Abstract
The aim of this study was to investigate the role of uric acid (UA) in assessing rectal cancer metastasis.
There were 475 newly diagnosed patients with complete data in our study, a total of 475 cases were reviewed, and divided into patients with metastasis and without metastasis.
There were several statistical differences in age, tumor diameter, carcino-embryonic antigen (CEA), and C-reactive protein (CRP) between the 2 groups. Importantly, serum concentrations of UA in patients with lymphatic metastasis were found to be increased compared with patients without lymphatic metastasis (270.9 ± 52.99 vs 215.8 ± 43.55; P < 0.001). There were positive correlations of serum UA with creatinine (Cr), CRP, and CEA (r = 0.281, P = 0.023; r = 0.312, P = 0.001; r = 0.294, P = 0.017) in rectal cancer patients with metastasis. Multivariate analysis model revealed that elevated serum levels of UA were significant prognostic marker for lymphatic metastasis in patients with rectal cancer, independently of CRP, CEA, and tumor diameter (odds ratio 1.035, 95% CI 1.013–1.057, P = 0.002). In receiver-operating characteristic curve analysis, the area under the curve of serum UA in assessing metastatic rectal cancer patients was 0.803, with sensitivity of 0.864 and specificity of 0.739.
Our results suggest that serum UA may be a novel marker in assessing tumor metastasis in patients with rectal cancer.
Keywords: carcino-embryonic antigen, rectal cancer, serum uric acid, tumor metastasis
1. Introduction
Colorectal cancer is the third most common noncutaneous malignancy, and accounts for the second most frequent cause in cancer-related deaths.[1] Oncologists have been aware that, compared with colon tumor, the diagnosis, staging and, treatment for rectal cancer have significant difference. Fortunately, local control and survival rate in colorectal cancer has been significantly improved with the improvement of operation and chemotherapy.[2–4] In clinical laboratory, the levels of carcino-embryonic antigen (CEA) have been severed as an independent prognostic factor in rectal cancer patients.[5] Several inflammatory biomarkers are valuable and easily available for the assessment of prognosis in patients with rectal cancer such as tumor necrosis factor (TNF), interleukin (IL)-6, and C-reactive protein (CRP).[6] Moreover, CRP has been involved with recurrence and prognosis in patients with rectal cancer.[7] However, other laboratory markers also have been needed to monitoring tumor recurrence, metastasis, and prognosis.
Uric acid (UA) is the product of purine metabolism in the body. In the past, we have focused on the relationship between UA and gout. Recently, serum UA was found to be associated with various diseases, such as cardiovascular disease, acute ischemic stroke, and lung cancer.[8–10] Increased serum UA concentrations were associated with mortality of cardiovascular disease.[11] A cross-sectional study found that elevated UA concentrations were not an independent risk factor of colorectal adenoma; it might be considered as a risk indicator for metabolic syndrome-related colorectal adenoma.[12] In fact, serum UA has been regarded to be an antioxidant in the body, and presents a compensatory mechanism against inflammation.[13] Adenoma is a developmental stage of rectal cancer, which is a progress from the adenoma-carcinoma sequence to invasive cancer, and inflammation may play a key role in metastasis of rectal cancer.[14] Considering this, we tend to provide a hypothesis that rectal cancer metastasis may be related to serum UA in patients with rectal cancer. Accordingly, the aim of this study was to investigate the role of UA in assessing rectal cancer metastasis.
2. Methods
2.1. Participants and materials
All patients who were diagnosed with rectal cancer at the First College of Clinical Medical Science, China Three Gorges University and Yichang Central People's Hospital during the period 2013 to 2016 were selected from a prospective colorectal cancer database. A total of 569 potential cases with rectal cancer were considered as candidates; finally, there were 475 newly diagnosed patients with complete data in our study. Exclusion criteria included hyperuricemia, gout, cardiovascular disease, hypertension, diabetes, infectious disease, hepatic or renal insufficiency, and other malignancy.
2.2. Data collection
We reviewed electronic patient records retrospectively; clinical and laboratory data were extracted, including sex, age, imaging reports, histopathological records, total protein (TP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (Cr), urea nitrogen (UN), CEA, CRP, erythrocyte sedimentation rate (ESR), and UA. These laboratory parameters were evaluated when patients with rectal cancer experienced first laboratory tests in our hospital. The study was approved by the Ethics Committee of The First College of Clinical Medical Science, China Three Gorges University and Yichang Central People's Hospital, and all patients provided informed consent.
2.3. Statistical analysis
All data were analyzed by using SPSS16.0 (SPSS Inc., Chicago, IL). Continuous variables were shown as mean ± standard deviation (SD), and categorical variables as percentages. Before analysis, Kolmogorov–Smirnov test was conducted to identify variable normality. Continuous variables with normal distribution were analyzed by independent-sample t test, and non-normal distribution data were compared by rank-sum test. Correlation analysis was carried out by Spearman approach. Further, multiple logistic regression analysis was used to analyze factors possibly associated with rectal cancer metastasis. The receiver-operating characteristic (ROC) curve was used to ascertain the sensitivity and specificity of serum UA as an estimator of rectal cancer metastasis. P < 0.05 was determined as statistically significant.
3. Results
Clinical and laboratory data of this study are given in Table 1. A total of 475 cases were reviewed, and divided into patients with metastasis and without metastasis. No patient received radiotherapy or chemotherapy before entering this study. There were several statistical differences in age, tumor diameter, CEA, and CRP between the 2 groups. Importantly, serum concentrations of UA in patients with lymphatic metastasis were found to be increased compared with patients without lymphatic metastasis (270.9 ± 52.99 vs 215.8 ± 43.55; P < 0.001).
Table 1.
Demographic and laboratory characteristics in metastatic nonmetastatic rectal cancer patients.
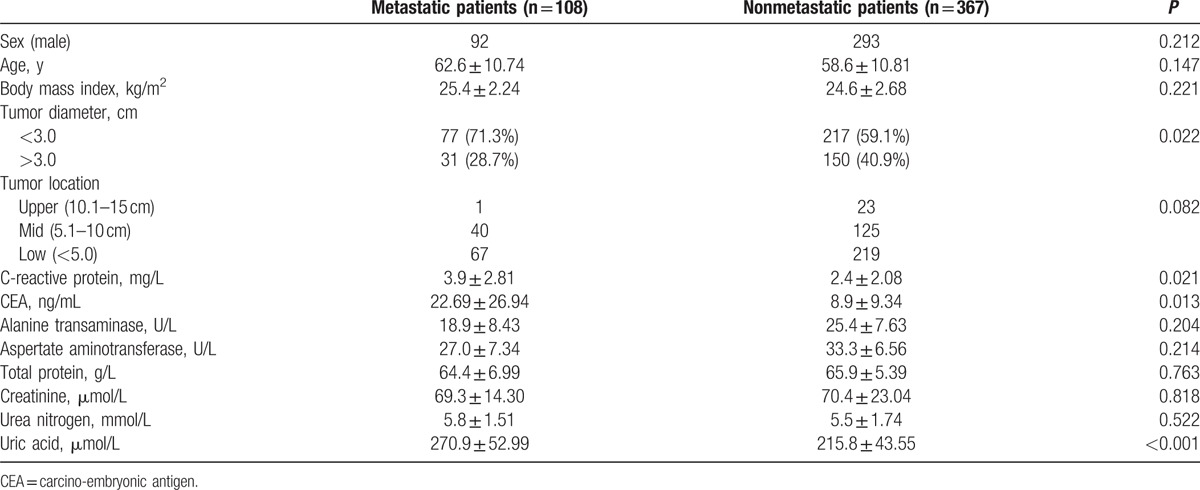
Among these cases, correlation analysis showed that serum UA concentrations were positively correlated with Cr, UN, CRP, and CEA (r = 0.327, p < 0.001; r = 0.298, P = 0.018; r = 0.305, P = 0.002; r = 0.217, P = 0.038) in all patients with rectal cancer. In addition, there were positive correlations of serum UA with Cr, CRP, and CEA (r = 0.281, P = 0.023; r = 0.312, P = 0.001; r = 0.294, P = 0.017) in rectal cancer patients with metastasis. However, the correlations between increased serum UA concentrations and CRP or CEA were not found in rectal cancer patients without metastasis.
After extensive univariate analysis, in comparison between the 2 groups, some significant variables that might be associated with tumor metastasis, to exclude other factors that might influence the association between UA and tumor metastasis in rectal cancer patients, all variables were included in the multivariable analysis to identify whether serum UA levels were related with tumor metastasis in rectal cancer patients. Therefore, age, sex, body mass index, tumor diameter, tumor location, ALT, AST, TP, Cr, UN, CRP, and CEA were included in multiple logistic regression analysis, and the results found that increase in CRP and CEA concentrations was associated with metastasis, and tumor diameter also was related to lymphatic metastasis in patients with rectal cancer. Interesting, multivariate analysis model revealed that elevated serum levels of UA were significant prognostic marker for lymphatic metastasis in patients with rectal cancer, independently of CRP, CEA, and tumor diameter (odds ratio [OR] 1.035, 95% confidence interval [CI] 1.013–1.057, P = 0.002; Table 2). ROC curve, shown in Fig. 1, was performed to estimate performance in identifying metastasis in rectal cancer patients; the area under the curve of serum UA in assessing metastatic rectal cancer patients was 0.803, with sensitivity of 0.864 and specificity of 0.739.
Table 2.
Some factors associated with metastatic rectal cancer patients in multiple logistic regression analysis.
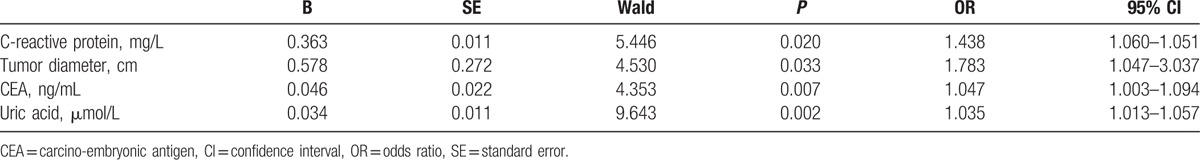
Figure 1.
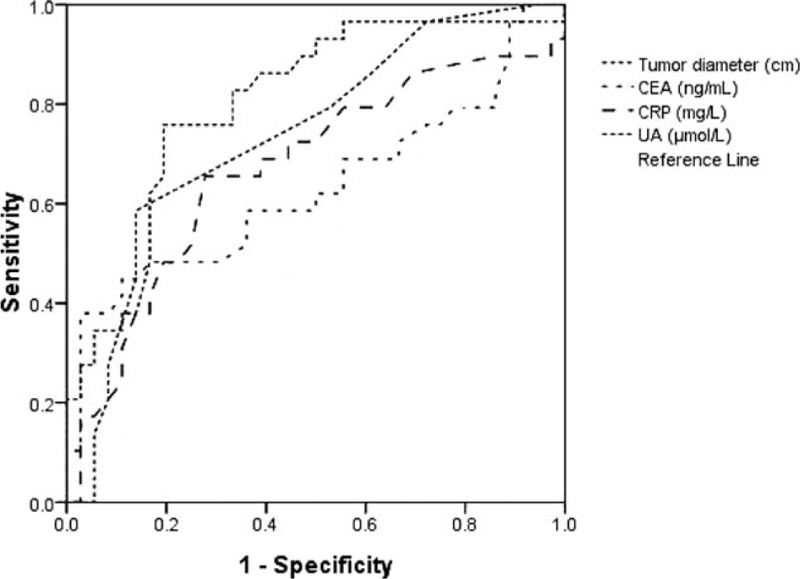
Receiver-operating characteristic (ROC) curve of tumor diameter, CEA, CRP, and UA in identifying metastatic rectal cancer patients. CEA = carcino-embryonic antigen, CRP = C-reactive protein, UA = uric acid.
4. Discussion
In this study, we examined pretreatment serum UA level in rectal cancer patients. We found that serum UA, CEA, and CRP was increased in rectal cancer patients with metastasis compared with those without metastasis, and higher serum UA levels were associated with metastatic rectal cancer patients in multiple logistic regression analysis. However, we did not find the association between tumor location and tumor metastasis in patients with rectal cancer.
A large prospective study suggested that higher serum UA levels are associated with the outcome of more serious prognostic indication in patients with cancer.[15] Serum UA level has been considered as an independent predictor of the prognosis in some cancer such as esophageal squamous cell carcinoma, nasopharyngeal carcinoma, and oral squamous cell carcinoma.[16–18] In addition, higher serum UA concentrations have been observed in cancer patients since nucleic acid turnover in proliferating diseased tissue.[19] Indeed, serum UA concentrations have been demonstrated to be related to inflammation and oxidative stress in some non-neoplastic diseases.[20,21] Mori et al[22] found that serum levels of CRP and CEA were biological markers in the prognosis of patients with colorectal cancer. To our knowledge, there is no study that directly confirmed the association between serum UA level and tumor metastasis in patients with rectal cancer. In the present study, we observed that serum UA concentrations were positively correlated with CRP and CEA level in rectal cancer patients with metastasis, and increased serum concentrations of UA may predict metastasis in patients with rectal cancer. Presence of more severe inflammatory response may contribute to increased serum UA concentration in rectal cancer patients with metastasis. Growing data demonstrated that nuclear factor-kappa B signaling pathway and Notch-1 play important roles in the UA-induced inflammatory response, and Notch-1 is able to decrease inflammation and oxidative stress induced by UA.[23] There were evidences that inflammation and oxidative stress can promote tumor cell proliferation and angiogenesis, and favor invasion and metastasis.[24] Thus, the values of serum UA may be a good predictor of metastasis in patients with rectal cancer.
In the current study, there are, however, several limitations. First, a relatively small sample size in this cross-sectional design is a major limitation. Moreover, some confounders associated with UA, such as diet, exercise, and alcohol consumption, were not included as variables in multiple regression analysis, and more clinical parameters for the severity of the disease were needed to explain the relationship between serum UA and metastasis status in multiple regression analysis. Finally, the association between UA and clinical prognostic significance was analyzed in patients with rectal cancer. Despite these limitations, our results suggest that serum UA may be a novel marker in assessing tumor metastasis in patients with rectal cancer.
4.1. Ethical review
The study was approved by the Ethics Committee of The First College of Clinical Medical Science, China Three Gorges University and Yichang Central People's Hospital, and all patients provided informed consent.
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, CEA = carcino-embryonic antigen, Cr = creatinine, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, IL-6 = interleukin-6, ROC = receiver-operating characteristic, SD = standard deviation, TNF = tumor necrosis factor, TP = total protein, UA = uric acid, UN = urea nitrogen.
Funding: This study was funded by Natural Science Foundation of Hubei Province, China (grant number 2014CFB312).
The authors declare that they have no conflict of interest.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- [2].Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–40. [DOI] [PubMed] [Google Scholar]
- [3].Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638–46. [DOI] [PubMed] [Google Scholar]
- [4].Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet (London, England) 2009;373:811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Huang CS, Lin JK, Wang LW, et al. Assessment of the value of carcinoembryonic antigen reduction ratio as a prognosis factor in rectal cancer. Am J Surg 2014;208:99–105. [DOI] [PubMed] [Google Scholar]
- [6].Zhou B, Shu B, Yang J, et al. C-reactive protein, interleukin-6 and the risk of colorectal cancer: a meta-analysis. Cancer Causes Control 2014;25:1397–405. [DOI] [PubMed] [Google Scholar]
- [7].Toiyama Y, Inoue Y, Saigusa S, et al. C-reactive protein as predictor of recurrence in patients with rectal cancer undergoing chemoradiotherapy followed by surgery. Anticancer Res 2013;33:5065–74. [PubMed] [Google Scholar]
- [8].Wu AH, Gladden JD, Ahmed M, et al. Relation of serum uric acid to cardiovascular disease. Int J Cardiol 2016;213:4–7. [DOI] [PubMed] [Google Scholar]
- [9].Wang Z, Lin Y, Liu Y, et al. Serum uric acid levels and outcomes after acute ischemic stroke. Molec Neurobiol 2016;53:1753–9. [DOI] [PubMed] [Google Scholar]
- [10].Horsfall LJ, Nazareth I, Petersen I. Serum uric acid and the risk of respiratory disease: a population-based cohort study. Thorax 2014;69:1021–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wu CY, Hu HY, Chou YJ, et al. High serum uric acid levels are associated with all-cause and cardiovascular, but not cancer mortality in elderly adults. J Am Geriatr Soc 2015;63:1829–36. [DOI] [PubMed] [Google Scholar]
- [12].Kim HJ, Kim JE, Jung JH, et al. Uric acid is a risk indicator for metabolic syndrome-related colorectal adenoma: results in a korean population receiving screening colonoscopy. Kor J Gastroenterol 2015;66:202–8. [DOI] [PubMed] [Google Scholar]
- [13].Nieto FJ, Iribarren C, Gross MD, et al. Uric acid and serum antioxidant capacity: a reaction to atherosclerosis? Atherosclerosis 2000;148:131–9. [DOI] [PubMed] [Google Scholar]
- [14].Shah MS, Fogelman DR, Raghav KP, et al. Joint prognostic effect of obesity and chronic systemic inflammation in patients with metastatic colorectal cancer. Cancer 2015;121:2968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Strasak AM, Rapp K, Hilbe W, et al. The role of serum uric acid as an antioxidant protecting against cancer: prospective study in more than 28 000 older Austrian women. Ann Oncol 2007;18:1893–7. [DOI] [PubMed] [Google Scholar]
- [16].Chen YF, Li Q, Chen DT, et al. Prognostic value of pre-operative serum uric acid levels in esophageal squamous cell carcinoma patients who undergo R0 esophagectomy. Cancer Biomarkers 2016;17:89–96. [DOI] [PubMed] [Google Scholar]
- [17].Lin H, Lin HX, Ge N, et al. Plasma uric acid and tumor volume are highly predictive of outcome in nasopharyngeal carcinoma patients receiving intensity modulated radiotherapy. Radiat Oncol (London, England) 2013;8:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ara SA, Ashraf S, Patil BM. Evaluation of serum uric acid levels in patients with oral squamous cell carcinoma. Indian J Dental Res 2016;27:178–83. [DOI] [PubMed] [Google Scholar]
- [19].Sevanian A, Davies KJ, Hochstein P. Serum urate as an antioxidant for ascorbic acid. Am J Clin Nutr 1991;546 suppl:1129s–34s. [DOI] [PubMed] [Google Scholar]
- [20].Liu J, Xu C, Ying L, et al. Relationship of serum uric acid level with non-alcoholic fatty liver disease and its inflammation progression in non-obese adults. Hepatol Res 2016;doi: 10.1111/hepr.12734. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [21].Hulsegge G, Herber-Gast GC, Spijkerman AM, et al. Obesity and age-related changes in markers of oxidative stress and inflammation across four generations. Obesity (Silver Spring, Md) 2016;24:1389–96. [DOI] [PubMed] [Google Scholar]
- [22].Mori K, Toiyama Y, Saigusa S, et al. Systemic analysis of predictive biomarkers for recurrence in colorectal cancer patients treated with curative surgery. Digest Dis Sci 2015;60:2477–87. [DOI] [PubMed] [Google Scholar]
- [23].Xie H, Sun J, Chen Y, et al. EGCG attenuates uric acid-induced inflammatory and oxidative stress responses by medicating the NOTCH pathway. Oxidative Med Cell Longevity 2015;2015:214836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mantovani A, Bussolino F, Dejana E. Cytokine regulation of endothelial cell function. FASEB J 1992;6:2591–9. [DOI] [PubMed] [Google Scholar]


