Abstract
Background and Purpose
Co-morbidity of diabetes mellitus and stroke results in worse functional outcome, poor long term recovery and extensive vascular damage. We investigated the neurorestorative effects and mechanisms of stroke treatment with human bone marrow derived mesenchymal stromal cells (hMSCs) in type two diabetes mellitus (T2DM) rats.
Methods
Adult male Wistar rats were induced with T2DM, subjected to 2 hours of middle cerebral artery occlusion (MCAo) and treated via tail-vein injection with: 1) PBS (n=8); 2) hMSCs (n=10, 5×106) at 3 days after MCAo.
Results
In T2DM rats, hMSCs administered at 3 days after MCAo significantly improves neurological function without affecting blood glucose, infarct volume and incidence of brain hemorrhage in comparison to T2DM-MCAo PBS treated rats. Delayed hMSC treatment of T2DM stroke significantly improves blood brain barrier integrity, increases vascular and arterial density and cerebral vascular perfusion, and promotes neuroblast cell migration and white matter remodeling as indicated by increased doublecortin, axon, myelin and neurofilament density, respectively. Delayed hMSC treatment significantly increases platelet-derived growth factor (PDGF) expression in the ischemic brain, decreases pro-inflammatory M1 macrophage and increases anti-inflammatory M2 macrophage compared to PBS treated T2DM-MCAo rats. In vitro data show that hMSCs increase sub-ventricular zone explant cell migration and primary cortical neuron neurite outgrowth while inhibition of PDGF decreases hMSCs induced SVZ cell migration and axonal outgrowth.
Conclusion
In T2DM stroke rats, delayed hMSC treatment significantly improves neurological functional outcome, and increases neurorestorative effects and M2 macrophage polarization. Increasing brain PDGF expression may contribute to hMSC induced neurorestoration.
Keywords: Stroke, ischemia, type 2 diabetes mellitus, mesenchymal stromal cells, platelet-derived growth factor
Introduction
Diabetes mellitus (DM) is a high risk factor for ischemic stroke and stroke patients with DM battle higher mortality rates and poor long term recovery than non-DM stroke patients1. In non-DM rodents, marrow stromal cell (MSC) therapy for stroke facilitates functional recovery by stimulating angiogenesis, vascular stabilization and white matter (WM) remodeling in the injured brain2, 3. However, MSCs therapy for stroke in non-DM rats does not successfully translate to DM stroke, and type one DM (T1DM) rats subjected to stroke and treated with MSCs 24 hours later, suffer from significantly increased brain hemorrhage, blood brain barrier (BBB) leakage and treated rats do not show functional improvement compared to T1DM stroke control rats4. Since nearly 90% of DM patients suffer from type 2 DM (T2DM), in this study, we investigated the therapeutic effects of delayed (3 days) human MSC (hMSC) treatment in T2DM stroke rats.
MSCs secrete several angiogenic, trophic and growth factors5. Platelet-derived growth factor (PDGF) in particular, is a potent neuroprotective molecule, and is secreted abundantly by MSCs6. PDGF is highly expressed in WM, and can induce axonal regeneration7. PDGF-BB and its receptors are also expressed on endothelial cells, and may play an important role in post stroke angiogenesis7. Macrophages are major regulators of vascularization and axonal remodeling8. Macrophages can be classified broadly into two types, the pro-inflammatory and anti-angiogenic M1 phenotype, and the anti-inflammatory and pro-angiogenic M2 phenotype8. Whether hMSC treatment regulates PDGF expression and M2 macrophage polarization in T2DM-stroke rats has not been investigated.
In this study, we investigate the therapeutic effects and underlying mechanisms of delayed hMSC treatment of stroke in T2DM rats. We hypothesize that delayed hMSC treatment of T2DM stroke significantly improves functional outcome, and induces neurorestorative effects and PDGF and M2 macrophage polarization may partially contribute to hMSC induced neurorestoration.
Materials and methods
All experiments were conducted in accordance with the standards and procedures of the American Council on Animal Care and Institutional Animal Care and Use Committee of Henry Ford Health System.
Diabetes induction
Adult Male Wistar rats (175–200g, Charles River) were induced with T2DM using a combination of high-fat diet (HFD) and low dose of Streptozotocin (STZ)9, 10. Body weight, blood glucose, high density lipoprotein (HDL), and total cholesterol were measured before and 10 days after STZ injection. Rats with fasting (8 hours) plasma glucose ≥300 mg/dl were considered diabetic.
MCAo model and experiment groups
T2DM rats were subject to transient (2 hours) middle cerebral artery occlusion (MCAo) by intraluminal suture model3, 10. Rats were randomized (n=10/group) and treated 3 days after MCAo via tail vein injection with: 1) phosphate-buffered saline (PBS); 2) 5×106 hMSCs (Cognate Bioservices, Inc.). T2DM stroke rats treated with PBS or hMSCs at 3 day after stroke were sacrificed at 4 weeks after MCAo to evaluate long term effects and blood serum and brain tissues were collected for ELISA and immunostaining. Mortality rate was 20% in each group. The sample size, 10 per group, was predefined to detect an effect size of 1.33 with a power of 80%.
Functional tests
To assess neurological functional outcome, a battery of tests including a modified neurological severity score test (mNSS)11, adhesive removal test3 and foot-fault test10 were performed before MCAo and after MCAo on days 1, 7, 14, 21 and 28 by an investigator who was blinded to the experimental groups.
Histological and immunohistochemical assessment
Rats were transcardially perfused with 0.9% saline, brains immediately removed and fixed in 4% paraformaldehyde. A standard paraffin block was obtained from the center of the lesion (bregma −1mm to +1mm). Every 10th coronal section for a total of 5 sections (6µm thick) was used for immunohistochemical staining. Antibody against Von Willebrand Factor (vWF, an endothelial cell marker, 1:400; Dako), α-smooth muscle actin (αSMA, smooth muscle cell marker, mouse monoclonal IgG 1:800, Dako), ED1 (microglia/macrophages marker, 1:30; AbD Serotec), CD163 (M2 macrophage marker, 1:500, Abcam), Doublecortin (DCX, a protein expressed in migrating neurons, 1:200, Santa Cruz), SMI-31 (phosphorylated neurofilament marker, 1:1000, Covance), PDGFRa (platelet derived growth factor receptor, 1:400, Santa Cruz) and PDGFRb (1:100, R&D systems) were employed. Bielschowsky silver (BS) and luxol fast blue (LFB) staining was used to demonstrate axons and myelin respectively. Antibody against albumin (albumin-FITC, polyclonal, 1:500, Abcam) was used to demonstrate BBB leakage. Gomori One-Step Trichrome Stain was used to evaluate arteriosclerosis. For control experiments non-immune serum was substituted for the primary antibody.
Quantification analysis
The ischemic border zone (IBZ) is defined as the area surrounding the lesion. For quantitative measurements, five slides from each brain, with each slide containing 4 fields from striatum bundle of the IBZ (BS and LFB) or 8 fields from striatum and cortex of the IBZ (ED1, CD163, PDGFRa, PDGFRb, DCX, albumin, αSMA, vWF and SMI) were digitized under a 20× or 40× objective (Olympus BX40) using a 3-CCD color video camera (Sony DXC-970MD) interfaced with an MCID image analysis system (Imaging Research)10. Data were analyzed in a blinded manner and positive areas or positive cell numbers were measured in the IBZ.
Arterial density, wall thickness, and diameter and occluded artery measurement
The number of arteries stained with αSMA were counted and analyzed with regard to small and large vessels (≥10µm diameter). The arterial density in the IBZ, and the 10 largest arterial wall thicknesses and diameter were measured.
Trichrome immunostaining and measurement
Using Gomori One-Step Trichrome Stain (Sigma), brain sections were post fixed in Bouin fixative. Nuclei were stained with Weigert hematoxylin and then stained in Gomori trichrome stain followed by a 0.5% acetic water rinse. Connective tissue and collagen are stained blue, nuclei are stained dark red/purple, and cytoplasm is stained red/pink. Artery intimae, media, and artery diameter (minimum diameter) were measured in the internal carotid artery (ICA).
Cerebral blood perfusion measurement
To test cerebral vascular perfusion, an additional set of animals (n=5/group) were prepared and FITC-dextran (FD2000S, Sigma) 50mg/rat in 2ml PBS was injected intravenously 5 minutes before sacrifice12, 13. Brain tissues were fixed by 4% paraformaldehyde for 48 hours then were processed to acquire adjacent 100µm thick coronal sections using a vibratome. Five sections from the bregma (−1mm to +1mm) were imaged using a fluorescence microscope (Zeiss Axiophot 2) and FITC-dextran labeled vessels were quantification using ImageJ.
Angiogenesis ELISA array assay
Mouse angiogenesis antibody array kit (R&D Systems), including 55 angiogenesis-associated proteins and cytogeneses, was employed to test angiogenic protein level in ischemic brain tissue according to the manufacturer's instructions.
SVZ cell migration assay
Rats were subjected to 2 hours of MCAo and SVZ explants were isolated from the ipsilateral side at 24 hours after stroke. To further test whether hMSCs affect neuroblast migration, we used a previously described in vitro SVZ explant culture model14. The cultured SVZ explants were treated as per the following experimental groups: 1) control; 2) +50% hMSCs conditioned media; 3) +50% hMSCs conditioned media + PDGF inhibitor (20µg). The average linear distance of cell migration from the edge of the SVZ explant was measured using the MCID software.
Primary cortical neuron axonal outgrowth assay
Axonal outgrowth was measured, as previously described15. Briefly, primary cortical neurons were harvested from pregnant embryonic (day 18) Wistar rats. A microfluidic chamber (Standard Neuron Device, Xona Microfluidics) was used to separate axons from neuronal soma16. The experimental groups included: 1) control no treatment; 2) +50% hMSC conditioned media; 3) +50% hMSC conditioned medium +PDGF inhibitor (20µg).
Statistical analysis
All measurements and analyses were performed by normality of distribution, and the homogeneity of variances was tested including the functional tests, biochemistry and immunostaining. The Global test using GEE were employed to test effects on functional recovery measured from three behavioral tests at each time point. The analysis began with testing hMSC effect on the overall functional recovery, followed by testing the treatment effect on the individual test. One-way analysis of variance (ANOVA) was used for the immunostaining analysis. All data are presented as mean ± standard error (SE).
Results
hMSC treatment of stroke in T2DM rats significantly improves functional outcome but does not affect blood glucose level, lesion volume and brain hemorrhage transformation
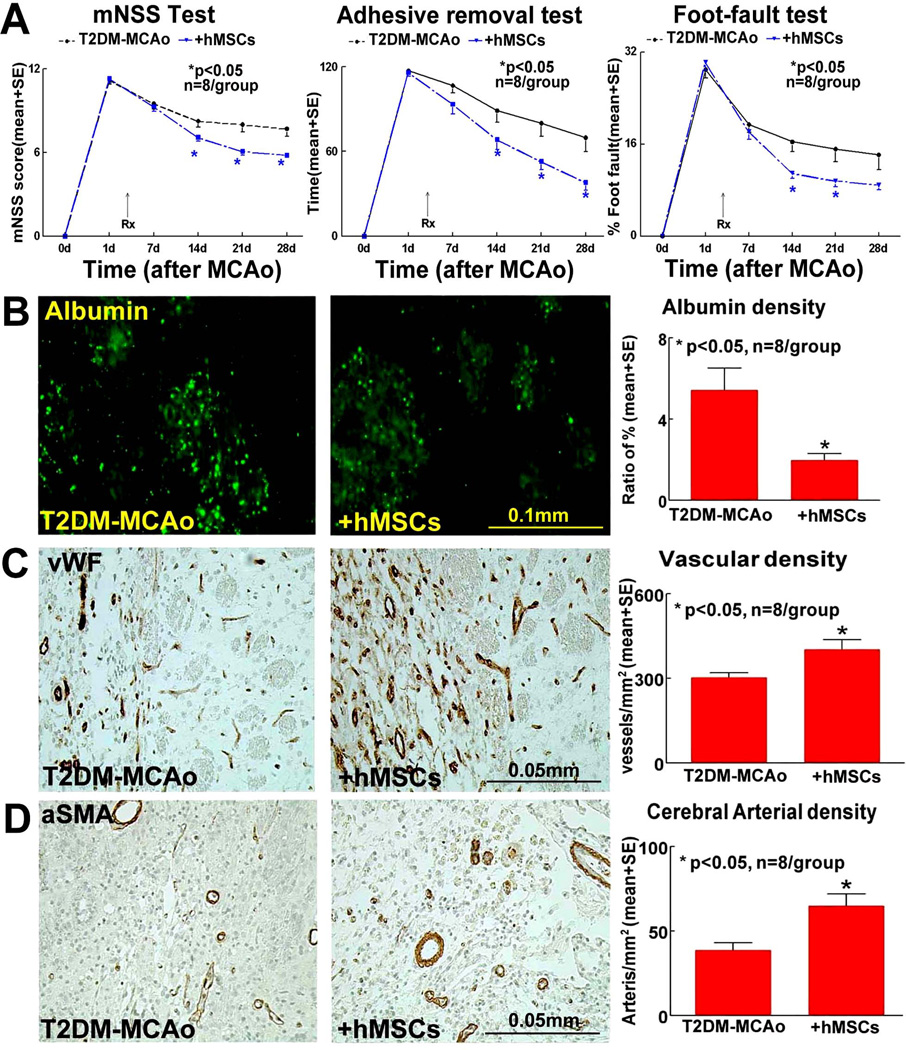
Delayed hMSC treatment initiated at 3 days after MCAo significantly improves long term neurological function compared to T2DM MCAo control rats as indicated by mNSS test, foot-fault test and adhesive removal test (p<0.05, n=8/group, Figure 1A). Data were evaluated for normality and ranked data were used for the analysis because the data were not normally distributed. The data show that the overall group effect was significant at 14, 21 and 28 days after stroke (p<0.05). However, hMSC treatment of stroke in T2DM rats does not significantly decrease lesion volume and brain hemorrhage as well as the blood glucose/lipid levels compared to non-treatment T2DM stroke controls (p>0.05) (Supplementary Table 1).
Figure 1.
In T2DM-MCAo rats, compared to PBS treatment, hMSC treatment 3 days post stroke significantly improves functional outcome indicated by A) mNSS Test, Adhesive removal test, Foot-fault test; significantly decreases BBB leakage as indicated by B) FITC-albumin staining, and improves vascular remodeling as indicated by: C) vWF and D) αSMA immunostaining and quantification data in the IBZ.
hMSC treatment of stroke in T2DM rats promotes neurovascular remodeling
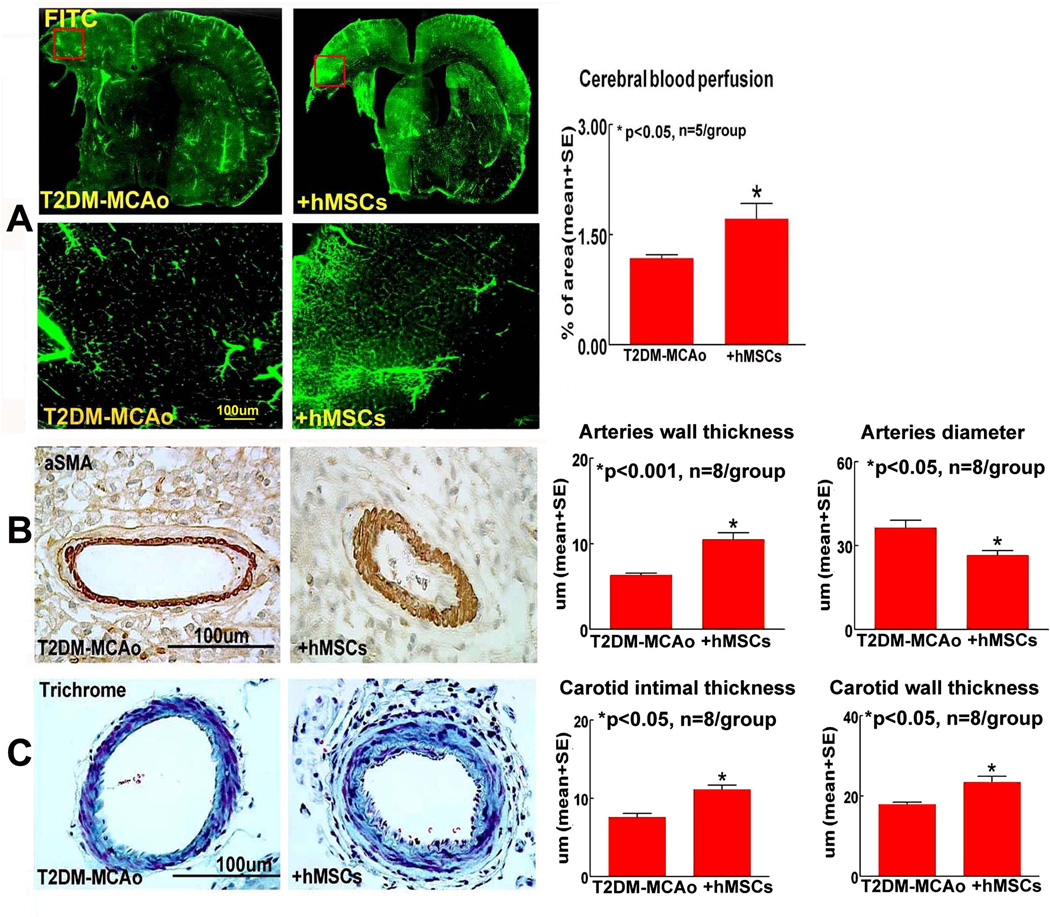
To test the mechanisms of hMSC treatment induced improvement of functional outcome after stroke in T2DM rats, vascular remodeling and cerebral perfusion were evaluated. We found that hMSC treatment of stroke initiated at 3 days after MCAo significantly decreases BBB leakage (Figure 1B), and increases cerebral vascular and cerebral arterial density (Figures 1C–D) in the IBZ compared to T2DM-MCAo rats after stroke (p<0.01). Figure 2A shows that the cerebral vascular perfusion is significantly increased in the T2DM-hMSCs rats compared with control group.
Figure 2.
In T2DM-MCAo rats, delayed hMSC therapy significantly A) improves cerebral perfusion, B) increases cerebral artery wall thickness (αSMA) and decreases artery diameter (αsMA); C) increases intimae thickness and increases internal carotid wall thickness (Trichrome) compared to PBS treated T2DM-MCAo rats.
hMSC treatment of stroke in T2DM rats may have potential adverse effects such as arteriosclerosis-like changes
hMSC treatment significantly increases cerebral artery wall thickness, artery intimae thickness and ICA wall thickness; and significantly decreases cerebral artery diameter compared to vehicle control in T2DM rats after stroke (Figure 2B–C). The data are consistent with our previous study that MSC treatment in T1DM MCAo rats increases atherosclerotic-like vascular changes4.
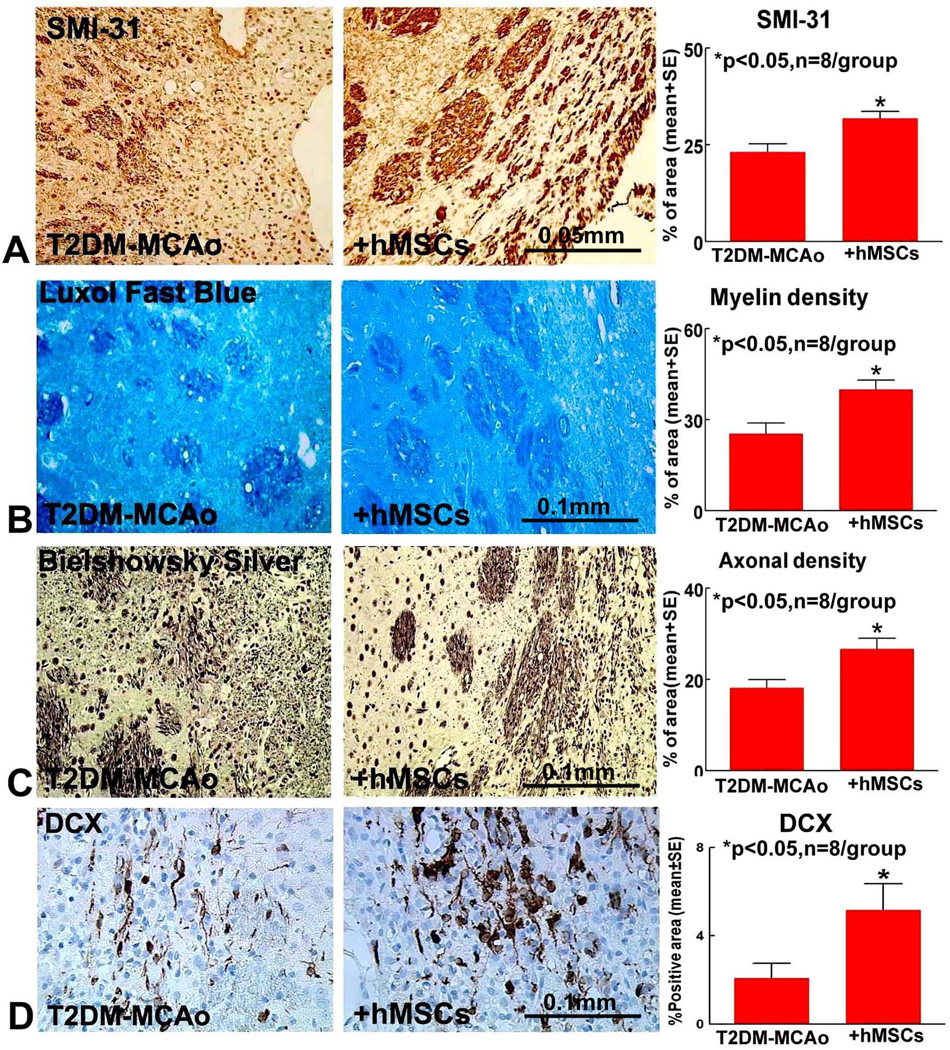
hMSC treatment of stroke in T2DM rats promotes neural progenitor cell migration and axonal/WM remodeling in IBZ
To test whether hMSC treatment affects axon/WM remodeling, BS (axon), LFB (myelin), SMI-31 (phosphorylated neurofilament), staining were performed. To test whether hMSC treatment regulates neural progenitor cell migration, DCX immunostaining was performed. Figure 3 show that hMSC significantly increases SMI-31 (A), myelin (B) and axon (C) density in the striatum bundle of the IBZ as well as increases neural progenitor cell migration (D) after T2DM-MCAo.
Figure 3.
Compared to PBS treatment, hMSC treatment 3 days post stroke in T2DM MCAo rats significantly promotes WM remodeling indicated by A) SMI-31; B) Luxol fast blue; C) Bielschowsky silver immunostaining and quantification analysis. hMSC therapy also promotes neural progenitor cell migration indicated by D) DCX immunostaining.
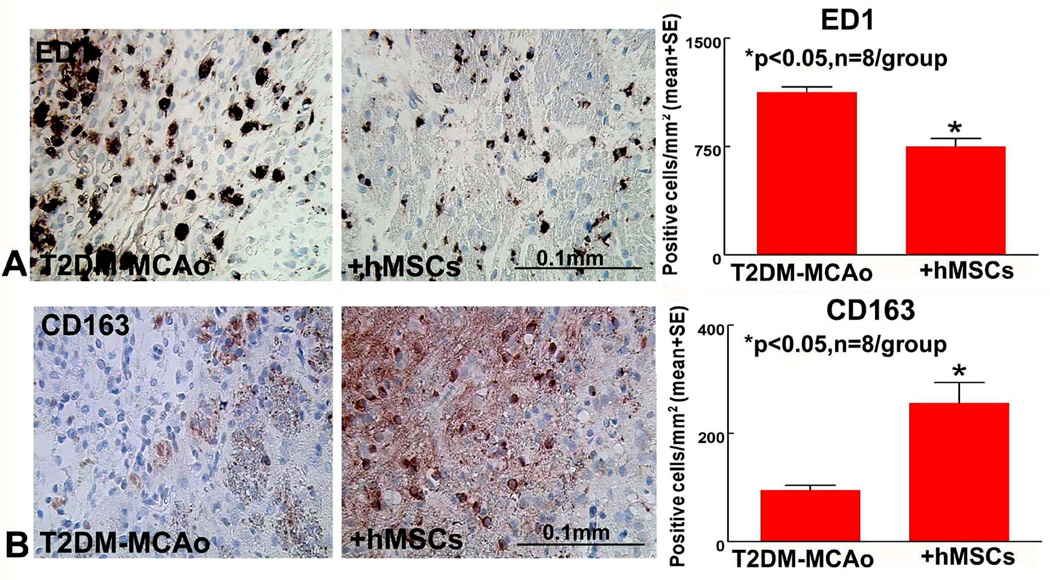
hMSC treatment of T2DM stroke rats promotes M2 macrophage polarization in the ischemic brain
To investigate underlying mechanisms of hMSC treatment induced neurorestorative effects in T2DM-MCAo rats, M2 macrophage polarization was evaluated. Figure 4 shows that hMSC treatment significantly decreases M1 macrophage ED1 (A) and significantly increases M2 macrophage CD163 (B) expression compared to PBS treated T2DM MCAo rats. The data suggest that hMSC treatment initiated at 3 days after MCAo in T2DM promotes M2 polarization and decreases pro-inflammatory effects post ischemia.
Figure 4.
Compared to PBS treatment, hMSC treatment 3 days post stroke in T2DM MCAo rats significantly promotes macrophage polarization indicated by: A) decreasing M1 macrophage ED1 and B) increasing M2 macrophage CD 163.
hMSC treatment of stroke in T2DM increases PDGF and its receptor expression in the ischemic brain
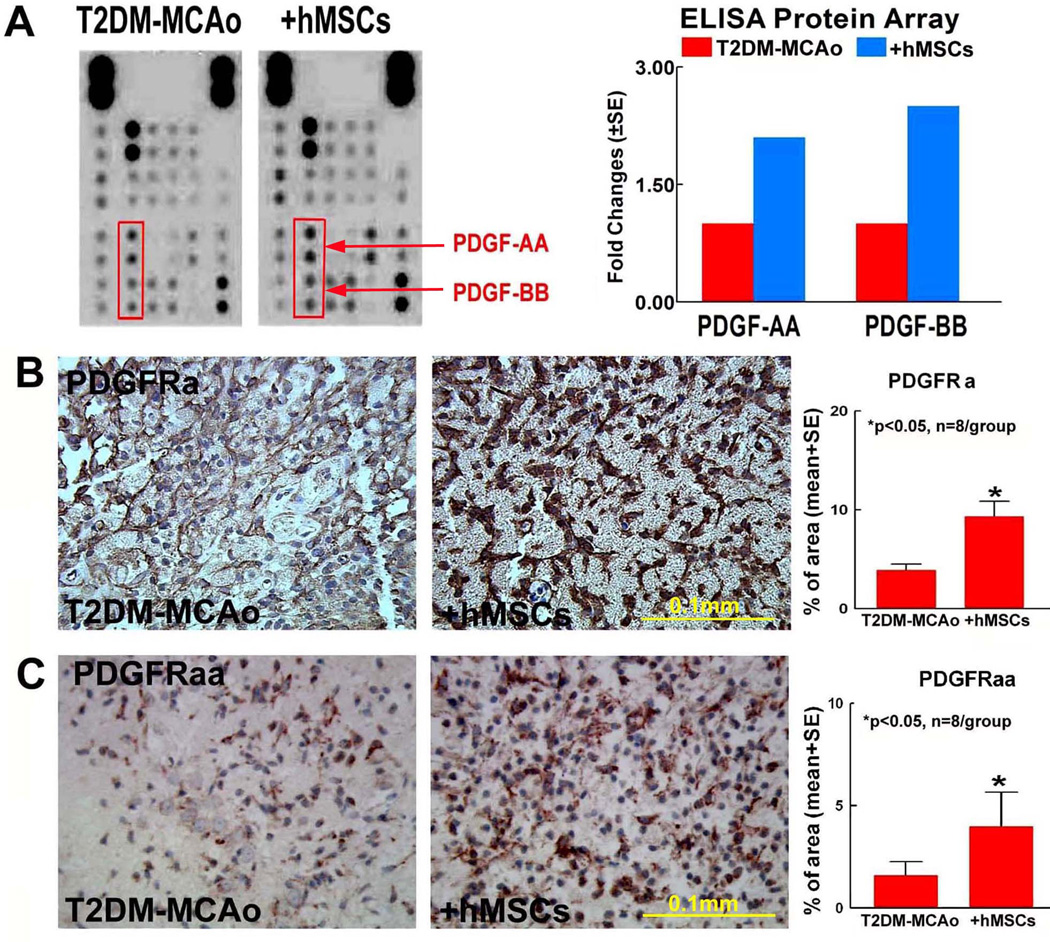
To identify potential molecular mechanism underlying hMSC induced dual effects of neurorestorative and atherosclerotic-like vascular damage, an angiogenic antibody array was performed. We found that hMSCs treatment of T2DM-MCAo rat’s increases PDGF-AA and PDGF-BB levels compared to PBS treated T2DM-MCAo rats (Figure 5A). Consistent with the ELISA array, immunostaining (Figure 5B–C) show that PDGFRa and PDGFRaa expression are significantly increased in hMSC treated T2DM-MCAo rats compared to PBS treated T2DM-MCAo control.
Figure 5.
A) Angiogenesis ELISA protein array shows that hMSCs treatment of T2DM stroke increases PDGF-AA and PDGF-BB, and these results are confirmed by: B) PDGFRa and C) PDGFRaa immunostaining and quantification analysis.
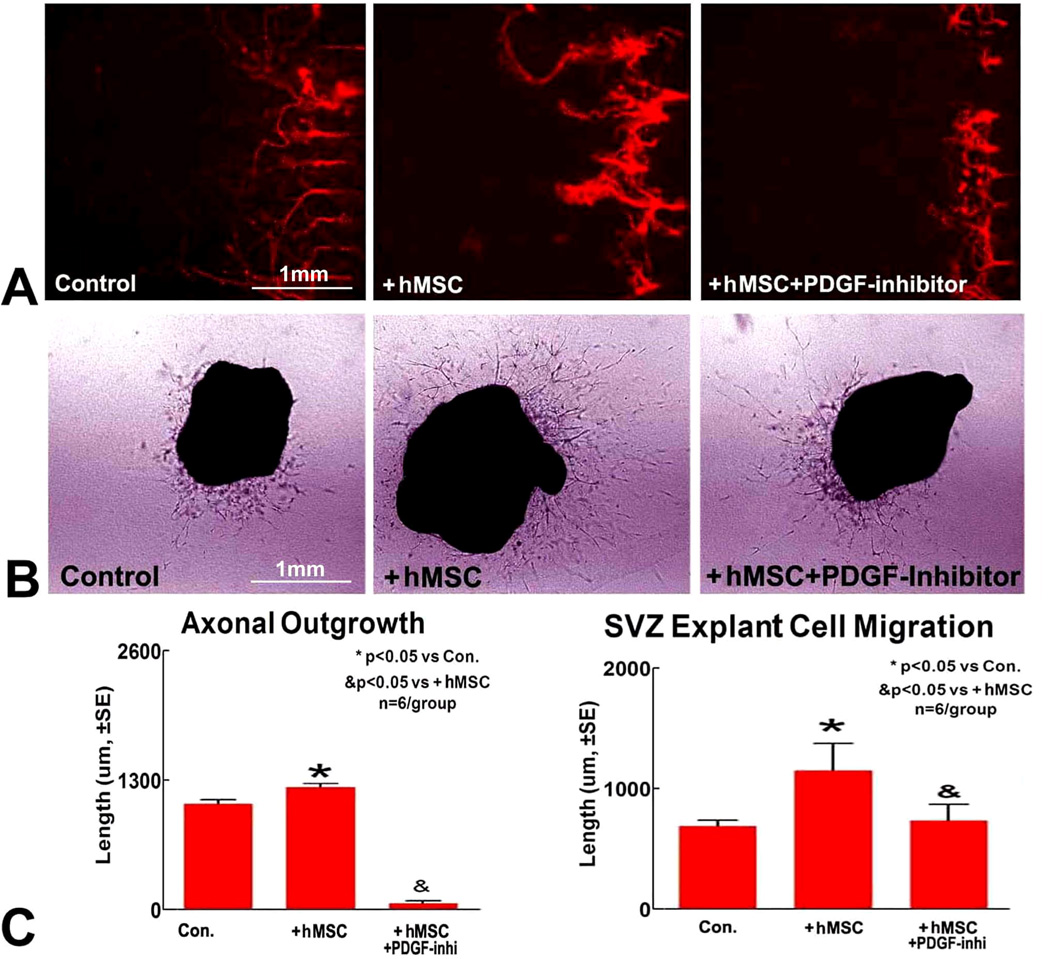
Inhibition of PDGF attenuates hMSC induced axonal/WM remodeling and decreases SVZ explant cell migration
To test whether PDGF plays an important role in hMSC treatment derived neurorestorative effects, in vitro studies were performed. We found that hMSC treatment increases PCN axonal outgrowth and SVZ explant cell migration (Figure 6). Inhibition of PDGF decreases hMSC induced axonal outgrowth changes and SVZ explant cell migration. These data indicate that increase in PDGF may play a key role in hMSC-induced neurorestorative effects.
Figure 6.
A) Primary cortical neuron axonal outgrowth assay indicates that hMSC enhances axon outgrowth, and inhibition of PDGF attenuates hMSC induced axon outgrowth. B) SVZ explant cell migration assay shows that hMSC enhances SVZ cell migration, and inhibition of PDGF attenuates hMSC induced axon outgrowth. C) Quantification analysis.
Discussion
In this study, we present for the first time that delayed (3 day) treatment for T2DM stroke using hMSCs not only increases angiogenesis, axonal/white matter remodeling, but also increases neuroblast cell migration in the ischemic brain. We also specifically demonstrate the contribution of PDGF in hMSC-induced axonal/white matter remodeling as well as in SVZ explant migration.
Diabetes triggers a cascade of events leading to vascular endothelial cell dysfunction, increased vascular permeability, vigorous angiogenesis but dysfunctional neovascularization, and poor recovery after ischemic stroke4, 17. We have previously shown that MSCs secrete several growth and trophic factors, one of which is VEGF (Vascular endothelial growth factor)5. It has been previously demonstrated that acute (1 hr after stroke) administration of VEGF enhances cerebral microvascular perfusion, increases BBB leakage, cerebral hemorrhage, infarction volume after stroke, while delayed (48 hours) administration of VEGF enhances angiogenesis in the ischemic penumbra and significantly improves neurological functional recovery13. Since diabetic rats suffer from vascular damage18 which is aggravated after an ischemic insult12, the effects of VEGF may be exacerbated in diabetic stroke rats compared to non-diabetic stroke rats. Our data show that VEGF is significantly increased in the ischemic brain of T2DM rats compared to wild type (WT) non-DM rats at 1 day after stroke (Supplementary figure 2). VEGF is significantly decreased by 3 days after stroke in the ischemic brain of T2DM stroke rats (Supplementary figure 3). In T1DM rats, when MSC therapy was initiated at 1 day after stroke, it induced brain hemorrhage and BBB leakage4, 19. In the acute phase after stroke, loss of BBB integrity and brain hemorrhage may override the beneficial effects of MSC treatment such as vascular and axonal/WM remodeling4. In the current study, we have demonstrated that treatment initiation at a delayed time point i.e. 3 days after stroke, in T2DM rats significantly improves neurological functional outcome, does not increase BBB leakage and brain hemorrhage, and significantly promotes vascular remodeling, as demonstrated by increased vascular and arterial density and vascular perfusion. Hence, for cell therapy for DM subjects, treatment initiation time point is extremely critical and should be considered when treating diabetic stroke. Since the time point of hMSC treatment in the current T2DM stroke study (3 days after stroke) differs from that of the T1DM stroke treatment (I day after stroke), we are unable to compare the apparent divergent outcomes between T1DM and T2DM MSC treatments. However, comparison of response to cell-based therapy performed under the identical treatment protocols in T1DM and T2DM rats with stroke warrants investigation.
Angiogenesis plays an important role in improving post stroke neurological function20. Angiogenic events increase blood supply to the ischemic brain tissue, and are also tightly coupled to neurogenesis21, 22. In this study, we found that hMSC treatment of stroke increases vascular density and cerebral blood perfusion in T2DM rats. Post ischemia, there is an exuberant expansion of neural progenitor cells in the SVZ, differentiation into mature neurons, astrocytes, and oligodendrocytes and migration to the IBZ20. Neural progenitor cell migration is closely associated with blood vessels which serve as scaffolds, and guide the migration of neural progenitor cells from the SVZ towards damaged brain regions23. Our results show that hMSC therapy significantly increases SVZ explant cell migration and neural progenitor cell migration identified by increased DCX density in the IBZ. These data suggest that hMSC treatment of stroke in T2DM rats promotes neurovascular remodeling which may partially contribute to the functional outcome after stroke.
Stroke and diabetes cause axonal/WM damage which induces long-term disability due to the brain’s limited capacity of axonal regeneration and inhibitory environment for axon regrowth, sprouting and remyelination24. Post stroke protection of neurons in the gray matter is not sufficient, as WM damage would still hinder neuronal connectivity and functioning. Therefore, targeting WM remodeling is crucial for improving long term functional outcome after stroke. In this study, we found that hMSC treatment of stroke initiated at 3 days after MCAo in addition to promoting neurovascular remodeling, also increases axonal/WM remodeling in the ischemic brain.
M1 macrophages are neurotoxic, while M2 macrophages promote a regenerative growth response in adult sensory axons8. M2 phenotype polarization creates a conducive environment for axonal extension and functional recovery25. Post ischemia, the local and infiltrating microglia and macrophages assume an anti-inflammatory and protective M2 phenotype26. Extending this M2 phase of these macrophages and microglia and delaying their transition into the pro-inflammatory M1 phenotype is a desirable effect. Macrophage invasion typically starts around 24 hours post stroke and increases by 3 to 7 days after stroke; however, recent studies have indicated that the increased level of macrophage accumulation in the brain persists to at least 28 days after stroke27. Our data show that hMSC treatment of stroke in T2DM rats significantly induces M2 macrophage polarization as well as increases vascular integrity and axonal/WM remodeling in the ischemic brain. MSCs secrete several growth and trophic factors which may mediate several pathways and contribute towards neurorestoration after stroke in T2DM rats. Using the experimental design and methods used in this study, it is difficult to dissect whether M2 macrophage polarization has a direct or indirect (by creating a hospitable environment for brain repair) role in hMSC induced neurorestorative effects after stroke in T2DM rats. This is an important question and future studies are warranted.
Accumulating evidence suggests that the neurorestorative effects of cell therapy is primarily derived from the secretion of trophic and growth factors that stimulate endogenous brain repair and remodeling to induce neurological recovery20. We acknowledge the similarities observed in treatment derived benefits from our other cell-based treatment strategies such as human umbilical cord blood cells (HUCBCs). Accordingly, we have shown that for T2DM stroke, delayed (3 day) cell therapy with HUCBCs10, 28 and hMSCs significantly promote long term neurological functional outcome without affecting blood glucose and infarction volume. Similar to HUCBCs10, 20, hMSC treatment also promotes neurorestorative effects such as vascular and white matter remodeling and induces anti-inflammatory effects such as M2 macrophage polarization (Supplementary figure 2). However, the mechanism of action is different for HUCBC and hMSCs. We have reported that HUCBC treatment in T2DM stroke promotes serum, brain and brain endothelial cell microRNA-126 (miR-126) expression which stimulates vascular and white matter remodeling in the ischemic brain28. Compared to HUCBCs, hMSCs secrete significantly less miR-126 in-vitro, and in T2DM stroke rats, treatment with hMSCs stimulate significantly less circulating miR-126 compared to treatment with HUCBCs (Supplementary figure 3). In the present study, we demonstrate that hMSC treatment of T2DM stroke significantly increases PDGF expression, which then plays a key role in promoting neurovascular and WM remodeling.
PDGF signaling mediates vascular smooth muscle cell (SMC) and pericyte function and regulates vessel integrity. PDGF treatment decreases axonal abnormalities and improves remyelination by promoting proliferation of oligodendrocytes and oligodendrocyte progenitor cells29. Our data show that hMSC treatment of stroke in T2DM rats significantly increases PDGF expression in the ischemic brain and promotes neurovascular and WM remodeling. Inhibition of PDGF attenuates hMSC induced SVZ cell migration and PCN axonal sprouting. However, PDGF signaling is also a key mediator of SMC proliferation and plays an important role in arteriosclerosis30. PDGF triggers the switching of SMC from a quiescent, contractile phenotype to a proliferative, migratory, and synthetic phenotype31. PDGF is a major stimulus for the abnormal migration and proliferation of SMCs and contributes critically to vascular disease. Our data show that hMSC therapy in T2DM stroke rats not only increases neurovascular and axonal/WM remodeling but also increases arteriosclerosis-like changes compared to PBS treatment. Therefore, increasing PDGF by hMSC treatment of stroke in T2DM rats not only promote neurovascular and axonal/WM remodeling, but also may induce vascular arteriosclerosis-like changes.
Conclusions
T2DM stroke treatment using hMSCs initiated 3 days post the ischemic insult significantly improves neurological functional recovery by promoting neurovascular and axonal/WM remodeling in the ischemic brain. Our data suggest that M2 macrophage polarization and increase of PDGF may contribute to the mechanisms underlying hMSC treatment derived neurorestorative and atherosclerotic-like effects.
Supplementary Material
Acknowledgments
The authors wish to thank Qinge Lu and Sutapa Santra for the technical assistance. hMSCs were provided by Cognate Bioservices, Inc. Baltimore, MD.
Sources of Funding
This work was supported by National Institute of Neurological Disorders and Stroke RO1 NS083078 (J.C.) NS088656 (M.C.) and 41NS064708 (J.C.), American Heart Association grant 14GRNT20460026 (J.C.). The National Natural Science Foundation of China (81300993, 81571145)(T.Y.).
Footnotes
Disclosures: None
References
- 1.Paciaroni M, Agnelli G, Caso V, Corea F, Ageno W, Alberti A, et al. Acute hyperglycemia and early hemorrhagic transformation in ischemic stroke. Cerebrovasc Dis. 2009;28:119–123. doi: 10.1159/000223436. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Ye X, Yan T, Zhang C, Yang XP, Cui X, et al. Adverse effects of bone marrow stromal cell treatment of stroke in diabetic rats. Stroke. 2011;42:3551–3558. doi: 10.1161/STROKEAHA.111.627174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zacharek A, Chen J, Cui X, Li A, Li Y, Roberts C, et al. Angiopoietin1/tie2 and vegf/flk1 induced by msc treatment amplifies angiogenesis and vascular stabilization after stroke. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27:1684–1691. doi: 10.1038/sj.jcbfm.9600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Windmolders S, De Boeck A, Koninckx R, Daniels A, De Wever O, Bracke M, et al. Mesenchymal stem cell secreted platelet derived growth factor exerts a pro-migratory effect on resident cardiac atrial appendage stem cells. J Mol Cell Cardiol. 2014;66:177–188. doi: 10.1016/j.yjmcc.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Krupinski J, Issa R, Bujny T, Slevin M, Kumar P, Kumar S, et al. A putative role for platelet-derived growth factor in angiogenesis and neuroprotection after ischemic stroke in humans. Stroke. 1997;28:564–573. doi: 10.1161/01.str.28.3.564. [DOI] [PubMed] [Google Scholar]
- 8.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52:313–320. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Yan T, Venkat P, Chopp M, Zacharek A, Ning R, Cui Y, et al. Neurorestorative therapy of stroke in type 2 diabetes mellitus rats treated with human umbilical cord blood cells. Stroke. 2015;46:2599–2606. doi: 10.1161/STROKEAHA.115.009870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 12.Prakash R, Li W, Qu Z, Johnson MA, Fagan SC, Ergul A. Vascularization pattern after ischemic stroke is different in control versus diabetic rats: Relevance to stroke recovery. Stroke. 2013;44:2875–2882. doi: 10.1161/STROKEAHA.113.001660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, et al. Vegf enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. Journal of Clinical Investigation. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Cui X, Zacharek A, Chopp M. Increasing ang1/tie2 expression by simvastatin treatment induces vascular stabilization and neuroblast migration after stroke. Journal of Cellular and Molecular Medicine. 2009;13:1348–1357. doi: 10.1111/j.1582-4934.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Ueno Y, Liu XS, Buller B, Wang X, Chopp M, et al. The microrna-17-92 cluster enhances axonal outgrowth in embryonic cortical neurons. J Neurosci. 2013;33:6885–6894. doi: 10.1523/JNEUROSCI.5180-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor AM, Blurton-Jones M, Rhee SW, Cribbs DH, Cotman CW, Jeon NL. A microfluidic culture platform for cns axonal injury, regeneration and transport. Nat Methods. 2005;2:599–605. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li PA, Gisselsson L, Keuker J, Vogel J, Smith ML, Kuschinsky W, et al. Hyperglycemia-exaggerated ischemic brain damage following 30 min of middle cerebral artery occlusion is not due to capillary obstruction. Brain Res. 1998;804:36–44. doi: 10.1016/s0006-8993(98)00651-9. [DOI] [PubMed] [Google Scholar]
- 18.Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: Effects on angiogenesis, vascular remodeling, and wound healing. International Journal of Vascular Medicine. 2012;2012:918267. doi: 10.1155/2012/918267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan T, Ye X, Chopp M, Zacharek A, Ning R, Venkat P, et al. Niaspan attenuates the adverse effects of bone marrow stromal cell treatment of stroke in type one diabetic rats. PLoS One. 2013;8:e81199. doi: 10.1371/journal.pone.0081199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Venkat P, Zacharek A, Chopp M. Neurorestorative therapy for stroke. Frontiers in Human Neuroscience. 2014;8:382. doi: 10.3389/fnhum.2014.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teng H, Zhang ZG, Wang L, Zhang RL, Zhang L, Morris D, et al. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab. 2008;28:764–771. doi: 10.1038/sj.jcbfm.9600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chopp M, Zhang ZG, Jiang Q. Neurogenesis, angiogenesis, and mri indices of functional recovery from stroke. Stroke. 2007;38:827–831. doi: 10.1161/01.STR.0000250235.80253.e9. [DOI] [PubMed] [Google Scholar]
- 23.Kojima T, Hirota Y, Ema M, Takahashi S, Miyoshi I, Okano H, et al. Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells. 2010;28:545–554. doi: 10.1002/stem.306. [DOI] [PubMed] [Google Scholar]
- 24.Singh B, Singh V, Krishnan A, Koshy K, Martinez JA, Cheng C, et al. Regeneration of diabetic axons is enhanced by selective knockdown of the pten gene. Brain. 2014;137:1051–1067. doi: 10.1093/brain/awu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakajima H, Uchida K, Guerrero AR, Watanabe S, Sugita D, Takeura N, et al. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012;29:1614–1625. doi: 10.1089/neu.2011.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- 27.Michalski D, Heindl M, Kacza J, Laignel F, Kuppers-Tiedt L, Schneider D, et al. Spatio-temporal course of macrophage-like cell accumulation after experimental embolic stroke depending on treatment with tissue plasminogen activator and its combination with hyperbaric oxygenation. Eur J Histochem. 2012;56:e14. doi: 10.4081/ejh.2012.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Ning R, Zacharek A, Cui C, Cui X, Yan T, et al. Mir-126 contributes to human umbilical cord blood cell-induced neurorestorative effects after stroke in type-2 diabetic mice. Stem Cells. 2016;34:102–113. doi: 10.1002/stem.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jean I, Allamargot C, Barthelaix-Pouplard A, Fressinaud C. Axonal lesions and pdgf-enhanced remyelination in the rat corpus callosum after lysolecithin demyelination. Neuroreport. 2002;13:627–631. doi: 10.1097/00001756-200204160-00018. [DOI] [PubMed] [Google Scholar]
- 30.Keramati AR, Singh R, Lin A, Faramarzi S, Ye ZJ, Mane S, et al. Wild-type lrp6 inhibits, whereas atherosclerosis-linked lrp6r611c increases pdgf-dependent vascular smooth muscle cell proliferation. Proc Natl Acad Sci U S A. 2011;108:1914–1918. doi: 10.1073/pnas.1019443108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Louis SF, Zahradka P. Vascular smooth muscle cell motility: From migration to invasion. Experimental & Clinical Cardiology. 2010;15:e75–e85. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.