Abstract
Objective
To review cases and increase awareness in clinicians treating patients who may be taking biotin.
Methods
We describe the presentation and workup of a woman with secondary progressive multiple sclerosis on high dose biotin with laboratory studies suggestive of thyrotoxicosis.
Results
Plasma samples showed laboratory evidence of elevated thyroid hormone levels with elevated free thyroxine >7.8 ng/dl (reference interval (RI) 0.9-1.7 ng/dl) and decreased thyroid stimulating hormone <0.02 uIU/ml (RI 0.50-5.70 uIU/ml). Laboratory values normalized when biotin was withheld prior to repeat testing.
Conclusions
Our case report demonstrates that ingestion of high dose biotin in multiple sclerosis patients can cause interference with laboratory assessment of thyroid function. This interference causes laboratory values suggestive of thyrotoxicosis and can lead to unnecessary evaluation. Clinicians should be aware of the risk of laboratory interference in this patient demographic.
Keywords: High dose biotin, interference, competitive immunoassay, thyrotoxicosis, multiple sclerosis
Biotin is a decarboxylase enzyme cofactor readily bioavailable from nutritional sources and synthesized by gut bacteria with an adequate adult intake of 35 ug/day (1). Biotin is widely available as a vitamin supplement in formulations of 0.6 mg to 10 mg per tablet. It is advertised for the treatment of alopecia but it is unknown if biotin supplementation is effective except in rare metabolic disorders such as multiple carboxylase and biotinidase deficiencies (1-3). Biotin engages in a high affinity noncovalent interaction with the protein streptavidin and has been incorporated as an affinity tag for many in vitro assays (4). Interference of free biotin from patient samples with biotin-based thyroid laboratory assays has been described in infants and young children receiving high dose biotin therapy for suspected inborn metabolic disorders (5,6). Low doses of biotin in vitamin supplements are not thought to produce serum levels high enough to interfere with laboratory assays though some manufacturers such as Roche Diagnostics Inc. mention the potential for interference in anyone taking >5 mg biotin per day (7-9).
High dose biotin (100 mg to 300 mg per day which is 10,000 times the daily adequate intake) is being investigated as a treatment for progressive multiple sclerosis (MS)(10). Biotin is thought to be an important factor in myelin production by the mechanism of acetyl CoA carboxylase activation (10). High dose biotin has been tested in an initial uncontrolled pilot study in which patients showed reduced clinical progression of MS after a mean follow-up of 9 months (10). In secondary progressive MS, where there are currently no approved treatment options to halt disease progression, high dose biotin is seen as a promising therapy with little risk as it is widely available as a supplement with good oral absorption and few known side-effects (10). The following case describes a woman on high dose biotin for the treatment of MS referred to endocrinology because of laboratories suggestive of thyrotoxicosis.
Case description
A 74-year-old woman with a history of MS and chronic autoimmune thyroiditis on stable levothyroxine replacement therapy for 20 years had routine screening results suggestive of severe thyrotoxicosis; thyroid stimulating hormone (TSH) 0.02 uIU/ml (Reference Interval (RI) 0.5-5.70 uIU/ml) and free thyroxine (free T4) >7.8 ng/dl (RI 0.9-1.7 ng/dl) by immunoassay (Roche Diagnostics Inc, Indianapolis, IN) (as all laboratories herein except where noted). The patient suspended her standard thyroxine dose for 8 weeks but her free T4 did not decrease (Table 1). She was referred to an endocrinologist. On further evaluation, vitals were a blood pressure of 158/77 mm Hg, pulse 78 beats/min, and temperature of 36.7 °C (98.0 °F). Clinical evaluation by the endocrinologist did not reveal signs or symptoms of thyrotoxicosis. Additional thyroid function testing revealed a markedly elevated total T4 15.3 ug/dl (RI 4.6-10.7 ug/dl), and total T3 >651 (RI 80-200 ng/dl) that were also suggestive of a thyrotoxicosis. The patient was prescribed atenolol 25 mg daily pending further evaluation. She underwent a radioactive I123 thyroid uptake and scan that demonstrated homogenous uptake of 7.2% and 16.9% at 6 and 24 hrs respectively. She had positive anti-thyroglobulin and thyroperoxidase antibodies confirming autoimmune thyroid disease. Thyroid stimulating immunoglobulin was negative. The clinician also ordered a free T4 by dialysis-mass spectrometry which was normal at 1.7 ng/dl (RI 0.8-2.0 ng/dl) and more consistent with the patient’s clinical presentation. The discrepancy in the T4 values by the two methods spurred laboratory workup for interference with the immunoassays. After ruling out heterophile antibodies (antibodies that bind capture and signal antibodies in the absence of analyte) as a source of interference (11), the patient stopped taking her biotin 100 mg TID for 3 days and all values normalized; TSH 4.54 uIU/mL (RI 0.5-5.70 uIU/ml), free T4 1.5 ng/dl (RI 0.9-1.7 ng/dl), total T4 9.0 ug/dl (RI 4.6-10.7 ug/dl), and total T3 111 ng/dl (RI 80-200 ng/dl). In retrospect, it was determined that the initiation of biotin therapy for her MS coincided with the emergence of her abnormal thyroid function tests. This was confirmed by normalization of thyroid function tests after holding biotin prior to repeat laboratory assessment.
Table 1.
Patient Laboratory Values.
| 9 months prior | At Presentation | Off levothyroxine, 2 weeks | Off levothyroxine, 4 weeks | Off levothyroxine, 7-8 weeks | Off biotin, 3 days Off levothyroxine, 14 weeks | |
|---|---|---|---|---|---|---|
| TSH | 3.58 | 0.02 ↓ | 0.02 ↓ | 4.54 | ||
| Free T4 | >7.8 ↑ | >7.8 ↑ | >7.8 ↑ | >7.8 ↑ | 1.5 | |
| Total T4 | 15.3 ↑ | 9.0 | ||||
| Total T3 | >651 ↑ | 111.0 | ||||
| Thyroperoxidase Ab | 264.3 ↑ | |||||
| Thyroglublin Ab | 713.2 ↑ | |||||
| Free T4 by dialysis | 1.7 |
Reference intervals: TSH 0.5-5.70 uIU/ml, free T4 0.9-1.7 ng/dl, total T4 4.6-10.7 ug/dl, total T3 80-200 ng/dl, PTH 15-65 pg/ml Thyroperoxidase Ab 0-33.9 lU/ml, Thyrogloublin Ab <115 lU/ml, Free T4 by dialysis 0.8-2.0 ng/dl
Discussion
Although prior instances of biotin interference have been described in infants and young children with suspected inborn metabolic disorders, (5,6,12) it is important to alert clinicians to a new patient context. We describe one case of abnormal thyroid function tests mimicking severe thyrotoxicosis and have observed similar results in two other women taking high dose biotin for progressive MS.
Biotin is a common protein co-factor for biochemical assays because of its small size and highly avid interaction with streptavidin (4). High dose biotin is a known interfering agent with Roche and Ortho (Ortho Clinical Diagnostics, Raritan, NJ) immunoassays. Both Roche and Ortho assays employ a biotinylated monoclonal anti-TSH antibody however these two methods may have different sensitivities to biotin interference, owing to different signal detection methods and other assay design considerations. Users of Roche immunoassays are cautioned not to draw samples from patients taking more than 5 mg of biotin per day for at least 8 hours following the last biotin dose (7-9). Studies of biotin metabolism in humans and animals indicate it is rapidly cleared from plasma by urinary excretion with some fraction accounted for by renal metabolites (13,14). Healthy human subjects given biotin 100 to 300 mg capsules demonstrated elimination half-lives varying between 7.8 and 18.8 hours (15). One study shows a single oral dose of 30 mg may persistently interfere with T4 and TSH assays for slightly longer than 24 hours on 3 different assay platforms (12).
The Roche sandwich immunoassay employs a biotinylated antibody to the analyte therefore excess free biotin displaces antigen-antibody complexes which leads to less ruthenium-conjugated antibody binding to solid phase (Figure 1) (7,8). In the Roche competitive immunoassay, the reagent is a complex of biotinylated analyte and microparticles that displaces the sample analyte being measured from an antibody-conjugate with ruthenium (9). Therefore, excess free biotin simulates increased sample analyte by displacing biotinylated antigen from the microparticle, such that less ruthenium is then incorporated into the solid phase (9). Since TSH is a sandwich immunoassay while the free T4, total T4, and total T3 are competitive immunoassays, the expected derangement with biotin interference is false depression of TSH along with a false elevation of free T4, total T4, and total T3. Additionally, thyroperoxidase and thyroglobulin antibody assays are also competitive immunoassays subject to false elevation. The directionality of changes in these patient’s laboratories was consistent with high-dose biotin interference (Table 1). This mixed directionality of analyte measurement changes with high-biotin interference can further obscure the picture of laboratory interference as it mimics true hyperthyroidism. Thus, assessment of free T4 by equilibrium dialysis may be helpful in these cases as it does not show this interference. The normal free T4 value by dialysis was instrumental in alerting the clinician to the laboratory assay interference issue.
Figure 1.
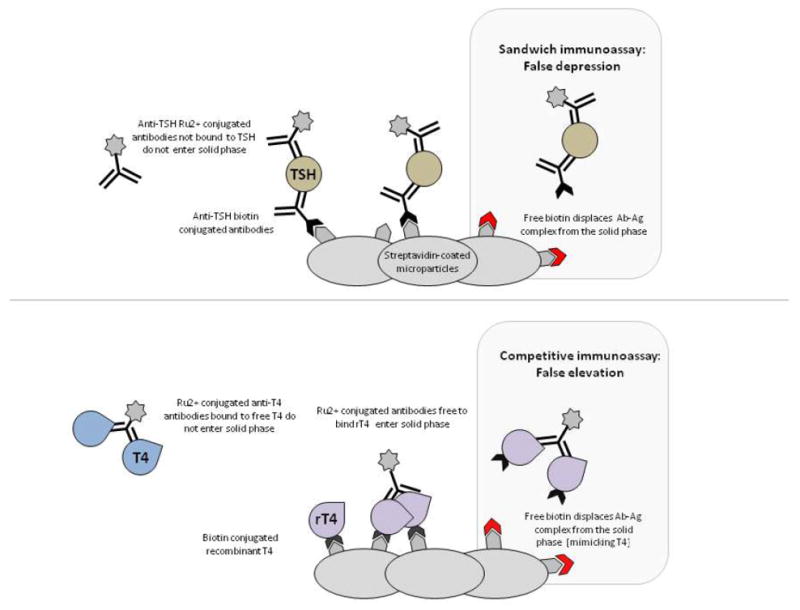
Mechanism of biotin interference in Roche immunoassays. The Roche sandwich immunoassay employs a biotinylated antibody to the analyte (TSH in this example) therefore excess free biotin displaces antigen-antibody complexes (Ab-Ag) which leads to less ruthenium (Ru2+)-conjugated antibody binding to solid phase and false depression of analyte measurement. The Roche competitive immunoassay utilizes a complex of biotinylated analyte (T4 in this example) and microparticles with recombinant T4 (rT4) that displace the sample analyte being measured from an antibody-conjugate with Ru2+. Excess free biotin also displaces Ab-Ag complex from the solid phase producing false elevations in sample analyte measurements.
This case illustrates that prompt recognition of biotin interference requires a high index of suspicion because the interference mimics the laboratory changes in thyrotoxicosis. This patient required additional evaluation, including repeated blood testing and endocrine specialty referrals. This additional evaluation added cost and inconvenience to both the patient and overall medical system. Despite laboratory assay manufacturer warnings, most clinicians may not be aware of their laboratory’s particular assay or this specific potential interference by biotin. Since not all laboratory assays use biotin containing components, it is important to keep open communication between laboratory and clinical providers to ensure that clinicians are aware of this potential inference. Another impediment to recognizing the interference is that biotin may not be listed in the medical record as a medication. Specific ascertainment of dosage and preparation is imperative to correctly identify the source of interference. In the three cases of high dose biotin interference that we ascertained, the formulations of high-dose biotin were prepared by a compounding pharmacy and were not detailed in the medical record. We anticipate more cases of biotin interference. In the month these cases were being reviewed, a local compounding pharmacy dispensed 370 one-month prescriptions of high dose biotin (personal communication). In order to raise awareness for this interference, we sent memos to clinicians and are taking steps to incorporate high-dose biotin interference as a banner warning in the electronic medical record. Close collaboration of the clinical laboratory and treating clinicians will be required to avoid unnecessary testing and potential morbidity from inaccurate laboratory measurements.
Conclusion
In the case we describe, the laboratory measurements suggestive of thyrotoxicosis were attributed to interference of the patient’s high dose biotin treatment with the biotin-streptavidin chemistry of the immunoassays. We observed normalization of TSH and free T4 measurements when patients withheld biotin for 2 to 5 days. As our case illustrates, early consideration of biotin interference minimizes unnecessary repeat laboratory and imaging studies. Since trials in MS are ongoing, we expect to see more patients on high dose biotin treatment with spurious laboratory measurements. Therefore we advise careful history-taking and close communication with the laboratory when the clinical picture does not match the laboratory results.
Abbreviations
- MS
Multiple sclerosis
- TSH
Thyroid stimulating hormone
- Free T4
Free thyroxine
- PTH
Parathyroid hormone
References
- 1.McMahon RJ. Biotin in Metabolism and Molecular Biology. Annu Rev Nutr. 2002;22:221–239. doi: 10.1146/annurev.nutr.22.121101.112819. [DOI] [PubMed] [Google Scholar]
- 2.Nyhan WL. Inborn errors of biotin metabolism. Arch Dermatol. 1987;123:1696–1698a. [PubMed] [Google Scholar]
- 3.Blumeyer A, Tosti A, Messenger A, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. J Dtsch Dermatol Ges. 2011:S1–57. doi: 10.1111/j.1610-0379.2011.07802.x. [DOI] [PubMed] [Google Scholar]
- 4.Diamandis EP, Christopoulos TK. The biotin-(strept)avidin system: principles and applications in biotechnology. Clin Chem. 1991;37:625–636. [PubMed] [Google Scholar]
- 5.Kwok JS, Chan IH, Chan MH. Biotin interference on TSH and free thyroid hormone measurement. Pathology (Phila) 2012;44:278–280. doi: 10.1097/PAT.0b013e3283514002. [DOI] [PubMed] [Google Scholar]
- 6.Henry JG, Sobki S, Arafat N. Interference by biotin therapy on measurement of TSH and FT4 by enzyme immunoassay on Boehringer Mannheim ES700 analyser. Ann Clin Biochem. 1996;33:162– 163. doi: 10.1177/000456329603300214. [DOI] [PubMed] [Google Scholar]
- 7.Package insert. Elecsys TSH Immunoassay. Mannheim, Germany: Roche Cobas; 2014. [Google Scholar]
- 8.Package insert. Elecsys PTH Immunoassay. Mannheim, Germany: Roche Cobas; 2014. [Google Scholar]
- 9.Package insert. Elecsys Free T4 Immunoassay. Mannheim, Germany: Roche Cobas; 2014. [Google Scholar]
- 10.Sedel F, Papeix C, Bellanger A, et al. High doses of biotin in chronic progressive multiple sclerosis: a pilot study. Mult Scler Relat Disord. 2015;4:159–169. doi: 10.1016/j.msard.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Després N, Grant AM. Antibody interference in thyroid assays: a potential for clinical misinformation. Clin Chem. 1998;44:440–454. [PubMed] [Google Scholar]
- 12.Wijeratne NG, Doery JC, Lu ZX. Positive and negative interference in immunoassays following biotin ingestion: a pharmacokinetic study. Pathology (Phila) 2012;44:674–675. doi: 10.1097/PAT.0b013e32835a3c17. [DOI] [PubMed] [Google Scholar]
- 13.Bitsch R, Salz I, Hötzel D. Studies on bioavailability of oral biotin doses for humans. Int J Vitam Nutr Res. 1989;59:65–71. [PubMed] [Google Scholar]
- 14.Wang KS, Kearns GL, Mock DM. The Clearance and Metabolism of Biotin Administered Intravenously to Pigs in Tracer and Physiologic Amounts Is Much More Rapid than Previously Appreciated. J Nutr. 2001;131:1271–1278. doi: 10.1093/jn/131.4.1271. [DOI] [PubMed] [Google Scholar]
- 15.Peyro Saint Paul L, Debruyne D, Bernard D, et al. Pharmacokinetics and pharmacodynamics of MD1003 (high-dose biotin) in the treatment of progressive multiple sclerosis. Expert Opin Drug Metab Toxicol. 2015;12:327–344. doi: 10.1517/17425255.2016.1136288. [DOI] [PubMed] [Google Scholar]
- 16.Loh TP, Kao SL, Halsall DJ, et al. Macro-thyrotropin: a case report and review of literature. J Clin Endocrinol Metab. 2012;97:1823–1828. doi: 10.1210/jc.2011-3490. [DOI] [PubMed] [Google Scholar]


