The amygdala and hippocampus have been implicated consistently in the pathophysiology of posttraumatic stress disorder (PTSD).1,2 While several studies have observed reduced hippocampal volume in PTSD, studies of amygdala volume and PTSD have been mixed.1–3
In addition to method differences, one reason for these mixed results is that most structural magnetic resonance imaging studies in PTSD have treated PTSD as a homogeneous entity instead of considering how amygdala volume may relate to its heterogeneous phenotypic expression.
Confirmatory factor analytic studies have revealed that PTSD is best represented by 5 symptom clusters: reexperiencing, avoidance, numbing, dysphoric arousal (eg, sleep difficulties), and anxious arousal (eg, hypervigilance).4 To our knowledge, no study has evaluated the relation between amygdala and hippocampal volume and this contemporary model of PTSD. Here, we evaluated these associations in combat veterans.
Methods
Forty-eight Iraq/Afghanistan combat veterans participated in this study. Recruitment was conducted to ensure a full dimensional range of DSM-IV PTSD symptoms (ie, including non/minimally symptomatic veterans and equal proportions of veterans with mild, moderate, and severe/extreme symptoms), with 23 veterans (47.9%) meeting diagnostic criteria for combat-related PTSD. Exclusion criteria included psychosis; bipolar disorder; drug abuse or dependence (current or lifetime); alcohol abuse in the past 30 days or alcohol dependence in the past 12 months; moderate and severe traumatic brain injury (ie, loss of consciousness >30 minutes); neurologic disorder (eg, stroke or seizure); learning disability or confirmed diagnosis of attention-deficit/hyperactivity disorder; use of antipsychotics, psychostimulants, or sedatives/hypnotics; antidepressant dose stable less than 30 days; and/or PTSD diagnosis prior to combat exposure. The VA Connecticut Healthcare System Human Subjects Subcommittee and Yale University Human Research Protection Program approved this study. All participants provided written informed consent.
Structural magnetic resonance imaging data were acquired on a Siemens Trio TIM 3T (MPRAGE; voxel size 1 × 1 × 1 mm; repetition time, 2.5 seconds; echo time, 2.77 milliseconds; flip angle, 7°). Blinded to the clinical status, image processing and segmentation were conducted using the fully automated Freesurfer recon-all pipeline (http://surfer.nmr.mgh.harvard.edu).
We computed partial correlations between independent variables and amygdala and hippocampal volumes adjusted for total intracranial volume and entered variables with associations at the P < .05 level into a multivariable linear regression analysis using total intracranial volume as a covariate. To evaluate subscales of the Clinician-Administered PTSD Scale associated with volumes, we conducted a post hoc multivariable linear regression analysis (α = .01). Finally, to evaluate interrelationships among variables related to regional volumes, exploratory path analyses were conducted using Mplus version 7.2 (http://www.statmodel.com).
Results
The Table shows sample characteristics and partial correlation results. After adjustment for intracranial volume, Combat Experiences Scale and total Clinician-Administered PTSD Scale scores were independently associated with right amygdala volume. Multivariable linear regression for right amygdala volume showed adjusted R2 = 0.46 (Combat Experiences Scale: β = −0.27; t = 2.34; P = .02; Clinician-Administered PTSD Scale: β = −0.24; t = 2.10; P = .04). Post hoc analysis revealed that anxious arousal was independently negatively related to right amygdala volume (β = −0.38; t = 3.33; P = .002); no other symptom cluster was significant (β > −0.08; t < 0.53; and P > .59 for all). The best-fitting model in path analyses showed right amygdala volume mediating the relationship between combat exposure and anxious arousal (χ2 = 0.03; P = .87; Bayesian Information Criterion = 921.38; Akaike Information Criterion = 906.41; root mean square error of approximation = 0.00 [0.00–0.20]; Comparative Fit Index = 1.00; Tucker-Lewis Index = 1.00; the other 2 models had χ2 = 3.17 or higher, P = .07 or lower, and higher root mean square error of approximation and lower Comparative Fit Index and Tucker-Lewis Index values, which indicate worse fit). The Figure shows standardized coefficients of the best-fitting model.
Table.
Sample Characteristics and Results of Total Intracranial Volume-Adjusted Partial Correlationsa
| Characteristic | No. (%) | Amygdala | Hippocampus | ||
|---|---|---|---|---|---|
| Right | Left | Right | Left | ||
| Age, mean (SD), y | 33.1 (7.8) | 0.00 | 0.05 | −0.05 | 0.17 |
| Male | 41 (85.4) | −0.02 | 0.20 | 0.05 | 0.08 |
| White race/ethnicity | 32 (66.7) | −0.07 | −0.20 | −0.17 | −0.11 |
| College or higher education | 13 (27.1) | 0.22 | 0.10 | 0.17 | 0.26 |
| Married/living with partner | 20 (41.7) | 0.01 | −0.04 | 0.08 | 0.17 |
| Lifetime traumatic events (TLEQ score), mean (SD) | 12.3 (6.1) | −0.27 | 0.08 | 0.09 | 0.00 |
| Childhood trauma (CTQ total score), mean (SD) | 42.6 (15.1) | −0.02 | −0.13 | −0.12 | −0.06 |
| Time in service, mean (SD), y | 10.4 (6.8) | 0.06 | 0.01 | 0.03 | 0.15 |
| No. of deployments, mean (SD) | 2.0 (1.3) | −0.05 | −0.10 | −0.03 | −0.01 |
| Combat exposure severity (CES score), mean (SD) | 5.6 (4.4) | −0.36b | −0.14 | −0.23 | −0.32c |
| CAPS total score, mean (SD) | 25.5 (27.0) | −0.39b | −0.16 | −0.26 | −0.20 |
| Reexperiencing | 6.5 (8.1) | −0.35c | −0.15 | −0.27 | −0.24 |
| Avoidance | 2.5 (3.9) | −0.35c | −0.18 | −0.27 | −0.24 |
| Numbing | 5.8 (7.2) | −0.31c | −0.17 | −0.26 | −0.15 |
| Dysphoric arousal | 7.0 (6.9) | −0.33c | −0.07 | −0.14 | −0.08 |
| Anxious arousal | 3.6 (4.0) | −0.45b | −0.16 | −0.25 | −0.20 |
| Chronic PTSD symptoms (≥3 mo) | 4 (8.3) | 0.01 | 0.09 | 0.14 | 0.08 |
| Depressive symptom severity (BDI–II score), mean (SD) | 14.1 (12.7) | −0.11 | −0.06 | 0.03 | −0.02 |
| Psychotropic medication | 12 (25.0) | 0.19 | −0.05 | −0.05 | 0.00 |
| Lifetime dependence | |||||
| Alcohol | 3 (6.3) | −0.17 | −0.09 | −0.18 | −0.21 |
| Nicotine | 5 (10.4) | −0.27 | −0.11 | −0.18 | −0.12 |
Abbreviations: BDI-II, Beck Depression Inventory–II; CAPS, Clinician-Administered PTSD Scale; CES, Combat Experiences Scale; CTQ, Childhood Trauma Questionnaire; PTSD, posttraumatic stress disorder; TLEQ, Traumatic Life Events Questionnaire
A total of 3 multivariable linear regression analyses were conducted: one with main effects of intracranial volume, combat exposure severity, and CAPS scores, and combat exposure severity by CAPS score interaction term; a second with only intracranial volume, combat exposure severity, and CAPS scores; and a third post hoc analysis to evaluate how the 5 PTSD symptom clusters were related to amygdala volume (results reported in the Results section). In the multivariable linear regression analysis, the combat exposure severity by CAPS interaction was not significant (β = −0.01; t = 0.02; P = .98); thus, the final model contained only main effects of total intracranial volume, combat exposure, and CAPS scores.
P < .01.
P < .05.
Figure. Path Model of Right Amygdala Volume as a Mediator of the Relation Between Combat Exposure and Anxious Arousal Symptoms.
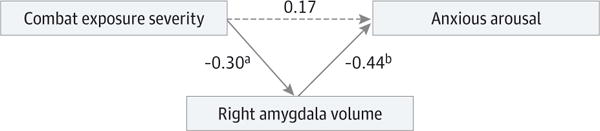
The values represent standardized β coefficients. The solid lines represent significant associations; dotted line, nonsignificant association. Right amygdala volume was additionally regressed on total intracranial volume in all path models. Association between combat exposure severity and anxious arousal was significant when right amygdala volume was excluded from the model (β = 0.31; t = 2.22; P = .03). The 95% CI for the association between combat exposure severity and anxious arousal when right amygdala volume was excluded from the model was −0.16 to 0.43; for combat exposure severity and right amygdala volume, −0.10 to −0.52; and for right amygdala volume and anxious arousal, −0.17 to −0.67.
aP < .01.
bP < .001.
Discussion
This study suggests that reduced right amygdala volume is most strongly associated with anxious arousal symptoms in combat veterans. This finding is consistent with experimental studies linking reduced amygdala volume to stress-evoked hyperresponsiveness.5,6 Right amygdala volume also fully mediated the relation between combat exposure severity and anxious arousal, suggesting that increased combat exposure may contribute to reduced amygdala volume, which in turn is associated with increased anxious arousal.
While this study was limited by the cross-sectional design and relatively small and predominantly male sample, the results underscore the potential utility of a dimensional approach to evaluating neurobiological factors associated with PTSD. Such an approach may be useful in informing etiologic models, as well as prevention and treatment approaches for this debilitating disorder.
Acknowledgments
Funding/Support: Preparation of this study was supported in part by the Clinical Neurosciences Division of the US Department of Veterans Affairs National Center for Posttraumatic Stress Disorder, grant K23 MH-101498 from the National Institutes of Health (Dr Abdallah), and a private donation.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Pietrzak had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs Pietrzak and Averill contributed equally.
Study concept and design: Pietrzak, Neumeister, Krystal, Harpaz-Rotem.
Acquisition, analysis, or interpretation of data: Pietrzak, Averill, Abdallah, Neumeister, Levy, Harpaz-Rotem.
Drafting of the manuscript: Pietrzak.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Pietrzak, Averill.
Obtained funding: Neumeister, Krystal, Harpaz-Rotem.
Administrative, technical, or material support: Pietrzak.
Study supervision: Abdallah, Neumeister, Krystal, Levy.
Conflict of Interest Disclosures: Dr Pietrzak is a scientific consultant to Cogstate Ltd. Dr Abdallah has received consultation fees from Genentech. Dr Krystal consults for several pharmaceutical and biotechnology companies. No other disclosures were reported.
Disclaimer: The views and opinions expressed in this report are those of the authors and should not be construed to represent the views of sponsoring organizations, agencies, or the US government.
References
- 1.Karl A, Schaefer M, Malta LS, Dörfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30(7):1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Woon FL, Hedges DW. Amygdala volume in adults with posttraumatic stress disorder: a meta-analysis. J Neuropsychiatry Clin Neurosci. 2009;21(1):5–12. doi: 10.1176/jnp.2009.21.1.5. [DOI] [PubMed] [Google Scholar]
- 3.Morey RA, Gold AL, LaBar KS, et al. Mid-Atlantic MIRECC Workgroup Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Arch Gen Psychiatry. 2012;69(11):1169–1178. doi: 10.1001/archgenpsychiatry.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armour C, Carragher N, Elhai JD. Assessing the fit of the Dysphoric Arousal model across two nationally representative epidemiological surveys: the Australian NSMHWB and the United States NESARC. J Anxiety Disord. 2013;27(1):109–115. doi: 10.1016/j.janxdis.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J Neurosci. 2008;28(4):990–999. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartley CA, Fischl B, Phelps EA. Brain structure correlates of individual differences in the acquisition and inhibition of conditioned fear. Cereb Cortex. 2011;21(9):1954–1962. doi: 10.1093/cercor/bhq253. [DOI] [PMC free article] [PubMed] [Google Scholar]


