Abstract
Objective
To test the hypothesis that an 8-year intensive lifestyle intervention (ILI) suppresses aging-dependent changes in regional lean (LM) and fat mass (FM) among obese or overweight persons with type 2 diabetes.
Methods
Regional body composition was measured by dual energy X-ray absorptiometry within a subset of 1019 volunteers (45–75 years old) in the Look AHEAD study randomized to ILI or diabetes support and education (DSE). The ILI goal was to achieve and maintain ≥7% weight loss through increased physical activity and reduced caloric intake.
Results
Over 8 years, the DSE group exhibited a linear loss of LM and FM. During Year 1, the ILI group lost LM and FM. Between Years 1 and 8, the ILI group regained most FM in all regions; regional LM converged with that of the DSE group; the percent of LM loss was greater for the leg than for the trunk. Among both groups, regional LM and FM change was proportional to the size of the region, trunk > leg > arm.
Conclusions
The ILI did not suppress aging-dependent LM losses, particularly in the leg region. The long term consequences of rapid LM and FM loss and subsequent regain mostly as fat are unknown.
Keywords: Obesity, diabetes, body composition, weight loss
Introduction
Obesity-linked type 2 diabetes and metabolic syndrome are risk factors for serious pathological sequelae, most prominently cardiovascular disease (CVD). Central obesity is also a CVD risk factor, and a large waist circumference is one of the diagnostic criteria for metabolic syndrome.(1) Moreover, as people age, their average weight shifts from the extremities to the trunk, and LM decreases. Over time (2, 3, 4), these changes can impair mobility due to the loss of strength associated with reduced muscle mass.(5, 6)
The Look AHEAD clinical trial of obese or overweight diabetic persons compared the long term effects of intentional weight loss via an intensive lifestyle intervention (ILI) with a group receiving diabetes support and education (DSE).(7, 8, 9, 10) Weight loss over one year in Look AHEAD ILI vs. DSE groups reduced adiposity and improved metabolic biomarkers.(11) Given that Look AHEAD has long term data on this aging group of diabetic participants,(12) it is ideal for testing the effects of intentional weight loss on regional body composition achieved via the ILI. Total body composition at baseline and at Years 1, 4, and 8 has been reported.(12, 13, 14) Over the eight years of the study, fat mass (FM) within the DSE group changed little whereas LM decreased linearly. In contrast, at the end of one year the ILI group lost weight as FM (~2/3) and LM (~1/3) but at the end of eight years regained weight mostly as FM while losing LM. Comparison of ILI and DSE showed that at the end of eight years, total body composition in the two groups was similar. Here we report changes in regional LM and FM of ILI vs. DSE groups at Years 1, 4, and 8 post baseline within a subgroup of 1,018 participants at Look AHEAD sites in Baton Rouge, Houston, Boston, and Seattle who performed dual-energy x-ray absorptiometry (DXA) scans to test the hypothesis that over the eight year intervention, the ILI will have less FM and more LM each of three body regions, leg, arm, and trunk.
Research Design and Methods
Participant Characteristics
Men and women (n = 5,145), 45 to 76 years of age with type 2 diabetes, and a body mass index (BMI) of ≥ 25 kg/m2 (or ≥ 27 kg/m2 if receiving insulin), were randomized to ILI or DSE groups with equal probability using a web-based data management system that verifies eligibility. Randomization was stratified by center and blocked with random block sizes of four and six. Enrollment criteria have been reported.(7) Protocol and consent forms were approved by institutional review boards at each site, and participants provided informed consent.
Study Design
The ILI was designed to achieve and maintain weight loss ≥ 7% through reduced caloric intake and increased physical activity.(15, 16) In Years 2–8, the ILI focused on maintaining weight loss and the duration of physical activity. Participants randomized to the DSE group received general information about healthy eating and physical activity but none of the comprehensive behavioral components of the ILI.(17) General medical and diabetes care were provided by participant’s non-study health care providers. The consort diagram for the DXA sub study has been reported.(14)
Study Measures
Body composition was measured by DXA using Hologic (QDR-4500A) fan beam densitometers, using 2-compartment models; details have been reported.(14) Regional cut-points were set by the Hologic software and adjusted by the DXA operator if the software failed to achieve the standard demarcations. The boundary for the arm is a line bisecting the shoulder joint, and the leg boundary is a line bisecting the femoral neck. Software upgrades were approved by a DXA quality assurance center. Results were corrected for underestimated fat mass using Hologic software(18), and scans were analyzed at a central reading center. The principles of the DXA methodology provide a 2-compartment measurement of fat and fat-free mass on the molecular level, and the fat-free component can be subdivided into bone mineral and soft tissue. The term lean tissue (LM) in our figure and tables refers to lean soft tissue.
Statistical Analysis
The analysis included 1019 (88%) participants (513 DSE group; 506 ILI group) who had a baseline DXA scan and at least one follow-up DXA measurement. For each region (arm, leg, and trunk), the percent total FM is computed as total regional FM divided by total FM times 100; percent LM is computed similarly. Least square mean estimates for each gender and intervention group were obtained using general linear models. Gender differences within each group were assessed using contrasts, and based on the observed gender differences, all subsequent analyses were stratified by gender. The effect of the intervention on longitudinal changes in body composition was determined. For the average arm, leg, and trunk FM and LM (kg), we fit separate linear mixed effects models (LMM) that included the main effect of intervention groups, time, and their interaction while adjusting for appropriate baseline measures. The intervention group effects were assessed for the overall follow-up period and at each follow-up visit. We also evaluated percent change in regional FM and LM between baseline and follow-up. The LMMs included the main effects of intervention groups, time, and region as well as all two-way and three-way interactions. These models allowed comparison of the overall change in different regions and change within groups at follow-up. Analyses were performed by SAS 9.3 (Cary, NC), and a p-value <0.05 was considered significant.
Results
Initial Body Compositions
Initial body composition expressed as a percent of LM and FM was similar for DSE and ILI groups with the distribution of FM and LM for females and males being Trunk ≫; Leg > Arm (Figure 1 A, B). The trunk was the predominant site of FM accumulation for both females (~53%) and males (~58%). As a percent of total FM, females presented with more leg and arm FM (32% and 13% respectively) than males (27% and 11% respectively; Figure 1 A). The percent leg LM in female and male was not different (32%) whereas females presented with less arm LM than males (11 vs. 12%) and slightly more trunk LM (52 vs. 51%; Figure 1 B).
Figure 1.
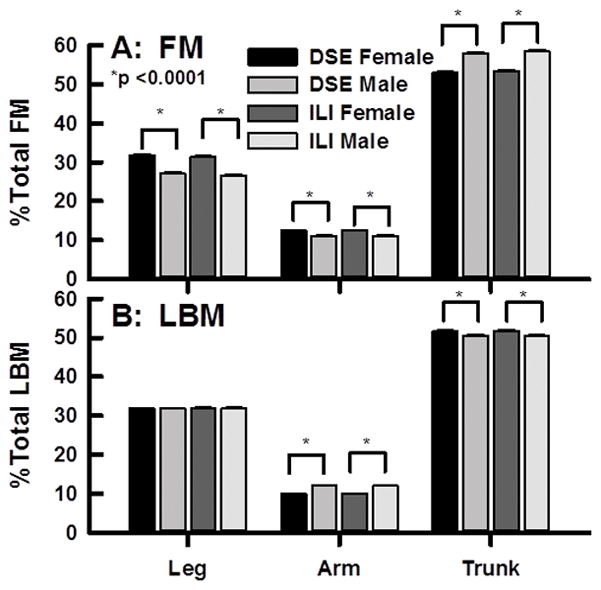
Baseline Distribution of FM (A) and LM (B) among males and females in the DXA sub study of body composition as labeled.
Longitudinal Changes in Regional Body Composition in the DSE Group
Over the eight years of the study, LM among DSE male and female participants declined linearly in all three body regions (Figures 2, 3 A–C). A preliminary analysis showed that leg LM (p=0.05) and trunk fat mass (p=0.0065) have significant intervention by gender interactions so the results are presented separately for males and females. Among DSE females, leg-, arm-, and trunk-LM losses from baseline to Year 8 were 0.418 ± 0.04, 0.1 ± 0.01, and 0.796 ± 0.09 kg respectively (p <0.0001; Table 1). Among DSE males, the respective LM losses were 0.536 ± 0.05, 0.248 ± 0.02, and 0.801 ± 0.12 kg (p <0.0001). Over the same time interval, regional FM among the DSE participants either declined linearly or was unchanged (Figures 2, 3 D–F). Respective baseline to Year 8 leg, arm and trunk FM changes for DSE females were −0.227 ± 0.06 (p<0.0001), 0.01 ± 0.03 (NS), and −0.646 ± 0.21 kg (p<0.002) (Table 1); the respective values for DSE males were −0.021 ± 0.05 (NS), 0.047 ± 0.02 (p<0.04), and 0.17 ± 0.26 kg (NS) (Table 2).
Figure 2.
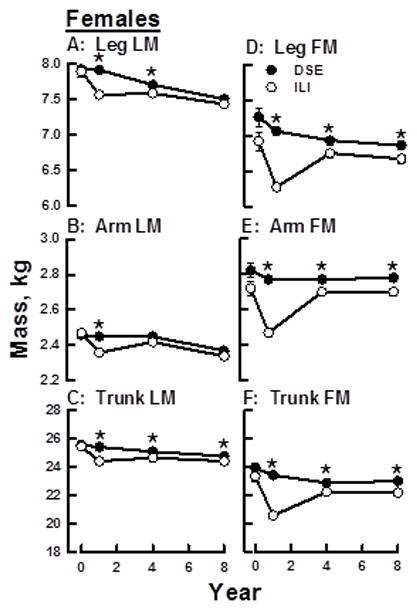
Changes in Regional LM and FM over Eight Years among Females within DSE (C) and ILI (I) Groups. A–C, FM Changes; D–F, LM Changes. *p <0.05.
Figure 3.
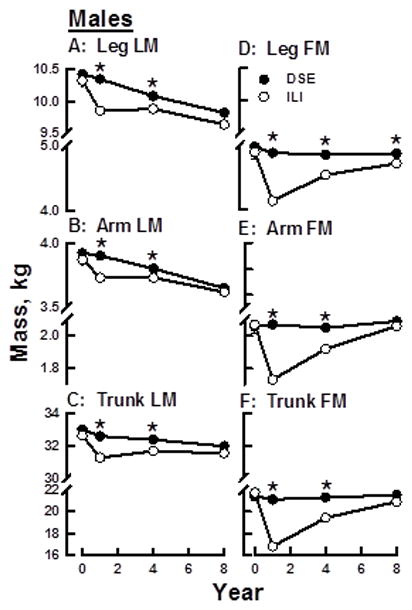
Changes in Regional LM and FM over Eight Years among Males within DSE (C) and ILI (I) Groups. A–C, FM Changes; D–F, LM Changes. *p <0.05.
Table 1.
FM and LM (kg) Change from Baseline to Years 1, 4, and 8 for Females.
| DSE | ILI | |||||
|---|---|---|---|---|---|---|
| Leg LM | Difference | SE | p-value | Difference | SE | p-value |
| Baseline to Year 1 | −0.003 | 0.04 | 0.9359 | −0.349 | 0.03 | <.0001 |
| Baseline to Year 4 | −0.214 | 0.04 | <.0001 | −0.331 | 0.04 | <.0001 |
| Baseline to Year 8 | −0.418 | 0.04 | <.0001 | −0.485 | 0.04 | <.0001 |
| Arm LM | DSE | ILI | ||||
| Baseline to Year 1 | −0.018 | 0.01 | 0.2082 | −0.102 | 0.01 | <.0001 |
| Baseline to Year 4 | −0.012 | 0.01 | 0.4059 | −0.049 | 0.01 | 0.0005 |
| Baseline to Year 8 | −0.1 | 0.01 | <.0001 | −0.127 | 0.01 | <.0001 |
| Trunk LM | DSE | ILI | ||||
| Baseline to Year 1 | −0.161 | 0.09 | 0.0754 | −1.149 | 0.09 | <.0001 |
| Baseline to Year 4 | −0.479 | 0.09 | <.0001 | −0.903 | 0.09 | <.0001 |
| Baseline to Year 8 | −0.796 | 0.09 | <.0001 | −1.145 | 0.09 | <.0001 |
| Leg FM | DSE | ILI | ||||
| Baseline to Year 1 | −0.029 | 0.06 | 0.6104 | −0.786 | 0.06 | <.0001 |
| Baseline to Year 4 | −0.158 | 0.06 | 0.0066 | −0.311 | 0.06 | <.0001 |
| Baseline to Year 8 | −0.227 | 0.06 | 0.0001 | −0.383 | 0.06 | <.0001 |
| Arm FM | DSE | ILI | ||||
| Baseline to Year 1 | 0.003 | 0.03 | 0.9130 | −0.282 | 0.02 | <.0001 |
| Baseline to Year 4 | −0.003 | 0.03 | 0.9084 | −0.057 | 0.03 | 0.0249 |
| Baseline to Year 8 | 0.01 | 0.03 | 0.6870 | −0.056 | 0.03 | 0.0317 |
| Trunk FM | DSE | ILI | ||||
| Baseline to Year 1 | −0.232 | 0.20 | 0.2570 | −2.984 | 0.20 | <.0001 |
| Baseline to Year 4 | −0.784 | 0.21 | 0.0002 | −1.325 | 0.21 | <.0001 |
| Baseline to Year 8 | −0.646 | 0.21 | 0.0022 | −1.381 | 0.21 | <.0001 |
P-values represent a comparison of change from baseline within the group and do not represent comparisons between the ILI and DSE groups.
Table 2.
FM and LM (kg) Change from Baseline to Years 1, 4, and 8 for Males
| DSE | ILI | |||||
|---|---|---|---|---|---|---|
| Leg LM | Difference | SE | p-value | Difference | SE | p-value |
| Baseline to Year 1 | −0.011 | 0.05 | 0.81 | −0.498 | 0.05 | <.0001 |
| Baseline to Year 4 | −0.274 | 0.05 | <.0001 | −0.47 | 0.05 | <.0001 |
| Baseline to Year 8 | −0.536 | 0.05 | <.0001 | −0.713 | 0.05 | <.0001 |
| Arm LM | DSE | ILI | ||||
| Baseline to Year 1 | 0.005 | 0.02 | 0.8213 | −0.157 | 0.02 | <.0001 |
| Baseline to Year 4 | −0.098 | 0.02 | <.0001 | −0.162 | 0.02 | <.0001 |
| Baseline to Year 8 | −0.248 | 0.02 | <.0001 | −0.273 | 0.02 | <.0001 |
| Trunk LM | DSE | ILI | ||||
| Baseline to Year 1 | −0.188 | 0.12 | 0.1039 | −1.485 | 0.12 | <.0001 |
| Baseline to Year 4 | −0.398 | 0.12 | 0.0008 | −1.069 | 0.12 | <.0001 |
| Baseline to Year 8 | −0.801 | 0.12 | <.0001 | −1.212 | 0.13 | <.0001 |
| Leg FM | DSE | ILI | ||||
| Baseline to Year 1 | −0.015 | 0.05 | 0.7610 | −0.785 | 0.05 | <.0001 |
| Baseline to Year 4 | −0.043 | 0.05 | 0.3817 | −0.362 | 0.05 | <.0001 |
| Baseline to Year 8 | −0.021 | 0.05 | 0.6795 | −0.17 | 0.05 | 0.0019 |
| Arm FM | DSE | ILI | ||||
| Baseline to Year 1 | 0.021 | 0.02 | 0.3296 | −0.318 | 0.02 | <.0001 |
| Baseline to Year 4 | 0.01 | 0.02 | 0.6565 | −0.126 | 0.02 | <.0001 |
| Baseline to Year 8 | 0.047 | 0.02 | 0.0397 | 0.014 | 0.02 | 0.5454 |
| Trunk FM | DSE | ILI | ||||
| Baseline to Year 1 | −0.216 | 0.24 | 0.3710 | −4.624 | 0.25 | <.0001 |
| Baseline to Year 4 | −0.033 | 0.25 | 0.8929 | −1.977 | 0.26 | <.0001 |
| Baseline to Year 8 | 0.17 | 0.26 | 0.5124 | −0.469 | 0.27 | 0.0863 |
P values represent a comparison of change from baseline within the group and do not represent comparisons between the ILI and DSE groups.
Longitudinal Changes in Body Composition – ILI vs. DSE Groups
Changes in regional FM and LM in the ILI and DSE groups varied over the 8-year study interval. Among ILI males and females, the majority of LM loss occurred during Year 1 resulting in less LM in the ILI group compared with the DSE group (Figures 2, 3 A–F). In the ILI group, leg-, arm-, and trunk-LM, respectively, decreased 0.349 ± 0.03, 0.102 ± 0.01, and 1.149 ± 0.09 kg for females and 0.498 ± 0.05, 0.157 ± 0.02, and 1.485 ± 0.12 kg for males (Tables 1, 2). Between Years 1 and 8, the rate of LM decline among ILI males and females markedly slowed in the arm and leg regions and was essentially unchanged for the trunk in females. For females in the ILI, LM was lower than that of the DSE group in all regions at Year 1 but was lower at Year 4 only in the leg and trunk. By Year 8, LM in ILI and DSE was essentially the same in the leg and arm but continued to be lower in the trunk (Figure 2, A–C). For ILI males, LM was lower than that of the DSE group in all regions at Years 1 and 4 but there were no regional differences by Year 8. Thus by Year 8, LM in all regions among ILI males and females were converging with those of the DSE group.
With respect to FM changes, at the end of Year 1, leg-, arm-, and trunk-FM among both males and females in the ILI group were lower than those at ILI baseline and the DSE group at Year 1 (Figure 2, 3; Tables 1, 2). Leg-, arm,- and trunk-FM respectively decreased 0.786 ± 0.06, 0.282 ± 0.02, and 2.984 ± 0.2 kg for females and 0.785 ± 0.05, 0.318 ± 0.02, and 4.624 ± 0.25 kg for males. From baseline to Year 1, percent mass lost as fat in the leg, arm, and trunk respectively were 69.3, 73.5, and 72.3 for ILI females and 60.9, 66.7, and 75.8 for ILI males. Between Years 1 and 8, leg-, arm-, and trunk-FM increased among females in the ILI group but remained lower than that of DSE group females at all time points (Figure 2 D–F). In contrast, regional FM among males in the ILI and DSE groups was different at all post-baseline time points except at Year 8 for arm and trunk (Figure 3 D–F). As with regional LM, at Year 8 regional FM in the ILI and DSE groups was converging or had converged for both males and females.
Percent Change in Regional LM and FM between Baseline and Year 1
Among DSE females and males, the percent regional LM and FM lost between baseline and Year 1 was small. In contrast, compared to the DSE group the ILI groups lost much more LM and FM from all three regions during the same time period (Figure 4 A, B; Figure 5 A, B). Within the ILI group, the percent change of arm-, leg-, and trunk-LM from baseline to Year 1 was similar among females (Figures 4 A) and males (Figures 5 A). However, FM loss from trunk was greater than that from leg and arm among all ILI members in the first year (Figures 4 B, 5 B). Among ILI females, 66, 69, and 73% of the weight loss from leg, arm, and trunk in the first year occurred as FM; the corresponding values for ILI males were 63, 71, and 79% (Figures 2 and 3).
Figure 4.
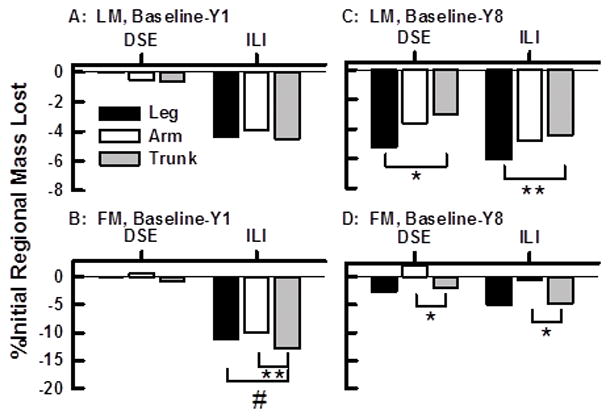
Percent Changes in Regional LM and FM between Baseline and Years One and Eight Among Females within the DSE (C) and ILI (I) Groups. A–C, FM Changes; D–F, LM Changes. *p <0.0001, **p <0.01, #p <0.05.
Figure 5.
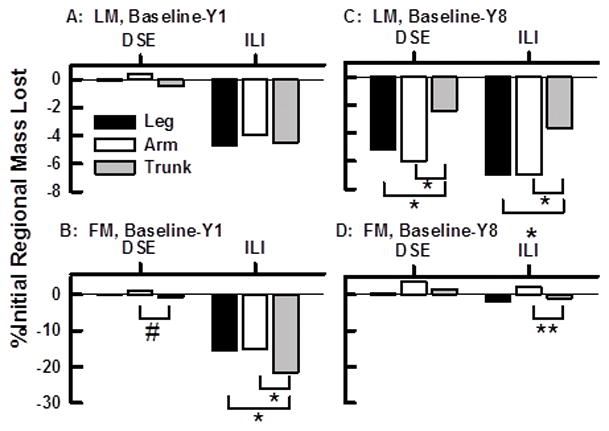
Percent Changes in Regional LM and FM between Baseline and Years One and Eight Among Males within the DSE (C) and ILI (I) Groups. A–C, FM Changes; D–F, LM Changes. Other details in Figure 3 legend.
Percent Change in Regional LM and FM by Year 8
By Year 8, regional changes in FM for females and males in the DSE and ILI groups were remarkably similar (Figures 4 D, 5 D). Slightly more FM was lost from the trunk than from the arm region of females in both groups (Figure 4 D) but this loss was observed among males only in the ILI group (Figure 5 D). Regional LM changes by Year 8 were also remarkably similar among females and males in the both groups (Figures 4C, 5 C). By Year 8 females and males in both the ILI and DSE groups lost LM from all three body regions (Figures 4 C, 5 C). However, the LM loss was uneven across regions with females losing more LM from the leg region than from the trunk (Figures 4 C); this effect was nearly the same in both groups. Among males a similar effect was observed; there was greater LM loss from both arm and leg regions than from the trunk within DSE and ILI groups (Figure 5 C). Remarkably, among both females and males, the weight regain as fat between Years 1 and 8 was 95 to 100% with a concurrent LM loss in all regions.
Discussion
At baseline, the ILI and DSE groups were similar with respect to numerous anthropomorphic measures,(14) and regional LM and FM (Figure 1). The greater regional LM observed among men is consistent with the greater skeletal muscle in men vs. women.(19) The magnitude of the changes in regional LM or FM change was proportional to the size of the region—arm < leg < trunk (Figure 1), supporting the hypothesis that changes in regional LM and FM are proportional to baseline whole body LM and FM respectively.
Like total body FM change,(14) regional changes in FM in the DSE group were nearly unchanged or declined slightly from baseline to Year 8. Although the ILI group lost more regional FM than the DSE group between baseline and Year 1, at the end of 8 years, regional FM among ILI male and female participants nearly converged with those for DSE. Thus, the effects of the ILI on regional FM were similar to what we reported for total body FM.(14) The DSE group lost LM from all regions over 8 years, an effect that we attribute to aging. Despite initial regional LM loss in the ILI group between baseline and 1 year, at the end of 8 years, regional LM for the ILI and DSE groups were converging. Thus not only did the ILI fail to maintain a reduced regional FM, it failed to suppress the aging-dependent effects on LM loss.
Differential Changes in Regional LM and FM
As a percent of regional mass change between baseline and Year 1, the ILI produced a preferential loss of fat from the trunk (13 and 22% for females and males, respectively) vs. leg (11 and 15%, females and males, respectively) and arm (10 and 16% for females and males, respectively). Given that waist circumference is one of the criteria for metabolic syndrome, this would be expected to shift some participants out of that category or at least towards normal whole body metabolism and is consistent with the Look AHEAD findings of improved glycemic control in the ILI group.
Changes in percent regional FM between baseline and Year 8 in both groups were different but small. In contrast, changes in regional LM between baseline and Year 8 were more profound and declined linearly within all three regions for the DSE group, again an effect we attribute to aging. Within both ILI and DSE groups, the percent regional LM loss was greater for leg than for trunk for females (Figures 4 C) and greater for leg and arm than for trunk for males (Figures 5 C). Given that independent living and mobility depend on extremity-skeletal muscle (leg) and that LM is ~80% skeletal muscle, the rapid LM loss during rapid weight loss and the failure of the ILI to prevent aging-dependent LM loss over 8 years is concerning, all the more so when superimposed on dynapenia, loss of muscle quality with age.(20) Although there are only limited data on physical activity in Look AHEAD, at Year 4 in the whole Look AHEAD cohort, ILI actually improved mobility(21) and in a another subgroup at Year 8 the ILI group exhibited better lower extremity physical function than the DSE group.(22) Thus, the ILI may have helped suppress the aging-dependent dynapenia despite the LM loss detected via the DXA scan. The body compositions of diabetic and non-diabetic persons differ, the latter presenting with less total fat, leg FM and leg LM and more trunk FM and LM.(12) Thus, it is unlikely that our findings can be generalized to a wider population that would include non-diabetic persons.
Although dietary and/or behavioral methods can produce clinically meaningful weight loss within the non-diabetic obese population, the long term success of these trials has lagged other more aggressive interventions such as bariatric surgery, which also produces weight-loss with improved glycemic control.(23) However, given its low cost and safety when compared to surgical and pharmacologic interventions, a behavioral approach to improving glycemic control and cardiovascular risk factors through weight loss remains an attractive therapeutic option. The retail food environment plays a growing and challenging role in food choice and eating patterns in ways that determine energy intake and ultimately body weight. The affordability(24) and availability of energy dense highly palatable foods,(25, 26, 27) large portion sizes,(28) and limited neighborhood access to healthy foods all seem to provide an environment that promotes obesity. Although some of these environmental factors are suited to behavioral approaches, additional research is needed. Reducing obesity in the U.S. also requires a comprehensive and coordinated approach using policy and environmental changes that support healthier communities that promote social change leading to healthier lifestyle choices.
Given that the ILI, like many other weight loss programs, had substantial weight regain after initial weight loss, additional research on weight loss maintenance is warranted. Like other chronic diseases, obesity management likely requires lifetime intervention with an intensity that is similar to that of the first year of the ILI group. New approaches are also needed to preserve LM during the weight loss and according to a recent meta-analysis exercise-induced weight loss without profound calorie restriction better improved bone mineral density than caloric restriction,(29) leading to the hypothesis that adding muscle strengthening to aerobic exercise better preserves LM during weight loss.
Strengths and Weaknesses of Study—Strengths
Regional body composition was assessed in a large, well-powered subsample of a randomized population of obese or overweight diabetic persons with >80% follow-up at eight years. The design included a standard protocol, vigilant staff training, and DXA calibration across sites. Weaknesses: DXA, though having no radiation risk, is a less precise measure of body composition than X-ray computed tomography and magnetic resonance imaging, which give better estimates of skeletal muscle. DXA does not accurately distinguish between types of fat in important depots, namely, subcutaneous abdominal vs. visceral fat. A further limitation is that physical function and body composition were not measured in the same subgroups, so that the correlation between these two outcomes could not be directly assessed.
Conclusions
Interventions with reduced caloric intake and a physical activity component such as walking do not appear to suppress the aging-dependent changes in lean mass, especially from the leg. The long term consequences of intentional weight loss leading initially to a more rapid loss of lean mass and weight regain as fat are unknown and require more investigation.
Supplementary Material
What is already known
Exercise and caloric restriction induce weight-loss from all body regions.
Weight-loss alters regional body composition.
Aging is associated with changes in whole body composition.
What does this study add?
An intensive lifestyle intervention (ILI) that included reduced calorie intake and increased physical activity among obese or overweight diabetic persons produced an initial weight loss from all body regions, two-thirds of which was fat.
Regional weight regain in the ILI group was nearly 100% fat; changes in regional fat and lean mass, gain or loss, were proportional to the size of the region, trunk > leg > arm.
The ILI did not suppress the aging-dependent preferential loss of leg-lean mass, an effect that could limit mobility during aging.
Acknowledgments
Funding: NIH cooperative agreements with the NIDDK: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992 and by NHLBI; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; NIH Office of Research on Women’s Health; and the Centers for Disease Control and Prevention. Other support was from the Intramural Research Program of NIDDK. Opinions expressed in this paper are those of the authors and do not necessarily reflect those of I.H.S. or other funding sources. Clinical sites, coordinating and central resources centers, and sponsors are cited in Supporting Information. Other support was from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (GCRC;M01RR02719); the Massachusetts General Hospital Mallinckrodt GCRC and the Massachusetts Institute of Technology GCRC (M01RR01066); University of Colorado Health Sciences GCRC (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); University of Tennessee at Memphis GCRC (M01RR0021140); University of Pittsburgh GCRC (M01RR000056), the Clinical Translational Research Center (UL1 RR 024153) and NIH grant (DK 046204); VA Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs; and the Frederic C. Bartter GCRC (M01RR01346).
Footnotes
Contributions to Look AHEAD were from FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST® (Nestle HealthCare Nutrition) Inc.; Hoffmann-La Roche Inc.; Abbott Nutrition; and Slim-Fast Brand (Unilever North America).
Trial Registration clinicaltrials.gov Identifier: NCT00017953
Drs. Pownall and Chen had access to all data and take responsibility for its integrity and analysis. Dr. Schwartz supervised DXA quality assurance and analysis. Dr. Pownall conceived the hypotheses and analyses and drafted the paper. Drs. Johnson, Schwartz and Chen refined interpretation and the final manuscript. Dr. Chen provided statistical analysis. All authors were involved in study design, interpretation, writing the paper, and approved the submitted version. Dr. Emily J. Gardner provided editorial assistance.
Author Contributions: Drs. Pownall and Chen had full access to all data in the study and take responsibility for its integrity and the accuracy of its analysis. Dr. Schwartz directed the centralized DXA quality assurance and supervised DXA data analysis. Dr. Pownall conceived the hypotheses and analytical tests and drafted the paper. Dr. Johnson and Schwartz refined the data interpretation and the final manuscript. Dr. Chen provided statistical analysis and aided in the data interpretation. All authors were involved in the study design, data interpretation, and writing the paper and approved the submitted version. Dr. Emily J. Gardner provided editorial assistance.
References Cited
- 1.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 2.Toth MJ, Beckett T, Poehlman ET. Physical activity and the progressive change in body composition with aging: current evidence and research issues. Med Sci Sports Exerc. 1999;31:S590–596. doi: 10.1097/00005768-199911001-00017. [DOI] [PubMed] [Google Scholar]
- 3.Dey DK, Bosaeus I, Lissner L, Steen B. Changes in body composition and its relation to muscle strength in 75-year-old men and women: a 5-year prospective follow-up study of the NORA cohort in Goteborg, Sweden. Nutrition. 2009;25:613–619. doi: 10.1016/j.nut.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 4.Sowers M, Zheng H, Tomey K, Karvonen-Gutierrez C, Jannausch M, Li X, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab. 2007;92:895–901. doi: 10.1210/jc.2006-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudman D. Growth hormone, body composition, and aging. J Am Geriatr Soc. 1985;33:800–807. doi: 10.1111/j.1532-5415.1985.tb04195.x. [DOI] [PubMed] [Google Scholar]
- 6.Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc. 1997;45:92–100. doi: 10.1111/j.1532-5415.1997.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 7.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 8.Look Ahead Research G. Bray G, Gregg E, Haffner S, Pi-Sunyer XF, WagenKnecht LE, et al. Baseline characteristics of the randomised cohort from the Look AHEAD (Action for Health in Diabetes) study. Diab Vasc Dis Res. 2006;3:202–215. doi: 10.3132/dvdr.2006.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Look ARG, Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Look ARG, Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170:1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallagher D, Heshka S, Kelley DE, Thornton J, Boxt L, Pi-Sunyer FX, et al. Changes in Adipose Tissue Depots and Metabolic Markers Following a One-Year Diet and Exercise Intervention in Overweight and Obese Patients With Type 2 Diabetes. Diabetes Care. 2014 doi: 10.2337/dc14-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heshka S, Ruggiero A, Bray GA, Foreyt J, Kahn SE, Lewis CE, et al. Altered body composition in type 2 diabetes mellitus. Int J Obes (Lond) 2008;32:780–787. doi: 10.1038/sj.ijo.0803802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallagher D, Kelley DE, Yim JE, Spence N, Albu J, Boxt L, et al. Adipose tissue distribution is different in type 2 diabetes. Am J Clin Nutr. 2009;89:807–814. doi: 10.3945/ajcn.2008.26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pownall HJ, Bray GA, Wagenknecht LE, Walkup MP, Heshka S, Hubbard VS, et al. Changes in body composition over 8 years in a randomized trial of a lifestyle intervention: The look AHEAD study. Obesity (Silver Spring) 2015;23:565–572. doi: 10.1002/oby.21005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadden TA, Neiberg RH, Wing RR, Clark JM, Delahanty LM, Hill JO, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring) 2011;19:1987–1998. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Look ARG, Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14:737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wesche-Thobaben JA. The development and description of the comparison group in the Look AHEAD trial. Clin Trials. 2011;8:320–329. doi: 10.1177/1740774511405858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoeller DA, Tylavsky FA, Baer DJ, Chumlea WC, Earthman CP, Fuerst T, et al. QDR 4500A dual-energy X-ray absorptiometer underestimates fat mass in comparison with criterion methods in adults. Am J Clin Nutr. 2005;81:1018–1025. doi: 10.1093/ajcn/81.5.1018. [DOI] [PubMed] [Google Scholar]
- 19.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol (1985) 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 20.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 21.Rejeski WJ, Ip EH, Bertoni AG, Bray GA, Evans G, Gregg EW, et al. Lifestyle change and mobility in obese adults with type 2 diabetes. N Engl J Med. 2012;366:1209–1217. doi: 10.1056/NEJMoa1110294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houston DK, Leng X, Bray GA, Hergenroeder AL, Hill JO, Jakicic JM, et al. A long-term intensive lifestyle intervention and physical function: the look AHEAD Movement and Memory Study. Obesity (Silver Spring) 2015;23:77–84. doi: 10.1002/oby.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014;370:2002–2013. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finkelstein EA, Strombotne KL, Zhen C, Epstein LH. Food prices and obesity: a review. Advances in nutrition. 2014;5:818–821. doi: 10.3945/an.114.007088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karl JP, Roberts SB. Energy density, energy intake, and body weight regulation in adults. Advances in nutrition. 2014;5:835–850. doi: 10.3945/an.114.007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon-Larsen P. Food availability/convenience and obesity. Advances in nutrition. 2014;5:809–817. doi: 10.3945/an.114.007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson F, Wardle J. Variety, palatability, and obesity. Advances in nutrition. 2014;5:851–859. doi: 10.3945/an.114.007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livingstone MB, Pourshahidi LK. Portion size and obesity. Advances in nutrition. 2014;5:829–834. doi: 10.3945/an.114.007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soltani S, Hunter GR, Kazemi A, Shab-Bidar S. The effects of weight loss approaches on bone mineral density in adults: a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int. 2016 doi: 10.1007/s00198-016-3617-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


