Abstract
Background and Purpose
The minimal scan duration needed to obtain reliable lesion volumes with CT perfusion (CTP) has not been well established in the literature.
Methods
We retrospectively assessed the impact of gradual truncation of the scan duration on acute ischemic lesion volume measurements. For each scan, we identified its “optimal scan time”, defined as the shortest scan duration that yields measurements of the ischemic lesion volumes similar to those obtained with longer scanning, and the relative height of the fitted venous output function at its optimal scan time (rVOF).
Results
We analyzed 70 CTP scans of acute stroke patients. An optimal scan time could not be determined in 11 (16 %) scans. For the other 59 scans, the median optimal scan time was 32.7s (90th percentile 52.6s; 100th percentile 68.9s) and the median rVOF of their optimal scan times was 0.39 (90th percentile 0.02; 100th percentile 0.00). Based on a linear model, the optimal scan time was T0 plus 1.6 times the width of the Venous Output Function plus (p < 0.001, R2 = 0.49).
Conclusions
This study shows how the optimal duration of a CTP scan relates to the arrival time and width of the contrast bolus of the scan. This knowledge can be used to optimize CTP scan protocols and to determine if a scan is of sufficient duration. Provided a baseline (T0) of 10s, a total scan duration of 60–70s, which includes the entire downslope of the VOF in most patients, is recommended.
Keywords: Computed Tomography, perfusion imaging, ischemic stroke, duration, volume
Introduction
CT perfusion (CTP) has emerged as a valuable tool in the management of acute stroke, providing essential quantitative information on the extent of the irreversibly injured ischemic core and the potentially salvageable ischemic penumbra.1, 2 These serve as critical proxies for selection of patients who are likely to benefit from reperfusion therapy.3, 4 In addition to its expanding use in determining eligibility in clinical trials,5, 6 CTP is being increasingly integrated into routine clinical practice to help select patients for intravascular thrombolysis and endovascular revascularization.7, 8
While MRI has been the reference standard for assessment of the ischemic core and penumbra for many years, recent studies have demonstrated comparable results with CTP and MR imaging.9–11 In comparison with MR, CTP has several advantages: it is quicker, cheaper, more widely available, and can be applied in patients who are claustrophobic or have other MR contraindications such as pacemakers.12–14
The broad use of CTP in the management of acute stroke notwithstanding, recommendations regarding the optimal scan acquisition parameters, such as scan duration, differ between studies.15–17 Consequently, CTP scan times vary considerably between imaging centers and are as short as 40s at some centers and as long as 90s at others.2, 11, 17–22 Short scan times can result in truncation of the concentration measurements and resultant inaccurate CTP results.23–25 On the other hand, long scan times can expose patients to unnecessary radiation with no additive accuracy of CTP results. The optimal minimum scan duration, one that avoids truncation-related errors whilst not exposing patients to excess radiation (i.e. As Low As Reasonably Achievable (ALARA) radiation dose), remains undefined.
Because the assessment of ischemic tissue on CTP is based on contrast bolus passage characteristics, it is intuitive that the optimal CTP scan duration depends on the arrival time and the shape of the contrast bolus, and is, therefore, case-specific. However, the exact relationship between the characteristics of the contrast bolus and the optimal scan duration has not yet been established. The goal of this study was to define this relationship and to use it to determine the optimal CTP scan duration, both for an individual patient and for our patient sample as a whole. In order to identify the optimal scan duration, we sequentially truncated the length of the acquired CTP scan in a cohort of patients with a relatively long scan duration (90s) and recorded the impact of truncation on measurements of the ischemic core and penumbra volumes.
Materials and Methods
Patients
Following approval by the Institutional Review Board, consecutive CTP studies, performed at our institution for suspected acute ischemic stroke between 2008 and 2013, were retrospectively reviewed. No informed consent was required because of the retrospective nature of the study. CTP scans were included if they met the following criteria: (1) CTP lesion pattern consistent with an occlusion of a cerebral artery in the anterior circulation; (2) scan duration of 90s; (3) CTP Tmax>6 (Time To Peak of the Residue function) lesion volume greater than 5ml; and (4) a minimum of 4 cm z-axis coverage per imaging slab. Scans were excluded for any of the following reasons: (1) when severe motion artifact resulted in degradation of the image data not amenable to correction with motion correction algorithms; (2) when the automatically segmented lesion volumes contained artifacts; (3) when there was evidence of intracerebral hemorrhage; or (4) when a venous output function (VOF) curve could not be properly fitted.
Image protocol
CTP imaging was performed on 64-slice multidetector CT scanners (GE Healthcare, Waukesha, WI). The CTP was acquired as 2 scans, each covering a 4 cm slab (8 slices × 5.0mm), which were acquired in axial burst mode, i.e. 0.8s gantry rotation and 1.0s delay between the acquisition of subsequent time points. The most caudal slice of the caudal CTP scan was positioned at the level of the orbital roof. The second CTP scan was positioned immediately above the first scan avoiding any overlap between both CTP slabs. The two CTP scans were acquired sequentially with separate injections of 35 ml of iodinated contrast material (Omnipaque 350 mg/ml, GE Healthcare) followed by a 20 ml saline chaser, injected into an antecubital vein at a flow rate of 4 ml/s. The duration of each scan was 90s, during which period 50 frames were acquired in axial burst mode (sampling rate 1.8s). Other acquisition parameters were tube voltage 80 kVp, tube current 125 mA, and exposure time 800 milliseconds, detector collimation 40mm, slice collimation 5mm. A delay of 5–8s between start of contrast injection and CTP acquisition (a.k.a. “prep delay”) was used for patients.
Image post-processing
Post-processing of CTP images was performed using a customized research version of the RAPID (iSchemaView, Menlo Park, CA) perfusion software program.5, 26–29 RAPID allows for fully automated processing of CTP images, including automatic AIF detection and motion correction.
We used the VOF as a representation for the bolus shape in each CTP study because it is the most easy-to-determine signal, and, due to the large venous dimensions, less susceptible to partial volume effects than the arterial input function (AIF). We measured the VOF in CTP source data using a custom designed interface in which we identified the venous location with the highest area under the curve in the full resolution data set (512×512). The VOF was selected from the superior sagittal sinus (SSS), straight sinus, or transverse sinus, whichever region displayed the highest area under the concentration time curve. We parameterized the VOF by curve fitting using a gamma variate function as described by Madsen.30 For each scan we used the fit to determine: 1) the bolus arrival time (T0), defined as the time at which 0.1% of the area under the VOF curve fit was reached, and 2) the width of the VOF, defined as the full width at half maximum of the fitted gamma variate function. Although T0 can also be determined directly from one of the fitted parameters, we empirically found that the 0.1% criterion yielded a more accurate measure of T0.31
Simulation of shorter scans
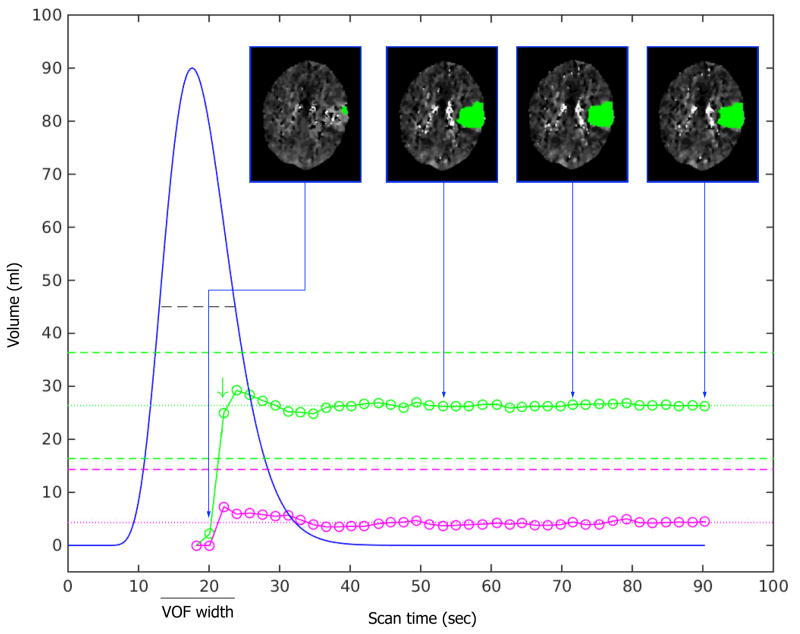
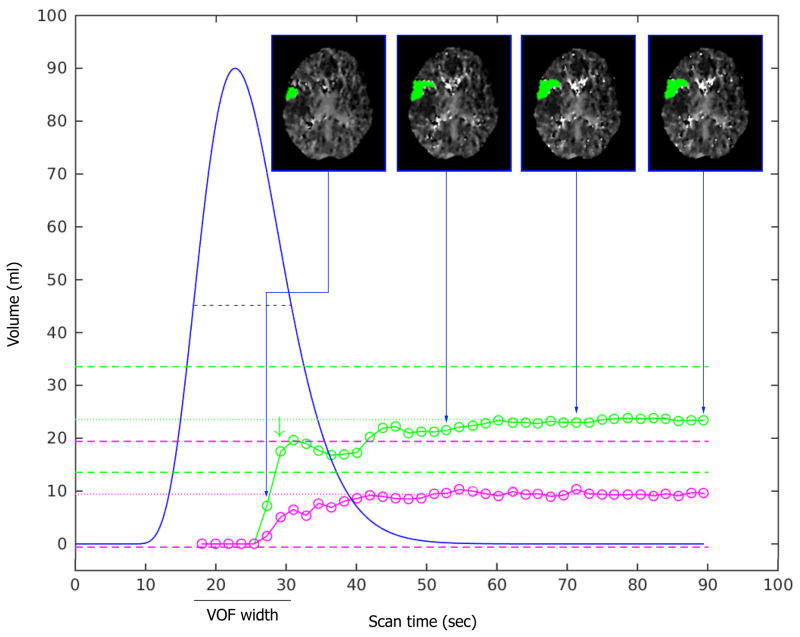
Shorter scan durations were simulated by incrementally eliminating frames starting from the end of the full scan duration of 50 frames (90s). Through this process, each full scan was truncated down to 10 frames (18s), yielding 40 versions of the original CTP scan (spanning 18 to 90s in scan duration). Each version was processed with RAPID to determine the volumes of the penumbra (Tmax >6s)32 and the ischemic core (relative Cerebral blood Flow (rCBF)<30%)4. The same AIF was used for processing of each version. Figure-1 shows examples of the lesion volumes as a function of scan duration.
Figure 1. Relationship between scan duration (seconds) and ischemic lesion volume (ml) estimates.
The VOF curve fit is shown as a blue curve. The volume estimates of critically hypoperfused tissue (Tmax>6s) are indicated with green circles. The dotted green line represents the mean volume of the Tmax>6 lesion for the last 6 frames (the “stability tail”). The dashed green lines represent the boundaries (mean volume ±10ml) beyond which the lesion volumes are deemed unreliable. For the scan shown in panel A, the Tmax>6s lesion volume estimate (green circles) is essentially unaffected by reductions in scan time from 90s down to 38s. With further reductions in scan time down to 23s (corresponds with the optimal scan time, green arrow), some fluctuations in the Tmax>6s lesion volume estimates is observed. When scan time is reduced below 23s, the Tmax>6s lesion volume estimate drops to nil and is clearly unreliable. A similar pattern is observed for the volume estimates of the ischemic core (rCBF<30%, pink circles), although no optimal scan time is noted given the comparatively small size of the ischemic core. The insets show axial CTP images corresponding to four select scan durations. The insets illustrate the marginal differences in lesion volume and lesion morphology when the scan time exceeds the optimal scan duration, but dramatic changes in lesion volume when the scan is shorter than its optimal duration. The graphs in Panel B show the same pattern as in panel A, but due to a later bolus arrival time (T0) and a wider VOF, the graphs of the ischemic lesion volume estimates and the optimal scan duration are shifted to the right.
Determination of optimal scan duration
Assessment of the optimal scan duration was only performed in scans that had converged on a stable lesion volume within the last 10s of the scan (79–90s). Specifically, this was defined as a scan for which the lesion volumes were not significantly impacted by truncation of the terminal 6 frames (defined as lesion volumes that deviated less than 2.5 mL from their average for the terminal six scan durations). Scans that met these criteria were considered “volumetrically stable”, and for each scan the average lesion volume of the terminal six scan durations was considered the “true” volume. For each scan, we then determined the optimal scan duration, defined as the minimal scan duration that would have provided an accurate estimate of the true volume. This was operationally defined as the shortest scan duration that yielded ischemic core and penumbra volumes that deviate <10% and <10mL from the true volumes and where, with longer scanning, the ischemic lesion volumes remained within these bounds. (figure-1). For each scan we also recorded the relative height of the fitted venous output function (rVOF), expressed as a fraction of peak height, at its optimal scan duration.
Statistical Analysis
Group comparisons were performed with Mann-Whitney U test and linear regression was used to evaluate the dependency of the of the optimal scan duration on VOF parameters. A p-value less than 0.05 was considered statistically significant. Statistical analyses were performed in MATLAB and R.
Results
Scan selection and VOF characteristics
Between 2008 and 2013, a total of 70 CTP scans of acute stroke patients were identified that were of 90s duration, had a sufficient contrast bolus allowing VOF selection, showed an acute Tmax>6 lesion of at least 5 ml, and were without significant motion artifact. Fifty-nine of the 70 scans (84.3%) were volumetrically stable during the terminal phase of the scan by our definition. Their median T0 was 16.4s (IQR 12.4 – 20.3) and their median VOF width was 15.4s (IQR 13.4 – 18.9). In 7 of the 59 scans (12%), T0 was shorter than 10s prior to adjusting T0. The 11 (16%) scans that were not volumetrically stable were analyzed individually to assess the reason for instability. Four had very late bolus arrival and wide VOFs, with truncation of the VOF during its downslope at the 90s mark. The remaining 7 cases were unstable despite adequate temporal VOF coverage. The volume estimates in these cases appear to have been affected by motion during the terminal phase of the scan.
Relation between Optimal Scan Duration and VOF parameters
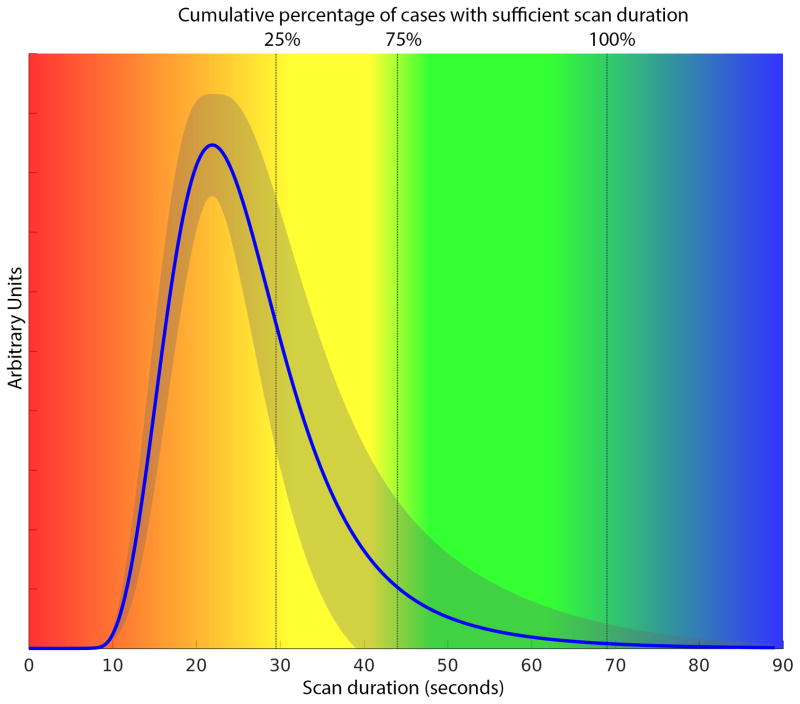
For the 59 scans that were volumetrically stable, the median optimal scan time was 32.7s (90th percentile 52.6s; 100th percentile 68.9s) and the median rVOF of the optimal scan times was 0.39 (90th percentile 0.02; 100th percentile 0.00). (Table 1) Figure-2 shows the averaged VOF curve fit (with a T0 normalized to 10s) and color-coded zones that indicate the reliability of CTP volumes as a function of scan duration for the 59 scans included in this analysis. Linear regression analysis demonstrated that the VOF width is significantly associated with the optimal scan duration:
Table 1.
Optimal CTP scan duration and rVOF height at scan completion for reliable CTP lesion estimation
| Percentile of patients with reliable CTP lesion volume estimates (n=59) | Optimal CTP scan duration (native T0) | Optimal CTP scan duration (T0 normalized to 10s) | rVOF height |
|---|---|---|---|
| 50th | 42.3 s | 32.7 s | 39% |
| 60th | 47.6 s | 37.2 s | 28% |
| 70th | 52.4 s | 41.0 s | 11% |
| 80th | 55.9 s | 45.9 s | 6% |
| 90th | 60.7 s | 52.6 s | 2% |
| 100th | 72.9 s | 68.9 s | 0% |
CTP indicates computed tomography perfusion; rVOF: height of the fitted venous output function expressed as a fraction of peak height; T0: bolus arrival time.
Figure 2. Cumulative percentage of patients with accurate ischemic lesion volume estimates.
For the 59 scans that had stable lesion volume estimates with 90s scan duration, the vertical color-coding corresponds to the cumulative proportion of scans that have reached their optimal scan duration: 25% of scans have an optimal scan duration <29.5s; 75% <44s, and 100% <69s. These data indicate that, with a uniform T0 of 10s, a total scan duration of 69s would have been sufficient for all 59 cases. A total scan duration of 60s, would have been sufficient for 93% of the cases (55 of 59). The averaged VOF for the 59 cases, normalized to a T0 of 10s, is shown (blue curve) with its standard deviation (grey shaded area).
The 95% prediction interval of this function ranged from −25.6 to 25.6 s.
Discussion
This study shows that the optimal CTP scan duration for assessment of ischemic lesion volumes in acute stroke patients is dependent on the bolus arrival time and the width of the venous outflow function. We demonstrate that the optimal scan duration coincides with the terminal part of the downslope of the VOF and can be estimated as the bolus arrival time (T0) plus 1.6 widths of the VOF. For the population included in this analysis, a scan duration of approximately 70s covers the entire downslope of the VOF and would have been sufficient to accurately assess the ischemic lesion volumes in all cases.
Relatively few studies have analyzed the impact of scan duration on lesion volume measurements and none have related the optimal scan duration to the shape of the contrast bolus. Prior recommendations were based on modeling of circulation times to the brain among normal patients, whereas our study used empirical data from acute stroke patients to determine the optimal scan duration.17 In our study, there was a predictable pattern by which the measured volume of the ischemic lesion changed depending on the total scan duration; volumes were nil with very short scan duration, fluctuated considerably with intermediate scan duration, and converged onto a fixed value when the scan was longer than the optimal scan duration (figure-1). These results are in contrast with prior studies that did not identify a scan duration beyond which lesion volumes stabilized. One MR study reported that the perfusion lesion volume becomes increasingly larger with lengthier scan durations.27 In a CTP study, lengthier scan durations were associated with smaller ischemic core but larger penumbra.25 Our results, which make intuitive sense, indicate that for each case there is an optimal scan duration beyond which additional scan time does not yield additional benefit.
There is wide variability among hospitals in terms of CTP scan duration. Driven by a desire to limit radiation exposure, some hospitals have shortened their CTP protocol to 40–45s. Our data demonstrate that these protocols are often too short to accurately estimate lesion volumes. With such short protocols, ischemic lesion volume estimates may be inaccurate in up to 50% of cases. In clinical practice, it may be difficult to recognize cases that are scanned too short, because the images are typically still of good quality and without artifact even though the lesion volume estimates are inaccurate. Some of the problems of short scanning protocols may be mitigated by the use of bolus arrival data from the CTA to determine the scan delay of the CTP.33 This technique can avoid unnecessarily long baselines of the CTP. However, even with a uniform baseline of 10s, a 40s protocol would still be too short for more than a quarter of the acute stroke population.
Some hospitals use relatively long CTP protocols that mimic typical MR perfusion acquisition. Our data demonstrate that 90s scan protocols are unnecessarily long. Among the patients included in our primary analysis, a scan duration of approximately 70s (given a uniform baseline of 10s) was sufficient to yield accurate lesion volumes for all patients, whereas a 60s scan duration was sufficiently long for 93% of the patients. Eleven patients were excluded from our primary analysis because, even at 90s scan time, the ischemic lesions had not converged onto a stable volume. However, in the majority of these cases the reason for instability was patient motion (n=7) rather than truncation of the VOF (n=4). The four cases with late bolus arrival and very wide VOFs may have benefitted from a scan duration that is even longer than 90s, but it is uncertain if lesion volumes would ever be accurate among such patients with very broad bolus passage. Accordingly, we suggest that 70s CTP protocols are optimal as they provide sufficient temporal coverage to accurately estimate ischemic lesion volumes in practically all cases. Shortening the CTP protocol to 60s may be acceptable, as this would still yield accurate lesion volume estimates in the vast majority of cases (93%). Longer scan times may be justified under certain circumstances to acquire the data necessary for alternative purposes, such as to generate permeability maps.
While a 60–70s scan protocol appears optimal for the acute stroke population as a whole, it may be excessive for any given individual patient. Figure-1 shows two examples of scans that could have been significantly shorter than 60s without impacting lesion volumes. In this study, we demonstrate that an individual patient’s minimum scan duration can be predicted based on the shape of their VOF. Specifically, from the time of bolus arrival, the CTP acquisition needs to continue for 1.6 widths of the VOF + 25s to have sufficient temporal coverage in 95% of cases. This knowledge can be used to develop novel individualized CTP acquisition protocols. For example, a “scout” bolus of similar volume but lower concentration combined with low-radiation customized CT acquisition could be obtained to determine a patient’s VOF characteristics.34–36 This information can then be used to determine the optimal CTP scan time for an individual patient. This would limit the radiation dose for that patient to the minimal amount necessary for accurate measurements, in keeping with the ALARA principle of radioprotection. While this type of protocol cannot be readily implemented on current scanners, future advances in CTP technology may make such individualized scan protocols feasible.
Knowledge of the link between a subject’s VOF characteristics and the optimal scan duration has potential applications beyond individualizing the duration of CTP scans. It could, for example, be used to optimize scanning protocols by replacing fixed sampling rates with smart adaptive sampling protocols that have relatively high temporal sampling during bolus passage and reduced sampling rates during the baseline and the tail end of the scan.18, 37–40 Combining modified temporal sampling with ‘saline bolus chasing’ and ‘smart preparation acquisition trigger threshold’ techniques might modify the requirements for contrast duration and quantity. Simply increasing the contrast injection rate, while keeping total volume constant, reduces the VOF width and could be another approach to reduce the required scan time and thus radiation exposure.36 This has the added benefit that it would increase bolus peak height, which would improve contrast to noise of the CTP image. Knowledge of the relationship between VOF characteristics and optimal scan duration can be used after CTP acquisition to determine if a scan is of sufficient duration to reliably estimate ischemic lesion volumes. In this setting, visual analysis of the VOF curve can be very informative, because complete coverage of the venous bolus passage (i.e. no truncation of the VOF peak) indicates that a scan is sufficiently long for reliable lesion volume estimation.
Our study has limitations. First, the results of our study may, to some extent, be dependent on the specific software used to calculate lesion volumes.4, 17, 41–43 However, the use of alternative software is unlikely to challenge the central conclusion of this study; namely, that the optimal CTP scan duration is dependent on the patient’s VOF characteristics and is, therefore, case-specific. Second, our recommendations follow from our specific analytic methods. Should other methods be used, such as the “initial slope” method to calculate CBF or the use of CBV core thresholds rather than CBF thresholds, variable results may be found. However, comparable changes to Tmax calculations are to be expected. Third, we chose the VOF rather than the AIF as a reference because the VOF is easier to determine and less subject to partial volume artifacts. Given the very close relationship between the VOF and the AIF, the results of the study would likely have been similar had the AIF been used as a reference, but the coefficients that describe the optimal scan duration as a function of the AIF would have been slightly different than those of the VOF that we report here. Fourth, the lesion volumes on 11 of 70 scans remained unstable, even with maximum (90s) scan duration. Four of these scans were characterized by a wide VOF. Whether the relationship between VOF characteristics and optimal scan duration is similar between these scans and the 59 included in our regression analysis could not be assessed. Future studies with even longer scan duration are required to answer this question. Finally, some of the definitions used in this study are arbitrary. For example, we defined lesion volumes as inaccurate if they deviated more than 10 mL from the ‘true’ volume. While we felt that 10 mL is likely clinically meaningful and exceeds the typical measurement error of lesion volume estimates, an alternative definition could have been used instead.
Summary
Our results describe the relationship between a patient’s VOF characteristics and their optimal CTP scan duration. Fixed-duration CTP scan protocols, which are currently the standard in clinical practice, and vary in length from 40–90s, are often either too short to accurately assess the ischemic lesion volume or too long, thus exposing patients to unnecessary radiation.2, 11, 15, 17–24 Future development of case-specific CTP protocols, informed by a low-radiation ‘scout’ with a test bolus, could mitigate the limitations of fixed duration scan protocols. A simple method to individualize the CTP acquisition, that can already be implemented, is to base the delay between contrast administration and start of CTP scanning on contrast arrival data from a CTA. When this strategy is used to avoid unnecessarily long baselines, a CTP scan duration of 60–70s appears optimal for the evaluation of acute stroke patients.
Acknowledgments
Funding. The study was funded by grants from the National Institute for Neurological Disorders and Stroke (NINDS). 1U10NS086487 (G. Albers) and 5 R01 NS075209 (M. Lansberg).
Footnotes
DISCLOSURES/CONFLICT OF INTEREST
G. Albers has received consulting fees and expenses from Lundbeck for Steering Committee work and consulting fees from Concentric for serving on a Data Safely and Monitory Board. G Albers and R Bammer are equity shareholders in iSchemaView and perform consulting work for iSchemaView. Soren Christensen performs consulting work for iSchemaView. All other authors report no conflicts of interest.
Reference List
- 1.Heiss WD, Sobesky J, Hesselmann V. Identifying thresholds for penumbra and irreversible tissue damage. Stroke. 2004;35:2671–4. doi: 10.1161/01.STR.0000143329.81997.8a. [DOI] [PubMed] [Google Scholar]
- 2.Wintermark M, Flanders AE, Velthuis B, Meuli R, van LM, Goldsher D, et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke. 2006;37:979–85. doi: 10.1161/01.STR.0000209238.61459.39. [DOI] [PubMed] [Google Scholar]
- 3.Bivard A, Spratt N, Levi C, Parsons M. Perfusion computer tomography: imaging and clinical validation in acute ischaemic stroke. Brain. 2011;134:3408–16. doi: 10.1093/brain/awr257. [DOI] [PubMed] [Google Scholar]
- 4.Campbell BC, Christensen S, Levi CR, Desmond PM, Donnan GA, Davis SM, et al. Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke. 2011;42:3435–40. doi: 10.1161/STROKEAHA.111.618355. [DOI] [PubMed] [Google Scholar]
- 5.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 6.Ma H, Parsons MW, Christensen S, Campbell BC, Churilov L, Connelly A, et al. A multicentre, randomized, double-blinded, placebo-controlled Phase III study to investigate EXtending the time for Thrombolysis in Emergency Neurological Deficits (EXTEND) Int J Stroke. 2012;7:74–80. doi: 10.1111/j.1747-4949.2011.00730.x. [DOI] [PubMed] [Google Scholar]
- 7.Burton KR, Dhanoa D, Aviv RI, Moody AR, Kapral MK, Laupacis A. Perfusion CT for selecting patients with acute ischemic stroke for intravenous thrombolytic therapy. Radiology. 2015;274:103–14. doi: 10.1148/radiol.14140728. [DOI] [PubMed] [Google Scholar]
- 8.Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 9.Campbell BC, Christensen S, Levi CR, Desmond PM, Donnan GA, Davis SM, et al. Comparison of computed tomography perfusion and magnetic resonance imaging perfusion-diffusion mismatch in ischemic stroke. Stroke. 2012;43:2648–53. doi: 10.1161/STROKEAHA.112.660548. [DOI] [PubMed] [Google Scholar]
- 10.Schaefer PW, Barak ER, Kamalian S, Gharai LR, Schwamm L, Gonzalez RG, et al. Quantitative assessment of core/penumbra mismatch in acute stroke: CT and MR perfusion imaging are strongly correlated when sufficient brain volume is imaged. Stroke. 2008;39:2986–92. doi: 10.1161/STROKEAHA.107.513358. [DOI] [PubMed] [Google Scholar]
- 11.Schramm P, Schellinger PD, Fiebach JB, Heiland S, Jansen O, Knauth M, et al. Comparison of CT and CT angiography source images with diffusion-weighted imaging in patients with acute stroke within 6 hours after onset. Stroke. 2002;33:2426–32. doi: 10.1161/01.str.0000032244.03134.37. [DOI] [PubMed] [Google Scholar]
- 12.Jackson D, Earnshaw SR, Farkouh R, Schwamm L. Cost-effectiveness of CT perfusion for selecting patients for intravenous thrombolysis: a US hospital perspective. AJNR Am J Neuroradiol. 2010;31:1669–74. doi: 10.3174/ajnr.A2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wintermark M, Meuli R, Browaeys P, Reichhart M, Bogousslavsky J, Schnyder P, et al. Comparison of CT perfusion and angiography and MRI in selecting stroke patients for acute treatment. Neurology. 2007;68:694–7. doi: 10.1212/01.wnl.0000255959.30107.08. [DOI] [PubMed] [Google Scholar]
- 14.Wintermark M, Sanelli PC, Albers GW, Bello J, Derdeyn C, Hetts SW, et al. Imaging recommendations for acute stroke and transient ischemic attack patients: A joint statement by the American Society of Neuroradiology, the American College of Radiology, and the Society of NeuroInterventional Surgery. AJNR Am J Neuroradiol. 2013;34:E117–E127. doi: 10.3174/ajnr.A3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirata M, Sugawara Y, Murase K, Miki H, Mochizuki T. Evaluation of optimal scan duration and end time in cerebral CT perfusion study. Radiat Med. 2005;23:351–63. [PubMed] [Google Scholar]
- 16.Lev MH. Perfusion imaging of acute stroke: its role in current and future clinical practice. Radiology. 2013;266:22–7. doi: 10.1148/radiol.12121355. [DOI] [PubMed] [Google Scholar]
- 17.Wintermark M, Albers GW, Alexandrov AV, Alger JR, Bammer R, Baron JC, et al. Acute stroke imaging research roadmap. Stroke. 2008;39:1621–8. doi: 10.1161/STROKEAHA.107.512319. [DOI] [PubMed] [Google Scholar]
- 18.Corcuera-Solano I, McLellan AM, Doshi AH, Pawha PS, Tanenbaum LN. Whole-brain adaptive 70-kVp perfusion imaging with variable and extended sampling improves quality and consistency while reducing dose. AJNR Am J Neuroradiol. 2014;35:2045–51. doi: 10.3174/ajnr.A4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho CY, Hussain S, Alam T, Ahmad I, Wu IC, O’Neill DP. Accuracy of CT cerebral perfusion in predicting infarct in the emergency department: lesion characterization on CT perfusion based on commercially available software. Emerg Radiol. 2013;20:203–12. doi: 10.1007/s10140-012-1102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy BD, Fox AJ, Lee DH, Sahlas DJ, Black SE, Hogan MJ, et al. Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke. 2006;37:1771–7. doi: 10.1161/01.STR.0000227243.96808.53. [DOI] [PubMed] [Google Scholar]
- 21.Schaefer PW, Mui K, Kamalian S, Nogueira RG, Gonzalez RG, Lev MH. Avoiding “pseudo-reversibility” of CT-CBV infarct core lesions in acute stroke patients after thrombolytic therapy: the need for algorithmically “delay-corrected” CT perfusion map postprocessing software. Stroke. 2009;40:2875–8. doi: 10.1161/STROKEAHA.109.547679. [DOI] [PubMed] [Google Scholar]
- 22.Turk AS, Magarick JA, Frei D, Fargen KM, Chaudry I, Holmstedt CA, et al. CT perfusion-guided patient selection for endovascular recanalization in acute ischemic stroke: a multicenter study. J Neurointerv Surg. 2013;5:523–7. doi: 10.1136/neurintsurg-2012-010491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borst J, Marquering HA, Beenen LF, Berkhemer OA, Dankbaar JW, Riordan AJ, et al. Effect of extended CT perfusion acquisition time on ischemic core and penumbra volume estimation in patients with acute ischemic stroke due to a large vessel occlusion. PLoS One. 2015;10:e0119409. doi: 10.1371/journal.pone.0119409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Copen WA, Deipolyi AR, Schaefer PW, Schwamm LH, Gonzalez RG, Wu O. Exposing hidden truncation-related errors in acute stroke perfusion imaging. AJNR Am J Neuroradiol. 2015;36:638–45. doi: 10.3174/ajnr.A4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mangla R, Ekhom S, Jahromi BS, Almast J, Mangla M, Westesson PL. CT perfusion in acute stroke: know the mimics, potential pitfalls, artifacts, and technical errors. Emerg Radiol. 2014;21:49–65. doi: 10.1007/s10140-013-1125-9. [DOI] [PubMed] [Google Scholar]
- 26.Campbell BC, Yassi N, Ma H, Sharma G, Salinas S, Churilov L, et al. Imaging selection in ischemic stroke: feasibility of automated CT-perfusion analysis. Int J Stroke. 2015;10:51–4. doi: 10.1111/ijs.12381. [DOI] [PubMed] [Google Scholar]
- 27.Inoue M, Mlynash M, Straka M, Lansberg MG, Zaharchuk G, Bammer R, et al. Patients with the malignant profile within 3 hours of symptom onset have very poor outcomes after intravenous tissue-type plasminogen activator therapy. Stroke. 2012;43:2494–6. doi: 10.1161/STROKEAHA.112.653329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue M, Mlynash M, Straka M, Kemp S, Jovin TG, Tipirneni A, et al. Clinical outcomes strongly associated with the degree of reperfusion achieved in target mismatch patients: pooled data from the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution studies. Stroke. 2013;44:1885–90. doi: 10.1161/STROKEAHA.111.000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging. 2010;32:1024–37. doi: 10.1002/jmri.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madsen MR. A simplified formulation of the gamma variate function. Physics in Medicine and Biology. 1992;37:1597–600. [Google Scholar]
- 31.Christensen S, Calamante F, Hjort N, Wu O, Blankholm AD, Desmond P, et al. Inferring origin of vascular supply from tracer arrival timing patterns using bolus tracking MRI. J Magn Reson Imaging. 2008;27:1371–81. doi: 10.1002/jmri.21386. [DOI] [PubMed] [Google Scholar]
- 32.Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol. 2012;11:860–7. doi: 10.1016/S1474-4422(12)70203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morhard D, Wirth CD, Reiser MF, Schulte-Altedorneburg G, Ertl-Wagner B. Optimal sequence timing of CT angiography and perfusion CT in patients with stroke. Eur J Radiol. 2013;82:e286–e289. doi: 10.1016/j.ejrad.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Bae KT. Comparison of moderate versus high concentration of contrast media injected at the same total iodine dose and fixed injection duration. Radiology. 2005;236:740–1. doi: 10.1148/radiol.2362050096. [DOI] [PubMed] [Google Scholar]
- 35.Bae KT. Test-bolus versus bolus-tracking techniques for CT angiographic timing. Radiology. 2005;236:369–70. doi: 10.1148/radiol.2361050123. [DOI] [PubMed] [Google Scholar]
- 36.Bae KT. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology. 2010;256:32–61. doi: 10.1148/radiol.10090908. [DOI] [PubMed] [Google Scholar]
- 37.Abels B, Klotz E, Tomandl BF, Villablanca JP, Kloska SP, Lell MM. CT perfusion in acute ischemic stroke: a comparison of 2-second and 1-second temporal resolution. AJNR Am J Neuroradiol. 2011;32:1632–9. doi: 10.3174/ajnr.A2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shankar JJ, Lum C, Sharma M. Whole-brain perfusion imaging with 320-MDCT scanner: Reducing radiation dose by increasing sampling interval. AJR Am J Roentgenol. 2010;195:1183–6. doi: 10.2214/AJR.10.4230. [DOI] [PubMed] [Google Scholar]
- 39.Wiesmann M, Berg S, Bohner G, Klingebiel R, Schopf V, Stoeckelhuber BM, et al. Dose reduction in dynamic perfusion CT of the brain: effects of the scan frequency on measurements of cerebral blood flow, cerebral blood volume, and mean transit time. Eur Radiol. 2008;18:2967–74. doi: 10.1007/s00330-008-1083-x. [DOI] [PubMed] [Google Scholar]
- 40.Wintermark M, Smith WS, Ko NU, Quist M, Schnyder P, Dillon WP. Dynamic perfusion CT: optimizing the temporal resolution and contrast volume for calculation of perfusion CT parameters in stroke patients. AJNR Am J Neuroradiol. 2004;25:720–9. [PMC free article] [PubMed] [Google Scholar]
- 41.Kamalian S, Kamalian S, Maas MB, Goldmacher GV, Payabvash S, Akbar A, et al. CT cerebral blood flow maps optimally correlate with admission diffusion-weighted imaging in acute stroke but thresholds vary by postprocessing platform. Stroke. 2011;42:1923–8. doi: 10.1161/STROKEAHA.110.610618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kudo K, Sasaki M, Yamada K, Momoshima S, Utsunomiya H, Shirato H, et al. Differences in CT perfusion maps generated by different commercial software: quantitative analysis by using identical source data of acute stroke patients. Radiology. 2010;254:200–9. doi: 10.1148/radiol.254082000. [DOI] [PubMed] [Google Scholar]
- 43.Zussman BM, Boghosian G, Gorniak RJ, Olszewski ME, Read KM, Siddiqui KM, et al. The relative effect of vendor variability in CT perfusion results: a method comparison study. AJR Am J Roentgenol. 2011;197:468–73. doi: 10.2214/AJR.10.6058. [DOI] [PubMed] [Google Scholar]