Abstract
IL2-inducible T-cell kinase (ITK), a member of the Tec family tyrosine kinases, is the predominant Tec kinase in T cells and natural killer (NK) cells mediating T cell receptor (TCR) and Fc receptor (Fc R) initiated signal transduction. ITK deficiency results in impaired T and NK cell functions, leading to various disorders including malignancies, inflammation, and autoimmune diseases. In this mini-review, the role of ITK in T cell signaling and the development of small molecule inhibitors of ITK for the treatment of T-cell related disorders is examined.
Keywords: IL2-inducible T-cell kinase (ITK), inhibitors of ITK, T cell signaling
Introduction
The Tec (tyrosine kinase expressed in hepatocellular carcinoma) family tyrosine kinases play important roles in mediating intracellular signaling in hematopoietic cells [1]. They consist of five members: Tec, Bruton’s tyrosine kinase (BTK), IL2-inducible T-cell kinase (ITK, also known as EMT or TSK), resting lymphocyte kinase (RLK, also known as TXK) and bone marrow-expressed kinase (BMX, also known as ETK). BTK is an essential regulator of B cell receptor (BCR)-initiated signaling pathways [2, 3]. Three Tec kinases including ITK, RLK and TEC are expressed in T lymphocytes. ITK is expressed at the highest level in naïve T cells and thymocytes, followed by RLK and then TEC [4]. Their expression levels possibly contribute to their relative importance in T cells. Itk-/- mice have profound defects in T-cell development and function; combined deletion of ITK and RLK worsens these defects [5-7]; however, no major defects in T cells have been described in Tec-/- mice [8]. During CD4+ T cell differentiation, ITK is expressed in both T helper type 1 (Th1) and T helper type 2 (Th2) cells with an upregulated level in Th2 cells, while RLK is expressed only in Th1 cells but not in Th2 cells, suggesting a critical role of ITK in the differentiation and function of Th2 cells [9]. ITK has also been shown to be important for the development of invariant natural killer T (NKT) αβ cells and NKT-like γδ T cells besides conventional T-cells [10, 11]. In natural killer (NK) cells, ITK positively regulates Fc receptor (FcR) - induced granule release, calcium mobilization, and cytotoxicity but negatively regulates natural killer group 2, member D (NKG2D)-initiated signaling [12]. These studies implicate an essential role of ITK in T and NK cell development and function, and therefore developing small molecular inhibitors of ITK for treatment of T and NK cell related diseases would be attractive.
Domain structure of ITK
The ITK gene, discovered in 1992 [13, 14], is chromosomally localized at 5q31-32 position in humans [15]. ITK protein is a 72 kDa kinase expressed in T cells, NK cells and mast cells, and it contains five distinct domains including pleckstrin homology (PH), Tec homology (TH), Src homology 3 (SH3), Src homology 2 (SH2) and Src homology 1 (SH1) (Figure 1) [16, 17]. The PH domain is at the amino terminal of ITK and helps the protein to bind phosphorylated lipids on the membrane [18, 19]. The TH domain exhibits the familial recognition of ITK and contains a proline rich region (PRR) which is necessary for binding the SH3 domain [20, 21]. The SH3 domain binds to the PRR in the TH domain, resulting in the auto-inhibited state of ITK, while the SH2 domain enables protein-protein interactions and allows ITK to bind with phospholipase Cγ1 (PLCγ1) [22-25]. Therefore, ITK is activated by mutations or deletions in the SH3 domain and inactivated by mutations and deletions in the SH2 domain. At the carboxyl terminus of ITK is the SH1 domain which contains ITK’s kinase activity resides and adenosine 5’-triphosphate (ATP) binding pocket [26]. Known targets of this domain include PLCγ1 [27], T-bet [28], Tim-3 [29] and TFII-I [30].
Figure 1.

Domain structure of ITK. ITK protein contains five distinct domains including pleckstrin homology (PH), Tec homology (TH), Src homology 3 (SH3), Src homology 2 (SH2) and Src homology 1 (SH1)
ITK-mediated TCR signaling pathways
ITK is a critical mediator of T cell receptor (TCR) signaling (Figure 2). Upon TCR stimulation, the TCR interacts with peptide-MHC complex presented on antigen presenting cells, leading to the activation of lymphocyte-specific protein tyrosine kinase (LCK) and phosphatidylinositol 3-kinase (PI3K), two important molecules for the activation of ITK [26, 31, 32]. Activation of LCK results in phosphorylation of the CD3 immunoreceptor tyrosine-based activation motifs (ITAMs) and the recruitment of Zeta-chain-associated protein kinase 70 (ZAP70) to activated ITAMs [33], and then these two kinases phosphorylate downstream adaptors linker for activation of T cells (LAT) and SH2 domain-containing leukocyte protein of 76 kDa (SLP-76) [34, 35]. Following PI3K activation, ITK is brought from the cytoplasm to the plasma membrane via the interaction between its PH domain and the PI3K phosphorylated phospholipids in the plasma membrane [36, 37]. There, ITK interacts with the activated complex of SLP-76 and LAT adaptors via its SH2 and SH3 domains, leading to its phosphorylation on the activation loop (Y511) by Lck [26, 38-40].
Figure 2. ITK-mediated T cell receptor (TCR) signaling pathway.
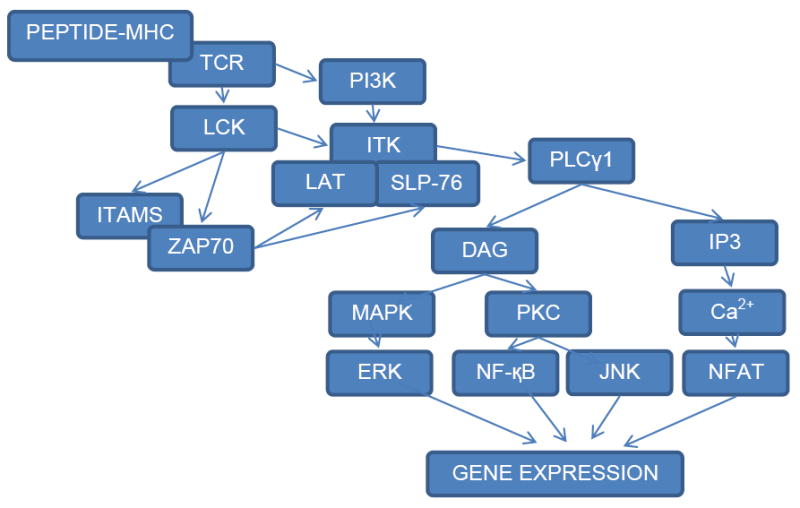
Upon TCR stimulation, TCR interacts with peptide-MHC complex presented on antigen presenting cells, leading to the activation of LCK and PI3K. Activation of LCK results in phosphorylation of the CD3 immunoreceptor tyrosine-based activation motifs (ITAMs) as well as ZAP70 and downstream adaptors LAT and SLP-76. Following PI3K activation, ITK is brought from the cytoplasm to the plasma membrane. There, ITK interacts with the activated complex of SLP-76 and LAT adaptors, leading to its phophorylation on the activation loop (Y511) by Lck. Activated ITK autophosphorylates Y180 in its SH3 domain and phosphorylates its downstream target PLCγ1. Activated PLCγ1 hydrolyzes PIP2 to produce inositol-3-phosphate (IP3) and diacylglycerol (DAG). IP3 binds to receptors on intracellular organelles and cause Ca2+ release from the intracellular store, leading to sustained Ca2+ influx and downstream activation of transcription factors including NFAT. DAG activates two signal pathways. One is mitogen activated protein kinase (MAPK) pathway which leads to the activation of extracellular signal regulated kinase (ERK) and the other one is Protein Kinase C (PKC). pathway which activates nuclear factor-kappaB (NF-κB) and c-Jun amino-terminal kinase (JNK).
Activated ITK autophosphorylates Y180 in its SH3 domain and phosphorylates its downstream target PLCγ1 (Figure 2) [41, 42]. Autophosphorylated Y180 modulates the binding of ITK to different protein targets. Activated PLCγ1 hydrolyzes phosphatidylinositol biphosphate (PIP2) to produce inositol-3-phosphate (IP3) and diacylglycerol (DAG), two critical second messengers in TCR signaling [43]. IP3 binds to receptors on intracellular organelles and causes Ca2+ release from the intracellular store, leading to sustained Ca2+ influx and downstream activation of transcription factors including nuclear factor of activated T-cells (NFAT) [6, 7, 44]. DAG activates two signal pathways. One is the mitogen activated protein kinase (MAPK) pathway which leads to the activation of extracellular signal regulated kinase (ERK) and the other is the Protein Kinase C (PKC) pathway which activates nuclear factor-kappaB (NF-κB) and c-Jun amino-terminal kinase (JNK) [45, 46].
The role of ITK in T cell development and differentiation
Numerous studies have shown that ITK deficiency results in defects in T cell development. Itk knockout mice have impaired T cell activation, decreased numbers of mature thymocytes, reduced cell proliferation, lower ratios of CD4+:CD8+ T cells, and defects in thymic selection [5, 7, 44]. These phenotypes are worsened in Itk/Rlk double knockout mice, indicating functional compensation between these Tec kinases [7]. ITK deficiency affects not only conventional T lymphocytes but also innate T cells [47, 48]. Studies show that though total CD4+ T cells and conventional CD8+ T cells are reduced in Itk-/- mice, the total number of CD8+ T cells remains normal due to the existence of innate CD8+ T cells emanating from Itk knockout mice [49, 50].
ITK also regulates the differentiation of T helper cells including Th1, Th2, T helper 17 (Th17) and relatively normal levels of Th1 cytokines such as interferon gamma (IFNγ) but reduced Th2 cytokine including interleukin (IL)-4, IL-5 and IL-13, therefore, T cells in these mice preferentially develop into Th1 cells [9, 51, 52]. Itk-/- T cells also produce reduced IL-17A which regulates Th17 differentiation [53]. In addition, a new study identifies ITK as a critical regulator of the balance between Th17 and Treg cells by showing that Itk-/- CD4+ T cells preferentially develop into Treg cells [54].
ITK and T cell disorders
Due to the critical role of ITK in T cell development and differentiation, disregulated ITK causes T cell related disorders.
Allergy and hypersensitivity
Itk knockout mice display reduced Th2 cells and Th2-type cytokines, and Th2 cells are important in the pathogenesis of inflammatory diseases including allergic asthma and atopic dermatitis, making ITK a potential therapeutic target in these diseases [55, 56]. Patients with allergic asthma have increased Th2 cells and Th2 cytokines which lead to lung inflammation [55]; Atopic dermatitis is caused by an excess of Th2 response and patients with atopic dermatitis have an increased expression level of ITK [57, 58].
Infection
Itk knockout mice have been shown to effectively clear parasites such as Leishmania major, Nippostrongylus brasiliensis and Schistosome mansonii. Infections with these pathogens elicit a strong Th2 response and are not cleared because of the lack of Th1 response. Mice without ITK display strong Th1 responses and produce normal levels of Th1 cytokines including IFN-γ, therefore, they efficiently clear the infections by these pathogens [7, 44, 51].
Itk-/- mice have increased memory like CD8+ T cells with innate function, and thereby they have increased anti-bacterial responses to infection with Listeria monocytogenes [50]. However, Itk regulatory T cells (TReg). Itk deficient CD4+ T cells produce deficiency also affects cytotoxic CD8+ T cells, leading to impaired anti-viral immune responses [59, 60]. Itk mutations in the SH2 domain have been reported in patients with Epstein - Barr virus related lymphoproliferative diseases [61, 62], which may also be related to impaired cytotoxic CD8+ T cell response.
Human immunodeficiency virus (HIV) is a retrovirus causing acquired immunodeficiency syndrome (AIDS). ITK has been shown to be an important factor in regulating the infection and replication of HIV [63]. Critical activators of HIV transcription including NFAT, NFκB and activator protein 1 (AP-1) are regulated by ITK [64]. In addition, the assembly and release of HIV viral particles is influenced by ITK via its regulation on actin cytoskeleton rearrangement [65-67].
Autoimmune diseases
Autoimmune diseases are caused by the activation of self-reactive T cells resulting in impaired organ function. ITK positively regulates Th17 cells which mediate autoimmune disorders including experimental autoimmune encephalomyelitis (EAE) [68, 69]. As a downstream mediator of the B7-CD28 co-stimulatory pathway, ITK also plays an important role in controlling the migration of auto-reactive T cells [70].
T cell malignancies
ITK has been shown to be up-regulated and aberrantly activated in T-cell malignancies [71, 72], and its downstream targets including NFAT, NFκB and MAPK are involved in the pathogenesis of T-cell malignancies [73]. Targeting the ITK-dependent IL-2 receptor (CD25) signaling pathway in T- cell lymphomas / leukemias with anti-CD25 monoclonal antibodies has shown promising efficiency [74]. These results indicate that ITK is a potential therapeutic target for T cell malignancies. Recently, a chromosomal translocation between Itk and spleen tyrosine kinase (Syk) leading to T-cell lymphoma was identified [75-77]. This translocation fuses the PH and TH domain from ITK to the SYK kinase domain, resulting in activated SYK. The activation of ITK-SYK fusion protein is dependent on PI3k signaling [78], suggesting that PI3K inhibitors may be effective in treating ITK-SYK initiated T-cell lymphoma [79, 80].
ITK inhibitors
Numerous lines of evidence suggest ITK as a potential therapeutic target in various diseases and demonstrate the importance of developing small molecule inhibitors of ITK. With the exception of Ibrutinib, the first FDA approved ITK inhibitor; the majority of small molecule ITK inhibitors are still in the early stages of development
Aminothiazole based ITK inhibitors
By screening compound libraries and analyzing structure and activity relationships (SAR), two aminothiazole based molecule inhibitors of ITK, BMS-488516 and BMS-509744, were identified [81-83]. They are ATP competitive inhibitors, indicating their binding to the ATP binding site of the ITK kinase domain. These two ITK inhibitors had more than 200-fold selectivity versus other Tec family tyrosine kinases and potently inhibited ITK with half maximal inhibitory concentration (IC50) values of 96 nM and 19 nM, respectively. Both compounds inhibited TCR-induced PLCγ1 phosphorylation, T-cell proliferation, calcium mobilization and IL-2 production. BMS-509744 efficiently suppressed lung inflammation in a mouse model of allergic asthma.
Benzimidazole based ITK inhibitors
The benzimidazole series of compound 1 was identified by high throughput screening [84]. It is an ATP competitive and has an IC50 value of 12 nM in the ITK enzyme assay. Further modifications improved the selectivity, potency and drug-like properties including stability and oral administration. Compound 10n and 10o inhibited IL-2 production with an IC50 of 240 nM and 690 nM, respectively, in a human whole blood assay. Oral administration of compound 10n inhibited the production of IL-2 and IL-4 in a mouse model of T cell activation induced by anti-CD3 [85].
Aminopyrimidine based ITK inhibitors
Compound 44 is a potent small molecule inhibitor of ITK identified by high-throughput screening of a library containing 468,462 compounds [58]. It has an IC50 of 65 nM in inhibiting ITK kinase activity. Compound 44 inhibited the secretion of IL-2 and IFN-γ and the proliferation of activated T cells. In two models of inflammatory skin diseases, compound 44 significantly reduced skin inflammation.
6.4 3-aminopyride-2-ones based ITK inhibitors
This is a new series of ITK inhibitors identified by structure-based design, starting from 3-aminopyride-2-ones, a fragment designed de novo [86]. Among various derivatives, the compound 7v represented the best ITK inhibitor with good potency and selectivity.
Indolylndazole based ITK inhibitors
ITK inhibitor 11o was identified based on indolylndazole libraries [87]. It had enzymatic activity at 11 nM and cellular activity at 20 nM. In an anti-CD3-induced IL-2 mouse model, intravenous or oral administration of 11o (10mg/kg) inhibited IL-2 secretion, and this drug was well tolerated without obvious side effects.
CTA056
CTA056, 7-benzyl-1-(3-(piperidin-1-yl)propyl)-2-(4-(pyridin-4-yl)phenyl)-1H-imidazo[4,5-g]quinoxalin-6(5H)-one, was developed through screening a library comprising 9600 compounds, followed by molecular modeling and analysis of SAR [88]. It showed the highest inhibition toward ITK with an IC50 of 100 nM, followed by BTK with an IC50 of 400 nM. CTA056 treatment in T cells inhibited the phosphorylation of ITK and its downstream targets including PLCγ1 and reduced the secretion of IL-2 and IFN-γ. CTA056 selectively targeted malignant T cells expressing ITK, including acute lymphoblastic T-cell leukemia and cutaneous T-cell lymphoma. In a xenograft model of T cell leukemia, CTA056 treatment (5 mg/kg, twice a week, intratumoral injection) prevented tumor growth.
6.7 ITK inhibitors targeting cysteine-442 in the ATP pocket
Ibrutinib is an irreversible inhibitor of BTK and ITK [89, 90]. It binds to cysteine-481 residue in BTK or cysteine-442 residue in ITK and inhibits downstream activation of BCR or TCR, respectively. After TCR stimulation in primary CD4+ T cells and Jurkat T cells, Ibrutinib inhibited activation of PLCγ1, NFAT, JunB and IKBα which are ITK downstream targets [89]. Interestingly, ibrutinib specifically inhibited Th2 T cell activation and Th2-type cytokine release and provided a selective advantage to Th1 and CD8+ T cells which express RLK beside ITK. The inhibitory effect of ibrunitib on ITK was further validated in primary CLL samples and several murine models of CLL, parasitic infection (Leishmania major) and infectious disease (Listeria monocytogenes) [89]. Ibrutinib has shown significant clinical activity in the treatment of mantel cell lymphoma (MCL) and CLL [91-93], and been approved by the U.S. Food and Drug Administration (FDA) for the treatment of these two malignancies.
Compound 12 is another irreversible ITK inhibitor targeting Cysteine-442 in the ATP pocket [94]. In activated human peripheral blood mononucleated cells (PBMCs), compound 12 had the most potency at inhibiting IL-12, followed by IFNγ, IL-13 and IL-17. The inhibition of T cell activation by compound 12 was demonstrated in a rat model by inhaled delivery of this drug. The inhibitory effect of compound 12 on Th1, Th2 and Th17 T cells suggests that it might also inhibit RLK, which needs verification by further study.
Other ITK inhibitors
ITK inhibitors based on pyrazolyl-indole [95] or thienopyrazole [33] were described in previous studies. Rosmarinic acid, a natural compound, was also shown to inhibit activation of ITK, PLCγ1, NFAT and Ca2+ mobilization [35]. Instead of targeting the ATP site in ITK, inhibitors targeting ITK allosteric sites were discovered and characterized [96].
Conclusion
Intensive studies have demonstrated the essential role of ITK in T cell development and differentiation, implicating ITK as a potent therapeutic target in various diseases including Th2 cell related immunodeficiency and inflammation. The success of ibrutinib, a BTK/ITK-targeting inhibitor, in the treatment of B-cell leukemia and lymphoma encourages the development of such targeted therapy in T-cell related diseases. Though a number of ITK inhibitors have been discovered, most of them are still in their early stages of development and more effort is needed before their application in clinic. In addition, due to a compensatory role of RLK to ITK in Th1 T cells, development of dual inhibitors targeting both ITK and RLK will be important.
Acknowledgments
This work was supported by the National Cancer Institute (grants 5 T32 CA009338-33-03, P01 CA095426, K12 CA133250-05, and P50 CA140158), the American Cancer Society (grant 125039-PF-13-246-01-LIB), the Leukemia & Lymphoma Society, the American Society of Hematology, Mr and Mrs Michael Thomas, the Harry Mangurian Foundation, and the D. Warren Brown Family Foundation.
References
- 1.Berg LJ, et al. Tec family kinases in T lymphocyte development and function. Annu Rev Immunol. 2005;23:549–600. doi: 10.1146/annurev.immunol.22.012703.104743. [DOI] [PubMed] [Google Scholar]
- 2.Buggy JJ, Elias L. Bruton tyrosine kinase (BTK) and its role in B-cell malignancy. Int Rev Immunol. 2012;31(2):119–32. doi: 10.3109/08830185.2012.664797. [DOI] [PubMed] [Google Scholar]
- 3.Zhong Y, Yeh Y-Y. Validated Critical Roles of Bruton’s Tyrosine Kinase (BTK) in Chronic Lymphocytic Leukemia (CLL) Journal of Postdoctoral Research. 2014;2(2):43–45. [Google Scholar]
- 4.Readinger JA, et al. Tec kinases regulate T-lymphocyte development and function: new insights into the roles of Itk and Rlk/Txk. Immunol Rev. 2009;228(1):93–114. doi: 10.1111/j.1600-065X.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao XC, Littman DR. Altered T cell receptor signaling and disrupted T cell development in mice lacking Itk. Immunity. 1995;3(6):757–69. doi: 10.1016/1074-7613(95)90065-9. [DOI] [PubMed] [Google Scholar]
- 6.Liu KQ, et al. T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J Exp Med. 1998;187(10):1721–7. doi: 10.1084/jem.187.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaeffer EM, et al. Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science. 1999;284(5414):638–41. doi: 10.1126/science.284.5414.638. [DOI] [PubMed] [Google Scholar]
- 8.Ellmeier W, et al. Severe B cell deficiency in mice lacking the tec kinase family members Tec and Btk. J Exp Med. 2000;192:1611–24. doi: 10.1084/jem.192.11.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller AT, et al. Signaling through Itk promotes T helper 2 differentiation via negative regulation of T-bet. Immunity. 2004;21(1):67–80. doi: 10.1016/j.immuni.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Qi Q, Kannan AK, August A. Tec family kinases: Itk signaling and the development of NKT alphabeta and gammadelta T cells. FEBS J. 2011;278(12):1970–9. doi: 10.1111/j.1742-4658.2011.08074.x. [DOI] [PubMed] [Google Scholar]
- 11.Felices M, Berg LJ. The Tec kinases Itk and Rlk regulate NKT cell maturation, cytokine production, and survival. J Immunol. 2008;180(5):3007–18. doi: 10.4049/jimmunol.180.5.3007. [DOI] [PubMed] [Google Scholar]
- 12.Khurana D, et al. Differential regulation of human NK cell-mediated cytotoxicity by the tyrosine kinase Itk. J Immunol. 2007;178(6):3575–82. doi: 10.4049/jimmunol.178.6.3575. [DOI] [PubMed] [Google Scholar]
- 13.Siliciano JD, Morrow TA, Desiderio SV. itk, a T-cell-specific tyrosine kinase gene inducible by interleukin 2. Proc Natl Acad Sci U S A. 1992;89(23):11194–8. doi: 10.1073/pnas.89.23.11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heyeck SD, Berg LJ. Developmental regulation of a murine T-cell-specific tyrosine kinase gene, Tsk. Proc Natl Acad Sci U S A. 1993;90(2):669–73. doi: 10.1073/pnas.90.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson S, et al. Identification, cloning, and characterization of a novel human T-cell-specific tyrosine kinase located at the hematopoietin complex on chromosome 5q. Blood. 1993;82(5):1561–72. [PubMed] [Google Scholar]
- 16.Yang WC, et al. Tec kinases: a family with multiple roles in immunity. Immunity. 2000;12(4):373–82. doi: 10.1016/s1074-7613(00)80189-2. [DOI] [PubMed] [Google Scholar]
- 17.Brown K, et al. Crystal structures of interleukin-2 tyrosine kinase and their implications for the design of selective inhibitors. J Biol Chem. 2004;279(18):18727–32. doi: 10.1074/jbc.M400031200. [DOI] [PubMed] [Google Scholar]
- 18.Ching KA, et al. Emt/Itk associates with activated TCR complexes: role of the pleckstrin homology domain. J Immunol. 1999;163(11):6006–13. [PubMed] [Google Scholar]
- 19.Huang YH, et al. Positive regulation of Itk PH domain function by soluble IP4. Science. 2007;316(5826):886–9. doi: 10.1126/science.1138684. [DOI] [PubMed] [Google Scholar]
- 20.Andreotti AH, et al. Regulatory intramolecular association in a tyrosine kinase of the Tec family. Nature. 1997;385(6611):93–7. doi: 10.1038/385093a0. [DOI] [PubMed] [Google Scholar]
- 21.Hao S, August A. The proline rich region of the Tec homology domain of ITK regulates its activity. FEBS Lett. 2002;525(1-3):53–8. doi: 10.1016/s0014-5793(02)03066-1. [DOI] [PubMed] [Google Scholar]
- 22.Ching KA, et al. TCR/CD3-Induced activation and binding of Emt/Itk to linker of activated T cell complexes: requirement for the Src homology 2 domain. J Immunol. 2000;165(1):256–62. doi: 10.4049/jimmunol.165.1.256. [DOI] [PubMed] [Google Scholar]
- 23.Nore BF, et al. Identification of phosphorylation sites within the SH3 domains of Tec family tyrosine kinases. Biochim Biophys Acta. 2003;1645(2):123–32. doi: 10.1016/s1570-9639(02)00524-1. [DOI] [PubMed] [Google Scholar]
- 24.Bunnell SC, et al. Identification of Itk/Tsk Src homology 3 domain ligands. J Biol Chem. 1996;271(41):25646–56. doi: 10.1074/jbc.271.41.25646. [DOI] [PubMed] [Google Scholar]
- 25.Pawson T, Gish GD, Nash P. SH2 domains, interaction modules and cellular wiring. Trends Cell Biol. 2001;11(12):504–11. doi: 10.1016/s0962-8924(01)02154-7. [DOI] [PubMed] [Google Scholar]
- 26.Heyeck SD, et al. Lck phosphorylates the activation loop tyrosine of the Itk kinase domain and activates Itk kinase activity. J Biol Chem. 1997;272(40):25401–8. doi: 10.1074/jbc.272.40.25401. [DOI] [PubMed] [Google Scholar]
- 27.Bogin Y, et al. SLP-76 mediates and maintains activation of the Tec family kinase ITK via the T cell antigen receptor-induced association between SLP-76 and ITK. Proc Natl Acad Sci U S A. 2007;104(16):6638–43. doi: 10.1073/pnas.0609771104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang ES, et al. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005;307(5708):430–3. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- 29.van de Weyer PS, et al. A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochem Biophys Res Commun. 2006;351(2):571–6. doi: 10.1016/j.bbrc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 30.Sacristan C, et al. Characterization of a novel interaction between transcription factor TFII-I and the inducible tyrosine kinase in T cells. Eur J Immunol. 2009;39(9):2584–95. doi: 10.1002/eji.200839031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bunnell SC, et al. Biochemical interactions integrating Itk with the T cell receptor-initiated signaling cascade. J Biol Chem. 2000;275(3):2219–30. doi: 10.1074/jbc.275.3.2219. [DOI] [PubMed] [Google Scholar]
- 32.Wilcox HM, Berg LJ. Itk phosphorylation sites are required for functional activity in primary T cells. J Biol Chem. 2003;278(39):37112–21. doi: 10.1074/jbc.M304811200. [DOI] [PubMed] [Google Scholar]
- 33.Samelson LE. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu Rev Immunol. 2002;20:371–94. doi: 10.1146/annurev.immunol.20.092601.111357. [DOI] [PubMed] [Google Scholar]
- 34.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jordan MS, Singer AL, Koretzky GA. Adaptors as central mediators of signal transduction in immune cells. Nat Immunol. 2003;4(2):110–6. doi: 10.1038/ni0203-110. [DOI] [PubMed] [Google Scholar]
- 36.Yang WC, et al. Tec kinase signaling in T cells is regulated by phosphatidylinositol 3-kinase and the Tec pleckstrin homology domain. J Immunol. 2001;166(1):387–95. doi: 10.4049/jimmunol.166.1.387. [DOI] [PubMed] [Google Scholar]
- 37.August A, et al. Src-induced activation of inducible T cell kinase (ITK) requires phosphatidylinositol 3-kinase activity and the Pleckstrin homology domain of inducible T cell kinase. Proc Natl Acad Sci U S A. 1997;94(21):11227–32. doi: 10.1073/pnas.94.21.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shan X, Wange RL. Itk/Emt/Tsk activation in response to CD3 cross-linking in Jurkat T cells requires ZAP-70 and Lat and is independent of membrane recruitment. J Biol Chem. 1999;274(41):29323–30. doi: 10.1074/jbc.274.41.29323. [DOI] [PubMed] [Google Scholar]
- 39.Su YW, et al. Interaction of SLP adaptors with the SH2 domain of Tec family kinases. Eur J Immunol. 1999;29(11):3702–11. doi: 10.1002/(SICI)1521-4141(199911)29:11<3702::AID-IMMU3702>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 40.Zhang W, et al. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92(1):83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 41.Joseph RE, Fulton DB, Andreotti AH. Mechanism and functional significance of Itk autophosphorylation. J Mol Biol. 2007;373(5):1281–92. doi: 10.1016/j.jmb.2007.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perez-Villar JJ, Kanner SB. Regulated association between the tyrosine kinase Emt/Itk/Tsk and phospholipase-C gamma 1 in human T lymphocytes. J Immunol. 1999;163(12):6435–41. [PubMed] [Google Scholar]
- 43.Schaeffer EM, Schwartzberg PL. Tec family kinases in lymphocyte signaling and function. Curr Opin Immunol. 2000;12(3):282–8. doi: 10.1016/s0952-7915(00)00088-1. [DOI] [PubMed] [Google Scholar]
- 44.Fowell DJ, et al. Impaired NFATc translocation and failure of Th2 development in Itk-deficient CD4+ T cells. Immunity. 1999;11(4):399–409. doi: 10.1016/s1074-7613(00)80115-6. [DOI] [PubMed] [Google Scholar]
- 45.Dower NA, et al. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat Immunol. 2000;1(4):317–21. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- 46.Roose JP, et al. A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Mol Cell Biol. 2005;25(11):4426–41. doi: 10.1128/MCB.25.11.4426-4441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berg LJ. Signalling through TEC kinases regulates conventional versus innate CD8(+) T-cell development. Nat Rev Immunol. 2007;7(6):479–85. doi: 10.1038/nri2091. [DOI] [PubMed] [Google Scholar]
- 48.Prince AL, et al. The Tec kinases Itk and Rlk regulate conventional versus innate T-cell development. Immunol Rev. 2009;228(1):115–31. doi: 10.1111/j.1600-065X.2008.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu J, August A. August, Naive and innate memory phenotype CD4+ T cells have different requirements for active Itk for their development. J Immunol. 2008;180(10):6544–52. doi: 10.4049/jimmunol.180.10.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu J, et al. Memory phenotype CD8+ T cells with innate function selectively develop in the absence of active Itk. Eur J Immunol. 2007;37(10):2892–9. doi: 10.1002/eji.200737311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaeffer EM, et al. Mutation of Tec family kinases alters T helper cell differentiation. Nat Immunol. 2001;2(12):1183–8. doi: 10.1038/ni734. [DOI] [PubMed] [Google Scholar]
- 52.Schwartzberg PL, Finkelstein LD, Readinger JA. TEC-family kinases: regulators of T-helper-cell differentiation. Nat Rev Immunol. 2005;5(4):284–95. doi: 10.1038/nri1591. [DOI] [PubMed] [Google Scholar]
- 53.Gomez-Rodriguez J, et al. Differential expression of interleukin-17A and -17F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity. 2009;31(4):587–97. doi: 10.1016/j.immuni.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gomez-Rodriguez J, et al. Itk-mediated integration of T cell receptor and cytokine signaling regulates the balance between Th17 and regulatory T cells. J Exp Med. 2014;211(3):529–43. doi: 10.1084/jem.20131459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferrara TJ, et al. Reduced airway hyperresponsiveness and tracheal responses during allergic asthma in mice lacking tyrosine kinase inducible T-cell kinase. J Allergy Clin Immunol. 2006;117(4):780–6. doi: 10.1016/j.jaci.2005.12.1330. [DOI] [PubMed] [Google Scholar]
- 56.Mueller C, August A. Attenuation of immunological symptoms of allergic asthma in mice lacking the tyrosine kinase ITK. J Immunol. 2003;170(10):5056–63. doi: 10.4049/jimmunol.170.10.5056. [DOI] [PubMed] [Google Scholar]
- 57.Matsumoto Y, et al. Identification of highly expressed genes in peripheral blood T cells from patients with atopic dermatitis. Int Arch Allergy Immunol. 2002;129(4):327–40. doi: 10.1159/000067589. [DOI] [PubMed] [Google Scholar]
- 58.von Bonin A, et al. Inhibition of the IL-2-inducible tyrosine kinase (Itk) activity: a new concept for the therapy of inflammatory skin diseases. Exp Dermatol. 2011;20(1):41–7. doi: 10.1111/j.1600-0625.2010.01198.x. [DOI] [PubMed] [Google Scholar]
- 59.Atherly LO, et al. The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity. 2006;25(1):79–91. doi: 10.1016/j.immuni.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 60.Bachmann MF, Littman DR, Liao XC. Antiviral immune responses in Itk-deficient mice. J Virol. 1997;71(10):7253–7. doi: 10.1128/jvi.71.10.7253-7257.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huck K, et al. Girls homozygous for an IL-2-inducible T cell kinase mutation that leads to protein deficiency develop fatal EBV-associated lymphoproliferation. J Clin Invest. 2009;119(5):1350–8. doi: 10.1172/JCI37901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stepensky P, et al. IL-2-inducible T-cell kinase deficiency: clinical presentation and therapeutic approach. Haematologica. 2011;96(3):472–6. doi: 10.3324/haematol.2010.033910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Readinger JA, et al. Selective targeting of ITK blocks multiple steps of HIV replication. Proc Natl Acad Sci U S A. 2008;105(18):6684–9. doi: 10.1073/pnas.0709659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gomez C, Hope TJ. The ins and outs of HIV replication. Cell Microbiol. 2005;7(5):621–6. doi: 10.1111/j.1462-5822.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 65.Dombroski D, et al. Kinase-independent functions for Itk in TCR-induced regulation of Vav and the actin cytoskeleton. J Immunol. 2005;174(3):1385–92. doi: 10.4049/jimmunol.174.3.1385. [DOI] [PubMed] [Google Scholar]
- 66.Grasis JA, Browne CD, Tsoukas CD. Inducible T cell tyrosine kinase regulates actin-dependent cytoskeletal events induced by the T cell antigen receptor. J Immunol. 2003;170(8):3971–6. doi: 10.4049/jimmunol.170.8.3971. [DOI] [PubMed] [Google Scholar]
- 67.Pontow SE, et al. Actin cytoskeletal reorganizations and coreceptor-mediated activation of rac during human immunodeficiency virus-induced cell fusion. J Virol. 2004;78(13):7138–47. doi: 10.1128/JVI.78.13.7138-7147.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aranami T, Yamamura T. Th17 Cells and autoimmune encephalomyelitis (EAE/MS) Allergol Int. 2008;57(2):115–20. doi: 10.2332/allergolint.R-07-159. [DOI] [PubMed] [Google Scholar]
- 69.Curtis MM, Way SS. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology. 2009;126(2):177–85. doi: 10.1111/j.1365-2567.2008.03017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jain N, et al. CD28 and ITK signals regulate autoreactive T cell trafficking. Nat Med. 2013;19(12):1632–7. doi: 10.1038/nm.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaukonen J, Savolainen ER, Palotie A. Human Emt tyrosine kinase is specifically expressed both in mature T-lymphocytes and T-cell associated hematopoietic malignancies. Leuk Lymphoma. 1999;32(5-6):513–22. doi: 10.3109/10428199909058409. [DOI] [PubMed] [Google Scholar]
- 72.Shin J, et al. Lesional gene expression profiling in cutaneous T-cell lymphoma reveals natural clusters associated with disease outcome. Blood. 2007;110(8):3015–27. doi: 10.1182/blood-2006-12-061507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao WL. Targeted therapy in T-cell malignancies: dysregulation of the cellular signaling pathways. Leukemia. 2010;24(1):13–21. doi: 10.1038/leu.2009.223. [DOI] [PubMed] [Google Scholar]
- 74.Dancey G, et al. A Phase I Clinical Trial of CHT-25 a 131I-Labeled Chimeric Anti-CD25 Antibody Showing Efficacy in Patients with Refractory Lymphoma. Clin Cancer Res. 2009;15(24):7701–7710. doi: 10.1158/1078-0432.CCR-09-1421. [DOI] [PubMed] [Google Scholar]
- 75.Streubel B, et al. Novel t(5;9)(q33;q22) fuses ITK to SYK in unspecified peripheral T-cell lymphoma. Leukemia. 2006;20(2):313–8. doi: 10.1038/sj.leu.2404045. [DOI] [PubMed] [Google Scholar]
- 76.Pechloff K, et al. The fusion kinase ITK-SYK mimics a T cell receptor signal and drives oncogenesis in conditional mouse models of peripheral T cell lymphoma. J Exp Med. 2010;207(5):1031–44. doi: 10.1084/jem.20092042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rigby S, et al. The lymphoma-associated fusion tyrosine kinase ITK-SYK requires pleckstrin homology domain-mediated membrane localization for activation and cellular transformation. J Biol Chem. 2009;284(39):26871–81. doi: 10.1074/jbc.M109.034272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hussain A, et al. Phosphatidylinositol-3-kinase-dependent phosphorylation of SLP-76 by the lymphoma-associated ITK-SYK fusion-protein. Biochem Biophys Res Commun. 2009;390(3):892–6. doi: 10.1016/j.bbrc.2009.10.070. [DOI] [PubMed] [Google Scholar]
- 79.Herman SE, Johnson AJ. Molecular pathways: targeting phosphoinositide 3-kinase p110-delta in chronic lymphocytic leukemia. Clin Cancer Res. 2012;18(15):4013–8. doi: 10.1158/1078-0432.CCR-11-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhong Y, Liu T-M, Johnson AJ. A Breakthrough Therapy in Relapsed Chronic Lymphocytic Leukemia: the Combination of Idelalisib and Rituximab. Journal of Postdoctoral Research. 2014;2(3):41–43. [Google Scholar]
- 81.Das J, et al. Discovery and SAR of 2-amino-5-(thioaryl)thiazoles as potent and selective Itk inhibitors. Bioorg Med Chem Lett. 2006;16(14):3706–12. doi: 10.1016/j.bmcl.2006.04.060. [DOI] [PubMed] [Google Scholar]
- 82.Das J, et al. Discovery and SAR of 2-amino-5-[(thiomethyl)aryl]thiazoles as potent and selective Itk inhibitors. Bioorg Med Chem Lett. 2006;16(9):2411–5. doi: 10.1016/j.bmcl.2006.01.115. [DOI] [PubMed] [Google Scholar]
- 83.Lin TA, et al. Selective Itk inhibitors block T-cell activation and murine lung inflammation. Biochemistry. 2004;43(34):11056–62. doi: 10.1021/bi049428r. [DOI] [PubMed] [Google Scholar]
- 84.Snow RJ, et al. Hit-to-lead studies on benzimidazole inhibitors of ITK: discovery of a novel class of kinase inhibitors. Bioorg Med Chem Lett. 2007;17(13):3660–5. doi: 10.1016/j.bmcl.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 85.Riether D, et al. 5-Aminomethylbenzimidazoles as potent ITK antagonists. Bioorg Med Chem Lett. 2009;19(6):1588–91. doi: 10.1016/j.bmcl.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 86.Charrier JD, et al. Discovery and structure-activity relationship of 3-aminopyrid-2-ones as potent and selective interleukin-2 inducible T-cell kinase (Itk) inhibitors. J Med Chem. 2011;54(7):2341–50. doi: 10.1021/jm101499u. [DOI] [PubMed] [Google Scholar]
- 87.Herdemann M, et al. Optimisation of ITK inhibitors through successive iterative design cycles. Bioorg Med Chem Lett. 2011;21(6):1852–6. doi: 10.1016/j.bmcl.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 88.Guo W, et al. Molecular characteristics of CTA056, a novel interleukin-2-inducible T-cell kinase inhibitor that selectively targets malignant T cells and modulates oncomirs. Mol Pharmacol. 2012;82(5):938–47. doi: 10.1124/mol.112.079889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dubovsky JA, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122(15):2539–49. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pan Z, et al. Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase. ChemMedChem. 2007;2(1):58–61. doi: 10.1002/cmdc.200600221. [DOI] [PubMed] [Google Scholar]
- 91.Byrd JC, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang ML, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507–16. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Woyach JA, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood. 2014;123(12):1810–7. doi: 10.1182/blood-2013-09-527853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Harling JD, et al. Discovery of novel irreversible inhibitors of interleukin (IL)-2-inducible tyrosine kinase (Itk) by targeting cysteine 442 in the ATP pocket. J Biol Chem. 2013;288(39):28195–206. doi: 10.1074/jbc.M113.474114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Velankar AD, et al. Synthesis and biological evaluation of novel (4 or 5-aryl)pyrazolyl-indoles as inhibitors of interleukin-2 inducible T-cell kinase (ITK) Bioorg Med Chem. 2010;18(12):4547–59. doi: 10.1016/j.bmc.2010.04.056. [DOI] [PubMed] [Google Scholar]
- 96.Han S, et al. Selectively targeting an inactive conformation of IL-2-induced T cell kinase by allosteric inhibitors. Biochem J. 2014 doi: 10.1042/BJ20131139. [DOI] [PubMed] [Google Scholar]


