Abstract
Leading a mentally stimulating life may build up a reserve of neural and mental resources that preserve cognitive abilities in late life. Recent autopsy evidence links neuronal density in the locus coeruleus (LC), the brain's main source of norepinephrine, to slower cognitive decline before death, inspiring the idea that the noradrenergic system is a key component of reserve. Here, we tested this hypothesis using neuromelanin-sensitive magnetic resonance imaging to visualize and measure LC signal intensity in healthy younger and older adults. Established proxies of reserve, including education, occupational attainment, and verbal intelligence, were linearly correlated with LC signal intensity in both age groups. Results indicated that LC signal intensity was significantly higher in older than younger adults and significantly lower in women than in men. Consistent with the LC-reserve hypothesis, both verbal intelligence and a composite reserve score were positively associated with LC signal intensity in older adults. LC signal intensity was also more strongly associated with attentional shifting ability in older adults with lower cognitive reserve. Together these findings link in vivo estimates of LC neuromelanin signal intensity to cognitive reserve in normal aging.
Keywords: Locus coeruleus, Aging, Norepinephrine, Cognitive reserve, Brain reserve, Sex, Gender
1. Introduction
Brain and cognitive reserve refer to an individual's capacity to cope with cognitive decline or pathology in late adulthood (Stern, 2002, 2006, 2009). The concept of reserve has generated considerable interest in the field of aging, particularly regarding Alzheimer's disease (AD), as factors believed to promote reserve not only predict lowered risk of dementia in older adults but also mask the severity of underlying brain pathology (Valenzuela and Sachdev, 2006).
Reserve capacity is theorized to rely on both passive and active factors. The “passive” model, referred to as “brain reserve capacity,” is characterized by the availability of physical neural resources (Stern, 2009). For instance, larger regional gray matter volumes, such as in the prefrontal and parietal cortices, are associated with higher cognitive reserve scores (e.g., Bartrés-Faz et al., 2009). In contrast, the “active model,” otherwise known as “cognitive reserve,” refers to an individual's capacity to invoke compensatory abilities developed over a lifetime of mentally enriching experiences (e.g., education). Epidemiological studies characterize years of education (Bennett et al., 2003; Le Carret et al., 2003), occupational attainment (Richards and Sacker, 2003; Stern et al., 1995), and IQ (Alexander et al., 1997) as 3 of the most common elements of cognitive reserve (Steffener and Stern, 2012; Stern et al., 1994; Whalley et al., 2004).
A critical question is how and why these cognitive reserve factors can protect brain function to either stave off or compensate for neurodegeneration. One possibility is that cognitive reserve proxies do not causally affect cognition but are instead indicative of a shared genetic factor that shields certain individuals from age-related pathology. This does not seem to be the case, however, because identical twins with different levels of education (Gatz et al., 2007) or occupational complexity (Andel et al., 2005) exhibit different risk of dementia. Thus, cognitive reserve proxies more likely promote healthy cognitive aging by modulating underlying brain systems.
Supporting this view, active and passive factors of reserve are far from mutually exclusive; for instance, neuroplasticity represents a critical intersection of both factors, as greater availability of neuronal resources might not only enhance healthy cognition but also affords more opportunities for compensation to occur in response to pathology (see Bartrés-Faz and Arenaza-Urquijo, 2011). Thus, the ideal biomarker of overall reserve is one that captures their interaction, representing a quantifiable neuronal resource that is modified by mentally enriching experiences; in turn, this substrate should be associated with better cognitive outcomes, even in healthy older individuals (Robertson, 2013, 2014). Identifying a shared neuromechanism of active and passive reserve is important because it could (1) quantify an individual's level of reserve and susceptibility to cognitive decline; (2) become the target of therapeutic, cognitive, or pharmacological intervention; and (3) serve as a biomarker of the efficacy of such interventions.
According to a hypothesis by Robertson (2013), the locus coeruleus-norepinephrine (LC-NE) system is a candidate neuromechanism of reserve based on its key role in recruiting mental resources to promote executive function (functional) and in regulating neuroplasticity (physical) throughout the brain (Berridge and Waterhouse, 2003; Sara, 2009). The LC is a small bilateral nucleus located in the dorsal pontine tegmentum (PT) and is the primary source of NE for the brain. Via its widespread release under arousal, NE enhances learning (Ahissar et al., 1996; Chamberlain et al., 2006; Harley, 1987), regulates synaptic plasticity (Ahissar et al., 1996; Neuman and Harley, 1983; Salgado et al., 2012), and optimizes high-order cognitive processes, including working memory (Arnsten and Li, 2005; Wang et al., 2007). NE is also neuroprotective and can lower toxicity in neurons (Counts and Mufson, 2010) and buffer neurons from oxidative stress (Troadec et al., 2001). Aging is characterized by decreased LC volume and cell density (Chan-Palay and Asan, 1989; German et al., 1988, 1992; Lohr and Jeste, 1988; Manaye et al., 1995; Vijayashankar and Brody, 1979), suggesting that altered noradrenergic structure or signaling in late adulthood is likely to correspond with cognitive decline.
Indeed, new evidence reveals an important link between LC functional/neuronal integrity and cognitive function. For example, a human functional magnetic resonance imaging (fMRI) study found that greater functional connectivity between the LC and parahippocampal gyrus was associated with higher memory performance in healthy older adults, and this connectivity was lower in older adults with mild cognitive impairment (MCI) (Jacobs et al., 2015). In another fMRI study, healthy older adults with higher levels of cognitive reserve showed reduced activity in an LC-related brainstem cluster while participants encoded scenes, which the authors interpreted as increased neuronal efficiency (Solé-Padullés et al., 2009). Perhaps most compellingly, a recent autopsy study found reduced postmortem neuronal density in the LC was associated with cognitive decline ~6 years before death, even after controlling for neuron density in other aminergic nuclei, including the dorsal raphe, ventral tegmental area, and substantia nigra (Wilson et al., 2013). In the study of Wilson et al. (2013), controlling for LC neuronal density diminished the association between Lewy body pathology and cognitive decline, suggesting that, like cognitive reserve variables (Ritter et al., 2008; Roe et al., 2007, 2008), LC structural integrity could account for discrepancies between the amount of brain pathology and apparent cognitive decline (Wilson et al., 2013). Thus, via its regulation of healthy cognition and central neuronal function, the LC-NE system may mediate the protective effects of reserve on cognitive aging processes (Robertson, 2013, 2014).
Of relevance to the LC-reserve hypothesis, LC neurodegeneration has received increasing attention as a possible contributor to the pathophysiology of AD (Braak and Del Tredici, 2011; Braak et al., 2011; Chalermpalanupap et al., 2013; Grudzien et al., 2007; Mravec et al., 2014). LC tau pathology is also visible in early adulthood, and there are some indications that it might even precede one of the hallmark signs of AD–amyloid pathology–in the medial temporal lobe (Braak et al., 2011). The findings of Braak et al. (2011) also indicate that pathologic tau processes are not exclusive to older age but instead are present at low levels even among younger adults, with the extent of pathology progressing across the lifespan at different rates for different individuals. Consistent with this observation, LC neurofibrillary tangles and abnormal tau show an age-MCI-AD continuum, such that higher levels of these cytopathologies are associated with greater cognitive impairment (Grudzien et al., 2007). From the perspective that both LC cytopathology and the transition from normal cognitive aging to clinical dementia occur along a continuum, it might be possible to link estimates of LC structure to reserve within a healthy population.
The aim of this neuroimaging study was to link variations in LC neuromelanin signal intensity, or contrast-to-noise ratio (CNR), to proxies of cognitive reserve in normal aging. To this end, we used neuromelanin-sensitive weighted MRI to visualize and measure the mean signal intensity of the LC in healthy younger and older adults. Until recently, imaging the human LC was notoriously difficult because of its small size (approximately 2–15 mm, Chan-Palay and Asan, 1989) and low MR signal in conventional T1-weighted anatomic images. However, a growing number of studies have taken advantage of the fact that–unlike most other structures in the brainstem–the LC contains neuromelanin, a byproduct of NE metabolism (Sasaki et al., 2006). Using MR sequences sensitive to neuromelanin, the LC can be visualized effectively (Shibata et al., 2006). For instance, 3-T fast spin-echo (FSE) T1-weighted MRI sequences can enhance MR signal contrast between the LC and neighboring brainstem tissue (Keren et al., 2009; Sasaki et al., 2006; Shibata et al., 2006; Takahashi et al., 2015). Estimating neuromelanin signal intensity is, therefore, a promising in vivo biomarker of LC structure in humans.
To test the LC-reserve hypothesis, we examined the association between LC signal intensity and 3 proxies of cognitive reserve: years of education, occupational attainment scores, and verbal intelligence. Human neuroimaging studies have also identified that gray matter volume in several brain regions, including the prefrontal and parietal cortices, are associated with a composite measure of cognitive reserve (Bartrés-Faz et al., 2009). Thus, to determine the specificity of the predicted LC-reserve association, we also used voxel-based morphometry (VBM) to examine whether proxies of cognitive reserve were related to regional variations in brain structure more generally.
We hypothesized that education, occupational complexity, and verbal intelligence–and, in particular, an additional cognitive reserve factor score capturing shared variance among these 3 variables–would be positively associated with LC signal intensity in older adults. Additionally, we examined age and sex differences in LC signal intensity based on evidence that human LC neuromelanin concentration changes across the lifespan (Shibata et al., 2006) and that LC volume differs by sex in rodents (Babstock et al., 1997). As suggested by Christensen et al. (2007), the most meaningful test of the reserve hypothesis examines how the relationship between biomarkers and cognitive measures varies as a function of reserve level. Thus, to test this possibility, we performed a moderation analysis to determine whether cognitive reserve factor scores moderated the relationship between LC signal intensity and the “shifting” subscale of the Attentional Control Scale (ACS) (Derryberry and Reed, 2002) in healthy older adults.
2. Methods
2.1. Participant characteristics
Twenty-eight healthy older and 35 healthy younger adults were recruited to participate in this study. All participants had normal or corrected-to-normal vision and hearing, and self-reported no history of chronic illness or cognitive impairment. One male older adult participant was taking a beta-blocker.1 All participants provided written informed consent approved by the University of Southern California Institutional Review Board. For all analyses, 5 older adults (2 women) were excluded: 3 participants had extreme motion artifacts in their FSE images, 1 participant had an incidental finding on a separate radiological MRI scan, and an FSE scan was not collected for 1 participant. Two younger men were excluded because they did not have FSE scans collected. Thus, the LC signal intensities of 23 healthy older (9 women; age: mean [M] = 67, standard deviation [SD] = 5; range = 58–75) and 33 healthy younger (14 women; age: M = 24, SD = 5; range = 18–34) adults were analyzed in this study (Table 1).
Table 1.
Demographic and cognitive performance data
| Measures | Young adults | Older adults |
|---|---|---|
| n (F) | 33 (14) | 23 (9) |
| Age (SD) | 24 (5) | 67 (5) |
| Education (SD) | 16 (3) | 17 (2) |
| WTAR (SD) | 43 (6) | 41 (7) |
| Occupation (SD) | N/A | 81 (11) |
| ACS | N/A | 60 (6) |
| ACS—shifting (SD) | N/A | 14 (3) |
| ACS—focusing (SD) | N/A | 23 (2) |
| State anxiety (STAI, SD) | 39 (11) | 27 (6) |
| Trait anxiety (STAI, SD) | 36 (10) | 28 (7) |
Not all sample sizes for the behavioral/demographic variables are equal—see Section 2.2 for specific exclusions and scoring procedures. Most young adults were full-time students; so, they do not have an occupational attainment score. Age and education were measured in years.
Key: ACS, Attention Control Scale; F, female; N/A, not applicable; SD, standard deviation; STAI, State-Trait Anxiety Inventory; WTAR, Wechsler Test of Adult Reading.
2.2. Cognitive reserve variables
Before scanning, participants completed a demographic questionnaire measuring age, sex, and 2 environmental factors that have been previously linked to cognitive reserve: education and occupational complexity (Table 1). For occupation, participants provided their job title along with a detailed description of their profession. This information was converted into an “occupational attainment” variable using a scale reported in Nam and Boyd (2004), which scores a wide range of occupations based on their intellectual complexity and difficulty (Nam and Boyd, 2004). Occupational attainment was scored by 2 separate raters (DC and AP: intraclass coefficient [ICC] = 0.85) and then averaged together. Occupational attainment scores were only determined for older adults because most of the younger adults were students.
Participants also completed the Wechsler Test of Adult Reading (Wechsler, 2001), which served as a proxy for verbal IQ (Armstrong et al., 2012; Leeson et al., 2011). On the Wechsler Test of Adult Reading (WTAR), participants were prompted by an experimenter to pronounce 50 irregularly spelled words. WTAR performance was measured as the number of correct word pronunciations out of a total of 50. Independent sample t tests revealed no significant age difference in WTAR scores or years of education between young and older adults (ps > 0.1).
To test for a relationship between LC signal intensity and reserve, each of these cognitive reserve variables was individually correlated with LC signal intensity in each age group, separately. Robust correlations were performed using a Theil-Sen estimator, a robust linear correlation technique that is less sensitive to outliers than parametric regressions (Wilcox, 2004). One additional male older adult missed >12 consecutive words on the WTAR, resulting in the test being discontinued; therefore, this participant was excluded. Additionally, WTAR data were not collected for 3 other older adults, yielding a total n of 19 for older adult LC-WTAR correlation analyses. WTAR data were not collected for 2 young adults (1 woman) because they were non-native English speakers. Thus, a total n of 31 young adults were analyzed in the LC-WTAR correlation analyses.
2.3. Cognitive reserve factor analysis in older adults
The idea of cognitive reserve refers to an amalgamation of mentally stimulating factors that confer resilience to cognitive impairment. Thus, a factor analysis was performed in the older adults to identify shared variance among education, occupational attainment, and WTAR scores. We performed a principal component analysis of a correlation matrix with an unrotated factor solution. Factor extraction was constrained to eigenvalues >1. This analysis produced a single composite “cognitive reserve” score that was then linearly correlated with LC signal intensity using a Theil-Sen estimator. A total of 19 older adults were analyzed based on the criteria of having all 3 reserve measures. The factor loadings were high for all 3 variables, indicating they were highly intercorrelated: WTAR (0.85), occupational attainment (0.84), and education (0.91).
2.4. Executive attention measures
To examine the relationship between LC structure and cognitive ability in older adults, we administered the ACS (Table 1). The ACS is a 20-item self-report measure assessing an individual's ability to switch attention between tasks and focus attention. Although this cognitive outcome measure is based on self-report rather than performance, previous studies demonstrate that the ACS is highly correlated with performance on a Go/No-Go task (Judah et al., 2014).
Consistent with the proposal of Derryberry and Reed (2002), one large behavioral study validated and confirmed that ACS data have a 2-factor structure, such that the item responses can be dissociated into separate aspects of attention: focusing and shifting (Judah et al., 2014). In a validation factor analysis, the 2-factor solution included 7 items loading on the focusing factor and 5 items loading on the shifting factor. The item with the highest loading score for the focusing subscale was “When I need to concentrate and solve a problem, I have trouble focusing my attention,” whereas the item that had the largest loading for the shifting subscale was “It is easy for me to alternate between two different tasks.”
The same items identified in this factor analysis were used to calculate a focusing and shifting score for each of the older adults in our dataset. One older adult was not administered the ACS, so was excluded from the moderation analysis (resulting in an n = 18). Based on much evidence that the noradrenergic system regulates set switching and cognitive flexibility via its modulation of the prefrontal cortex (Robbins and Arnsten, 2009), we expected that shifting–which is also associated with working memory performance (Derryberry and Reed, 2002)–would be significantly associated with LC signal intensity in the older adults.
Additionally, to control for the confounding effects of state anxiety on ACS attentional shifting (Judah et al., 2014), we also administered the 40-question State-Trait Anxiety Inventory (Spielberger, 1983) to the participants before their MRI scan so that we could model state anxiety (20 of the questions) as a nuisance covariate in the moderation analysis (see Table 1 and Section 2.5). In the absence of brain damage in healthy older individuals, the brain reserve model posits that neural reserve estimates should be directly associated with better cognitive outcomes. To test for this, ACS shifting scores were linearly correlated with LC signal intensity using a Theil-Sen estimator. One female older adult had missing values on her ACS questionnaire; so, her incomplete data were excluded from this analysis (n = 22).
2.5. Cognitive reserve moderation analysis
It was recently suggested that a fundamental test of the cognitive reserve hypothesis tests whether or not reserve scores moderate the strength of the relationship between brain structure and healthy cognitive outcomes (Christensen et al., 2007). Thus, to determine whether older adults’ cognitive reserve scores moderated the strength of the association between LC signal intensity and ACS shifting scores, we performed a multiple linear regression.
In a stepwise regression, continuous values for age, state anxiety, cognitive reserve loading score, LC signal intensity, and sex (only categorical variable) were mean centered and modeled as predictors of shifting in the first step of the regression. An interaction term for LC signal intensities and cognitive reserve factor loading scores was computed by multiplying their centered values together. This moderation term was then modeled in the second step of the regression, and the reserve moderation effect was tested by examining whether this interaction term in step 2 made a significant change to the adjusted R2 with a nominal α = 0.05.
2.6. MRI data acquisition
Neuroimaging data were acquired with a 3-T Siemens Magnetom Trio scanner at the USC Dana & David Dornsife Cognitive Neuroscience Imaging Center. One neuromelanin-sensitive–weighted MRI scan was collected using a T1-weighted FSE imaging sequence (repetition time = 750 ms, echo time = 12 ms, flip angle = 120°, 1 average to increase signal-to-noise ratio (SNR), 11 axial slices, field of view = 220 mm, bandwidth = 220 Hz/Px, slice thickness = 2.5 mm, slice gap = 3.5 mm, in-plane resolution = 0.429 × 0.429 mm2, and scan duration = 1 minute and 53 seconds). To examine the relationship between regional gray matter volumes and cognitive reserve scores, we also collected a high-resolution T1-weighted anatomic image for each participant (repetition time = 2,300 ms, echo time = 2.26 ms, inversion time = 1,060 ms, flip angle = 9°, 176 sagittal slices, field of view = 256 mm, bandwidth = 200 Hz/Px, voxel resolution = 1 mm3 isotropic, and scan duration = 4 minutes and 44 seconds).
2.7. LC neuromelanin signal intensity analysis
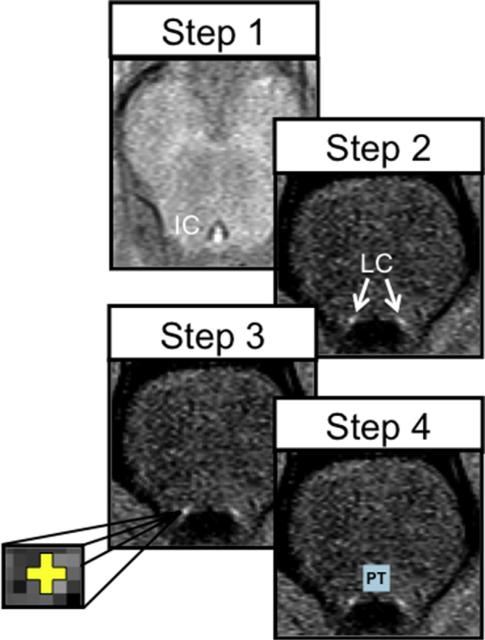
To measure LC neuromelanin signal intensity, left and right LC regions of interest (ROIs) were hand drawn by 2 separate researchers (DC and EM) on each participant's FSE T1-weighted images using the FSLVIEW tool in FSL, version 5.0.4 (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Both researchers were blind to all other participant information. The ICC ranged from 0.81 to 0.88, indicating high interrater reliability in estimating the location and mean signal intensity of the LC. For an overview of the anatomic tracing protocol, see Fig. 1.
Fig. 1.
Locus coeruleus (LC) anatomic tracing protocol. Step 1: in the axial plane, the inferiormost slice of the inferior colliculus (IC) was located; we then moved down 7 mm (2 slices) into a slice where LC signal intensities were most apparent. Step 2: left and right LC regions of interest (ROIs) appeared as high (bright) signal intensities neighboring the corners of the fourth ventricle. Step 3: a small cross (3 × 3 voxels, yellow) of approximately the width of the LC (~1–2 mm) was placed on the voxels with peak signal intensity. Step 4: a dorsal pontine tegmentum reference ROI (light blue) was defined as a 10 × 10 voxel square located 6 voxels above the more ventral (higher in the magnetic resonance axial image) of the 2 LCs and equidistantly between them. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Consistent with earlier LC-tracing procedures, LC ROIs were defined in the axial slice located 7 mm below the inferior boundary of the inferior colliculus where signal consistent with the neuroanatomic location of the LC is most evident (Shibata et al., 2006). Within this axial slice, 2 LC ROIs were manually delineated as a cross that was ~1.29 mm wide and ~1.29 mm high (i.e., 3 × 3 voxels, see Fig. 1) to approximate the 1- to 2-mm distribution of LC neurons in this slice (German et al., 1988). These ROIs were centered on the left and right voxels with the highest signal intensities in locations that were anatomically consistent with the LC. In some instances, the peak voxel was located immediately adjacent to the fourth ventricle. Thus, to avoid low signal value in the ventricle (i.e., partial volume effects), the center of the ROI was placed one voxel further away from the ventricle; that way, the LC ROI still captured the peak voxel while avoiding underestimating overall signal intensity. The mean signal intensities from the left and right LC ROIs were then extracted and averaged to increase the SNR of the intensity estimates.
To control for noise variability in the FSE images, a reference ROI was drawn in the dorsal PT using a 10 × 10 voxel square (18.4 mm2). This size was chosen to avoid capturing low signal intensities in the ventral tip of the fourth ventricle and the darker medial leminiscus/pons. The dorsal boundary of the PT ROI was determined by moving 6 voxels (2.57 mm) from the center of the more ventral of the 2 LC ROIs toward the pons. The PT was drawn equidistantly between the left and right LC ROIs. If there was an odd number of voxels between the left and right LC ROIs, the PT square was drawn 1 voxel closer to the left LC ROI (right side in MNI space, see Fig. 1).
LC CNRs were calculated based on the mean LC signal intensity relative to the reference PT signal intensity using the following formula: LCCNR = (LCintensity – PTintensity)/PTintensity (Sasaki et al., 2006; Shibata et al., 2006). The 2 raters’ estimates of LC CNR were averaged together to increase the SNR. A 2 × 2 analysis of variance (ANOVA) was performed with age (young vs. old) and sex (female vs. male) modeled as between-subject factors and LC CNR–henceforth simply referred to in the text as LC signal intensity–as the dependent variable. Follow-up Bonferonni-corrected independent samples t tests were performed to test for main effects of sex within each age group, separately.
2.8. VBM analysis
Next, to determine whether cognitive reserve was specifically associated with LC signal intensity or whether it was related to brain structural variability more broadly, we performed a VBM analysis. FSLVBM (http://www.fmrib.ox.ac.uk/fsl/fslvbm) was used to identify brain regions that showed a significant correlation between regional brain volume and cognitive reserve factor loading scores (Good et al., 2002). First, the high-resolution T1-weighted anatomic images were brain extracted. These images were then prepared for VBM by (1) segmenting the unsmoothed volumes into partial-volume probabilistic cerebrospinal fluid, white matter, and gray matter masks; (2) transforming these native-space masks into MNI space using affine and subsequent nonlinear transformations and averaging these images together to create a study-specific gray matter template; (3) modulating MNI-space gray matter masks for each older participant using the Jacobian of the warp field; and (4) smoothing these images with a Gaussian kernel with a sigma = 3 mm.
The gray matter volumes for older adults with a reserve loading score (n = 19) were analyzed voxelwise using permutation-based nonparametric testing (i.e., using FSL's randomize tool) with 5,000 permutations and threshold-free cluster enhancement to correct for multiple comparisons (Smith and Nichols, 2009). The association between regional gray matter volume and cognitive reserve scores was tested using a general linear model with demeaned composite reserve scores as the covariate of interest. Demeaned values for age, sex, and total intracranial volume were also included in the general linear model as nuisance variables. In the resulting regression maps, clusters of “activation” would signify regions that had a significantly positive or negative partial correlation with cognitive reserve values.
3. Results
3.1. The effects of age and sex on LC signal intensity
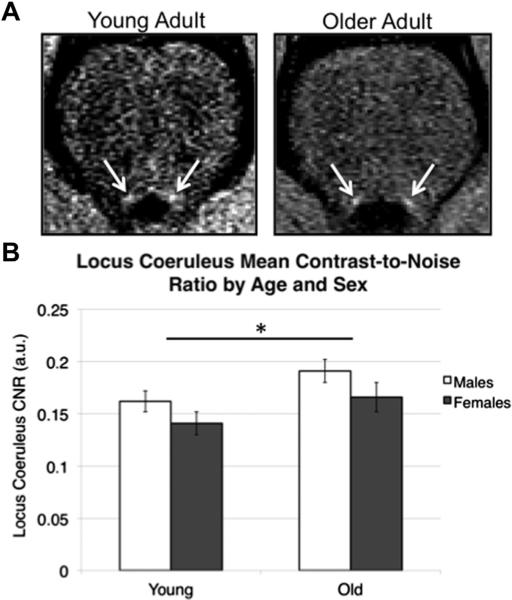
A 2 × 2 between-subjects ANOVA was used to determine the effects of age and sex on mean LC signal intensity (Fig. 2). The results indicated a significant main effect of age such that older adults exhibited higher mean LC signal intensity than younger adults, F(1,52) = 5.47, p = 0.023, η2 = 0.095 (old: M = 0.18, standard error of the mean [SEM] = 0.009; young: M = 0.15, SEM = 0.007). Women also showed significantly attenuated LC signal intensity compared with men, F(1,52) = 4.08, p = 0.049, η2 = 0.073 (women: M = 0.15, SEM = 0.009; men: M = 0.18, SEM = 0.007). There was no significant age × sex interaction effect or any main effect of sex within either age group (ps > 0.1). Follow-up Bonferroni-corrected independent sample t tests revealed that this sex difference in LC signal intensity was not significant within either age group alone (ps > 0.1).
Fig. 2.
Age and sex differences in locus coeruleus (LC) signal intensity. (A) An example of a left and right LC in the neuromelanin-sensitive T1-weighted fast-spin echo images of one younger and one older adult. For illustrative purposes only, the intensity thresholds have been adjusted to maximize the visual contrast between the LC and adjacent brainstem tissue because the mean LC signal intensities differ significantly between age groups. Because each participant's fast spin-echo image is normalized relative to the noise in his/her image, such adjustments are only meant to demonstrate that the LC is clearly delineable in both age groups. (B) Age and sex differences in LC signal intensity are displayed as bars (means) for each subgroup. Bars reflect standard errors of the means. *p < 0.05.
To acquire a consistent comparison between the LC signal intensities analyzed in the between-subject ANOVA and their linear associations with the demographic/behavioral data, we performed a follow-up analysis using only the participants that had complete data for both, YA: n = 31 (13 women) and OA: n = 19 (9 women). Across this subsample, the main effects of age and sex were even more significant, age: F(1,46) = 8.42, p = 0.006, η2 = 0.16 and sex: F(1,46) = 5.72, p = 0.021, η2 = 0.11. Although there was still no significant age × sex interaction effect (p > 0.1), 2 independent samples t tests with Bonferonni correction revealed a marginally significant effect of sex on LC signal intensity in the older adult subgroup (men > women, p = 0.057) but not in the young adult subgroup (men > women, p = 0.23).
Overall brainstem signal intensity differed across age groups, as older adults showed significantly lower PT signal intensity than younger adults, F(1,52) = 24.04, p < 0.001, η2 = 0.32, and significantly lower raw (non-normalized) LC signal intensity, F(1,52) = 12.09, p = 0.001, η2 = 0.19. But, for our purposes, the critical factor was the degree to which LC has greater contrast than the PT control regions, which is a reference for idiosyncrasies in within-participant image noise variability.
3.2. Relationship between LC signal intensity and cognitive reserve variables by age group
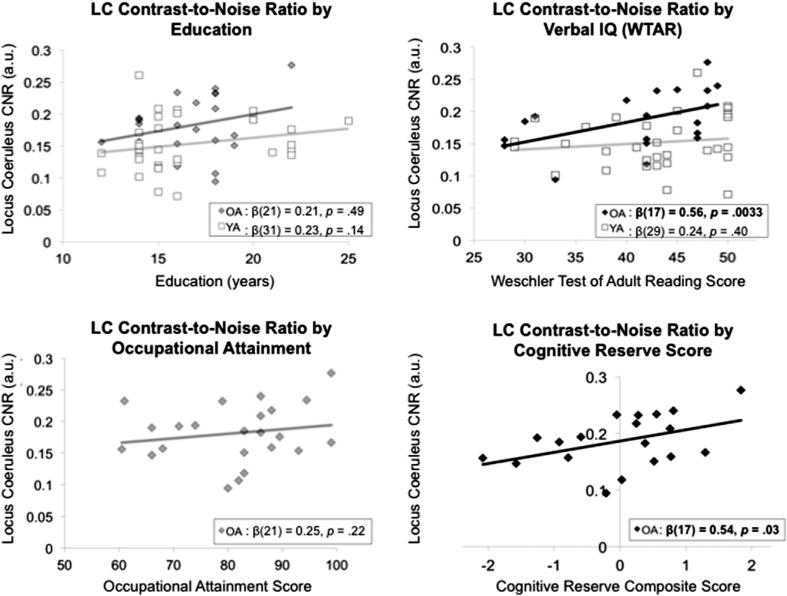
To test our main hypothesis that LC signal intensity is positively associated with cognitive reserve, we performed robust linear correlations in each age group, separately (Fig. 3). Among the 3 cognitive reserve proxies, verbal intelligence was the only variable significantly positively associated with LC signal intensity in older adults, β(17) = 0.56, p = 0.0033. Thus, older adults with higher LC signal intensity also exhibited better verbal performance on the WTAR. This LC-WTAR relationship was still significant after controlling for the effects of age and sex, β(14) = 0.56, p = 0.013. Neither of the reserve measures collected in younger adults (education and WTAR) was significantly associated with LC signal intensity (ps > 0.05).
Fig. 3.
Correlations between education, Wechsler Test of Adult Reading (WTAR) (index of verbal intelligence), and occupational attainment displayed for each age subgroup, separately. In older adults, a cognitive reserve composite score–calculated as the shared variance among the 3 reserve variables–was also correlated with locus coeruleus (LC) signal intensity. Darker bars indicate statistically significant correlation.
As predicted, LC signal intensity was positively associated with the composite cognitive reserve score in older adults, β(17) = 0.54, p = 0.03, which was calculated based on the shared variance among years of education, occupational attainment, and WTAR scores. However, the strength of this LC-reserve relationship diminished after controlling for the effects of age and sex, β(14) = 0.43, p = 0.077.
Unlike the mean LC CNR analysis, PT signal intensity did not significantly correlate with continuous age values in either age group (ps > 0.1). Thus, underlying differences in the reference ROI did not confound these LC-reserve relationships. As further validation, we examined whether raw LC signal intensities (i.e., mean values not normalized by PT intensity) also showed the same associations. Indeed, the LC-WTAR and LC-reserve correlations were still significant within the older adult group, indicating that these relationships were not artifacts of variability in PT intensity reference values.
A core postulate of the brain reserve hypothesis is that, in healthy individuals, brain reserve estimates should be correlated with better cognitive outcomes. Our LC-shifting correlation analysis did not completely validate this prediction, with higher LC signal intensity trending toward a positive association with ACS shifting scores, β(20) = 0.31, p = 0.13. Accounting for the effects of age, sex, and state anxiety had little effect on this relationship, β(17) = 0.33, p = 0.18. The same LC-shifting correlations were also not significant in the smaller group of older participants with both cognitive reserve loading and ACS scores available (n= 18, ps > 0.1).
3.3. VBM results
In the older adults, there were no brain regions where ICV-normalized gray matter volume was significantly correlated with cognitive reserve factor scores or LC signal intensity when controlling for sex and age. Thus, within our dataset, cognitive reserve scores were only significantly associated with LC signal intensity.
3.4. Cognitive reserve moderation analysis results
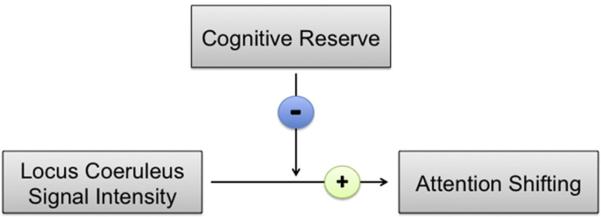
Although the main effect of LC signal intensity on attentional shifting was marginally significant, the idea of reserve is more readily invoked by the strength of this relationship differing as a function of cognitive reserve levels. Indeed, consistent with our main prediction that reserve factor loadings would moderate the LC-shifting relationship, the cognitive reserve × LC signal intensity interaction term explained a significant increase in variance in shifting scores above and beyond the influence of any individual predictor, ΔR2 = 0.26, F(1,11) = 5.17, p = 0.044. The standardized regression coefficient for the moderation term was negative, β = −0.59, indicating that, as cognitive reserve levels linearly decreased, the relationship between LC signal intensity and attentional shifting became stronger (Fig. 4).
Fig. 4.
A schematic of cognitive reserve's moderating effect on the locus coeruleus (LC)-attention association in healthy older adults. Continuous values for cognitive reserve factor scores moderated the strength of the positive relationship between LC signal intensity and attentional shifting. The negative standardized regression coefficient of the reserve × LC intensity interaction term indicated that as reserve levels linearly decreased, the association between LC signal intensity and attentional shifting strengthened significantly.
4. Discussion
In the present study, we tested the recent proposal that the LC-NE system is an important component of neural reserve (Robertson, 2013). Our results supported this hypothesis by providing the first in vivo evidence that variations in human LC neuromelanin signal intensity were associated with established proxies of cognitive reserve in healthy older adults. Cognitive reserve loading scores moderated the strength of the relationship between LC signal intensity and a self-report measure of attention shifting, such that this association became stronger in individuals with lower levels of cognitive reserve. This finding suggests that low-reserve older adults who may be more vulnerable to cognitive decline might also rely more heavily on normal noradrenergic system function to promote or maintain executive function. Together, our results support a growing literature implicating the noradrenergic system in cognitive aging.
Consistent with postmortem (Zecca et al., 2004; Zucca et al., 2006) and neuroimaging studies of LC structure in humans (Shibata et al., 2006), we found that older adults had higher LC CNR than younger adults. This finding accords with evidence that LC neuromelanin accrues in an inverted U pattern across the lifespan, with peak concentrations occurring around the age of 60 before rapidly decreasing (Manaye et al., 1995; Shibata et al., 2006). It is noteworthy, however, that the age-related increase in LC CNR we observed appeared to be an artifact of a substantial age-related decrease (~9.6%) in raw PT signal intensity. This finding is consistent with an earlier human neuromelanin MRI study at 3 T that found significant age-related decreases in PT signal intensity (Keren et al., 2009). Unfortunately, age differences in PT reference intensity were not reported in Shibata et al. (2006); so, it is unclear whether their estimates of LC CNR were similarly inflated by age-related decreases in the reference signal.
Notably, there are some mixed findings concerning LC neuronal changes with age. In some human histological studies, there were no significant age-related changes in the quantity of neuromelanin-containing LC neurons (Mouton et al., 1994; Ohm et al., 1997), whereas in other studies, LC neuron count was lower in older than younger adults (Manaye et al., 1995). Combined with our current finding of reduced PT signal intensity with age, we conclude that age-related changes in LC CNR should be interpreted with caution. Nonetheless, fast spin-echo MRI remains a highly effective method for localizing and quantifying neuromelanin-containing LC neurons in vivo (Keren et al., 2009, 2015).
Importantly, our main goal of identifying LC-reserve associations was not confounded by issues with baseline PT intensity because these relationships did not change when we used raw LC signal intensities. Our key finding was that, in older adults, cognitive reserve–as operationalized as shared variance among education, occupational complexity, and verbal intelligence–was positively correlated with LC intensity. Among the 3 individual reserve variables examined in this study, verbal intelligence (WTAR performance) was the only variable significantly correlated with LC signal intensity in older adults. Human research concerning the relationship between cognitive reserve variables and LC-NE system function is sparse, but there are some indications that pupil dilation, an index of LC activity (Murphy et al., 2014), relates to IQ level. Individuals with higher than average IQ show greater pupil dilation to difficult analogies than individuals with average IQ (Bornemann et al., 2010; Van Der Meer et al., 2010). The cognitive-enhancing effects of modafinil administration, which alters LC activity (Minzenberg et al., 2008), also appear to vary as a function of IQ level, with lower IQ individuals’ performance benefiting more from its administration (Randall et al., 2005).
Our finding that low-reserve older adults showed a tighter LC-shifting relationship than older adults with higher cognitive reserve supports this notion that the noradrenergic system's influence on cognition varies according to cognitive reserve capacity. A previous behavioral study demonstrated that the shifting subscale of the ACS is also correlated with working memory performance, as measured by letter-number sequencing performance (Judah et al., 2014). Thus, our findings accord with the well-established role of the LC-NE system in regulating executive function, particularly during effortful cognition and attention (Arnsten and Li, 2005; Bouret and Richmond, 2015; Murphy et al., 2011).
In contrast, there were no significant LC-reserve relationships in the young adults. One potentially contributing factor is that younger adults have yet to accumulate their full neuromelanin levels. In the LC, neuromelanin pigment is a by-product of autophagic degradation of oxidized NE and NE metabolites (Wakamatsu et al., 2015). Thus, older adults’ neuromelanin reflects a longer span of exposure to NE than does that of younger adults. In addition, healthy younger adults have yet to cope with age-related neuronal degeneration; so, the compensatory function of the LC-NE system might not be as important and/or as apparent as in older adults. As our moderation analysis revealed, such compensation by the LC-NE system might only manifest in low-reserve older adults. In essence, LC neuromelanin signal intensity was more strongly associated with self-assessed impairments in cognitive ability in the context of lower cognitive reserve capacity. With respect to the other individual reserve proxies, most of the younger adults in this study were undergraduate college students who have not finished their education or have fulltime occupations; therefore, they have yet to build up their full reserve, which is accumulated over a lifetime of mentally stimulating experiences (Richards and Sacker, 2003).
Another key finding was a significant sex difference in LC signal intensity, such that, on average, women showed ~20% lower LC signal intensity than men. To our knowledge, this is the first in vivo evidence of sex differences in LC structure in humans. A similar finding was reported in Sprague-Dawley rates wherein dorsal LC neuronal volume was denser in men than in women (Babstock et al., 1997; Bangasser et al., 2011). Lower LC signal intensity in women is intriguing given that various pathologies that are more prevalent in women have also been linked to the LC-NE system. For example, women have an increased risk of AD compared with men (Andersen et al., 1999), and female versus male carriers of the APOE4 allele, the strongest genetic risk factor for sporadic AD, are more likely to develop AD (Altmann et al., 2014). Moreover, one large-scale histologic study demonstrated that increased brain amyloid pathology, which also encompassed measurements in the lower brainstem, was more tightly correlated with age in women than in men (Braak et al., 2011). We examined a cognitively healthy population; however, we can only speculate on this relationship.
As detailed in the LC-reserve hypothesis of Robertson (2013), activating the LC-NE system function via mentally stimulating experiences may improve cognitive outcomes by triggering a variety of neuroprotective and memory-enhancing effects. Much research indicates that central NE release protects target neurons throughout the brain, particularly those in regions that facilitate learning and memory. NE reduces neuroinflammation (Feinstein et al., 2002), helps ameliorate amyloid cellular toxicity (Counts and Mufson, 2010), and protects cultured neurons from metabolic (Madrigal et al., 2009) and oxidative stress (Troadec et al., 2001). LC-NE activation can increase BDNF production (Jurič et al., 2006) and hippocampal neurogenesis (Kulkarni et al., 2002; Masuda et al., 2012; Veyrac et al., 2009)–both of which promote healthy cognitive and memory function. Exploration of novel environments, one form of mental stimulation, activates the LC (Kitchigina et al., 1997; Sara et al., 1994) and enhances memory via an adrenergic mechanism (Straube et al., 2003; Veyrac et al., 2009). Furthermore, activating β2-adrenergic receptors through environmental enrichment also prevents memory impairments induced by Aβ oligomers in the hippocampus (Li et al., 2013). Thus, cognitive reserve factors may protect cognitive abilities by enhancing neuroprotective and neuroplastic processes regulated by the LC.
Of key importance to the present study, neuromelanin also protects healthy LC function. LC neuromelanin chelates various metal oxidants, including mercury, lead, and iron (Zecca et al., 2004, 2008; Zucca et al., 2006), and scavenges free radicals (Álvarez-Diduk and Galano, 2015). Such protection against cell damage is particularly important given the LC's widespread exposure to–and consequent regulation of–circulating toxicants in the bloodstream (Mann, 1983; Pamphlett and Jew, 2013; Pamphlett, 2014). This research has led to speculation that age-related disintegration of neuromelanin releases previously immobilized toxicants back into the cell, leading to impaired NE output (Pamphlett, 2014; Pamphlett and Jew, 2015). Signal intensity might, therefore, be an effective biomarker of neuromelanin-mediated protection over LC neurons because neuromelanin bound to iron and copper generates the paramagnetic T1 effect exploited by FSE T1 imaging (Enochs et al., 1997; Tosk et al., 1992). It is noteworthy that local iron accumulation also affects signal estimates in T1 FSE images (Vymazal et al., 1999). However, unlike the substantia nigra, iron levels in the LC remain relatively stable across the lifespan (Zucca et al., 2006). Thus, we conclude that neuromelanin buffering rather than iron concentration most likely contributed to overall LC signal intensity measured in this study.
Taken together, our results are consistent with a rapidly growing literature implicating the noradrenergic system in healthy cognitive aging. However, there are several important limitations to address. Our sample sizes were modest, which limited our investigation of sex differences in the relationship between LC signal intensity and different reserve variables. Another important limitation is that we had a limited set of cognitive outcome measures. Although previous work shows that ACS shifting is correlated with executive function, this is not a 1:1 relationship; thus, self-assessed cognitive ability might not fully approximate actual performance. Future investigations should include a battery of neuropsychological assessments to more thoroughly measure cognitive performance and confirm its relationship with LC signal intensity in older adults. Because this was a cross-sectional study, we were unable to determine whether the reserve variables causally increase LC neuromelanin concentration or vice versa and whether or not variability in neuromelanin concentration causally relates to different cognitive outcomes. Thus, additional longitudinal studies are needed to confirm whether cognitive changes over time correspond with the degree of LC neuromelanin signal attenuation in later adulthood. In particular, it would be useful to test whether LC neuromelanin still relates to cognitive reserve variables in MCI or Alzheimer's patients, which would provide additional support that the LC is key mediator of the protective influence of environmental enrichment on cognition.
Cognitive reserve is complex and is associated with an array of environmental factors, including time spent doing mentally engaging activities (e.g., puzzles), that share variance with the variables used in our study. The WTAR, in particular, might not be an ideal measure of cognitive reserve because it relates to crystallized intelligence, which is relatively stable across the lifespan, rather than fluid intelligence, which is more closely related to cognitive flexibility and decreases with age (Schaie and Willis, 1993). Additionally, IQ is heavily determined by genetic factors; so, WTAR itself may more likely be a proxy for other experience-dependent factors, such as openness to learning, more directly involved. Although the WTAR might fail to fully explain the variance in reserve, there are indications that WTAR performance can predict incidence of PD-related MCI above and beyond the influence of education (Armstrong et al., 2012). Nonetheless, the implications of the WTAR-LC association identified in this study should be interpreted with caution, as other mechanisms/proxies related to verbal intelligence may be at play.
Finally, combining fMRI measures of LC activity during a cognitive task with FSE imaging would help establish whether LC structure and proxies of cognitive reserve are also associated with increased neural efficiency in the LC. The role of the LC in cognitive reserve is also theorized to involve its modulation of a broader, right hemisphere–biased executive attention network (Robertson, 2014). Thus, fMRI would help elucidate whether this network's processing efficiency is differentially associated with the functional (blood oxygen level–dependent) and structural (FSE) integrity of the noradrenergic system based on one's cognitive and brain reserve capacity.
5. Conclusions
Using neuromelanin-sensitive MRI in healthy older adults, we demonstrated that an in vivo measure of LC neuromelanin signal intensity is associated cognitive reserve, particularly verbal intelligence. We also found that older adults with lower levels of cognitive reserve showed a stronger association between LC signal intensity and attentional shifting, suggesting that–particularly for elders potentially vulnerable to cognitive decline–the integrity of the noradrenergic system helps support cognitive flexibility. Together, these findings support the idea that, by augmenting LC-NE system function, the same intellectually engaging experiences that help enrich one's life might also protect cognitive health in later adulthood.
Acknowledgements
We thank Jiancheng Zhuang, PhD, for helping adjust and optimize the fast spin-echo scan protocol for our Siemens MRI scanner and Rico Velasco for his assistance with scanning participants. We also thank Ringo Huang for his input regarding the anatomic tracing protocol and Drs Carolyn Harley and Caleb Finch for providing insightful comments on early drafts of the manuscript. This project was funded by federal National Institutes of Health grants R01AG038043 and K02AG032309.
Footnotes
After removing the older adult on atenolol, the results of all analyses in this study not only remained the same but also – in some instances – became more statistically significant. For instance, in Section 3.1, the new mixed ANOVA revealed larger main effects of Age, F(1,51) = 5.69, p = 0.021, and Sex, F(1,51) = 4.29, p = 0. 043, on LC signal intensity. Furthermore, whereas the LC-Education, LC-Occupation, and LC Shifting correlations in Section 3.2 remained non-significant, the relationship between LC signal intensity and composite reserve score became stronger both when not controlling for, β(16) = .57, p = 0.0067, and controlling for the influence of sex and age, β(13) = .51, p = 0.02. In the original LC-reserve analysis, controlling for the effects of sex and age made this relationship non-significant (p = 0.077; see Section 3.2). Finally, removing this older adult also increased the strength of the moderation effect of cognitive reserve reported in Section 3.4, ΔR2 = .31, F(1,10) = 6. 96, p = 0.025.
Disclosure statement
The authors declare no conflicts of interest.
References
- Ahissar E, Haidarliu S, Shulz D. Possible involvement of neuromodulatory systems in cortical Hebbian-like plasticity. J. Physiol. Paris. 1996;90:353–360. doi: 10.1016/s0928-4257(97)87919-3. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Furey ML, Grady CL, Pietrini P, Brady DR, Mentis MJ, Schapiro MB. Association of premorbid intellectual function with cerebral metabolism in Alzheimer's disease: implications for the cognitive reserve hypothesis. Am. J. Psychiatry. 1997;154:165–172. doi: 10.1176/ajp.154.2.165. [DOI] [PubMed] [Google Scholar]
- Altmann A, Tian L, Henderson VW, Greicius MD. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann. Neurol. 2014;75:563–573. doi: 10.1002/ana.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez-Diduk R, Galano A. Adrenaline and noradrenaline: protectors against oxidative stress or molecular targets? J. Phys. Chem. B. 2015;119:3479–3491. doi: 10.1021/acs.jpcb.5b00052. [DOI] [PubMed] [Google Scholar]
- Andel R, Crowe M, Pedersen NL, Mortimer J, Crimmins E, Johansson B, Gatz M. Complexity of work and risk of Alzheimer's disease: a population-based study of Swedish twins. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2005;60:P251–P258. doi: 10.1093/geronb/60.5.p251. [DOI] [PubMed] [Google Scholar]
- Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland J, Dartigues JF, Kragh-Sorensen P, Baldereschi M, Brayne C, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A. Gender differences in the incidence of AD and vascular dementia the EURODEM Studies. Neurology. 1999;53:1992–1997. doi: 10.1212/wnl.53.9.1992. [DOI] [PubMed] [Google Scholar]
- Armstrong M, Naglie G, Duff-Canning S, Meaney C, Gill D, Eslinger PJ, Zadikoff C, Mapstone M, Chou KL, Persad C, Litvan I, Mast BT, Fox S, Tang-Wai DF, Marras C. Roles of education and IQ in cognitive reserve in Parkinson's disease-mild cognitive impairment. Dement. Geriatr. Cogn. Disord. Extra. 2012;2:343. doi: 10.1159/000341782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Li B-M. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol. Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Babstock D, Malsbury CW, Harley CW. The dorsal locus coeruleus is larger in male than in female SpragueeDawley rats. Neurosci. Lett. 1997;224:157–160. doi: 10.1016/S0304-3940(97)13462-0. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Zhang X, Garachh V, Hanhauser E, Valentino RJ. Sexual dimorphism in locus coeruleus dendritic morphology: a structural basis for sex differences in emotional arousal. Physiol. Behav. 2011;103:342–351. doi: 10.1016/j.physbeh.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartrés-Faz D, Arenaza-Urquijo EM. Structural and functional imaging correlates of cognitive and brain reserve hypotheses in healthy and pathological aging. Brain Topogr. 2011;24:340–357. doi: 10.1007/s10548-011-0195-9. [DOI] [PubMed] [Google Scholar]
- Bartrés-Faz D, Solé-Padullés C, Junqué C, Rami L, Bosch B, Bargalló N, Molinuevo JL. Interactions of cognitive reserve with regional brain anatomy and brain function during a working memory task in healthy elders. Biol. Psychol. 2009;80:256–259. doi: 10.1016/j.biopsycho.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson R, Schneider J, Evans D, De Leon CM, Arnold S, Bienias J. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60:1909–1915. doi: 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bornemann B, Foth M, Horn J, Ries J, Warmuth E, Wartenburger I, van der Meer E. Mathematical cognition: individual differences in resource allocation. ZDM. 2010;42:555–567. [Google Scholar]
- Bouret S, Richmond BJ. Sensitivity of locus ceruleus neurons to reward value for goal-directed actions. J. Neurosci. 2015;35:4005–4014. doi: 10.1523/JNEUROSCI.4553-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. The pathological process underlying Alzheimer's disease in individuals under thirty. Acta Neuropathol. 2011;121:171–181. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- Chalermpalanupap T, Kinkead B, Hu WT, Kummer MP, Hammerschmidt T, Heneka MT, Levey AI. Targeting norepinephrine in mild cognitive impairment and Alzheimer's disease. Alzheimers Res. Ther. 2013;5:21. doi: 10.1186/alzrt175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Müller U, Blackwell AD, Robbins TW, Sahakian BJ. Noradrenergic modulation of working memory and emotional memory in humans. Psychopharmacology. 2006;188:397–407. doi: 10.1007/s00213-006-0391-6. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V, Asan E. Quantitation of catecholamine neurons in the locus coeruleus in human brains of normal young and older adults and in depression. J. Comp. Neurol. 1989;287:357–372. doi: 10.1002/cne.902870307. [DOI] [PubMed] [Google Scholar]
- Christensen H, Anstey KJ, Parslow RA, Maller J, Mackinnon A, Sachdev P. The brain reserve hypothesis, brain atrophy and aging. Gerontology. 2007;53:82–95. doi: 10.1159/000096482. [DOI] [PubMed] [Google Scholar]
- Counts SE, Mufson EJ. Noradrenaline activation of neurotrophic pathways protects against neuronal amyloid toxicity. J. Neurochem. 2010;113:649–660. doi: 10.1111/j.1471-4159.2010.06622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. J. Abnormal Psychol. 2002;111:225. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Enochs WS, Petherick P, Bogdanova A, Mohr U, Weissleder R. Para-magnetic metal scavenging by melanin: MR imaging. Radiology. 1997;204:417–423. doi: 10.1148/radiology.204.2.9240529. [DOI] [PubMed] [Google Scholar]
- Feinstein DL, Heneka MT, Gavrilyuk V, Russo CD, Weinberg G, Galea E. Noradrenergic regulation of inflammatory gene expression in brain. Neurochem. Int. 2002;41:357–365. doi: 10.1016/s0197-0186(02)00049-9. [DOI] [PubMed] [Google Scholar]
- Gatz M, Mortimer JA, Fratiglioni L, Johansson B, Berg S, Andel R, Pedersen NL. Accounting for the relationship between low education and dementia: a twin study. Physiol. Behav. 2007;92:232–237. doi: 10.1016/j.physbeh.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German D, Walker B, Manaye K, Smith W, Woodward D, North A. The human locus coeruleus: computer reconstruction of cellular distribution. J. Neurosci. 1988;8:1776–1788. doi: 10.1523/JNEUROSCI.08-05-01776.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German DC, Manaye KF, White CL, Woodward DJ, McIntire DD, Smith WK, Mann D. Disease-specific patterns of locus coeruleus cell loss. Ann. Neurol. 1992;32:667–676. doi: 10.1002/ana.410320510. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Fristen K, Frackowiak R. A voxel-based morphometric study of ageing in 465 normal adult human brains.. Paper Presented at the Biomedical Imaging, 2002. 5th IEEE EMBS International Summer School on.; 2002. [DOI] [PubMed] [Google Scholar]
- Grudzien A, Shaw P, Weintraub S, Bigio E, Mash DC, Mesulam MM. Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer's disease. Neurobiol. Aging. 2007;28:327–335. doi: 10.1016/j.neurobiolaging.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Harley C. Norepinephrine in arousal, emotion and learning? Limbic modulation by norepinephrine and the Kety hypothesis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1987;11:419–458. doi: 10.1016/0278-5846(87)90015-7. [DOI] [PubMed] [Google Scholar]
- Jacobs HI, Wiese S, van de Ven V, Gronenschild EH, Verhey FR, Matthews PM. Relevance of parahippocampal-locus coeruleus connectivity to memory in early dementia. Neurobiol. Aging. 2015;36:618–626. doi: 10.1016/j.neurobiolaging.2014.10.041. [DOI] [PubMed] [Google Scholar]
- Judah MR, Grant DM, Mills AC, Lechner WV. Factor structure and validation of the attentional control scale. Cogn. Emot. 2014;28:433–451. doi: 10.1080/02699931.2013.835254. [DOI] [PubMed] [Google Scholar]
- Jurič DM, Miklič Š, Čarman-Kržan M. Monoaminergic neuronal activity up-regulates BDNF synthesis in cultured neonatal rat astrocytes. Brain Res. 2006;1108:54–62. doi: 10.1016/j.brainres.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Keren NI, Lozar CT, Harris KC, Morgan PS, Eckert MA. In vivo mapping of the human locus coeruleus. Neuroimage. 2009;47:1261–1267. doi: 10.1016/j.neuroimage.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren NI, Taheri S, Vazey EM, Morgan PS, Granholm AC, Aston-Jones GS, Eckert MA. Histologic validation of locus coeruleus MRI contrast in postmortem tissue. Neuroimage. 2015;113:235–245. doi: 10.1016/j.neuroimage.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchigina V, Vankov A, Harley C, Sara SJ. Novelty-elicited, noradrenaline-dependent enhancement of excitability in the dentate gyrus. Eur. J. Neurosci. 1997;9:41–47. doi: 10.1111/j.1460-9568.1997.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Kulkarni VA, Jha S, Vaidya VA. Depletion of norepinephrine decreases the proliferation, but does not influence the survival and differentiation, of granule cell progenitors in the adult rat hippocampus. Eur. J. Neurosci. 2002;16:2008–2012. doi: 10.1046/j.1460-9568.2002.02268.x. [DOI] [PubMed] [Google Scholar]
- Le Carret N, Lafont S, Letenneur L, Dartigues J-F, Mayo W, Fabrigoule C. The effect of education on cognitive performances and its implication for the constitution of the cognitive reserve. Dev. Neuropsychol. 2003;23:317–337. doi: 10.1207/S15326942DN2303_1. [DOI] [PubMed] [Google Scholar]
- Leeson VC, Sharma P, Harrison M, Ron MA, Barnes TR, Joyce EM. IQ trajectory, cognitive reserve, and clinical outcome following a first episode of psychosis: a 3-year longitudinal study. Schizophr. Bull. 2011;37:768–777. doi: 10.1093/schbul/sbp143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Jin M, Zhang D, Yang T, Koeglsperger T, Fu H, Selkoe DJ. Environmental novelty activates β 2-adrenergic signaling to prevent the impairment of hippocampal LTP by Aβ oligomers. Neuron. 2013;77:929–941. doi: 10.1016/j.neuron.2012.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr JB, Jeste DV. Locus ceruleus morphometry in aging and schizophrenia. Acta Psychiatr. Scand. 1988;77:689–697. doi: 10.1111/j.1600-0447.1988.tb05189.x. [DOI] [PubMed] [Google Scholar]
- Madrigal JL, Leza JC, Polak P, Kalinin S, Feinstein DL. Astrocyte-derived MCP-1 mediates neuroprotective effects of noradrenaline. J. Neurosci. 2009;29:263–267. doi: 10.1523/JNEUROSCI.4926-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaye KF, McIntire DD, Mann D, German DC. Locus coeruleus cell loss in the aging human brain: a non-random process. J. Comp. Neurol. 1995;358:79–87. doi: 10.1002/cne.903580105. [DOI] [PubMed] [Google Scholar]
- Mann DM. The locus coeruleus and its possible role in ageing and degenerative disease of the human central nervous system. Mech. Ageing Dev. 1983;23:73–94. doi: 10.1016/0047-6374(83)90100-8. [DOI] [PubMed] [Google Scholar]
- Masuda T, Nakagawa S, Boku S, Nishikawa H, Takamura N, Kato A, Koyama T. Noradrenaline increases neural precursor cells derived from adult rat dentate gyrus through beta2 receptor. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;36:44–51. doi: 10.1016/j.pnpbp.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Watrous AJ, Yoon JH, Ursu S, Carter CS. Modafinil shifts human locus coeruleus to low-tonic, high-phasic activity during functional MRI. Science. 2008;322:1700–1702. doi: 10.1126/science.1164908. [DOI] [PubMed] [Google Scholar]
- Mouton PR, Pakkenberg B, Gundersen HJG, Price DL. Absolute number and size of pigmented locus coeruleus neurons in young and aged individuals. J. Chem. Neuroanat. 1994;7:185–190. doi: 10.1016/0891-0618(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Mravec B, Lejavova K, Cubinkova V. Locus (coeruleus) minoris resistentiae in pathogenesis of Alzheimer's disease. Curr. Alzheimer Res. 2014;11:992–1001. doi: 10.2174/1567205011666141107130505. [DOI] [PubMed] [Google Scholar]
- Murphy PR, O'Connell RG, O'sullivan M, Robertson IH, Balsters JH. Pupil diameter covaries with BOLD activity in human locus coeruleus. Hum. Brain Mapp. 2014;35:4140–4154. doi: 10.1002/hbm.22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PR, Robertson IH, Balsters JH, O'Connell RG. Pupillometry and P3 index the locus coeruleusdnoradrenergic arousal function in humans. Psychophysiology. 2011;48:1532–1543. doi: 10.1111/j.1469-8986.2011.01226.x. [DOI] [PubMed] [Google Scholar]
- Nam CB, Boyd M. Occupational status in 2000; over a century of census-based measurement. Popul. Res. Policy Rev. 2004;23:327–358. [Google Scholar]
- Neuman RS, Harley CW. Long-lasting potentiation of the dentate gyrus population spike by norepinephrine. Brain Res. 1983;273:162–165. doi: 10.1016/0006-8993(83)91106-x. [DOI] [PubMed] [Google Scholar]
- Ohm T, Busch C, Bohl J. Unbiased estimation of neuronal numbers in the human nucleus coeruleus during aging. Neurobiol. Aging. 1997;18:393–399. doi: 10.1016/s0197-4580(97)00034-1. [DOI] [PubMed] [Google Scholar]
- Pamphlett R. Uptake of environmental toxicants by the locus ceruleus: a potential trigger for neurodegenerative, demyelinating and psychiatric disorders. Med. Hypotheses. 2014;82:97–104. doi: 10.1016/j.mehy.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Pamphlett R, Jew SK. Heavy metals in locus ceruleus and motor neurons in motor neuron disease. Acta Neuropathol. Commun. 2013;1:81. doi: 10.1186/2051-5960-1-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamphlett R, Kum Jew S. Different populations of human locus ceruleus neurons contain heavy metals or hyperphosphorylated Tau: implications for amyloid-β and tau pathology in Alzheimer's disease. J. Alzheimer's Dis. 2015;45:437–447. doi: 10.3233/JAD-142445. [DOI] [PubMed] [Google Scholar]
- Randall DC, Shneerson JM, File SE. Cognitive effects of modafinil in student volunteers may depend on IQ. Pharmacol. Biochem. Behav. 2005;82:133–139. doi: 10.1016/j.pbb.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Richards M, Sacker A. Lifetime antecedents of cognitive reserve. J. Clin. Exp. Neuropsychol. 2003;25:614–624. doi: 10.1076/jcen.25.5.614.14581. [DOI] [PubMed] [Google Scholar]
- Ritter P, Freyer F, Curio G, Villringer A. High-frequency (600 Hz) population spikes in human EEG delineate thalamic and cortical fMRI activation sites. Neuroimage. 2008;42:483–490. doi: 10.1016/j.neuroimage.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu. Rev. Neurosci. 2009;32:267. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson IH. A noradrenergic theory of cognitive reserve: implications for Alzheimer's disease. Neurobiol. Aging. 2013;34:298–308. doi: 10.1016/j.neurobiolaging.2012.05.019. [DOI] [PubMed] [Google Scholar]
- Robertson IH. A right hemisphere role in cognitive reserve. Neurobiol. Aging. 2014;35:1375–1385. doi: 10.1016/j.neurobiolaging.2013.11.028. [DOI] [PubMed] [Google Scholar]
- Roe CM, Mintun MA, D'Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Arch. Neurol. 2008;65:1467–1471. doi: 10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe CM, Xiong C, Miller JP, Morris JC. Education and Alzheimer disease without dementia support for the cognitive reserve hypothesis. Neurology. 2007;68:223–228. doi: 10.1212/01.wnl.0000251303.50459.8a. [DOI] [PubMed] [Google Scholar]
- Salgado H, Kohr G, Trevino M. Noradrenergic “tone” determines dichotomous control of cortical spike-timing-dependent plasticity. Sci. Rep. 2012;2:7. doi: 10.1038/srep00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Vankov A, Hervé A. Locus coeruleus-evoked responses in behaving rats: a clue to the role of noradrenaline in memory. Brain Res. Bull. 1994;35:457–465. doi: 10.1016/0361-9230(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Shibata E, Tohyama K, Takahashi J, Otsuka K, Tsuchiya K, Takahashi S, Ehara S, Terayama Y, Sakai A. Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson's disease. Neuroreport. 2006;17:1215–1218. doi: 10.1097/01.wnr.0000227984.84927.a7. [DOI] [PubMed] [Google Scholar]
- Schaie KW, Willis SL. Age difference patterns of psychometric intelligence in adulthood: generalizability within and across ability domains. Psychol. Aging. 1993;8:44. doi: 10.1037//0882-7974.8.1.44. [DOI] [PubMed] [Google Scholar]
- Shibata E, Sasaki M, Tohyama K, Kanbara Y, Otsuka K, Ehara S, Sakai A. Age-related changes in locus ceruleus on neuromelanin magnetic resonance imaging at 3 Tesla. Magn. Reson. Med. Sci. 2006;5:197–200. doi: 10.2463/mrms.5.197. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Solé-Padullés C, Bartrés-Faz D, Junqué C, Vendrell P, Rami L, Clemente IC, Jurado MA. Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer's disease. Neurobiol. Aging. 2009;30:1114–1124. doi: 10.1016/j.neurobiolaging.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE, Vagg PR, Jacobs GA. Manual for State and Trait Anxiety Inventory (Form Y) Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Steffener J, Stern Y. Exploring the neural basis of cognitive reserve in aging. Biochim. Biophys. Acta. 2012;1822:467–473. doi: 10.1016/j.bbadis.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2006;20:112–117. doi: 10.1097/01.wad.0000213815.20177.19. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer's disease. JAMA. 1994;271:1004–1010. [PubMed] [Google Scholar]
- Stern Y, Tang MX, Denaro J, Mayeux R. Increased risk of mortality in Alzheimer's disease patients with more advanced educational and occupational attainment. Ann. Neurol. 1995;37:590–595. doi: 10.1002/ana.410370508. [DOI] [PubMed] [Google Scholar]
- Straube T, Korz V, Balschun D, Frey J. Requirement of β-adrenergic receptor activation and protein synthesis for LTP-reinforcement by novelty in rat dentate gyrus. J. Physiol. 2003;552:953–960. doi: 10.1113/jphysiol.2003.049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi J, Shibata T, Sasaki M, Kudo M, Yanezawa H, Obara S, Terayama Y. Detection of changes in the locus coeruleus in patients with mild cognitive impairment and Alzheimer's disease: high-resolution fast spin-echo T1-weighted imaging. Geriatr. Gerontol. Int. 2015;15:334–340. doi: 10.1111/ggi.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosk JM, Holshouser BA, Aloia RC, Hinshaw DB, Hasso AN, Macmurray JP, Bozzetti LP. Effects of the interaction between ferric iron and L-dopa melanin on T1 and T2 relaxation times determined by magnetic resonance imaging. Magn. Reson. Med. 1992;26:40–45. doi: 10.1002/mrm.1910260105. [DOI] [PubMed] [Google Scholar]
- Troadec JD, Marien M, Darios F, Hartmann A, Ruberg M, Colpaert F, Michel PP. Noradrenaline provides long-term protection to dopaminergic neurons by reducing oxidative stress. J. Neurochem. 2001;79:200–210. doi: 10.1046/j.1471-4159.2001.00556.x. [DOI] [PubMed] [Google Scholar]
- Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychol. Med. 2006;36(04):441–454. doi: 10.1017/S0033291705006264. [DOI] [PubMed] [Google Scholar]
- Van Der Meer E, Beyer R, Horn J, Foth M, Bornemann B, Ries J, Kramer J, Warmuth E, Wartenburger I. Resource allocation and fluid intelligence: Insights from pupillometry. Psychophysiology. 2010;47(1):158–169. doi: 10.1111/j.1469-8986.2009.00884.x. [DOI] [PubMed] [Google Scholar]
- Veyrac A, Sacquet J, Nguyen V, Marien M, Jourdan F, Didier A. Novelty determines the effects of olfactory enrichment on memory and neurogenesis through noradrenergic mechanisms. Neuropsychopharmacology. 2009;34:786–795. doi: 10.1038/npp.2008.191. [DOI] [PubMed] [Google Scholar]
- Vijayashankar N, Brody H. Quantitative study of the pigmented neurons in the nuclei locus coeruleus and subcoeruleus in man as related to aging. J. Neuropathol. Exp. Neurol. 1979;38:490–497. doi: 10.1097/00005072-197909000-00004. http://dx.doi.org/10.1097/00005072-197909000-00004. [DOI] [PubMed] [Google Scholar]
- Vymazal J, Righini A, Brooks RA, Canesi M, Mariani C, Leonardi M, Pezzoli G. T1 and T2 in the brain of healthy subjects, patients with Parkinson disease, and patients with multiple system atrophy: relation to iron Content 1. Radiology. 1999;211:489–495. doi: 10.1148/radiology.211.2.r99ma53489. [DOI] [PubMed] [Google Scholar]
- Wakamatsu K, Tabuchi K, Ojika M, Zucca FA, Zecca L, Ito S. Norepinephrine and its metabolites are involved in the synthesis of neuromelanin derived from the locus coeruleus. J. Neurochem. 2015 doi: 10.1111/jnc.13237. http://dx.doi.org/10.1111/jnc.13237. [DOI] [PMC free article] [PubMed]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, Nou E. α2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading: WTAR. Psychological Corporation; 2001. [Google Scholar]
- Whalley LJ, Deary IJ, Appleton CL, Starr JM. Cognitive reserve and the neurobiology of cognitive aging. Ageing Res. Rev. 2004;3:369–382. doi: 10.1016/j.arr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Wilcox RR. Some results on extensions and modifications of the TheildSen regression estimator. Br. J. Math. Stat. Psychol. 2004;57:265–280. doi: 10.1348/0007110042307230. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Nag S, Boyle PA, Hizel LP, Yu L, Buchman AS, Bennett DA. Neural reserve, neuronal density in the locus ceruleus, and cognitive decline. Neurology. 2013;80:1202–1208. doi: 10.1212/WNL.0b013e3182897103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca L, Bellei C, Costi P, Albertini A, Monzani E, Casella L, Turro NJ. New melanic pigments in the human brain that accumulate in aging and block environmental toxic metals. Proc. Natl. Acad. Sci. 2008;105:17567–17572. doi: 10.1073/pnas.0808768105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca L, Stroppolo A, Gatti A, Tampellini D, Toscani M, Gallorini M, Fariello RG. The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. Proc. Natl. Acad. Sci. U. S. A. 2004;101:9843–9848. doi: 10.1073/pnas.0403495101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucca F, Bellei C, Giannelli S, Terreni M, Gallorini M, Rizzio E, Zecca L. Neuromelanin and iron in human locus coeruleus and substantia nigra during aging: consequences for neuronal vulnerability. J. Neural Transm. 2006;113:757–767. doi: 10.1007/s00702-006-0453-2. [DOI] [PubMed] [Google Scholar]