Abstract
Background and Purpose
Aortic arch plaque (AAP) is a risk factor for ischemic stroke, but its association with subclinical cerebrovascular disease is not established. We investigated the association between AAP and subclinical cerebrovascular disease in an elderly stroke-free community-based cohort.
Methods
The Cardiovascular Abnormalities and Brain Lesions (CABL) study was designed to investigate cardiovascular predictors of silent cerebrovascular disease in the elderly. AAPs were assessed by suprasternal transthoracic echocardiography (TTE) in 954 participants. SBI and white matter hyperintensity volume (WMHV) were assessed by brain MRI. The association of AAP thickness with SBI and WMHV was evaluated by logistic regression analysis.
Results
Mean age was 71.6 ± 9.3 years, 63% were women. AAP was present in 658 (69%) subjects. Silent brain infarcts (SBI) were detected in 138 participants (14.5%). In multivariate analysis adjusted for potential confounders, AAP thickness and large AAP (≥4mm in thickness) were significantly associated with the upper quartile of WMHV (WMHV-Q4) (OR=1.17; 95% CI, 1.04–1.32; P=0.009 and OR=1.79; 95% CI, 1.40–3.09; P=0.036, respectively), but not with SBI (OR=1.08; 95% CI, 0.94–1.23; P=0.265 and OR=1.46; 95% CI, 0.77–2.77; P=0.251, respectively).
Conclusions
Aortic arch atherosclerosis was associated with WMHV in a stroke-free community-based elderly cohort. This association was stronger in subjects with large plaques and independent of cardiovascular risk factors. Aortic arch assessment by TTE may help identify subjects at higher risk of subclinical cerebrovascular disease, who may benefit from aggressive stroke risk factors treatment.
Keywords: Atherosclerosis, cerebrovascular diseases, stroke, silent brain infarct, white matter hyperintensity
Introduction
White matter hyperintensities (WMHs) and silent brain infarcts (SBIs), both manifestations of subclinical cerebrovascular disease, are commonly seen on brain magnetic resonance imaging (MRI) scans of older adults 1. In the general population, the prevalence of SBI ranges from 7% to 28% 2, 3 with the elderly population at the higher end of the range. With respect to WMH, the prevalence ranges from 11–21% at age 64, to 94% at age 82 4, 5. It has been shown that SBI and WMH share common risk factors with stroke 6, 7 and are strong predictors of future stroke 4, 8, cognitive impairment 9, 10 and dementia 9. Because of its wide variability in prevalence among older adults from different cohorts, and its association with cardiovascular disease risk factors and prior stroke, subclinical cerebrovascular disease is believed to be at least partially preventable through detection and treatment of modifiable risk factors 1.
Aortic arch plaque (AAP) is an established risk factor for ischemic stroke. The association between AAP and stroke risk, initially established in autopsy studies 11, was subsequently confirmed by in vivo studies that used transesophageal echocardiography (TEE) with a case-control 12 or prospective 13 design. Large plaques (defined as ≥ 4 mm in thickness in most studies) were proven to be strongly associated with first stroke 11, 12, recurrent stroke 13 and death 14. In a previous study, aortic arch atherosclerosis was found to be associated with SBI in patients with atrial fibrillation (AF) 15, and the authors suggested that microembolization of small thrombi might be the mechanism for SBI. However, little is known on the relationship between aortic arch atherosclerosis and subclinical brain disease in the general population, especially in the elderly, who have the greatest frequency of both AAPs and subclinical brain lesions. Accordingly, the aim of the present study was to investigate the association between aortic arch atherosclerosis and subclinical cerebrovascular disease in an elderly community-based cohort without prior stroke.
Methods
Study Population
The Cardiovascular Abnormalities and Brain Lesion (CABL) study is a community-based epidemiological study designed to investigate the cardiovascular predictors of silent cerebrovascular disease in the community. CABL based its recruitment on the Northern Manhattan Study (NOMAS), a population-based prospective study that enrolled 3298 participants from the community living in northern Manhattan between 1993 and 2001. The study design and recruitment details of NOMAS have been described previously 16. Beginning in 2003, participants were invited to participate in an MRI substudy if they (1) were at least 55 years of age, (2) had no contraindications to MRI, and (3) did not have a previous diagnosis of stroke. From September 2005 to July 2010, NOMAS MRI participants that voluntarily agreed to undergo a more extensive cardiovascular evaluation including transthoracic echocardiography (TTE) were included in CABL. Participants for whom both echocardiography and brain MRI information were available constitute the sample of the present study. Participants in CABL were representative of the NOMAS population with respect to gender distribution (63% female), were slightly older (mean age 71.6 vs. 69.3 years) and more of Hispanic race-ethnicity (68.1% vs. 52.4%). Written informed consent was obtained from all study participants. The study was approved by the Institutional Review Boards of Columbia University Medical Center and of the University of Miami.
Risk Factor Assessment
Cardiovascular risk factors, ascertained through direct examination and interview by trained research assistants, and blood tests were also performed at the time of TTE or MRI. Hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, or the participant’s self-reported history of hypertension or use of antihypertensive medication. Diabetes mellitus was defined as fasting blood glucose ≥126 mg/dL or the participant’s self-reported history of diabetes mellitus or use of diabetes mellitus medications. Hypercholesterolemia was defined as total serum cholesterol >240 mg/dL, a patient’s self-report of hypercholesterolemia or use of lipid-lowering treatment. Cigarette smoking, either at the time of the interview or in the past, was recorded. Coronary artery disease was defined as a history of myocardial infarction, coronary artery bypass grafting, percutaneous coronary intervention, typical angina, or use of anti-ischemic medications. AF was defined from ECG at the time of echocardiography or from self-reported history. The race-ethnicity classification was based on self-identification, and categorized as non-Hispanic black, non-Hispanic white, and Hispanic, according to federal guidelines.
Detection of aortic plaques
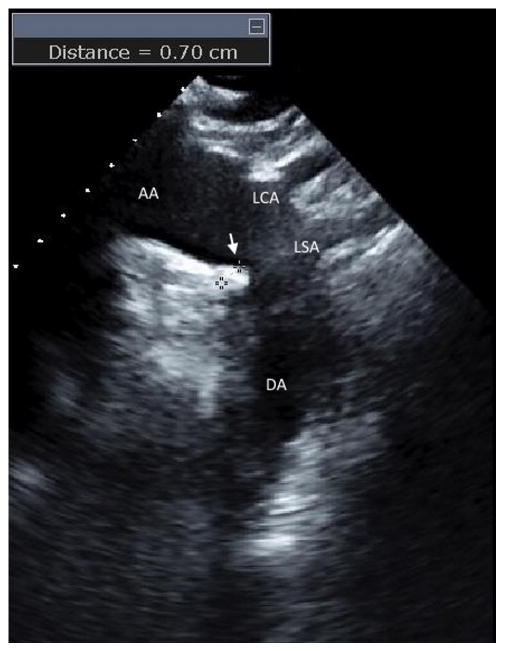
Two-dimensional transthoracic images of the aortic arch with real time three-dimensional confirmation from a suprasternal window were obtained by a registered cardiac sonographer following a standardized protocol using a commercially available system (iE33; Philips Medical Systems, Andover, MA) equipped with a 2.5–3.5-MHz transducer. All the tests were stored on digital media for subsequent analysis. The aortic arch was defined as the portion of aorta between the curve at the end of the ascending portion and the takeoff of the left subclavian artery. A plaque was defined as a discrete protrusion of the intimal surface of the vessel at least 1 mm in thickness, different in appearance and echogenicity from the adjacent intact intimal surface (Fig. 1, arrow). AAP were characterized according to previously described criteria 13, as large (≥ 4 mm in thickness), small (<4 mm in thickness), or not present. In case of multiple plaques, the most advanced lesion was considered. All images were interpreted by a single experienced echocardiographer (MDT) blinded to participant’s characteristics and risk factors.
Figure 1.
Two-dimensional echocardiographic image from the suprasternal window of a large atherosclerotic plaque (arrow) in the distal portion of the aortic arch. The measurement of the plaque thickness (7mm) is shown. AA=aortic arch, DA=descending aorta, LCA=left carotid artery, LSA= left subclavian artery.
Brain MRI
A detailed description of the assessment of subclinical cerebrovascular lesions has been published previously. 17, 18 In brief, brain imaging was performed on a 1.5-T MRI system (Philips Medical Systems). SBIs were rated by 2 of the authors (C.D. and M.Y.) and defined as either a cavitation on the fluid-attenuated inversion recovery sequence of at least 3 mm in size, distinct from a vessel (owing to the lack of signal void on T2 sequence), and of equal intensity to cerebrospinal fluid in the case of lacunar infarction, or as a wedge shaped cortical or cerebellar area of encephalomalacia with surrounding gliosis consistent with infarction attributable to distal arterial branch occlusion. Interobserver agreement for SBI detection was 93.3% 18. White matter hyperintensity volume (WMHV) analysis was based on a fluid attenuated inversion recovery image and performed by using the Quantum 6.2 package on a Sun Microsystems Ultra 5 workstation. WMHV was expressed as proportion of total cranial volume to correct for differences in head size. The time difference between MRI and TTE was < 90 days in 591 subjects (62%). All measurements were performed blinded to participant identifying and clinical information.
Statistics
WMHV was categorized in quartiles of the observed distribution, and the upper quartile (WMHV-Q4) was considered as the primary outcome in the analyses. Data are presented as mean±standard deviation for continuous variables and as percentage for categorical variables. Comparison between two groups (presence/absence of SBI; WMHV-Q4/WMHV-Q1-3) was carried using the Student two-sided t-test for continuous variables and the Chi-square test for categorical variables. One-way ANOVA was used to compare the differences between mean values across AAP groups (no plaque, small plaque and large plaque). Univariate and multivariate logistic regression analysis were used to assess the association between the presence of AAP thickness and SBI, or the upper quartile (WMHV-Q4). No plaque was used as a reference for the AAP thickness category analysis. Variables that were associated with SBI or WMHV-Q4 in univariate analysis at the 0.1 level were entered as covariates in the multivariate analyses. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. For all statistical analyses, a 2-tailed P<0.05 was considered significant. Statistical analyses were performed by using SAS software version 9.3 (SAS Institute Inc, Cary, NC).
Results
Study Population
Mean age of the study population was 71.6 ± 9.3 years, 63% were women. AAP was present in 658 (69%) subjects; the plaque size was small (< 4 mm) in 543 (57%) cases, and large (≥ 4 mm) in 115 (12%) cases. SBI was present in 138 cases (14.5%). Mean WMHV was 0.65±0.82% (median= 0.34%, interquartile range=0.52%). Clinical characteristics of the study population according to plaque presence and size are shown in Table 1. Age, gender, body mass index, hypertension, smoking history and hypertension medication were significantly different across the groups.
Table 1.
Characteristics of the study population according to plaque presence and size
| (N=954) | No Plaque N=296 |
Small Plaque N=543 |
Large plaque N=115 |
P |
|---|---|---|---|---|
| Age (years) | 68.4 ± 9.0 | 72.3 ± 9.0 | 76.8 ± 8.2 | <0.001 |
| Male, n (%) | 97(32.8) | 222(40.9) | 32 (27.8) | 0.007 |
| Race-Ethnicity | ||||
| White, n (%) | 30 (10.1) | 83 (15.3) | 21 (18.3) | |
| Hispanic, n (%) | 210 (71.0) | 365 (67.2) | 75 (65.2) | 0.173 |
| Black, n (%) | 48 (16.2) | 82 (15.1) | 19 (16.5) | |
| Other, n (%) | 8 (2.7) | 13 (2.4) | 0 (0) | |
| Body mass index, kg/m2 | 29.4 ± 5.0 | 27.7 ± 4.7 | 28.0 ± 5.0 | <0.001 |
| Hypertension, n (%) | 220 (74.3) | 436 (80.3) | 102 (88.7) | 0.004 |
| Hypercholesterolemia, n (%) | 198 (66.9) | 365 (67.2) | 87 (76.7) | 0.182 |
| Diabetes mellitus, n (%) | 93 (31.4) | 162 (29.8) | 28 (24.4) | 0.368 |
| Coronary Artery Disease, n (%) | 11 (3.7) | 35 (6.5) | 11 (9.6) | 0.063 |
| History of MI, n (%) | 10 (3.4) | 28 (5.2) | 7 (6.1) | 0.388 |
| History of AF, n (%) | 17 (5.7) | 32 (5.9) | 11 (9.6) | 0.303 |
| Smoking history, n (%) | 141 (47.6) | 292 (53.8) | 70 (60.9) | 0.041 |
| Hypertension medication, n (%) | 198 (66.9) | 398 (73.3) | 94 (81.7) | 0.008 |
| SBI, n (%) | 26 (9.6) | 81 (15.6) | 22 (19.1) | 0.02 |
| WMHV/total cranial volume (%) | 0.44 ± 0.48 | 0.69 ± 0.86 | 0.98 ± 1.13 | <0.001 |
SBI=silent brain infarct
WMHV=white matter hyperintensity volume
MI=myocardial infarction
AF=atrial fibrillation
Subjects with SBI were significantly older, more frequently male and had higher frequencies of hypertension, diabetes mellitus, AF history, and use of hypertension medication (Table 2). Subjects in the WMHV-Q4 were also older, more often hypertensive, and had higher frequencies of AF history and use of hypertension medication (also Table 2).
Table 2.
Characteristics of subjects with and without SBI and WMH in the upper quartile (WMHV-Q4)
| SBI (−) (N=816) | SBI (+) (N=138) | P | WMHV-Q1-3 (N=716) | WMHV-Q4 (N=238) | P | |
|---|---|---|---|---|---|---|
| Age (years) | 70.9 ± 9.1 | 76.1 ± 8.8 | <0.001 | 69.6 ± 8.7 | 77.7 ± 8.1 | <0.001 |
| Male, n (%) | 288 (35.3) | 63 (45.7) | 0.020 | 268 (37.4) | 83 (34.9) | 0.479 |
| Race-Ethnicity | ||||||
| White, n (%) | 111 (13.6) | 23 (16.7) | 106 (14.8) | 28 (11.8) | ||
| Hispanic, n (%) | 568 (69.6) | 82 (59.4) | 0.114 | 503 (70.3) | 147 (61.8) | 0.001 |
| Black, n (%) | 120 (14.7) | 29 (21.0) | 93 (13.0) | 56 (23.5) | ||
| Other, n (%) | 17 (2.1) | 4 (2.4) | 14 (2.0) | 7 (2.9) | ||
| Body mass index, kg/m2 | 28.4 ± 4.9 | 27.8 ± 4.6 | 0.159 | 28.5 ± 4.9 | 27.6 ± 4.8 | 0.012 |
| Hypertension, n (%) | 634 (77.7) | 124 (89.9) | 0.001 | 542 (75.7) | 216 (90.8) | <0.001 |
| Hypercholesterolemia, n (%) | 554 (67.9) | 96 (69.6) | 0.697 | 490 (68.4) | 160 (67.2) | 0.729 |
| Diabetes mellitus, n (%) | 232 (28.4) | 51 (37.0) | 0.043 | 204 (28.5) | 79 (33.2) | 0.169 |
| Coronary artery disease, n (%) | 45 (5.5) | 12 (8.7) | 0.145 | 40 (5.6) | 17 (7.1) | 0.380 |
| History of MI, n (%) | 36 (4.4) | 9 (6.5) | 0.280 | 32 (4.5) | 13 (5.5) | 0.531 |
| History of AF, n (%) | 41 (5.0) | 19 (13.8) | <0.001 | 35 (4.9) | 25 (10.5) | 0.002 |
| Smoking history, n (%) | 429 (52.6) | 74 (53.6) | 0.819 | 372 (52.0) | 131 (55.0) | 0.409 |
| Hypertension medication, n (%) | 571 (70.0) | 119 (86.2) | <0.001 | 489 (68.3) | 201 (84.5) | <0.001 |
| Aortic plaque thickness (mm) | 1.9 ± 1.5 | 2.3 ± 1.4 | 0.007 | 1.8 ± 1.4 | 2.4 ± 1.4 | <0.001 |
SBI=silent brain infarct
WMHV=white matter hyperintensity volume
MI=myocardial infarction
AF=atrial fibrillation
AAP, SBI and WMH
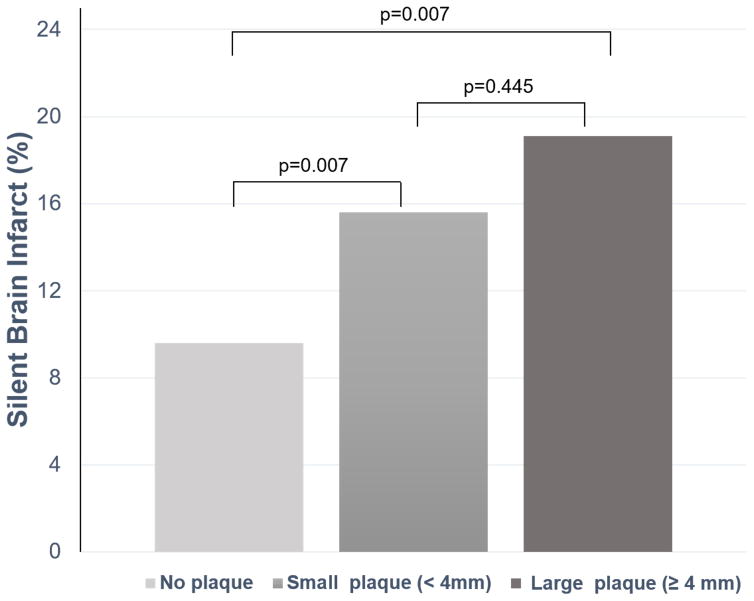
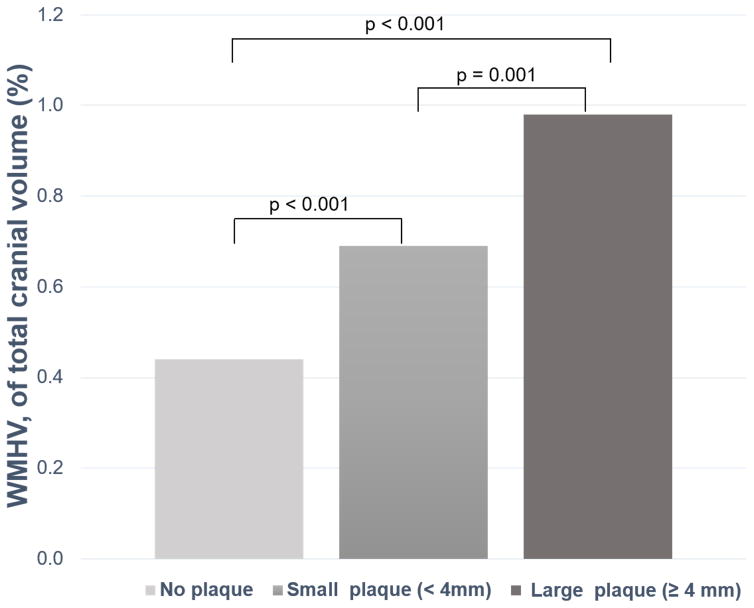
The prevalence of SBI was higher in subjects with small and large plaques compared to subjects with no plaque (Table 1). Mean WMHV percentage of total cranial volume increased significantly with increasing arch plaque thickness category (Table 1). The intergroup differences across AAP categories for presence of SBI and mean WMHV are shown in Figure 2A and 2B, respectively. The mean AAP thickness was also significantly higher in participants with SBI than those without (2.3 ± 1.4 mm vs. 1.9 ± 1.5 mm, P=0.007), and in the WMHV-Q4 group than in the WMHV-Q1-3 groups (2.4 ± 1.4 mm vs. 1.8 ± 1.4 mm, P<0.001) (Table 2). Data on the association between AAP thickness categories and the presence of SBI and WMHV-Q4 are shown in Table 3. Plaque thickness was significantly associated with both SBI and WMHV-Q4 in univariate analysis. After adjustment for relevant covariates, the significance of the association was lost for SBI but not for WMHV-Q4. Similarly, the presence of small or large AAP was significantly associated with SBI in univariate analysis, but not in the multivariate models. In univariate analysis, both small and large plaques were also significantly associated with WMHV-Q4. After adjusting for covariates, large plaque remained significantly associated with WMHV-Q4, whereas small plaque showed a non-significant trend. The subgroup analysis in the 591 subjects with an MRI and TTE time difference < 90 days showed a similar trend as in the entire population. Please see http://stroke.ahajournals.org for the online supplemental table (Supplemental Table I).
Figure 2.
SBI prevalence (A) and WMHV, expressed as a percentage of the total cranial volume (B), in subjects with no, small and large AAP.
AAP= aortic arch plaque
Table 3.
Association of AAP with SBI and WMHV in the upper quartile (WMHV-Q4)
| SBI | WMHV-Q4 | |||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted OR* (95% CI) | Unadjusted OR (95% CI) | Adjusted OR* (95% CI) | |
| AAP thickness (mm) | 1.19 (1.05–1.35)† | 1.08 (0.94–1.23) | 1.34 (1.21–1.49)† | 1.17 (1.04–1.32)† |
| AAP thickness category | ||||
| No plaque | Reference | Reference | Reference | Reference |
| Small plaque (<4mm) | 1.85 (1.18–2.90)† | 1.47 (0.92–2.34) | 2.02 (1.40–2.91)† | 1.49 (0.10–2.23) |
| Large plaque (≥ 4 mm) | 2.26 (1.24–4.15)† | 1.46 (0.77–2.77) | 3.49 (2.14–5.70)† | 1.79 (1.04–3.09)‡ |
AAP=aortic arch plaque
SBI=silent brain infarct
WMHV=white matter hyperintensity volume
Values in tables are odds ratios (OR) and 95% confidence intervals (CI)
Adjusted for age, sex, hypertension, diabetes, history of atrial fibrillation, and hypertension medication in SBI analyses; and for age, race-ethnicity, hypertension, history of atrial fibrillation, body mass index, and hypertension medication in WMHV analyses.
P < 0.01
P < 0.05
Discussion
In this study, we investigated the relationship between aortic arch atherosclerosis and subclinical cerebrovascular disease in an elderly community-based cohort without prior stroke. We found that AAP thickness was independently associated with WMHV, but not with SBI. The association with WMHV was stronger in subjects with large plaques.
Numerous case-control 13, 19 and prospective studies 13, 20 have confirmed the role of proximal arch plaques as risk factors for stroke and other embolic events; however, the mechanism underlying the association between AAP and WMHV is not immediately clear. The prevalence of severe AAP in stroke patients has been reported to be between 14% and 21% 19, 21, and is similar to other recognized causes of embolic stroke, such as carotid artery disease, present in 9% to 17% 22 of stroke patients, and AF, present in 11% to 20% 23. With respect to subclinical cerebrovascular disease, SBIs and WMHs are considered to be related but somewhat different expressions of brain disease. While SBIs are focal areas of infarcts, presumed to result from the occlusion of a single small perforating artery supplying the subcortical areas of the brain 24, WMHs are considered areas of leukoaraiosis (loss of white matter) due to chronic hypoperfusion of the white matter and disruption of the blood-brain barrier, leading to chronic leakage of plasma into the white matter 25. Several studies showed that both conditions are associated with atherosclerotic and cardiovascular risk factors, such as hypertension, left ventricular hypertrophy, cigarette smoking, hyperhomocysteinemia, carotid plaque, arterial stiffness, and intima-media thickness 2, 3, 17, 26, 27.
Epidemiological studies have hypothesized that some silent cerebrovascular disease might have a microembolic etiology 28, 29. In a previous study, the association between unstable carotid plaques and WMH was examined in patients referred for carotid endarterectomy 30. Unstable plaques were associated on average with an over 2-fold increase in number of WMH lesions in the ipsilateral hemisphere compared to stable plaques. The authors suggested that microembolism may contribute to the development of WMH lesions and in particular to the development of small isolated lesions. However, the sample size was small, with the risk of unbalanced confounding risk factors between groups. Another study investigated the predictors of SBI in patients with nonvalvular AF 15. Complex aortic plaques, defined by TEE as large plaques, plaques with ulceration, or plaques with mobile components, were independently associated with the presence of SBI on brain MRI. The authors speculated that microembolization from the left atrium or advanced aortic lesions might be the cause of SBI. However, the sample size was again small (103 patients, with only 31 patients with SBI) and included only patients with nonvalvular AF, an important independent cause for embolic stroke. In line with our findings, a report from the Rotterdam Scan Study showed that aortic atherosclerosis in mid-life, assessed by presence of aortic calcification on abdominal radiographs, was associated with WMHs detected 20 years later 31; in that study, however, the relationship between aortic atherosclerosis and WMHs was lost when evaluated cross-sectionally in the elderly subjects, possibly because of the coexistence of several cardiovascular risk factors, which may decrease the discriminative power of atherosclerotic plaques. Additionally, the study derived its plaque information from the abdominal aorta, whose link with cerebrovascular disease is less strong and direct than for the proximal aorta.
The association between AAP and WMH found in this study seems unlikely to be of thromboembolic origin. AAP thickness was not associated with SBI after adjusting for other pertinent covariates. In addition, the presence of large plaque, which is known to carry the strongest association with thromboembolic stroke 14, was not associated with SBIs. On the contrary, AAP thickness was significantly associated with WMH severity, defined by the upper quartile of WMHV, independent of other confounders. The association was the stronger in subjects with large plaques. One potential mechanism explaining the association of AAP with WMHV, but not with SBI, might be through arterial stiffness. Aortic atherosclerosis is known to be strongly associated with arterial stiffness and increased aortic pulse wave velocity 32. Originally proposed by Fazekas et al as the “water hammer effect” 33, and recently described by Saji et al as the “Tsunami effect” 34, increased pulse wave velocity may contribute to cerebral microcirculatory damage, which in turn may induce disruption of vascular dynamics and complicate perivascular flow leading to WMH in the brain. Aortic arch atherosclerosis may also be a marker of diffuse atherosclerosis rather than an etiologic mechanism for subclinical cerebrovascular disease, and may have a common pathogenic pathway with WMH that portends the higher stroke risk associated with either condition.
Our study has potential clinical implications. We showed that the presence of AAPs is independently associated with WMH severity, which in turn is a strong predictor of stroke, cognitive impairment, and dementia 4, 8–10. Thus, aortic arch assessment by TTE in the elderly might contribute to identifying patients at higher risk of cerebrovascular disease, for whom more aggressive evaluation to confirm cerebrovascular disease (e.g., MRI) or risk factor modification might be warranted.
Strengths and Limitations
The main strengths of our study are the large number of subjects studied with advanced imaging techniques (brain MRI), the wide range of cardiovascular risk profiles present in our study population, and the confirmation of our findings after adjustment for pertinent potential confounders. However, our study also has limitations. The study sample included subjects > 55 years of age with a large representation of Hispanic ethnicity, which might preclude the generalization of our findings to populations with different demographic composition. However, because subclinical cerebrovascular disease is more commonly found in older adults, our cohort represented an ideal setting for this study. Furthermore, the cross-sectional design of our study only allows to document associations that do not necessarily imply cause-effect relationships. We used TTE rather than TEE for AAP detection and plaque measurements. Therefore, we may have underestimated the prevalence of plaques, and could not assess plaque morphology (ulceration or small mobile components), which is better assessed by TEE. However, TTE is a non-invasive technique that is better suited than TEE for use as a screening technique in asymptomatic individuals, and has proven to be accurate for assessing the presence of protruding AAP in patients evaluated for detection of a source of embolism, with positive and negative predictive values of 91% and 98%, respectively 35. Finally, we cannot exclude that unmeasured confounders may have been involved in the observed associations.
Conclusions
Aortic atherosclerosis was independently associated with WMH severity in a stroke-free community-based cohort. This association was stronger in subjects with large plaques. Whether a more aggressive risk factor control in this subset of patients may improve their cardiovascular and cerebrovascular outcomes requires further investigation.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by the National Institute of Neurological Disorders and Stroke (grant R01 NS36286 to Dr. Di Tullio and R37 NS29993 to Drs. Sacco and Elkind).
Footnotes
Disclosures
None
References
- 1.Manolio TA, Kronmal RA, Burke GL, Poirier V, O’Leary DH, Gardin JM, et al. Magnetic resonance abnormalities and cardiovascular disease in older adults. The cardiovascular health study. Stroke. 1994;25:318–327. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- 2.Prabhakaran S, Wright CB, Yoshita M, Delapaz R, Brown T, DeCarli C, et al. Prevalence and determinants of subclinical brain infarction: The northern manhattan study. Neurology. 2008;70:425–430. doi: 10.1212/01.wnl.0000277521.66947.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vermeer SE, Den Heijer T, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM, et al. Incidence and risk factors of silent brain infarcts in the population-based rotterdam scan study. Stroke. 2003;34:392–396. doi: 10.1161/01.str.0000052631.98405.15. [DOI] [PubMed] [Google Scholar]
- 4.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM, et al. Silent brain infarcts and white matter lesions increase stroke risk in the general population: The rotterdam scan study. Stroke. 2003;34:1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 5.Garde E, Mortensen EL, Krabbe K, Rostrup E, Larsson HB. Relation between age-related decline in intelligence and cerebral white-matter hyperintensities in healthy octogenarians: A longitudinal study. Lancet. 2000;356:628–634. doi: 10.1016/S0140-6736(00)02604-0. [DOI] [PubMed] [Google Scholar]
- 6.Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Prevalence and risk factors of silent brain infarcts in the population-based rotterdam scan study. Stroke. 2002;33:21–25. doi: 10.1161/hs0102.101629. [DOI] [PubMed] [Google Scholar]
- 7.Das RR, Seshadri S, Beiser AS, Kelly-Hayes M, Au R, Himali JJ, et al. Prevalence and correlates of silent cerebral infarcts in the framingham offspring study. Stroke. 2008;39:2929–2935. doi: 10.1161/STROKEAHA.108.516575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernick C, Kuller L, Dulberg C, Longstreth WT, Manolio T, Beauchamp N, et al. Silent mri infarcts and the risk of future stroke: The cardiovascular health study. Neurology. 2001;57:1222–1229. doi: 10.1212/wnl.57.7.1222. [DOI] [PubMed] [Google Scholar]
- 9.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 10.Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, et al. Association of mri markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: The framingham offspring study. Stroke. 2010;41:600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amarenco P, Duyckaerts C, Tzourio C, Hénin D, Bousser MG, Hauw JJ. The prevalence of ulcerated plaques in the aortic arch in patients with stroke. N Engl J Med. 1992;326:221–225. doi: 10.1056/NEJM199201233260402. [DOI] [PubMed] [Google Scholar]
- 12.Di Tullio MR, Sacco RL, Gersony D, Nayak H, Weslow RG, Kargman DE, et al. Aortic atheromas and acute ischemic stroke: A transesophageal echocardiographic study in an ethnically mixed population. Neurology. 1996;46:1560–1566. doi: 10.1212/wnl.46.6.1560. [DOI] [PubMed] [Google Scholar]
- 13.Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. The french study of aortic plaques in stroke group. N Engl J Med. 1996;334:1216–1221. doi: 10.1056/NEJM199605093341902. [DOI] [PubMed] [Google Scholar]
- 14.Di Tullio MR, Russo C, Jin Z, Sacco RL, Mohr JP, Homma S, et al. Aortic arch plaques and risk of recurrent stroke and death. Circulation. 2009;119:2376–2382. doi: 10.1161/CIRCULATIONAHA.108.811935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugioka K, Takagi M, Sakamoto S, Fujita S, Ito A, Iwata S, et al. Predictors of silent brain infarction on magnetic resonance imaging in patients with nonvalvular atrial fibrillation: A transesophageal echocardiographic study. Am Heart J. 2015;169:783–790. doi: 10.1016/j.ahj.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Sacco RL, Khatri M, Rundek T, Xu Q, Gardener H, Boden-Albala B, et al. Improving global vascular risk prediction with behavioral and anthropometric factors. The multiethnic nomas (northern manhattan cohort study) J Am Coll Cardiol. 2009;54:2303–2311. doi: 10.1016/j.jacc.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright CB, Paik MC, Brown TR, Stabler SP, Allen RH, Sacco RL, et al. Total homocysteine is associated with white matter hyperintensity volume: The northern manhattan study. Stroke. 2005;36:1207–1211. doi: 10.1161/01.STR.0000165923.02318.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willey JZ, Moon YP, Paik MC, Yoshita M, Decarli C, Sacco RL, et al. Lower prevalence of silent brain infarcts in the physically active: The northern manhattan study. Neurology. 2011;76:2112–2118. doi: 10.1212/WNL.0b013e31821f4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amarenco P, Cohen A, Tzourio C, Bertrand B, Hommel M, Besson G, et al. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med. 1994;331:1474–1479. doi: 10.1056/NEJM199412013312202. [DOI] [PubMed] [Google Scholar]
- 20.Fujimoto S, Yasaka M, Otsubo R, Oe H, Nagatsuka K, Minematsu K. Aortic arch atherosclerotic lesions and the recurrence of ischemic stroke. Stroke. 2004;35:1426–1429. doi: 10.1161/01.STR.0000127788.32550.d4. [DOI] [PubMed] [Google Scholar]
- 21.Jones EF, Donnan GA. The proximal aorta: A source of stroke. Baillieres Clin Neurol. 1995;4:207–220. [PubMed] [Google Scholar]
- 22.Jonas DE, Feltner C, Amick HR, Sheridan S, Zheng ZJ, Watford DJ, et al. Screening for asymptomatic carotid artery stenosis: A systematic review and meta-analysis for the u.S. Preventive services task force. Ann Intern Med. 2014;161:336–346. doi: 10.7326/M14-0530. [DOI] [PubMed] [Google Scholar]
- 23.Tsang TS, Petty GW, Barnes ME, O’Fallon WM, Bailey KR, Wiebers DO, et al. The prevalence of atrial fibrillation in incident stroke cases and matched population controls in rochester, minnesota: Changes over three decades. J Am Coll Cardiol. 2003;42:93–100. doi: 10.1016/s0735-1097(03)00500-x. [DOI] [PubMed] [Google Scholar]
- 24.Bamford JM, Warlow CP. Evolution and testing of the lacunar hypothesis. Stroke. 1988;19:1074–1082. doi: 10.1161/01.str.19.9.1074. [DOI] [PubMed] [Google Scholar]
- 25.Pantoni L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 26.Howard G, Wagenknecht LE, Cai J, Cooper L, Kraut MA, Toole JF. Cigarette smoking and other risk factors for silent cerebral infarction in the general population. Stroke. 1998;29:913–917. doi: 10.1161/01.str.29.5.913. [DOI] [PubMed] [Google Scholar]
- 27.Okada Y, Kohara K, Ochi M, Nagai T, Tabara Y, Igase M, et al. Mechanical stresses, arterial stiffness, and brain small vessel diseases: Shimanami health promoting program study. Stroke. 2014;45:3287–3292. doi: 10.1161/STROKEAHA.114.006539. [DOI] [PubMed] [Google Scholar]
- 28.de Leeuw FE, de Groot JC, Bots ML, Witteman JC, Oudkerk M, Hofman A, et al. Carotid atherosclerosis and cerebral white matter lesions in a population based magnetic resonance imaging study. J Neurol. 2000;247:291–296. doi: 10.1007/s004150050586. [DOI] [PubMed] [Google Scholar]
- 29.Streifler JY, Eliasziw M, Benavente OR, Alamowitch S, Fox AJ, Hachinski V, et al. Development and progression of leukoaraiosis in patients with brain ischemia and carotid artery disease. Stroke. 2003;34:1913–1916. doi: 10.1161/01.STR.0000080939.39414.83. [DOI] [PubMed] [Google Scholar]
- 30.Futrell N. Lacunar infarction: Embolism is the key. Stroke. 2004;35:1778–1779. doi: 10.1161/01.STR.0000131930.41057.48. [DOI] [PubMed] [Google Scholar]
- 31.de Leeuw FE, De Groot JC, Oudkerk M, Witteman JC, Hofman A, van Gijn J, et al. Aortic atherosclerosis at middle age predicts cerebral white matter lesions in the elderly. Stroke. 2000;31:425–429. doi: 10.1161/01.str.31.2.425. [DOI] [PubMed] [Google Scholar]
- 32.van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, et al. Association between arterial stiffness and atherosclerosis: The rotterdam study. Stroke. 2001;32:454–460. doi: 10.1161/01.str.32.2.454. [DOI] [PubMed] [Google Scholar]
- 33.Fazekas F, Kleinert R, Offenbacher H, Payer F, Schmidt R, Kleinert G, et al. The morphologic correlate of incidental punctate white matter hyperintensities on mr images. AJNR Am J Neuroradiol. 1991;12:915–921. [PMC free article] [PubMed] [Google Scholar]
- 34.Saji N, Toba K, Sakurai T. Cerebral small vessel disease and arterial stiffness: Tsunami effect in the brain? Pulse (Basel) 2016;3:182–189. doi: 10.1159/000443614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwammenthal E, Schwammenthal Y, Tanne D, Tenenbaum A, Garniek A, Motro M, et al. Transcutaneous detection of aortic arch atheromas by suprasternal harmonic imaging. J Am Coll Cardiol. 2002;39:1127–1132. doi: 10.1016/s0735-1097(02)01730-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.