Abstract
Background and Purpose
Therapeutic hypothermia (TH) is a potent neuroprotectant approved for cerebral protection after neonatal hypoxia-ischemia and cardiac arrest. TH for acute ischemic stroke is safe and feasible in pilot trials. We designed a study protocol to provide safer, faster TH in stroke patients.
Methods
Safety procedures and 4°C saline infusions for faster cooling were added to the Intravascular Cooling Treatment in Acute Stroke (ICTuS) trial protocol. A femoral venous intravascular cooling catheter following intravenous rt-PA in eligible patients provided 24 hours cooling followed by a 12 hour re-warm. Serial safety assessments and imaging were performed. The primary endpoint was 3-month modified Rankin score 0,1.
Results
Of the intended 1600 subjects, 120 were enrolled before the study was stopped. Randomly, 63 were to receive hypothermia (HY) plus anti-shivering treatment and 57 normothermia (NT). Compared to prior studies, cooling rates were improved with a cold saline bolus, without fluid overload. The intention-to-treat primary outcome of 90-day mRS 0,1 occurred in 33% HY and 38% NT subjects, OR (95% CL) of 0.81 (0.36, 1.85). Serious adverse events occurred equally. Mortality was 15.9% HY and 8.8% NT subjects, OR (95% CL) of 1.95 (0.56, 7.79). Pneumonia occurred in 19% HY vs. 10.5% in NT subjects, OR (95% CL) of 1.99 (0.63, 6.98).
Conclusion
Intravascular TH was confirmed to be safe and feasible in rt-PA treated acute ischemic stroke patients. Protocol changes designed to reduce pneumonia risk appeared to fail, although the sample is small.
Clinical trial registration
clinicaltrials.gov NCT 01123161.
Therapeutic hypothermia (TH) is the most potent neuroprotectant ever studied in experimental cerebral ischemia, with multiple effects at several stages of the ischemic cascade1, 2. Clinical trials confirmed powerful protection with TH after accidental neonatal hypoxic-ischemic injury3, 4 and global cerebral ischemia after cardiac arrest5, 6 and national and international guidelines recommend TH for these patients7. Clinical trials of TH for acute ischemic stroke are less well developed, but pilot studies have suggested benefit8. Early pilot trials used surface cooling technology in stroke patients who were intubated due to severe strokes, and therefore pharmacologically paralyzed, facilitating shivering control9–11. More rapid cooling and tighter temperature control may be facilitated by intravascular cooling catheter technology, but anti-shivering measures are required.
We have been developing intravascular cooling for acute ischemic stroke over the course of several trials. The initial study, Intravascular Cooling Trial of Acute Stroke or ICTuS, demonstrated safety of 12 or 24 hours of intravascular cooling in stroke patients12. Subsequently, the ICTuS-L trial confirmed the safety and feasibility of intravascular TH in patients who received intravenous rt-PA13. We developed an anti-shivering regimen and we determined that both cooling speed and target-temperature control depended on the patient’s body mass index (BMI) primarily, but also on effective shivering control14. Unfortunately, there was a significantly elevated incidence of pneumonia in the ICTuS-L patients who received hypothermia and the antishivering regimen15. The antishivering regimen includes two drugs, buspirone and meperidine, known to cause sedation and swallowing-reflex impairment so we hypothesized that the increased pneumonia could be due to aspiration. On the other hand, in ICTuS-L there appeared to be surveillance bias and no precise pneumonia definition, both of which may have led to over-call of pneumonia in TH treated patients.
We sought to test the hypothesis that pneumonia risk could be reduced by implementing a precise definition of pneumonia, rigorous surveillance, nasogastric drainage, and minimal use of meperidine in the ICTuS 2 study. During the application to renew funding for the trial, however, several publications established the efficacy of intra-arterial neurothrombectomy for selected patients16. Given that the target patient population in ICTuS-2 largely overlapped that included in these trials, the Steering Committee elected to halt ICTuS-2, examine the data, and redesign the trial to include neurothrombectomy. Here we report whether the pneumonia rate was in fact lowered in ICTuS-2 and whether the cold saline bolus was safe and effective.
Methods
The ICTuS-2/3 program was part of an NINDS funded Specialized Program Of Translational Research In Acute Stroke (SPOTRIAS), a collaboration among the NINDS, University of California at San Diego, and University of Texas at Houston. The ICTuS-2/3 protocol (NCT 01123161), including the statistical analysis plan, has been published17. In brief, the study sought to determine whether the combination of thrombolysis and hypothermia/antishivering regimen is superior to thrombolysis alone for the treatment of acute ischemic stroke. ICTuS-2/3 was a prospective, randomized, single-blind, multi-center Phase 2/3 study to include 1600 ischemic stroke patients treated within 3 hours of symptom onset with intravenous rt-PA, NIHSS ≥7 and ≤20 (left brain stroke) or ≤24 (right brain stroke), age 22–82. Intra-arterial recanalization procedures were disallowed. The study was to be conducted in two stages: a Phase 2 study (ICTuS-2) to assess the safety and feasibility of various protocol changes, to demonstrate sufficient recruitment, and to allow an interim analysis for futility; then a Phase 3 efficacy study (ICTuS-3) if pre-specified milestones were achieved. Phase 2 was to include 400 patients who would have been included in the total 1600-patient Phase 3 study. All patients received intravenous rt-PA. Patients were randomly assigned to either hypothermia (HY) targeted to 33°C or normothermia (NT) with a web-based application that used a random permuted block design stratified by site. Patients randomized to HY immediately received 2 liters of normal (0.9%) saline (4°C temperature) as fast as possible; NT patients received the same volume of room temperature saline. Hypothermia patients were then cooled with an intravascular cooling device (Celsius Catheter, Innercool, Carlsbad) for 24 hours, followed by controlled re-warming for 12 hours. Antishivering measures (meperidine, buspirone, skin warming) were utilized for all 36 hours. Shivering was assessed with a validated clinical rating scale18. Breakthrough shivering was treated with escalating intravenous meperidine. If shivering could not be controlled without respiratory compromise, the target temperature was to be increased in 0.5°C increments until shivering stopped (called “permissive hypothermia”). All patients underwent daily surveillance for pneumonia using the Centers for Disease Control (CDC) definition, with prompt initiation of antibiotic therapy in suspected cases19. The primary endpoint was favorable outcome, defined as a 90-day Modified Rankin Score (mRS) of 0 or 1. Additionally, we tested the hypothesis that an NIHSS 7 points improvement by 7 days after stroke could accurately detect favorable treatment response20. A further pre-specified analysis was a hypothesis that pneumonia risk could be predicted by the initial 24-hour rise in hs-CRP as a biomarker.
Recruitment was suspended on December 31, 2014. Final follow up visits were completed by April 30, 2015. The results are reported here according to our pre-specified statistical analysis plan for an intention-to-treat (ITT) and per protocol (PP) analysis.
Results
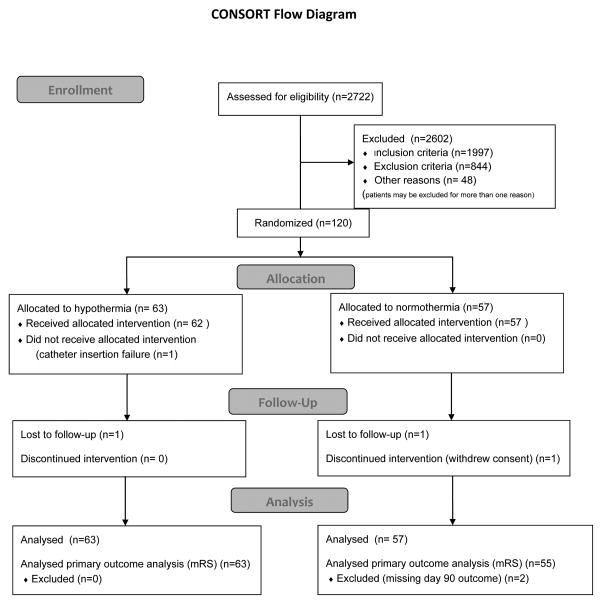
The CONSORT flow diagram21 is presented in Figure 1. Of the projected 1600 patients, 120 patients were enrolled (63 assigned to hypothermia (HY) and 57 to normothermia (NT)), so no statistically significant primary results were expected. Of the 120 enrolled patients, 16 (13%) exhibited rapid reversal of their deficit before study procedures could be initiated, making them “early responders”. The early responders (9 HY and 7NT) were included in the ITT population but not the PP analysis. Additionally, one HY patient suffered catheter insertion failure and in one HY patient the team did not attempt catheter placement: for the PP analysis they were both re-assigned to the NT group. Four additional patients were excluded from the PP analysis due to disallowed procedures (n=3, intra-arterial embolectomy) and pre-morbid mRS > 1 (n=1). This resulted in a PP population of 51 HY and 49 NT patients.
Figure 1. CONSORT flow diagram.
The flow of patients into the trial is summarized according to CONSORT guidelines. Patients could be excluded for more than one reason. A total of 3 patients were lost to follow up, 2 NT and 1HY. However, the HY patient was available by telephone, and for the 3 month mRS (primary endpoint) only, a score was recorded.
The groups were well balanced with respect to variables that influence outcome in acute stroke trials (Table 1). The ITT primary outcome of 90-day mRS 0,1 was observed in 33% in the HY and 38% in the NT group, for an OR (95% CL) of 0.81 (0.36, 1.85), Fisher’s Exact test not significant (NS). Similarly, in the PP population 90-day mRS 0,1 was seen in 24% HY and 38% NT patients, OR (95% CL) of 0.50 (0.19, 1.30). We analyzed several alternative outcome measures (secondary and sensitivity analyses), all of which were pre-specified in the statistical analysis plan. Using severity-adjusted outcomes based on initial NIHSS, the ITT adjusted OR (95% CL) for a good outcome was 1.37 (0.60, 3.19). The PP adjusted OR (95% CL) was 0.89 (0.34, 2.30). Using an ordinal regression model, the ITT OR for mRS 2–6 in the hypothermia group was 1.03 (0.55 to 1.95) and the PP OR was 1.39 (0.69 to 2.80).
Table 1. Baseline Demographics.
The ITT population baseline characteristics are compared between treatment groups. There were no statistically significant differences and summary values are within ranges typically seen in clinical trials of acute ischemic stroke patients. Categorical variables are summarized as mean±SD; proportions are summarized as a percent. Temperature at baseline (pre-intervention) is shown. Time from stroke symptom onset (or last known well) to initiation of the rt-PA bolus is summarized.
| Variable | Hypothermia (n=63) | Normothermia (n=57) |
|---|---|---|
| Age (years) | 65.5±10.3 | 67.5±11.1 |
| Male (%) | 54 | 61 |
| Weight (kg) | 87.1±19.7 | 85.0±23.7 |
| Diabetes Mellitus (%) | 25 | 30 |
| Hypertension (%) | 81 | 72 |
| Heart Rate (bpm) | 83±15 | 80±15 |
| Temperature (°C) | 36.6±0.46 | 36.4±0.50 |
| NIHSS | 14.1±4.8 | 14.5±4.9 |
| Onset to rt-PA (min) | 105±37 | 114±37 |
The sample size was insufficient to properly assess the benefit of TH; for exploratory purposes, a pre-specified multi-variable analysis was constructed using variables well-established to influence stroke outcome: age, baseline NIHSS, diabetes history, admission glucose, and time from stroke onset to start of rt-PA (details provided as a supplement). The OR (95% CL) for a good outcome (ITT population) in the HY group was 0.62 (0.26 to 1.51). The OR (95% CL) for a good outcome (PP population) in the HY group was 0.29 (0.10 to 0.85), Fisher’s Exact test p<0.05. Time to reach target temperature did not appear to change outcome: using the PP population, 33 patients achieved a core body temperature below 35°C within 6 hours of cooling onset; in these more rapidly cooled patients the proportion with 90-day mRS 0,1 in the HY group was 24%, compared to 38% in 47 NT patients, OR 0.52 (0.16,1.52).
The range of adverse events was similar between groups (see supplemental data) and consistent with prior large stroke trials22, 23. The ITT incidence of serious adverse events was 41% in the HY group and 35% NT, OR (95% CL) 1.30 (0.58, 2.92). Mortality was 15.9% in the HY group vs. 8.8% in the NT, for an OR (95% CL) of 1.95 (0.56, 7.79). Pneumonia was confirmed in 19% in the HY group vs. 10.5% in the NT group, for an OR (95% CL) of 1.99 (0.63, 6.98). No serious adverse event trends in any other specific organ class appeared in the ICTuS-2 data. Most importantly, no adverse events related to the intravascular cooling catheter, such as hematoma or arterial injury, were detected. No statistically significant increase in fluid overload was noted in either group: (3/63 (4.8%) HY vs 7/57 (12.3%) NT). The incidence of symptomatic ICH was low in both groups, 3.5% for NT and 1.6% HY, OR (95% CL) of 0.45 (0.01, 8.80).
In exploratory univariate analyses, without corrections for multiple comparisons, the following laboratory test results showed statistically significant 24–36 hour elevations or {decreases} from baseline in the hypothermia group (Wilcoxon rank sum test, p<0.05): white blood cell count, {platelets}, BUN, AST, ALT, CPK. These changes all resolved by discharge, except AST and ALT elevations were still statistically significant at Day 7. The changes in liver and kidney function were noted in the prior ICTuS-L trial, and none were considered clinically significant. The increase in white blood count may reflect the increased risk of pneumonia in the HY group, although the strength of this association was not tested.
As expected due to the small sample size, none of the secondary endpoints were statistically significantly different between the study groups. The NIHSS score at 7 days was 10.6±11.3 in the NT group (n=55), vs. 10.4±10.3 in the HY group (n=58). The Barthel index at 90 days was not different between the groups, in either the ITT population or the PP population, ITT Odds Ratio: 0.81 (0.33,1.95). The mRS at 60 days was not different between the groups in either the PP or the ITT populations.
To identify markers of efficacy, we tested several derived variables for any impact on outcome. For these analyses we selected only HY patients who successfully received the intravascular catheter. To determine if TH benefited patients with larger infarctions, we selected patients who were classified as “large vessel occlusion” by the 7-day TOAST coding: there was no trend toward benefit in these patients. Similarly, there was no sign of benefit in patients who showed a hyperdense MCA sign or early ischemic change on baseline CT scan.
We confirmed that greater than 7-point decrease in the NIHSS at 7 days following stroke significantly correlated with 90-day mRS. For patients who exhibited such an improvement compared to those patients without such improvement the ITT OR (95% CL) for a 90-day mRS 2–6 was 0.06 (0.02,0.21), Fisher’s Exact test p <0.0001. This finding is important because the use of a short-term surrogate endpoint that accurately predicts 3-month Rankin could allow significantly shorter development cycles at lower cost.
Among PP patients, baseline hs-CRP measurements did not differ between groups (Table 2). At 24 hours there was a significant increase in hs-CRP in both groups. Overall (both groups combined n=82) the increase was mean±SD 19.52±25.06. The 24h increase in all PP patients (both treatment groups combined) who developed pneumonia (n=16) was 27.40±31.07, compared to 17.61±23.21 in those who did not (n=66). The difference was not statistically significant (Wilcoxon rank sum p=0.17). However, there was a significant difference between the treatment groups, as summarized in Table 2: in NT patients the 24h increase in hs-CRP indicated patients at risk for developing pneumonia but in HY patients the 24h increase in hs-CRP was the same in patients with or without pneumonia.
Table 2. Effect of hypothermia on hs-CRP and pneumonia.
For both the ITT and PP population, the 24-hour delta hs-CRP (24h value – baseline value) is summarized as mean±SD. Differences between the groups were tested with a Wilcoxon Rank Sum test without correction for multiple comparisons.
| Group | With pneumonia | No pneumonia | Significance |
|---|---|---|---|
| Normothermia PP | 27.2±15.67 (n=4) | 11.18±23.13 (n=35) | P<0.05 |
| Hypothermia PP | 27.46±35.35 (n=12) | 24.88±21.40 (n=31) | NS |
| Normothermia ITT | 29.43±21.29 (n=6) | 10.08±21.40 (n=39) | P<0.05 |
| Hypothermia ITT | 27.458±35.35 (n=12) | 23.37±21.43 (n=40) | NS |
Time to key study events are summarized for the HY group (ITT n=53) in Figure 2. Time from arrival to rt-PA treatment was about 60 min, which was excellent at the time this study was being conducted. Time from symptom onset (or last known well time) to onset of cooling was 287.6±65.8min (range 175 to 477min). Both groups received equal volume saline infusions, 1970±457 ml (150 to 3000 ml). The time to completion of the infusions, although quite variable, was also equal between both groups: 132±219 min, (17 to 1650 min). The effect of a cold saline bolus on temperature is shown in Table 3, and there appears to be a statistically significant effect due to the 4°C infusion in the hypothermia patients (p<0.0001). In most cases, however, the intravascular cooling catheter was inserted during the cold saline infusion, so the recorded temperatures reflect the combined effect of both the saline and the cooling catheter.
Figure 2. Key Trial Milestones.
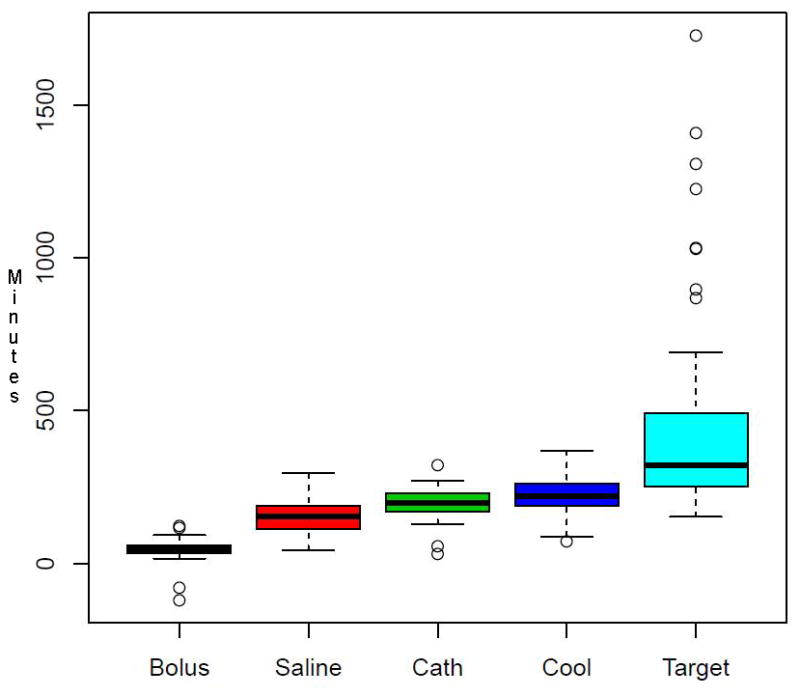
Time from arrival in the Emergency Department (ED) to key milestone events is summarized with box plots. Out of 63 ITT HY patients, no data was collected in 9 “early responder” patients; one patient crossed over from HY to NT; so the plots summarize data from the remaining 53 ITT HY patients. Each line represents the median, each box represents the upper and lower quartile, and whiskers are 1. 5* interquartile distance (IQD) from the quartile (upper, and lower, respectively). Outliers are shown as open circles. Bolus = time to rt-PA bolus. Saline = time to initiation of saline bolus. Cath = time to skin puncture for intravascular cooling catheter insertion. Cool = time to initiate cooling (start the cooling console) after successful catheter insertion. Target = time to first temperature in target range (<34 °C) or lowest temperature achieved if target was never reached.
Table 3. Effect of Cold Saline Bolus on Core Body Temperature.
For the PP population, the starting and ending temperatures are summarized as mean±SD for both groups. In the NT group the saline was administered at room temperature, while in the HY group the starting temperature of the saline was 4°C. Differences between the groups were tested with a Wilcoxon Rank Sum test without correction for multiple comparisons.
| N | Mean±SD | P | |
|---|---|---|---|
| Temperature at Start of Saline Bolus | |||
| Normothermia | 46 | 36.5±0.5 | |
| Hypothermia | 49 | 36.4±0.9 | NS |
| Temperature at End of Saline Bolus | |||
| Normothermia | 48 | 36.4±0.5 | |
| Hypothermia | 50 | 35.5±1.2 | <0.0001 |
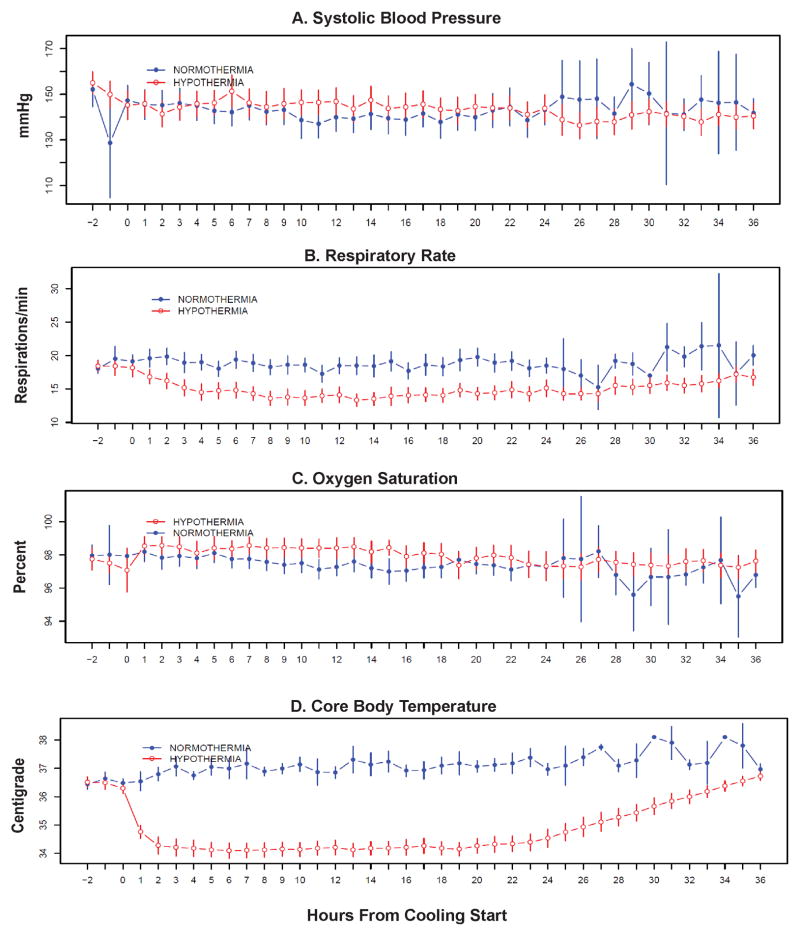
Hypothermia and the antishivering treatment had little effect on blood pressure or oxygen saturation (Figure 3). The total meperidine used in the HY patients (n=52) was mean±SD 1219.6± 424.5mg. Respiratory rate was significantly depressed, however, probably indicating over-treatment with the anti-shivering medications and failure of the permissive hypothermia protocol (Figure 3B). Cooling was rapid, with most patients at target within 2 hours (Figure 3D)
Figure 3. Vital Signs Throughout the Duration of Cooling and Rewarming.
At each time point the ITT population mean and 95% confidence intervals using standard errors is shown for each variable. Systolic blood pressure (Panel A) and oxygen saturation (Panel C) were not statistically different between groups. Respiratory rate (Panel B) was significantly suppressed in the hypothermia group (Wilcoxon Rank Sum test P<0.01). Core body temperature (Panel D) was rapidly and significantly reduced (Wilcoxon Rank Sum test P<0.01).
We explored a possible ‘dose-response’ effect of cooling by using the AUC method we published previously14. The 24 hour AUC was compared using 34, 35, and 36°C as the criterion for “target” temperature. The “dose” delivered below 34 (i.e. any temperature <34.0) was relatively little. On the other hand, there was a significant AUC under 35°C, affording moderate hypothermia. Thus, the efficacy results in this small study reflect the effect of mild (<35°C) hypothermia.
Discussion
ICTuS-2 was intended to include 400 patients and test whether pneumonia risk in HY vs NT patients could be reduced by certain protocol changes after ICTuS-L, and whether a cold-saline infusion enhanced cooling speed without causing fluid overload. We further tested whether pneumonia risk could be predicted from 24-hour changes in hs-CRP levels and whether 7-day improvements in NIHSS predicted 90-day mRS. The trial was stopped early due to the approval of intra-arterial neurothrombectomy and the expiration of the initial funding period, yet the two study groups were well matched (Table 1). Due to the small sample size there was little possibility of seeing statistically significant indications of benefit or harm; in confirmation of this, we examined various subsets of the data, including per-protocol, large vessel stroke, and early treated patients; we found no effects. In a single pre-specified PP analysis, we found a statistically significant result: in a multivariable regression that included age, baseline NIHSS, and other important variables, TH appeared to decrease significantly the odds of a good recovery (Fisher exact p<0.05), but this result must be qualified as we did not correct for multiple comparisons. Additional, properly powered clinical trials must be completed.
As for safety, the final sample size is insufficient to confirm or refute any risk. Nominally, there was a trend toward higher mortality in the HY group, perhaps related to a trend toward more pneumonia. Although the increased pneumonia rate was not statistically significant, the magnitude of the increase was similar to that seen in the previous ICTuS-L trial, suggesting that the protocol changes developed for ICTuS-2 did not succeed in limiting pneumonia during TH in awake patients. In contrast to the previous ICTuS-L, in the ICTuS-2 protocol there were precise definitions of pneumonia and careful surveillance of both treatment groups using Centers for Disease Control standards to overcome perceived case ascertainment bias in the previous study. Thus, having eliminated case ascertainment bias, the elevated pneumonia rate in ICTuS-2 probably indicates a real side effect of prolonged (24 hours) cooling and antishivering treatment. In contrast, and more reassuring, the rate of SAE was similar in both groups without signs of fluid overload due to the saline loading bolus.
Stroke patients who develop pneumonia exhibit higher hs-CRP 24 hours after admission24, 25 and we confirmed this, however HY patients without pneumonia also exhibited a large rise in 24-hour hs-CRP (Table 2). Thus, while it may be possible to develop a biomarker using hs-CRP to predict pneumonia in normothermic patients, apparently this would not be reliable in patients treated with TH.
While none of the results of ICTuS-2 are statistically significant, the overall impression of the data is relatively negative, with a nearly 2-fold nominal increase in pneumonia rate and mortality in the treated group. These worrisome trends ought to be tested in a larger trial, but several observations must be considered. First and foremost, hypothermia in awake patients requires aggressive shivering control, and these measures—which include prolonged infusions of meperidine—likely increase susceptibility to complications such as aspiration15. To avoid over-sedation in shivering patients, ICTuS-2 introduced a “permissive” approach to target temperature: the investigator was to treat shivering with meperidine only to a point of early sedation and thereafter to titrate upward the target temperature as needed to control shivering. Our data suggest that this permissive approach failed to significantly impact the pneumonia risk compared to the prior ICTuS-L trial. Further, as illustrated in Figure 3B the two study groups differed significantly in terms of respiratory suppression, suggesting the investigators did not fully implement “permissive hypothermia”.
The cold saline bolus appeared to improve cooling rates (time needed to achieve target) and should be included in future treatment protocols (Table 3, Figure 3D). Cold saline boluses have been implemented in pre-hospital treatment of post-cardiac arrest patients26. In the future, more powerful heat-extraction methods will likely cool patients to target temperature much more quickly. For example, it appears that intra-carotid cold saline for a very brief exposure provides powerful neuroprotection without the need for whole body cooling27. On the other hand, extremely powerful cooling techniques that can lower whole body temperature—and presumably brain temperature—within 20 minutes may provide the same or greater benefit without the safety issues associated with intra-carotid infusions.
Other limitations of ICTuS-2 should be noted, in addition to the small sample size. The intervention is invasive, and sham control catheterizations were not used. Thus, the trial was single-blind (the outcome examination was performed by a blinded examiner). as has been used widely in recent interventional trials28. The antishivering protocol we designed included therapy of proven benefit: meperidine, buspirone, and surface skin warming. Many practitioners recommend additional treatment using unproven therapy such as magnesium and dexmedotomidate29. We could not find rigorous justification for including these agents, but if they are truly effective then prohibiting them may partially explain the negative result in this small trial.
In conclusion, the ICTuS-2 trial was stopped early due to secular, significant changes in standard care of acute ischemic stroke. While the data were insufficient to permit any statistically significant findings, it appears that protocol measures designed to prevent pneumonia failed. Whether the explanation lies with TH or the sedating, antishivering protocol is unclear. Future studies will be needed to develop an effective, safe TH regimen for acute stroke.
Supplementary Material
Acknowledgments
Sources of Funding: P50 NS044148 and P50 NS044227
Research reported in this publication was supported by the National Institute Of Neurological Disorders And Stroke of the National Institutes of Health under Award Number P50NS044148 and P50 NS044227. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix: Contributors
Steering Committee: Patrick Lyden, James Grotta, Thomas Hemmen, Karen Rapp, Mary Jane Hess, Anthony Mullin
UCSD and UT Coordinating Committee: Patrick Lyden, James Grotta, Thomas Hemmen, Karen Rapp, Julie Jurf, Mary Jane Hess, Stephanie Parker, Gabriela Muranevici, Teresa Rzesiewicz, Bonnie Piantadosi
Statistical Center: Rema Raman, Karin Ernstrom, Gustavo Jimenez-Maggiora, Jia-Shing So, Sonia Jain
Safety Monitor: Michael Diringer
DSMB: Colin Derdeyn, Barney Stern, Scott Hamilton
Scientific Advisory Committee: Dalton Dietrich, Kyra Becker, Midori Yenari, Ulrich Dirnagl, Christine Wijman, Ángel Chamorro.
NINDS: Scott Janis, Claudia Moy
Sites (in order of recruitment)
Cedars-Sinai Medical Center (23), Patrick Lyden (Principal Investigator, PI), Felice Lin (Coordinator, C); Shlee Song, Konrad Schlick, Pooja Khanolkar
University of Texas at Houston (23), James Grotta (PI), Nancy J. Edwards (PI), Ana Roldan (C)
Sarasota Memorial Hospital (12), Mauricio Concha (PI), Jeanette Wilson (C), Amy Little, Pam Lewis
University of California, San Diego (10), Thomas Hemmen (PI), Brett Meyer, William Neil, Nhu Bruce, Amy Guzik, Ajeet Sohdi, Nabeel Herial, Bruce Ovbiagele, Dawn Meyer, Royya Modir, Teresa Rzesiewicz, Karen Rapp, Julie Jurf, Ronelyn Chavez
Columbia University (8), Sachin Agarwal (PI), Angela Velazquez (C), Stephan Mayer, Jan Claassen, Cristina Falo
Scripps Mercy Hospital (7), Gilda Tafreshi (PI), Thomas Hemmen, Brett Meyer, William Neil, Nhu Bruce, Amy Guzik, Royya Modir, Teresa Rzesiewicz, Nancy Kelly, Karen Rapp, Julie Jurf, Ronelyn Chavez, Bruce Ovbiagele
Michigan State University (7), Syed Hussain (PI), Erin Shell (C)
Alexian Brothers Hospital (4), Guy Dugan (PI), Elizabeth Kim (C),
North Memorial Medical Center (3) Irfan Altafullah (PI), Amy Tanner (C),
Centre Hospitalier Universitaire (3) Patrik Michel (PI), Ashraf Eskandari (C), Mauro Oddo, Tamarah Suys, Suzette Remillard, Maria Cordier
Hartford Hospital (3) Robert Brown (PI), Sara Jasak (C), Louise McCullough, Robert Brautigam
University of Alabama, Mobile (2) Andrei Alexandrov (PI), April Sisson (C), Karen Albright
Medical University Innsbruck (2) Gregor Broessner (PI), Erich Schmutzhard, Elissanet Escioglou
University of Colorado, Denver (2) William Jones (PI), Sharon Poisson (C), Jennifer Simpson
Abington Memorial Hospital (2) Qaisar Shah (PI), Karin Jonczak (C), Patricia Bussinger
Henry Ford Hospital (2) Christopher Lewandowski (PI), Shannen Berry (C), AnneMarie Lundell, Joseph B. Miller
St. Louis University (1), Salvador Cruz-Flores (PI), Eve Holzer (C) Susan Torretta
Hoag Memorial Hospital (1) David Brown (PI), Laura Heim (C)
St. Joseph’s Hospital (1) Carlos Smith (PI), Chip Kelley (C)
Yale University/Yale-New Haven Hospital (1), David Greer (PI), Evadne G. Marcolini, Emily J. Gilmore, Austin Seton (1) Neil Rutledge (PI), Del McBee (C)
University of Florida, Gainesville (1), Anna Khanna (PI), Sonisha Warren (C), Christina Wilsom, Vishnumurthy Shushrutha Hedna, Christian Rosado, Rosie Kizza
University of Miami (1) Kristine O’Phelan (PI), Andrea Escobar (C), Amedeo Merenda, Juan Perez Barcena, Amer Malik
Footnotes
Disclosures: all authors contributed to the trial design, data collection, analysis, and manuscript writing. No authors have a financial conflict of interest to disclose. Dr. Lyden serves on the DSMB for an industry sponsored trial of therapeutic hypothermia for cardiac arrest (COOL ARREST).
Citations
- 1.Dumitrascu OM, Lamb J, Lyden PD. Still cooling after all these years: Meta-analysis of pre-clinical trials of therapeutic hypothermia for acute ischemic stroke. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16645112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu TC, Grotta JC. Hypothermia for acute ischaemic stroke. Lancet Neurol. 2013;12:275–284. doi: 10.1016/S1474-4422(13)70013-9. [DOI] [PubMed] [Google Scholar]
- 3.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 4.Johnston MV, Fatemi A, Wilson MA, Northington F. Treatment advances in neonatal neuroprotection and neurointensive care. Lancet Neurol. 2011;10:372–382. doi: 10.1016/S1474-4422(11)70016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernard SG, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. New Engl J Med. 2002;346:557. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 6.The Hypothermia After Cardiac Arrest Study G. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. New Engl J Med. 2002;346:549. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 7.Nolan JP, Morley PT, Vanden Hoek TL, Hickey RW, Kloeck WG, Billi J, et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation. 2003;108:118–121. doi: 10.1161/01.CIR.0000079019.02601.90. [DOI] [PubMed] [Google Scholar]
- 8.Wan YH, Nie C, Wang HL, Huang CY. Therapeutic hypothermia (different depths, durations, and rewarming speeds) for acute ischemic stroke: a meta-analysis. J Stroke Cerebrovasc Dis. 2014;23:2736–2747. doi: 10.1016/j.jstrokecerebrovasdis.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Krieger DW, De Georgia MA, Abou-Chebl A, Andrefsky JC, Sila CA, Katzan IL, et al. Cooling for acute ischemic brain damage (COOL AID): an open pilot study of induced hypothermia in acute ischemic stroke. Stroke. 2001;32:1847–1854. doi: 10.1161/01.str.32.8.1847. [DOI] [PubMed] [Google Scholar]
- 10.Schwab S, Schwarz S, Spranger M, Keller E, Bertram M, Hacke W. Moderate hypothermia in the treatment of patients with severe middle cerebral artery infarction. Stroke. 1998;29:2461–2466. doi: 10.1161/01.str.29.12.2461. [DOI] [PubMed] [Google Scholar]
- 11.Kammersgaard LP, Rasmussen BH, Jorgensen HS, Reith J, Weber U, Olsen TS. Feasibility and safety of inducing modest hypothermia in awake patients with acute stroke through surface cooling: A case-control study: the Copenhagen Stroke Study. Stroke. 2000;31:2251–2256. doi: 10.1161/01.str.31.9.2251. [DOI] [PubMed] [Google Scholar]
- 12.Lyden PD, Allgren RL, Ng K, Akins P, Meyer B, Al-Sanani F, et al. Intravascular Cooling in the Treatment of Stroke (ICTuS): early clinical experience. J Stroke Cerebrovasc Dis. 2005;14:107–114. doi: 10.1016/j.jstrokecerebrovasdis.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Hemmen TM, Raman R, Guluma KZ, Meyer BC, Gomes JA, Cruz-Flores S, et al. Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ICTuS-L): final results. Stroke. 2010;41:2265–2270. doi: 10.1161/STROKEAHA.110.592295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyden P, Ernstrom K, Cruz-Flores S, Gomes J, Grotta J, Mullin A, et al. Determinants of Effective Cooling During Endovascular Hypothermia. Neurocrit Care. 2012;16:7. doi: 10.1007/s12028-012-9688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyden P, Ernstrom K, Raman R. Determinants of Pneumonia Risk During Endovascular Hypothermia. Ther Hypothermia Temp Manag. 2013;3:24–27. doi: 10.1089/ther.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell BC, Donnan GA, Lees KR, Hacke W, Khatri P, Hill MD, et al. Endovascular stent thrombectomy: the new standard of care for large vessel ischaemic stroke. Lancet Neurol. 2015;14:846–854. doi: 10.1016/S1474-4422(15)00140-4. [DOI] [PubMed] [Google Scholar]
- 17.Lyden PD, Hemmen TM, Grotta J, Rapp K, Raman R. Endovascular therapeutic hypothermia for acute ischemic stroke: ICTuS 2/3 protocol. Int J Stroke. 2014;9:117–125. doi: 10.1111/ijs.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badjatia N, Strongilis E, Gordon E, Prescutti M, Fernandez L, Fernandez A, et al. Metabolic impact of shivering during therapeutic temperature modulation: the Bedside Shivering Assessment Scale. Stroke. 2008;39:3242–3247. doi: 10.1161/STROKEAHA.108.523654. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control. [Accessed 1/10/2009];Criteria for Defining Nosocomial Pneumonia. 2005 http://www.cdc.gov/ncidod/hip/nnis/members/pneumonia/final/pneumocriteriav1.pdf.
- 20.Broderick JP, Lu M, Kothari R, Levine SR, Lyden PD, Haley EC, et al. Finding the most powerful measures of the effectiveness of tissue plasminogen activator in the NINDS tPA stroke trial. Stroke. 2000;31:2335–2341. doi: 10.1161/01.str.31.10.2335. [DOI] [PubMed] [Google Scholar]
- 21.Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276:637–639. doi: 10.1001/jama.276.8.637. [DOI] [PubMed] [Google Scholar]
- 22.Hesse K, Fulton RL, Abdul-Rahim AH, Lees KR, Collaborators V. Characteristic adverse events and their incidence among patients participating in acute ischemic stroke trials. Stroke. 2014;45:2677–2682. doi: 10.1161/STROKEAHA.114.005845. [DOI] [PubMed] [Google Scholar]
- 23.Johnston KC, Li JY, Lyden PD, Hanson SK, Feasby TE, Adams RJ, et al. Medical and neurological complications of ischemic stroke: experience from the RANTTAS trial. Stroke. 1998;29:447–453. doi: 10.1161/01.str.29.2.447. [DOI] [PubMed] [Google Scholar]
- 24.Di Napoli M, Schwaninger M, Cappelli R, Ceccarelli E, Di Gianfilippo G, Donati C, et al. Evaluation of C-Reactive Protein Measurement for Assessing the Risk and Prognosis in Ischemic Stroke: A Statement for Health Care Professionals From the CRP Pooling Project Members. Stroke. 2005;36:1316–1329. doi: 10.1161/01.STR.0000165929.78756.ed. [DOI] [PubMed] [Google Scholar]
- 25.Elkind MS, Tai W, Coates K, Paik MC, Sacco RL. High-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2, and outcome after ischemic stroke. Arch Intern Med. 2006;166:2073–2080. doi: 10.1001/archinte.166.19.2073. [DOI] [PubMed] [Google Scholar]
- 26.Bernard S, Buist M, Monteiro O, Smith K. Induced hypothermia using large volume, ice-cold intravenous fluid in comatose survivors of out-of-hospital cardiac arrest: a preliminary report. Resuscitation. 2003;56:9–13. doi: 10.1016/s0300-9572(02)00276-9. [DOI] [PubMed] [Google Scholar]
- 27.Mattingly TK, Denning LM, Siroen KL, Lehrbass B, Lopez-Ojeda P, Stitt L, et al. Catheter based selective hypothermia reduces stroke volume during focal cerebral ischemia in swine. J Neurointerv Surg. 2016;8:418–422. doi: 10.1136/neurintsurg-2014-011562. [DOI] [PubMed] [Google Scholar]
- 28.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 29.Choi HA, Ko SB, Presciutti M, Fernandez L, Carpenter AM, Lesch C, et al. Prevention of shivering during therapeutic temperature modulation: the Columbia anti-shivering protocol. Neurocrit Care. 2011;14:389–394. doi: 10.1007/s12028-010-9474-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.