paritaprevir/ritonavir/ombitasvir (150 mg/100 mg/25 mg) plus twice-daily dasabuvir (250 mg) was administered for the treatment of chronic HCV infection. Quetiapine was held transiently before antiviral therapy to avoid drug–drug interactions. The HCV ribonucleic acid (RNA) became rapidly undetectable on day 1, followed by day 7 and day 14 after dosing. Unfortunately, she was admitted due to fever, progressive thrombocytopenia, and elevated liver enzymes. Dengue fever infection with dengue virus genotype 2 (SYBR Green-based quantitative RT-PCR) infection was impressed on day 25 of treatment. She received supportive treatment with antipyretics (paracetamol 500 mg/time as needed) and intravenous fluids supply during hospitalization. PROD were terminated due to severe thrombocytopenia (7000/mm3). The patient discharged after 7 days supportive inpatient care for dengue fever. Dramatically, the serial testing of serum HCV RNA showed that the patient achieved SVR4, SVR8, and SVR12 (Table 1). The patients provided written informed consent. The institutional review board in Kaohsiung Medical University Hospital approved the survey.
Table 1.
Serial change of laboratory data and HCV RNA level of the patient.
3. Discussion
To our knowledge, this is the first report of DAA prescription that encountered dengue fever, and the patient benefited from HCV eradication only by 4 weeks of all oral DAA regimens. The current evidence support 12 to 24 weeks of interferon-free DAAs in treating CHC.[1] An 8-week sofosbuvir/ledipasvir is recommended for naive, noncirrhotic HCV-1 patients with baseline viral loads <8 million IU/mL.[3] As observed in a phase 2b study that 8 weeks of PROD plus ribavirin had very high SVR rates for naive, noncirrhotic patients of HCV-1b (96%, 23/24) or with low viral loads (<800,000 IU/mL, 100%, 9/9).[4] The patient did not take any drug with potential drug–drug interaction with PROD. The achievement of SVR to only 25 days of PROD in this patient may attribute to HCV-1b with low baseline viral loads and extraordinarily rapid suppression of HCV after treatment Day 1. As with interferon-based therapy, the finding may shed light for possible response-guided-therapy for so-called ultra-super-responders in the DAA era.[5] The strategy may be particular imperative in resource constrained countries. Notably, viral interference might exist in the same host. There are reciprocal suppressions between hepatitis B virus and HCV infections.[6,7] Whether the dengue virus, the Flaviviridae family as with HCV, enhances the HCV clearance remains unclear and needs further exploration.
Footnotes
Abbreviations: DAA = direct antiviral agents, HCV = hepatitis C virus, PROD = paritaprevir/ritonavir/ombitasvir/dasabuvir, SVR = sustained virological response.
The authors have no funding and conflicts of interest to disclose.
References
- [1].AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 2015;62:932–54. [DOI] [PubMed] [Google Scholar]
- [2].Rizzo L, Calvaruso V, Cacopardo B, et al. Comparison of transient elastography and acoustic radiation force impulse for non-invasive staging of liver fibrosis in patients with chronic hepatitis C. Am J Gastroenterol 2011;106:2112–20. [DOI] [PubMed] [Google Scholar]
- [3].Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014;370:1879–88. [DOI] [PubMed] [Google Scholar]
- [4].Kowdley KV, Lawitz E, Poordad F, et al. Phase 2b trial of interferon-free therapy for hepatitis C virus genotype 1. N Engl J Med 2014;370:222–32. [DOI] [PubMed] [Google Scholar]
- [5].Yu ML, Chuang WL. New treatments for HCV: perspective from Asia. Clin Liver Dis 2015;5:17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yu ML, Dai CY, Huang CF, et al. High hepatitis B virus surface antigen levels and favorable interleukin 28B genotype predict spontaneous hepatitis C virus clearance in uremic patients. J Hepatol 2013;60:253–9. [DOI] [PubMed] [Google Scholar]
- [7].Huang CF, Dai CY, Lee JJ, et al. Hepatitis C viremia interferes serum hepatitis B virus surface antigen and DNA levels in hepatitis B uremics. Hepatol Int 2014;8:224–32. [DOI] [PubMed] [Google Scholar]


