Abstract
Background
It has been suggested that smokeless tobacco users have high levels of exposure to nicotine and some toxic substances as measured by biomarker concentrations, but studies with nationally representative data have been limited.
Methods
We analyzed biomarkers of tobacco exposure for 23,684 adult participants from the National Health and Nutrition and Examination Survey (NHANES) from 1999-2012. The biomarkers analyzed were serum cotinine, urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), blood lead, blood cadmium, blood mercury, urinary arsenic, and urinary N-acetyl-S-(2-cyanoethyl)-L-cysteine (CYMA). We calculated geometric mean concentrations for each biomarker by tobacco use category (exclusive smokeless tobacco use, exclusive cigarette smoking, dual cigarette and smokeless tobacco use, and non-cigarette and smokeless tobacco use) and geometric mean ratios adjusting for demographic factors.
Results
Exclusive smokeless tobacco users had higher geometric mean concentrations of cotinine (178.9 ng/mL, 95% CI = 145.5, 220.0) and NNAL (583.0 pg/mg creatinine, 95% CI = 445.2, 763.5) than exclusive cigarette smokers, (130.6 ng/mL, 95% CI = 122.3, 139.6 and 217.6 pg/mg creatinine, 95% CI = 193.0, 245.2, respectively). Smokeless tobacco users also had higher concentrations of blood lead compared with non-tobacco users (adjusted geometric mean ratio = 1.30, 95% CI = 1.21, 1.38). Differences in concentrations of cadmium, mercury, and CYMA between smokeless tobacco users and non-tobacco users were not observed. Based on limited sample sizes, NNAL concentrations for smokeless tobacco users appear to have declined from 2007-2008 (geometric mean = 1013.7 pg/mg creatinine, 95% CI = 738.9, 1390.8) to 2011-2012 (geometric mean = 325.7 pg/mg creatinine, 95% CI = 159.6, 664.9).
Conclusions
Smokeless tobacco users have higher observed levels of exposure to nicotine and carcinogenic tobacco-specific nitrosamines, as measured by cotinine and NNAL biomarker concentrations, than cigarette smokers.
Impact
High levels of exposure to known harmful constituents for smokeless tobacco users is a cause of concern for individual and public health.
Keywords: Biomarkers, smokeless tobacco, cotinine, NNAL
Introduction
Use of smokeless tobacco products is attracting increasing attention from the public health community. According to the US National Adult Tobacco Survey (NATS), 7.1% of US adult males were current users of chewing tobacco, snuff, dip, snus, or dissolvable tobacco products in 2012-2013, making smokeless tobacco the most commonly used tobacco product among US adults after cigarettes and cigars (1). Smokeless tobacco use is particularly common among young people. Among US high school students, 9.6% of males were current users of chewing tobacco, snuff, or dip and 2.7% were current users of snus in 2013 (2) according to the National Youth Tobacco Survey (NYTS) (3), again making smokeless tobacco the third most commonly used tobacco product among this group after cigarettes and cigars. Smokeless tobacco use prevalence among US youth has also remained relatively consistent over time since 2000 according to NYTS data (4), even as cigarette smoking prevalence continued to decline among US youth during this period (5).
Biomarkers of tobacco exposure have previously been analyzed for cigarette smokers (6-8) and, to some extent, for cigar smokers (9), but less is known about biomarker levels among smokeless tobacco users. It is known that tobacco-specific nitrosamine (TSNA) levels in smokeless tobacco products themselves can vary due to a variety of factors including tobacco type, growing conditions, curing and fermentation processes, and storage conditions (10, 11) and that TSNA levels in smokeless tobacco products can vary widely (12-14). It has also been suggested that levels of some biomarkers can be as high or higher among smokeless tobacco users as among cigarette smokers. For example, Hecht et al. (15) analyzed the urinary cotinine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) concentrations of 182 smokeless tobacco users and 420 smokers from six studies in the Twin Cities, Minnesota and Washington, DC metropolitan areas. The urine samples were collected at baseline as part of studies that were designed to reduce participants’ use of these tobacco products. Hecht et al. found that smokeless tobacco users had significantly higher concentrations of cotinine and NNAL compared with cigarette smokers. Hecht et al. (16) subsequently found, using data from the three studies of smokeless tobacco users in the Twin Cities area, that cotinine and NNAL concentrations were significantly associated with years of smokeless tobacco use. Naufal et al. (17), on the other hand, analyzed biomarkers of volatile organic compounds, halogenated aromatic hydrocarbons, polycyclic aromatic hydrocarbons, acrylamide, NNAL, and metals from 368 smokeless tobacco users, 5,040 cigarette smokers, and 16,443 nonconsumers of tobacco and nicotine replacement therapy products from US National Health and Nutrition Examination Survey (NHANES) participants from 1999-2008. The authors concluded that biomarker concentrations were generally significantly lower among smokeless tobacco users compared with smokers, with the exception of NNAL and some halogenated aromatic hydrocarbons. They also did not find significant differences between smokeless tobacco users and nonusers with the exception of NNAL and some polyaromatic hydrocarbons. More recently, Agaku and King (18) used 2003-2010 NHANES data to assess the relationship between self-reported smokeless tobacco use and serum cotinine concentrations and identify the optimal cutpoint for identifying smokeless tobacco users based on cotinine concentrations. Agaku, Vardavas, and Connolly (19) conducted a similar analysis for NNAL using 2007-2010 data. These authors did not, however, compare cotinine concentrations for smokeless tobacco users with cigarette smokers or users of other tobacco products.
In this study, we analyzed biomarkers of tobacco exposure in a large nationally representative sample of US tobacco users and nonusers from the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2012. We selected seven biomarkers for analysis based on their particular relevance to tobacco exposure and health outcomes: cotinine, NNAL, cadmium, lead, mercury, arsenic, and CYMA (N-Acetyl-S-(2-cyanoethyl)-L-cysteine). We estimated geometric mean biomarker concentrations for smokeless tobacco users, cigarette smokers, dual cigarette and smokeless tobacco users, and non-tobacco users. We also calculated geometric mean ratios using regression analysis to analyze the association between biomarker concentrations and tobacco use status, with and without adjustment for demographic and socioeconomic factors such as sex, age, race/ethnicity, and educational attainment.
Our study builds upon previous research in presenting estimates from a large and nationally representative study population for smokeless tobacco users for cotinine, which was not included in the previous analysis by Naufal et al., and NNAL, which was only available at the time of this previous study for 2007-2008 NHANES participants, as well as the other selected biomarkers. As such, we present estimates not only of biomarker concentrations by tobacco use status, but also of biomarker concentrations over time, thus allowing us to investigate whether differences in product characteristics or product use patterns have contributed to changes in biomarker exposure for tobacco users in recent years.
Materials and Methods
Study Population and Tobacco Use Status
We analyzed biomarker concentrations by tobacco use for adult NHANES participants from 1999-2012. NHANES is a health and examination survey that uses a complex multistage design to obtain a nationally representative sample of the US civilian non-institutionalized population (20). NHANES has been conducted on a continuous basis by the National Center for Health Statistics (NCHS) since 1999 and surveys approximately 10,000 participants of all ages in each two-year cycle. Survey participants complete health interviews in their homes that include a cigarette smoking history questionnaire for adults aged 20 years and older. Participants then complete an additional questionnaire on recent tobacco use including smokeless tobacco in a Mobile Examination Center (MEC), where they also receive a medical examination that includes the collection of biospecimens such as urine and blood.
We analyzed biomarker concentrations among the 38,024 adults aged 20 years and older who participated in NHANES between 1999 and 2012. We excluded 736 survey participants who reported use of tobacco or nicotine products other than cigarettes or chewing tobacco or snuff (i.e., cigars, snuff, pipes, nicotine replacement therapy products or any product containing nicotine) during the past five days as well as 5318 participants who did not provide information on past five day tobacco use. We then categorized study participants into four mutually exclusive groups based on their reported cigarette and smokeless tobacco use: 1) “non-tobacco users” reported having smoked fewer than 100 cigarettes in their lives and not having used cigarettes, chewing tobacco, or snuff in the past five days, 2) “smokeless tobacco users” reported using chewing tobacco or snuff in the past five days and currently not using cigarettes at all (228 smokeless tobacco users who reported being former cigarette smokers, having smoked at least 100 cigarettes but currently not smoking at all, were excluded from the analysis for cadmium due to its long biologic half-life, which can be upwards of 10 years (21)), 3) “cigarette smokers” reported having smoked at least 100 cigarettes in their lives and currently smoking every day or some days and not having used chewing tobacco of snuff in the past five days, and 4) “dual cigarette and smokeless tobacco users” reported having smoked at least 100 cigarettes in their lives, currently smoking every day or some days, and having used chewing tobacco or snuff in the past five days. We did not include former cigarette smokers who had not used chewing tobacco of snuff in the past five days in the analysis. The analysis included a total of 23,684 participants.
Biomarkers of Exposure
The biomarkers of exposure selected for this analysis were chosen due to their relevance to tobacco exposure and health outcomes. Cotinine is the primary proximate metabolite of nicotine (22, 23). NNAL is a metabolite of the TSNA 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), which has been identified as a known human carcinogen by the International Agency on Research on Cancer (24, 25). NNK itself is formed from the nitrosation of nicotine or the related alkaloid pseudooxynicotine (26). Lead, cadmium, mercury, and arsenic are elements known to have toxic effects that can be found in tobacco products as well as in other environmental sources (27-30). CYMA is a metabolite of acrylonitrile and a selective biomarker of exposure to smoke (31). Urinary arsenic concentrations were available for 2003-2012 NHANES participants, urinary NNAL concentrations were available for 2007-2012 NHANES participants, and urinary CYMA concentrations were available for 2005-2006 and 2011-2012 survey participants. Data for other biomarkers were available from 1999-2012.
The analytical methods used to obtain these data are available in NHANES documentation (20). Serum cotinine was measured by an isotope dilution - liquid chromatography / atmospheric pressure chemical ionization tandem mass spectrometry process. The half-life of cotinine is 15-20 hours, and its availability in blood, urine, and saliva makes it a commonly used biomarker of recent nicotine exposure (23, 32). Urinary total NNAL was measured using liquid chromatography linked to tandem mass spectrometry. The half-life of NNAL has been estimated to be 10–18 days (33). Blood cadmium, lead, and total mercury were measured using inductively coupled plasma mass spectrometry. Urinary total arsenic was measured using liquid chromatography coupled to plasma dynamic reaction cell mass spectrometry. Urinary CYMA was measured using liquid chromotography coupled with electro spray tandem mass spectrometry. For concentrations below the limit of detection (LOD), a value equal to the LOD divided by the square root of two was used in analysis.
Demographic Variables
NHANES participants reported information on sex, age, race/ethnicity, and educational attainment. Race/ethnicity was subsequently categorized as non-Hispanic White, non-Hispanic Black, Mexican-American, other Hispanic, and other race including multi-racial. Educational attainment was categorized as less than high school graduate or equivalent, high school graduate or equivalent, and more than high school graduate or equivalent. Body mass index (BMI) for survey participants was calculated as kg/m2 from their measured height and weight as a continuous variable.
Statistical Analysis
Demographic and tobacco use variables were characterized using means for continuous variables and percentages for categorical variables. Biomarker concentrations were log-transformed for the analysis to minimize the effects of skewness in the data on estimates, and geometric means of observed biomarker concentrations by tobacco use category were calculated. Univariate and multivariate linear regression analysis were also used to analyze the relationship between biomarkers of exposure and tobacco use category, adjusting for sex, age, race/ethnicity, educational attainment, and BMI. Geometric mean ratios and 95% confidence intervals (CIs), from these analyses were calculated by exponentiating the estimated coefficients and their standard errors. Geometric mean biomarker concentrations were also calculated for cotinine and NNAL by two-year NHANES survey cycle and plotted by time. Box plots were also created to show the unweighted distribution of cotinine and NNAL concentrations for smokeless tobacco users who reported having used chewing tobacco or snuff on 1-2, 3-4, or 5 of the past days; the small number of NHANES participants who reported using both products in the past five days (n=4) were excluded from this particular analysis. All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC), and all figures were constructed using R version 3.0.2 (R Core Team, Vienna, Austria). Analyses were conducted using the MEC sample weights with the exception of analyses for arsenic, which were conducted with environmental subsample weights, and analyses for CYMA, which were conducted with the 2005-2006 volatile organic compounds subsample weights and 2011-2102 smoking subsample weights. Analyses were conducted taking into account the complex survey design information on survey strata and primary sampling units provided by NCHS.
Results
Characteristics of the Study Population by Tobacco Use Status
Table 1 presents demographic and tobacco use information for the NHANES study participants according to tobacco use status. Of the 23,684 individuals, 488 were exclusive smokeless tobacco users, 6791 were exclusive cigarette smokers, 92 were dual cigarette and smokeless tobacco users, and 16,313 were non-tobacco users. Of the smokeless tobacco users, 309 individuals reported using chewing tobacco, 175 reported using snuff , and 4 reported using both chewing tobacco and snuff in the past five days (data not shown). Smokeless tobacco and dual users were overwhelmingly male, at 94.7% (95% CI = 92.1%, 97.2%) and 99.4% (98.6%-100.0%) respectively. Dual users tended to be younger than members of other tobacco use groups with a mean age of 33.1 years (95% CI = 30.3, 35.8). Smokeless tobacco and dual users were also more likely to be non-Hispanic whites than members of other tobacco use groups at 88.7% (95% CI = 85.3%-92.1%) and 94.2% (90.1%-98.2%) respectively. The estimated mean number of cigarettes that dual users smoked on days that they smoked cigarettes in the past five days was less than the estimated mean for cigarette smokers, at 11.9 compared with 14.8 cigarettes, but the difference was not statistically significant (p = 0.071). There was a slight difference in the average number of days that cigarette smokers and dual users had smoked cigarettes in the past five days at 4.6 and 4.2 days respectively (p = 0.042). Smokeless tobacco users tended to have consistently used smokeless tobacco in the past five days, with an average of 4.2 days (95% CI = 4.1-4.4) having used chewing tobacco for chewing tobacco users and an average of 4.3 days (95% CI = 4.1-4.5) having used snuff for snuff users.
Table 1.
Characteristics of National Health and Nutrition Examination Survey (NHANES) Participants by Tobacco Use Status: United States 1999-2012
| Characteristics | N | Non-Tobacco Users | Exclusive Smokeless tobacco users | Exclusive Cigarette Smokers | Dual Cigarette and Smokeless Tobacco Users |
|---|---|---|---|---|---|
| (N=16313) | (N=488) | (N=6791) | (N=92) | ||
| Age | 23684 | 45.9 (45.3, 46.5)* | 44.2 (42.7, 45.7) | 42.0 (41.5, 42.4) | 33.1 (30.3, 35.8) |
| Sex | |||||
| Male | 10571 | 40.1% (39.2%-41.0%) | 94.7% (92.1%-97.2%) | 53.9% (52.5%-55.4%) | 99.4% (98.6%-100.0%) |
| Female | 13113 | 59.9% (59.0%-60.8%) | 5.3% (2.8%-7.9%) | 46.1% (44.6%-47.5%) | 0.6% (0.0%-1.4%) |
| Race/Ethnicity | |||||
| Mexican -American | 4583 | 9.0% (7.6%-10.3%) | 2.5% (1.3%-3.7%) | 6.8% (5.6%-8.0%) | 2.1% (0.0%-4.2%) |
| Other Hispanic | 1765 | 5.9% (4.7%-7.2%) | 0.7% (0.0%-1.5%) | 4.9% (3.4%-6.4%) | 1.4% (0.0%-4.3%) |
| Non-Hispanic White | 10780 | 66.8% (64.2%-69.4%) | 88.7% (85.3%-92.1%) | 70.8%(67.90%-73.7%) | 94.2% (90.1%-98.2%) |
| Non-Hispanic Black | 5095 | 11.7% (10.3%-13.2%) | 5.7% (3.5%-7.8%) | 12.3% (10.7%-13.9%) | 1.1% (0.0%-2.4%) |
| Other | 1461 | 6.6% (5.7%-7.4%) | 2.5% (0.9%-4.1%) | 5.1% (4.2%-6.0%) | 1.2% (0.0%-3.3%) |
| Education | |||||
| < High School Graduate | 6936 | 15.4% (14.4%-16.4%) | 20.3% (15.9%-24.6%) | 27.7% (26.1%-29.3%) | 15.1% (7.6%-22.6%) |
| High School Graduate | 5587 | 21.1% (20.1%-22.2%) | 34.2% (28.8%-39.7%) | 31.5% (30.0%-32.9%) | 42.7% (30.0%-55.4%) |
| > High School Graduate | 11134 | 63.5% (62.0%-65.0%) | 45.5% (40.0%-51.0%) | 40.9% (39.0%-42.8%) | 42.3% (30.6%-54.0%) |
| Body Mass Index (BMI), kg/m2 | 23311 | 28.7 (28.5, 28.8) | 30.0 (29.3, 30.8) | 27.5 (27.3, 27.6) | 26.7 (25.5, 27.9) |
| Past Five Day Cigarette/Smokeless Tobacco Use | |||||
| # of Days Smoked Cigarettes | 4.6 (4.6, 4.6) | 4.2 (3.9, 5.6) | |||
| # of Cigarettes Smoked per Day on Days Smoking Cigarettes | 14.8 (14.4, 15.3) | 11.9 (8.6, 15.2) | |||
| # of Days Used Chewing Tobacco | 4.2 (4.1-4.4) | 3.7 (3.3-4.2) | |||
| # of Days Used Snuff | 4.3 (4.1-4.5) | 3.5 (3.0, 4.0) |
95% confidence intervals for means and percentages are shown in parentheses.
Note: Non-tobacco users reported not having smoked at least 100 cigarettes in their lives and not having used cigarettes, chewing tobacco, or snuff in the past five days.
Smokeless tobacco users reported having used chewing tobacco or snuff in the past five days and currently not smoking cigarettes at all.
Cigarette smokers reported having smoked at least 100 cigarettes in their lives and currently smoking every day or some days and not having used chewing tobacco of snuff in the past five days.
Dual cigarette and smokeless tobacco users reported having smoked at least 100 cigarettes in their lives, currently smoking every day or some days, and having used chewing tobacco or snuff in the past five days
Survey participants were excluded from each group if they reported having used pipes, cigars, or nicotine gum, patches, or other nicotine products in the past five days
Analysis of Biomarkers of Exposure by Tobacco Use Status
Table 2 presents geometric mean biomarker concentrations by tobacco use status. Mean serum cotinine concentrations were higher for smokeless tobacco users (178.9 ng/mL, 95% CI = 145.5, 220.0) than for cigarette smokers (130.6 ng/mL, 95% CI = 122.3, 139.6). Cotinine concentrations for dual users (184.1 ng/mL, 95% CI = 132.4, 256.0) were similar to concentrations for smokeless tobacco users. Mean urinary NNAL concentrations were higher for smokeless tobacco users (583.0 pg/mg creatinine, 95% CI = 445.2, 763.5) and dual users (430.3 pg/ mg creatinine, 95% CI = 284.8, 650.1) than for cigarette smokers (217.6 pg/mg creatinine, 95% CI = 193.0, 245.2). NNAL concentrations were also consistently higher for smokeless tobacco users compared with cigarette smokers when analyzed in terms of concentration categories. For example, 37.5% (95% CI = 28.6, 46.4%) of smokeless tobacco users had NNAL concentrations below 500 pg/mg creatinine and 62.2% (95% CI = 54.6%, 69.7%) had concentrations below 1000 pg/mg creatinine. In contrast, 74.2% (95% CI = 70.4%, 78.1%) of smokers had NNAL concentrations below 500 pg/mg creatinine and 94.1% (95% CI = 92.4%, 95.8%) had concentrations below 1000 pg/mg creatinine. Mean NNAL concentrations were generally comparable for chewing tobacco (402.3 pg/mg creatinine, 95% CI = 294.3, 549.9) and snuff (463.7 pg/mg creatinine, 95% CI = 315.6, 681.2) users. Exclusion of the relatively small proportion of current cigarette smokers who reported not having smoked cigarettes in the past five days (n=301 of 6791) in sensitivity analysis produced similar results. For example, the geometric mean concentration of cotinine for the remaining smokers was 156.7 ng/mL (95% CI = 150.3, 163.4) and the mean concentration for NNAL was 247.3 pg/mg creatinine (95% CI = 225.4, 271.3).
Table 2.
Geometric Mean Biomarker Concentrations by Tobacco Use Status, NHANES 1999-2012
| Biomarkers of Exposure | Non-Tobacco Users | Exclusive Smokeless Tobacco Users | Exclusive Cigarette Smokers | Dual Cigarette and Smokeless Tobacco Users |
|---|---|---|---|---|
| Serum cotinine, ng/mL | ||||
| Number of observations | 15424 | 476 | 6439 | 90 |
| Mean (95% CI) | 0.043 (0.041, 0.046) | 179.6 (145.8, 221.1) | 130.6 (122.3, 139.6) | 184.1 (132.4, 256.0) |
| Urinary NNAL, pg/mg creatinine | ||||
| Number of observations | 7243 | 210 | 2952 | 43 |
| Mean (95% CI) | 0.98 (0.92, 1.04) | 583.0 (445.2, 763.5) | 217.6 (193.0, 245.2) | 430.3 (284.8, 650.1) |
| Blood cadmium, μg/L | ||||
| Number of observations | 15687 | 254 | 6509 | 90 |
| Mean (95% CI) | 0.268 (0.262, 0.273) | 0.220 (0.201, 0.240) | 0.941 (0.916, 0.968) | 0.644 (0.515, 0.806) |
| Blood lead, μg/L | ||||
| Number of observations | 15687 | 477 | 6509 | 90 |
| Mean (95% CI) | 1.18 (1.16, 1.21) | 1.76 (1.62, 1.91) | 1.76 (1.71, 1.81) | 1.76 (1.55, 2.00) |
| Blood mercury, μg/L | ||||
| Number of observations | 12997 | 357 | 5258 | 64 |
| Mean (95% CI) | 1.02 (0.97, 1.06) | 0.82 (0.73, 0.93) | 0.77 (0.73, 0.81) | 0.63 (0.49, 0.80) |
| Urinary arsenic, ng/mg creatinine | ||||
| Number of observations | 3905 | 119 | 1538 | 27 |
| Mean (95% CI) | 9.53 (8.98, 10.11) | 6.43 (5.36, 7.71) | 7.65 (7.05, 8.30) | 6.73 (4.84, 9.37) |
| Urinary CYMA, ng/mg creatinine | ||||
| Number of observations | 1883 | 41 | 1202 | 14 |
| Mean (95% CI) | 1.47 (1.37, 1.58) | 2.21 (1.11, 4.39) | 117.3 (103.1, 133.4) | 35.4 (2.1, 606.8) |
Note: Urinary NNAL, arsenic, and CYMA concentrations were adjusted for creatinine. NNAL data were available for 2007-2012 NHANES participants, arsenic data were available for 2003-2012 NHANES participants, and CYMA data were available for 2005-2006 and 2011-2012 NHANES participants. Former cigarette smokers were excluded from smokeless tobacco users for the analysis for cadmium.
Mean concentrations of blood lead were higher among smokeless tobacco users (1.76 μg/L, 95% CI = 1.62, 1.91), dual users (1.76 μg/L, 95% CI = 1.55, 2.00), and cigarette smokers (1.76 μg/L, 95% CI = 1.71, 1.81) compared with non-tobacco users (1.18 μg/L, 95% CI = 1.16, 1.21). Mean concentrations of blood cadmium, blood mercury, and urinary arsenic were not elevated among smokeless tobacco users compared with non-tobacco users.
Mean CYMA concentrations were higher among cigarette smokers (117.3 ng/mg creatinine, 95% CI = 103.1, 133.4) and dual users (35.4 ng/mg creatinine, 95% CI = 2.1, 606.8) but not among smokeless tobacco users (2.21 ng/mg creatinine, 95% CI = 1.11, 4.39) compared with non-tobacco users (1.47 ng/mg creatinine, 95% CI = 1.37, 1.58).
Associations between Biomarkers of Exposure and Tobacco Use Status
Table 3 presents results from multivariate regression analyses conducted to analyze whether tobacco use status was associated with higher biomarker concentrations, adjusting for demographic and socioeconomic factors.
Table 3.
Geometric Mean Ratios for Biomarkers of Exposure by Tobacco Use Status, NHANES 1999-2012
| Biomarker | Tobacco Use Category | Unadjusted Geometric Mean Ratio (95% Cl) | Adjusted Geometric Mean Ratio (95% Cl) |
|---|---|---|---|
| Serum cotinine | Exclusive smokeless tobacco users | 4160 (3406, 5081) | 3194 (2623, 3888) |
| Exclusive cigarette smokers | 3027 (2801, 3270) | 2439 (2240, 2655) | |
| Dual cigarette/smokeless tobacco users | 4265 (3064, 5936) | 3009 (2174, 4164) | |
| Non-tobacco users (Ref) | 1 | 1 | |
| Urinary NNAL | Excluisve smokeless tobacco users | 760 (574, 1006) | 587 (451, 764) |
| Exclusive cigarette smokers | 229 (205, 255) | 190 (171, 210) | |
| Dual cigarette/smokeless tobacco users | 541 (313, 935) | 393 (252, 614) | |
| Non-tobacco users (Ref) | 1 | 1 | |
| Blood cadmium | Exclusive smokeless tobacco users | 0.82 (0.75, 0.90) | 1.00 (0.93, 1.08) |
| Exclusive cigarette smokers | 3.52 (3.41, 3.63) | 3.69 (3.57, 3.81) | |
| Dual cigarette/smokeless tobacco users | 2.41 (1.93, 3.00) | 3.10 (2.50, 3.85) | |
| Non-tobacco users (Ref) | 1 | 1 | |
| Blood lead | Exclusive smokeless tobacco users | 1.49 (1.37, 1.61) | 1.30 (1.21, 1.38) |
| Exclusive cigarette smokers | 1.48 (1.44, 1.53) | 1.46 (1.42, 1.49) | |
| Dual cigarette/smokeless tobacco users | 1.49 (1.31, 1.70) | 1.50 (1.34, 1.67) | |
| Non-tobacco users (Ref) | 1 | 1 | |
| Blood mercury | Exclusive smokeless tobacco users | 0.81 (0.71, 0.92) | 0.86 (0.75, 0.98) |
| Exclusive cigarette smokers | 0.76 (0.72, 0.80) | 0.83 (0.79, 0.87) | |
| Dual cigarette/smokeless tobacco users | 0.62 (0.48, 0.79) | 0.70 (0.55, 0.89) | |
| Non-tobacco users (Ref) | 1 | 1 | |
| Urinary arsenic | Exclusive smokeless tobacco users | 0.91 (0.75, 1.09) | 0.86 (0.73, 1.02) |
| Exclusive cigarette only smokers | 0.83 (0.76, 0.92) | 0.87 (0.80, 0.94) | |
| Dual cigarette/smokeless tobacco users | 0.83 (0.45, 1.55) | 0.81 (0.59, 1.10) | |
| Non-tobacco users (Ref) | 1 | 1 | |
| Urinary CYMA | Exclusive smokeless tobacco users | 2.04 (1.04, 4.01) | 1.62 (0.83, 3.18) |
| Exclusive cigarette only smokers | 84.9 (72.5, 99.3) | 75.3 (65.2, 87.1) | |
| Dual cigarette/cigarette smokers | 33.5 (0.8, 1398.5) | 18.4 (0.7, 463.5) | |
| Non-tobacco users (Ref) | 1 | 1 |
Note: NNAL data were available for 2007-2012 NHANES participants, arsenic data were available for 2003-2012 NHANES participants, and CYMA data were available for 2005-2006 and 2011-2012 NHANES participants. The adjusted geometric mean ratios control for age, sex, race/ethnicity, educational attainment, and body mass index For urinary arsenic, CYMA, and NNAL, the adjusted ratios further control for urinary creatinine.
After adjustment for age, sex, race/ethnicity, education, and BMI, smokeless tobacco users, cigarette smokers, and dual users had increased geometric mean ratios for serum cotinine compared with non-tobacco users. Smokeless tobacco users also had increased geometric mean ratios compared with cigarette smokers (p = 0.04). Smokeless tobacco users, cigarette smokers, and dual users also had increased geometric mean ratios for urinary NNAL compared with non-tobacco users, and smokeless tobacco users and dual users had increased geometric mean ratios compared with cigarette smokers.
Smokeless tobacco users, along with cigarette smokers and dual users, had increased geometric mean ratios for blood lead compared with non-tobacco users. Smokeless tobacco users did not have increased geometric mean ratios for any of the other biomarkers.
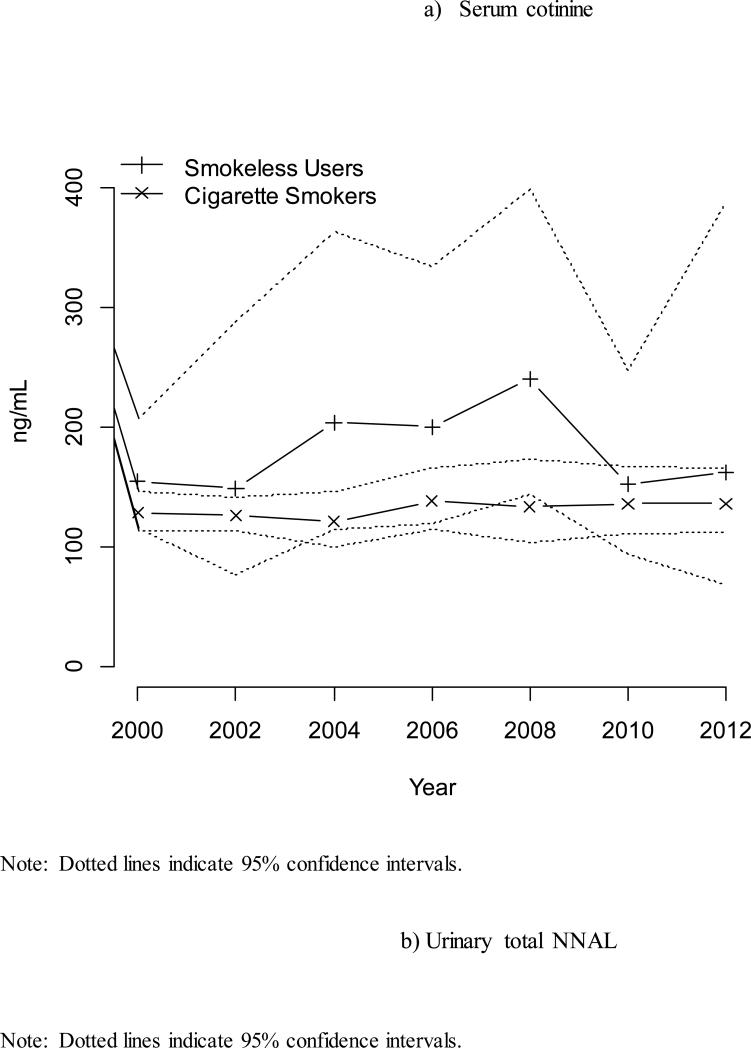
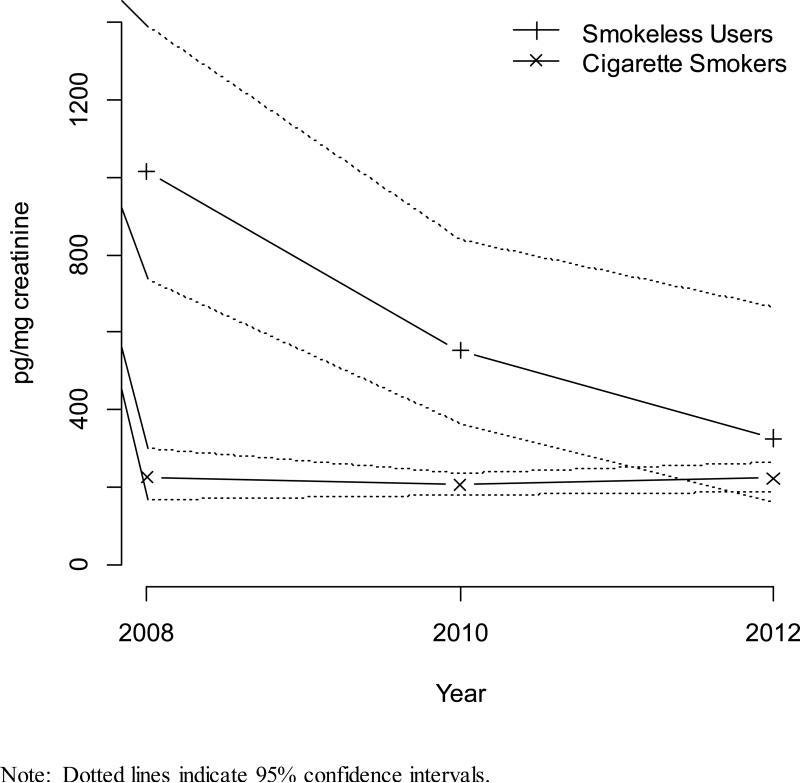
Trends in Tobacco-Specific Biomarkers Over Time
Figure 1 presents geometric mean serum cotinine and urinary NNAL concentrations for cigarette smokers and smokeless tobacco users over time. Cotinine concentrations for smokers and smokeless users were relatively consistent over time, although estimates for the smaller number of smokeless tobacco users showed more variability. Tests of trend for cotinine concentrations produced a p-value of 0.895 for smokers and 0.403 for smokeless tobacco users. Mean NNAL concentrations for smokers were relatively consistent from 2007-2008 to 2011-2012 but declined dramatically for smokeless tobacco users from a geometric mean of 1013.7 pg/mg creatinine (95% CI = 738.9, 1390.8, n = 81) in 2007-2008 to 328.6 pg/mg creatinine (95% CI = 164.7, 655.6, n = 53) in 2011-2012. Tests of trend for NNAL concentrations produced a p-value of 0.943 for smokers and 0.003 for smokeless tobacco users.
Figure 1.
Geometric mean biomarker concentrations by tobacco use status by year
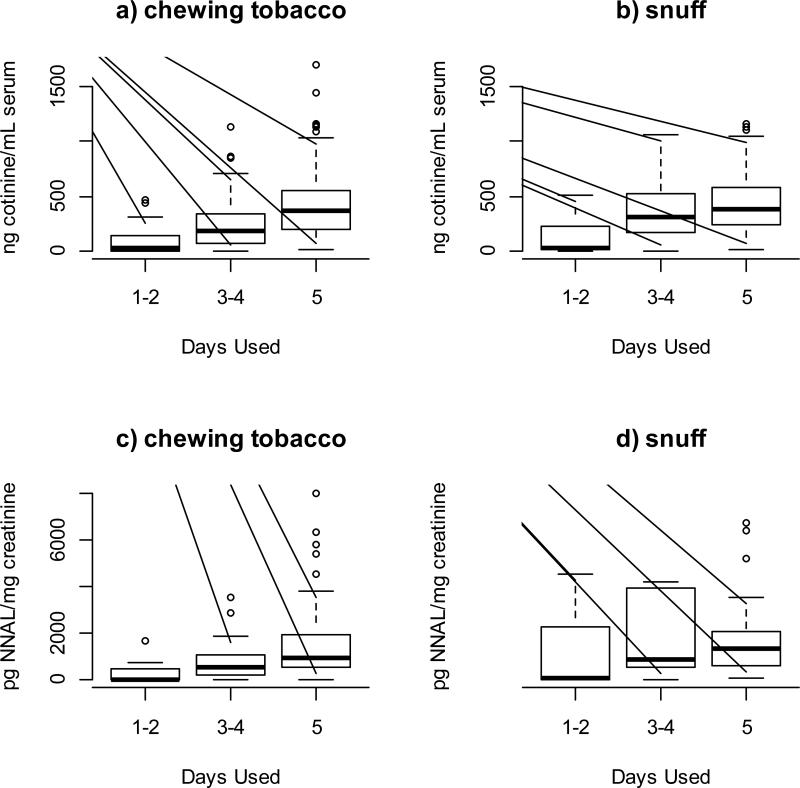
Analysis of Dose-Response Relationship for Tobacco-Specific Biomarkers
Figure 2 presents boxplots showing the distribution of cotinine and NNAL concentrations for chewing tobacco and snuff users by frequency of use in terms of the number of days that they had used the product in the past five days. The figure shows that concentrations consistently increased with number of days of use. Tests of trend for the association between biomarker concentrations and days using the product produced p-values less than 0.0001 for chewing tobacco and snuff for cotinine and equal to 0.003 for chewing tobacco and 0.03 for snuff for NNAL.
Figure 2.
Biomarker concentrations for smokeless tobacco users by number of days used chewing tobacco or snuff in the past 5 days
Discussion
We have analyzed biomarkers of tobacco exposure – serum cotinine, urinary total NNAL, blood cadmium, blood lead, blood total mercury, urinary arsenic, and urinary CYMA, from over 23,000 NHANES participants from 1999-2012. To our knowledge, this work provides the first estimates from a large, nationally representative US health survey population that compare serum cotinine and urinary NNAL concentrations for smokeless tobacco users with those of cigarette smokers and present trends in urinary NNAL concentrations over time. We have found higher cotinine concentrations and much higher NNAL concentrations for smokeless tobacco users compared with cigarette smokers as well as higher NNAL concentrations for dual users compared with smokers. We have also found evidence that NNAL concentrations among smokeless tobacco users are declining over time, although the sample sizesfor this analysis were limited due to the introduction of analysis of NNAL with the 2007-2008 NHANES cycle. We also found that smokeless tobacco users have higher concentrations of blood lead, but not blood cadmium, blood mercury, urinary arsenic, or urinary CYMA, compared with non-tobacco users.
The results for NNAL in this study are rather striking, both in terms of the magnitude of overall exposure for smokeless tobacco users as well as the apparent decrease in NNAL exposure over time. Our results confirm with a large and nationally representative survey sample previous findings that NNAL and cotinine concentrations were as high as or higher among smokeless tobacco users as among cigarette smokers (15). Relative concentrations for NNAL were particularly high, with concentrations for smokeless tobacco users being on average almost three times as high as concentrations for cigarette smokers. The causes of these differences in exposure between cigarette and smokeless tobacco users are not entirely understood. It has been previously suggested that higher urinary cotinine concentrations for smokeless tobacco users could be related to first pass clearance of swallowed tobacco juice, whereby constituents could to some extent be metabolized and excreted before they reach the systemic circulatory system (15). Similar issues related to metabolism and clearance of NNK and NNAL could also affect urinary NNAL levels among smokeless tobacco users in this study. Even so, results from this and previous research (15) suggest that nicotine and NNK exposure in smokeless tobacco users is at least as high as, if not higher than, exposure among cigarette smokers.
Although based on limited sample sizes, estimated NNAL concentrations for smokeless users fell by more than two thirds from 2007-2008 to 2011-2012, even though cotinine concentrations for these users were relatively unchanged during this period. The decrease in NNAL concentrations among smokeless tobacco users could be the result of a variety of factors including reductions in the quantity of smokeless tobacco used, although estimates from these NHANES do not show a decrease in the number of days that individuals used chewing tobacco or snuff in the past five days. For example, chewing tobacco users reported using the product on an average of 4.5 days (95% CI = 4.4, 4.7) in 2007-2008 and 4.3 days (95% CI = 3.7, 4.9) in 2011-2012. Moreover, cotinine concentrations among smokeless tobacco users were relatively consistent during the period. The decrease in NNAL concentrations could result in part from reductions in TSNAs in smokeless tobacco products generally. Borgerding et al. (34) analyzed toxicant concentrations in 43 US smokeless tobacco products sold in the US in 2006 and 2007 and found that TSNA concentrations observed for all of these commercial products were lower than historically reported values. Fisher et al. (10) found a decrease in average TSNAs for three commercial moist snuff products from 1997 to 2010, particularly in the period prior to 2005. The decrease in NNAL concentrations among smokeless tobacco users may also reflect a movement among users to smokeless products with lower levels of certain harmful constituents. Stepanov et al. (12), for example, analyzed total TSNAs in relatively new smokeless tobacco products such as Taboka, Marlboro Snus, Camel Snus, and Skoal Dry as compared with popular traditional brands of moist snuff such as Copenhagen Snuff, Skoal Lung Cut, and Kodiak Wintergreen that were purchased in 2006-2007. They found that total TSNAs averaged 1.97 μg/g dry weight tobacco in Taboka, Marlboro Snus, and Camel Snus, 4.54 μg/g dry weight tobacco in Skoal Dry, and 7.42 μg/g in the traditional moist snuff brands. Similar results were found specifically for NNK, the precursor of NNAL. These researchers subsequently analyzed novel smokeless tobacco products in 2010 (35) and 2011 (36) and found that TSNA levels in products such as Marlboro and Camel snus increased and decreased over time. Changes have also been observed in smokeless tobacco product use over time. Delnevo et al. (37) analyzed smokeless tobacco convenience store sales data from 2005 to 2011 and found changes in product market share durng this period. Market share for chewing tobacco, for example, decreased from 9.0% to 4.3% durng this time, and sales of snus increased from 0.0% to 3.7%. Approximately 90% of smokeless tobacco sold in convenience stores throughout the period was moist snuff, but the market share of portion pouches within this category increased from 5.5% to 14.5% during the period. Trends in NNAL concentrations among smokeless tobacco users should continue to be monitored and evaluated over time.
This analysis has also found that blood lead levels in smokeless tobacco users are comparable to those of cigarette smokers and higher than levels for non-tobacco users. This result is consistent with previous analysis of NHANES data (17). Further research on this topic is needed to establish that smokeless tobacco is the cause of these elevated lead levels among users and, if so, to identify the elements of smokeless tobacco production that contribute to these higher levels. CYMA concentrations were also higher among cigarette smokers and dual cigarette and smokeless tobacco users, but not among exclusive smokeless tobacco users, compared with non-tobacco users. This result is consistent with expectations, given that CYMA is a biomarker for smoke exposure.
Results in this study are subject to certain limitations, primarily due to the nature of the data being collected. First, we do not have detailed information on the type of smokeless tobacco product used, such as information on brand or product type such as snus, apart from chewing tobacco and snuff. Second, we do not have information on the quantity of product used, such as amount used per day, apart from the number of days using the product in the past five days. Finally, NHANES participants were only asked about past five day use of certain tobacco products other than cigarettes. We were thus unable to evaluate any effects of duration or former use of smokeless tobacco products. We also have no information on e-cigarette use in NHANES data, but e-cigarette use was minimal during much of the period of this analysis.
Our results have shown that smokeless tobacco use is significantly associated with high levels of exposure to known harmful and addictive constituents, in some cases greater than observed among cigarette smokers. This exposure is a cause of considerable concern for individual and public health. These findings also demonstrate the need for continuing study and surveillance of the toxic constituents of smokeless tobacco as well as their health effects on the individuals who use them.
Footnotes
Conflict of interest statement: The authors have no conflicts of interest to report.
Disclaimer: The views and opinions expressed in this article are those of the authors only and do not necessarily represent the views, official policy or position of the US Department of Health and Human Services or any of its affiliated institutions or agencies.
Reference List
- 1.Centers for Disease Control and Prevention Tobacco Product Use among Adults - United States, 2012-2013. Morbidity and Mortality Weekly Review. 2014;63:542–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Tobacco Use among Middle and High School Students - United States, 2013. Mor Mort Week Rep. 2014;63:1021–6. [PMC free article] [PubMed] [Google Scholar]
- 3.Tobacco product use among middle and high school students - United States, 2011 and 2012. Morb Mortal Wkly Rep. 2013;62:893–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Agaku IT, Vardavas CI, Ayo-Yusuf OA, Alpert HR, Connolly GN. Temporal trends in smokeless tobacco use among US middle and high school students, 2000-2011. JAMA. 2013;309:1992–4. doi: 10.1001/jama.2013.4412. [DOI] [PubMed] [Google Scholar]
- 5.Kann L, Kinchen S, Shanklin SL, Flint KH, Kawkins J, Harris WA, et al. Youth risk behavior surveillance--United States, 2013. MMWR Surveill Summ. 2014;63(Suppl 4):1–168. [PubMed] [Google Scholar]
- 6.Richter PA, Bishop EE, Wang J, Kaufmann R. Trends in tobacco smoke exposure and blood lead levels among youths and adults in the United States: the National Health and Nutrition Examination Survey, 1999-2008. Prev Chronic Dis. 2013;10:E213. doi: 10.5888/pcd10.130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richter PA, Bishop EE, Wang J, Swahn MH. Tobacco smoke exposure and levels of urinary metals in the U.S. youth and adult population: the National Health and Nutrition Examination Survey (NHANES) 1999-2004. Int J Environ Res Public Health. 2009;6:1930–46. doi: 10.3390/ijerph6071930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roethig HJ, Munjal S, Feng S, Liang Q, Sarkar M, Walk RA, et al. Population estimates for biomarkers of exposure to cigarette smoke in adult U.S. cigarette smokers. Nicotine Tob Res. 2009;11:1216–25. doi: 10.1093/ntr/ntp126. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Ketterman A, Rostron B, Day H. Biomarkers of Exposure among US Cigar Smokers: An Analysis of 1999-2012 National Health and Nutrition Examinatin Survey (NHANES) Data. Cancer Epidemiol Biomarkers Prev. 2014 doi: 10.1158/1055-9965.EPI-14-0849. [DOI] [PubMed] [Google Scholar]
- 10.Fisher MT, Bennett CB, Hayes A, Kargalioglu Y, Knox BL, Xu D, et al. Sources of and technical approaches for the abatement of tobacco specific nitrosamine formation in moist smokeless tobacco products. Food Chem Toxicol. 2012;50:942–8. doi: 10.1016/j.fct.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 11.Hatsukami DK, Lemmonds C, Zhang Y, Murphy SE, Le C, Carmella SG, et al. Evaluation of carcinogen exposure in people who used “reduced exposure” tobacco products. J Natl Cancer Inst. 2004;96:844–52. doi: 10.1093/jnci/djh163. [DOI] [PubMed] [Google Scholar]
- 12.Stepanov I, Jensen J, Hatsukami D, Hecht SS. New and traditional smokeless tobacco: comparison of toxicant and carcinogen levels. Nicotine Tob Res. 2008;10:1773–82. doi: 10.1080/14622200802443544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richter P, Hodge K, Stanfill S, Zhang L, Watson C. Surveillance of moist snuff: total nicotine, moisture, pH, un-ionized nicotine, and tobacco-specific nitrosamines. Nicotine Tob Res. 2008;10:1645–52. doi: 10.1080/14622200802412937. [DOI] [PubMed] [Google Scholar]
- 14.Lawler TS, Stanfill SB, Zhang L, Ashley DL, Watson CH. Chemical characterization of domestic oral tobacco products: total nicotine, pH, unprotonated nicotine and tobacco-specific N-nitrosamines. Food Chem Toxicol. 2013;57:380–6. doi: 10.1016/j.fct.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hecht SS, Carmella SG, Murphy SE, Riley WT, Le C, Luo X, et al. Similar exposure to a tobacco-specific carcinogen in smokeless tobacco users and cigarette smokers. Cancer Epidemiol Biomarkers Prev. 2007;16:1567–72. doi: 10.1158/1055-9965.EPI-07-0227. [DOI] [PubMed] [Google Scholar]
- 16.Hecht SS, Carmella SG, Edmonds A, Murphy SE, Stepanov I, Luo X, et al. Exposure to nicotine and a tobacco-specific carcinogen increase with duration of use of smokeless tobacco. Tob Control. 2008;17:128–31. doi: 10.1136/tc.2007.023242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naufal ZS, Marano KM, Kathman SJ, Wilson CL. Differential exposure biomarker levels among cigarette smokers and smokeless tobacco consumers in the National Health and Nutrition Examination Survey 1999-2008. Biomarkers. 2011;16:222–35. doi: 10.3109/1354750X.2010.546013. [DOI] [PubMed] [Google Scholar]
- 18.Agaku IT, King BA. Validation of Self-Reported Smokeless Tobacco Use by Measurement of Serum Cotinine Concentration Among US Adults. Am J Epidemiol. 2014 doi: 10.1093/aje/kwu182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agaku IT, Vardavas CI, Connolly G. Proposed cutoff for identifying adult smokeless tobacco users with urinary total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanonol: an aggregated analysis of NHANES 2007-2010 data. Nicotine Tob Res. 2013;15:1956–61. doi: 10.1093/ntr/ntt083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Center for Health Statistics [2014 Sep 9];National Health and Nutrition Examination Survey. 2012 http://www.cdc.gov/nchs/nhanes.htm. Available from: URL: http://www.cdc.gov/nchs/nhanes.htm.
- 21.Jarup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 2009;238:201–8. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Caraballo RS, Holiday DB, Stellman SD, Mowery PD, Giovino GA, Muscat JE, et al. Comparison of serum cotinine concentration within and across smokers of menthol and nonmenthol cigarette brands among non-Hispanic black and non-Hispanic white U.S. adult smokers, 2001-2006. Cancer Epidemiol Biomarkers Prev. 2011;20:1329–40. doi: 10.1158/1055-9965.EPI-10-1330. [DOI] [PubMed] [Google Scholar]
- 23.Jarvis MJ, Russell MA, Benowitz NL, Feyerabend C. Elimination of cotinine from body fluids: implications for noninvasive measurement of tobacco smoke exposure. Am J Public Health. 1988;78:696–8. doi: 10.2105/ajph.78.6.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia Y, Bernert JT, Jain RB, Ashley DL, Pirkle JL. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in smokers in the United States: NHANES 2007-2008. Biomarkers. 2011;16:112–9. doi: 10.3109/1354750X.2010.533288. [DOI] [PubMed] [Google Scholar]
- 25.Hecht SS, Yuan JM, Hatsukami D. Applying tobacco carcinogen and toxicant biomarkers in product regulation and cancer prevention. Chem Res Toxicol. 2010;23:1001–8. doi: 10.1021/tx100056m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23:907–22. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- 27.Agency for Toxic Substances and Disease Registry [2014 Feb 16];ToxFAQs for Cadmium. 2012 http://www.atsdr.cdc.gov/toxfaqs/tf.asp?id=47&tid=15. [PubMed]
- 28.Agency for Toxic Substances and Disease Registry [2014 Feb 16];ToxFAQs for Lead. 2012 http://www.atsdr.cdc.gov/toxfaqs/tf.asp?id=93&tid=22.
- 29.Agency for Toxic Substances and Disease Registry [2014 Sep 10];ToxFAQs for Mercury. 1999 http://www.atsdr.cdc.gov/toxfaqs/tf.asp?id=93&tid=22.
- 30.Agency for Toxic Substances and Disease Registry [2014 Feb 16];ToxFAQs for Arsenic. 2007 http://www.atsdr.cdc.gov/toxfaqs/tf.asp?id=19&tid=3. [PubMed]
- 31.Alwis KU, Blount BC, Britt AS, Patel D, Ashley DL. Simultaneous analysis of 28 urinary VOC metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI/MSMS). Anal Chim Acta. 2012;750:152–60. doi: 10.1016/j.aca.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avila-Tang E, Al-Delaimy WK, Ashley DL, Benowitz N, Bernert JT, Kim S, et al. Assessing secondhand smoke using biological markers. Tob Control. 2013;22:164–71. doi: 10.1136/tobaccocontrol-2011-050298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goniewicz ML, Havel CM, Peng MW, Jacob P, III, Dempsey D, Yu L, et al. Elimination kinetics of the tobacco-specific biomarker and lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Cancer Epidemiol Biomarkers Prev. 2009;18:3421–5. doi: 10.1158/1055-9965.EPI-09-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borgerding MF, Bodnar JA, Curtin GM, Swauger JE. The chemical composition of smokeless tobacco: a survey of products sold in the United States in 2006 and 2007. Regul Toxicol Pharmacol. 2012;64:367–87. doi: 10.1016/j.yrtph.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Stepanov I, Biener L, Knezevich A, Nyman AL, Bliss R, Jensen J, et al. Monitoring tobacco-specific N-nitrosamines and nicotine in novel Marlboro and Camel smokeless tobacco products: findings from Round 1 of the New Product Watch. Nicotine Tob Res. 2012;14:274–81. doi: 10.1093/ntr/ntr209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stepanov I, Biener L, Yershova K, Nyman AL, Bliss R, Parascandola M, et al. Monitoring tobacco-specific N-nitrosamines and nicotine in novel smokeless tobacco products: findings from round II of the new product watch. Nicotine Tob Res. 2014;16:1070–8. doi: 10.1093/ntr/ntu026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delnevo CD, Wackowski OA, Giovenco DP, Manderski MT, Hrywna M, Ling PM. Examining market trends in the United States smokeless tobacco use: 2005-2011. Tob Control. 2014;23:107–12. doi: 10.1136/tobaccocontrol-2012-050739. [DOI] [PMC free article] [PubMed] [Google Scholar]