MPK4 and MPK12 inhibit the activity of protein kinase HT1, which in turn controls SLAC1 anion channel activation by OST1 and GHR1 in stomatal CO2 signaling.
Abstract
Activation of the guard cell S-type anion channel SLAC1 is important for stomatal closure in response to diverse stimuli, including elevated CO2. The majority of known SLAC1 activation mechanisms depend on abscisic acid (ABA) signaling. Several lines of evidence point to a parallel ABA-independent mechanism of CO2-induced stomatal regulation; however, molecular details of this pathway remain scarce. Here, we isolated a dominant mutation in the protein kinase HIGH LEAF TEMPERATURE1 (HT1), an essential regulator of stomatal CO2 responses, in an ozone sensitivity screen of Arabidopsis thaliana. The mutation caused constitutively open stomata and impaired stomatal CO2 responses. We show that the mitogen-activated protein kinases (MPKs) MPK4 and MPK12 can inhibit HT1 activity in vitro and this inhibition is decreased for the dominant allele of HT1. We also show that HT1 inhibits the activation of the SLAC1 anion channel by the protein kinases OPEN STOMATA1 and GUARD CELL HYDROGEN PEROXIDE-RESISTANT1 (GHR1) in Xenopus laevis oocytes. Notably, MPK12 can restore SLAC1 activation in the presence of HT1, but not in the presence of the dominant allele of HT1. Based on these data, we propose a model for sequential roles of MPK12, HT1, and GHR1 in the ABA-independent regulation of SLAC1 during CO2-induced stomatal closure.
INTRODUCTION
Plant gas exchange with the surrounding environment occurs via stomata, small pores on the leaves and stems, which are formed by pairs of guard cells. These specialized cells have evolved to sense environmental as well as endogenous signals, and they integrate this information to optimize the balance between CO2 fixation in photosynthesis and water loss via transpiration. Stomata open in response to low CO2 concentrations to prevent a shortage of substrates for photosynthesis. However, higher than ambient CO2 concentrations lead to stomatal closure; this increases water use efficiency but can also cause increased leaf temperature and a reduced uptake of nutrients via decreases in the transpiration stream. Stomatal closure or opening in response to high or low CO2, respectively, can be studied by increasing or decreasing CO2 concentrations from ambient, which is ∼400 ppm. CO2-induced stomatal movements are an important area of research as they influence plant growth, water use efficiency, and carbon cycling in ecosystems.
Stomatal movements are regulated by the controlled uptake and release of osmotically active compounds across guard cell membranes. In Arabidopsis thaliana, the stomatal abscisic acid (ABA) signaling pathway has been extensively studied, whereas considerably less is known about guard cell CO2 signaling. However, several identified stomatal regulators are involved in both pathways. SLAC1, the guard cell anion channel that is involved in the regulation of stomatal closure in response to various stimuli, including ABA, was identified in mutant screens for regulators of stomatal CO2 and O3 sensitivity (Negi et al., 2008; Vahisalu et al., 2008). SLAC1 is activated by the protein kinase OPEN STOMATA1 (OST1) (Geiger et al., 2009; Lee et al., 2009), by GHR1 (Hua et al., 2012), and by calcium-dependent protein kinases (CPKs) (Geiger et al., 2010; Brandt et al., 2012), whereas the activity of these protein kinases is controlled by PYRABACTIN RESISTANCE1/REGULATORY COMPONENTS OF ABA RECEPTORS (PYR/RCAR)-dependent inhibition of PP2C protein phosphatases (Ma et al., 2009; Park et al., 2009; Umezawa et al., 2009; Geiger et al., 2010; Brandt et al., 2012; Hua et al., 2012).
In genetic studies, OST1, PYR/RCAR receptors, and PP2Cs have been shown to be involved in stomatal CO2 signaling (Xue et al., 2011; Merilo et al., 2013; Chater et al., 2015). Thus, ABA and CO2 signals largely activate similar components of stomatal regulation. Recent research indicates that although SLAC1 is essential for both ABA and CO2 signal transduction, SLAC1 activation is likely to occur via different mechanisms for these signals. The transmembrane region of SLAC1 was shown to be essential for CO2-induced but not for ABA-induced stomatal closure, suggesting an ABA-independent CO2-induced regulation of SLAC1 via the transmembrane domain (Yamamoto et al., 2016). However, as the stomatal response to CO2 was still partially impaired in slac1-4 plants transformed with SLAC1 lacking either the N terminus or both the N and C terminus (Yamamoto et al., 2016), the N-terminal region of SLAC1 may still contribute to CO2-induced SLAC1 activation.
Several CO2-specific guard cell regulators have also been identified. CARBONIC ANHYDRASE1 (CA1) and CA4 convert CO2 into bicarbonate, which plays an important role in the activation of SLAC1-dependent S-type anion channel currents in guard cell protoplasts (Hu et al., 2010, 2015; Xue et al., 2011). Recently, bicarbonate was also shown to enhance S-type anion currents in the heterologous Xenopus laevis oocyte system in the presence of the anion channel SLAC1 and a SLAC1-activating kinase (OST1, CPK6, or CPK23) (Wang et al., 2016). Thus, SLAC1 was proposed as a bicarbonate-responsive protein, contributing partially to the CO2 response.
Of the stomatal CO2 regulators identified to date, the protein kinase HT1 has a central role in CO2-induced stomatal regulation (Hashimoto et al., 2006). The ht1-2 mutant exhibits reduced stomatal conductance and displays completely abolished high CO2-induced stomatal closure and low CO2-induced stomatal opening. In comparison, in plants deficient in CA1/CA4, OST1, or SLAC1, stomatal CO2 responses remain partly functional (Hu et al., 2010; Xue et al., 2011; Merilo et al., 2013). Recently, additional mutant alleles for HT1 were isolated (Hashimoto-Sugimoto et al., 2016). All recessive ht1 alleles showed high leaf temperature in low and ambient CO2 and had point mutations or deletions of amino acids predicted to be essential for kinase activity (Hashimoto-Sugimoto et al., 2016). In addition, a dominant allele of HT1 with an arginine-to-lysine substitution at position 102 (R102K) was shown to retain kinase activity similar to HT1, but caused constitutively open stomata and a loss of stomatal responses to CO2 (Hashimoto-Sugimoto et al., 2016). While the recessive and dominant HT1 mutants were both insensitive to CO2 changes, they exhibited opposing stomatal conductances: high in the case of the dominant HT1(R102K) and low in the case of recessive mutations in HT1. Nevertheless, the molecular mechanisms that control the activation of HT1, as well as the substrates of this highly CO2-specific protein kinase, have not yet been elucidated.
The guard cell anion channel SLAC1 was identified from a screen for ozone-sensitive mutants (Overmyer et al., 2008; Vahisalu et al., 2008). Here, we characterize a dominant mutant from the same screen that has constitutively more open stomata and a complete guard cell CO2 insensitivity phenotype due to an alanine-to-valine substitution (A109V) in HT1. We show that MITOGEN-ACTIVATED PROTEIN KINASE4 (MPK4) and MPK12 can inhibit HT1 kinase activity and that the activity of HT1 with the dominant A109V mutation is inhibited significantly less by both MPKs. Furthermore, we show that while both HT1 and HT1(A109V) can suppress SLAC1 activation in oocytes, inhibition by HT1, but not by HT1(A109V), can be reversed by MPK12. These data suggest a model where CO2-induced stomatal closure is controlled by MPKs, which negatively regulate HT1 kinase and thus enable stomatal closure via SLAC1 activation.
RESULTS
O3 Sensitivity Screen in Arabidopsis Identifies a Dominant Mutation in HT1
The air pollutant ozone (O3) can be used to isolate mutants with impaired stomatal regulation (Overmyer et al., 2000, 2008; Vahisalu et al., 2008). Here, we characterize an O3-sensitive mutant that displayed both substantial O3-induced leaf injury and very high stomatal conductance, indicative of increased stomatal aperture (Figures 1A and 1B). Due to its constitutively high stomatal conductance, the mutant was named suu, which means “mouth” in both Estonian and Finnish. In accordance with enhanced transpiration, the dominant suu mutation also caused increased fresh weight loss of detached leaves (Figure 1C).
Figure 1.
The A109V Dominant Mutation in HT1 Causes Ozone Sensitivity and High Stomatal Conductance.
(A) Representative images of O3-treated (350 ppb for 6 h) and control plants, taken 24 h after the beginning of exposure. Bar = 1 cm.
(B) Stomatal conductance of intact plants. n = 5 to 6 plants; asterisk denotes statistically significant difference between genotypes (Student’s t test, P < 0.05).
(C) Leaf fresh weight loss in 2 h. n = 3 to 5 plants; statistically significantly different groups are denoted with different letters (ANOVA with Tukey unequal N HSD post hoc test, P < 0.05).
(D) Ion leakage from O3-treated (350 ppb for 6 h) and control (clean air [CA]) plants 10, 24, and 32 h after the beginning of O3 exposure. O3 exposure was repeated at least three times with similar results, and data are presented as mean ± sd (n = 4). O3-treated suu (ht1-8D) and ProHT1:HT1(A109V) in Col-0 plants were statistically significantly different from the wild type in all time points (ANOVA with Tukey HSD post hoc test, P < 0.05).
(E) Map of HT1 protein. Kinase domain, locations of suu (ht1-8D) mutation, ATP binding site, and deletion in ht1-2 are indicated.
Increased fresh weight loss in excised leaves and stomatal CO2 insensitivity were used for mapping the mutation in an F2 population of suu (Col-0) outcrossed to Ler. As the mutation was dominant, wild-type fresh weight loss and stomatal closure in response to elevated CO2 were used as the traits for selection. The mutation was mapped to the region between markers nga280 and nga111 on chromosome 1 (Supplemental Figure 1A). Among the candidate genes in this region was HT1, an essential regulator of stomatal CO2 responses (Hashimoto et al., 2006; Tian et al., 2015). A point mutation that resulted in an alanine-to-valine substitution in position 109 in HT1 [hereafter HT1(A109V)] was present in the suu mutant.
Stable transgenic lines expressing HT1(A109V) under the control of its native promoter [ProHT1:HT1(A109V)] conferred strong O3 sensitivity and high fresh weight loss to the wild-type Col-0 background (Figure 1D; Supplemental Figures 1B and 1C), showing that the phenotypes of the suu mutant were caused by the dominant A109V mutation in HT1. As seven independent mutant alleles for HT1 have been isolated to date (Hashimoto-Sugimoto et al., 2016), suu was renamed ht1-8D. The A109V mutation lies close to the ATP binding site of the HT1 kinase domain (Figure 1E). The kinase-dead HT1 in ht1-2 has a 14-amino acid deletion within the kinase domain, resulting from a point mutation in the donor splice site at nucleotide 448 (Hashimoto et al., 2006), which is also shown in Figure 1E.
There were no statistically significant differences between the stomatal densities of wild-type, ht1-8D, and ProHT1:HT1(A109V) plants (Supplemental Figure 1D), indicating that the high stomatal conductance of plants with the dominant A109V mutation in HT1 is caused by increased stomatal aperture.
The Dominant A109V Mutation in HT1 Causes High Stomatal Conductance and Impaired Responses to CO2
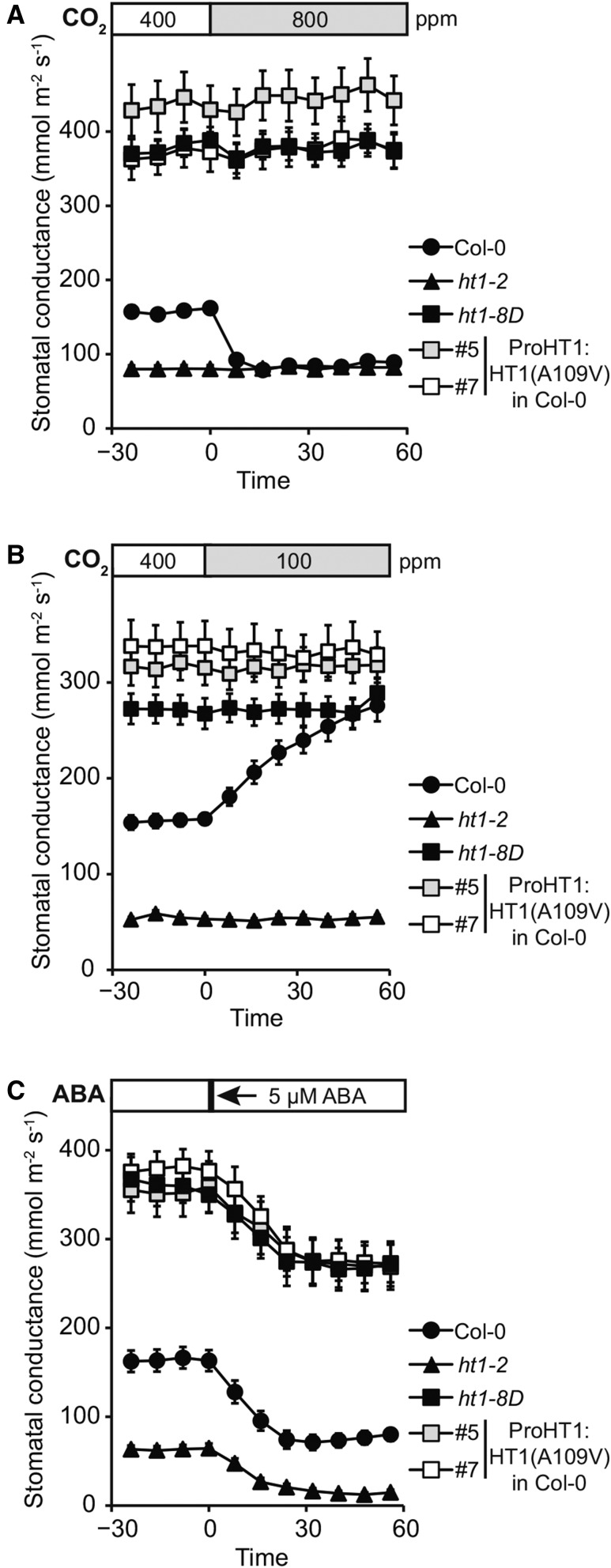
HT1 is a central regulator of CO2-induced stomatal movements; stomatal closure in response to high CO2 and stomatal opening in response to low CO2 were absent in loss-of-function ht1-2 plants, and these plants had closed stomata at all studied CO2 levels (Hashimoto et al., 2006). The dominant mutation in HT1 identified here caused an opposite, constitutively more open stomata phenotype. Therefore, we characterized the stomatal responses of ht1-8D, the kinase-dead ht1-2 allele, and two plant lines expressing HT1 with the dominant ht1-8D mutation [ProHT1:HT1(A109V) in Col-0 #5 and #7]. All these plant lines were completely insensitive to changes in CO2 concentration, both with regard to high CO2-induced stomatal closure and low CO2-induced stomatal opening (Figures 2A and 2B). Plants with the dominant HT1(A109V) mutation exhibited stomatal conductance twice as high as wild-type plants, whereas those with the recessive ht1-2 mutation had tightly closed stomata. ABA-induced stomatal closure and ABA-induced inhibition of stomatal opening were functional in plants with mutations in HT1, indicating intact ABA signaling and thus strong CO2 specificity of HT1 in stomatal regulation (Figure 2C; Supplemental Figures 2A to 2C). This was further supported by normal stomatal closure in response to darkness, reduced air humidity, and a short pulse of O3 in plants with the dominant A109V mutation in HT1 (Supplemental Figures 2D to 2F).
Figure 2.
Plants with A109V Mutation in HT1 Are CO2 Insensitive but Respond to ABA.
Stomatal response of intact plants to CO2 elevation (from 400 to 800 ppm), reduction in CO2 concentration (from 400 to 100 ppm), and 5 µM ABA, respectively. Stimulus was applied at time point zero, and pooled stomatal conductance data from three independent experiments are shown. Error bars indicate se (n = 12 to 21).
HT1 Interacts with MPK4 and MPK12
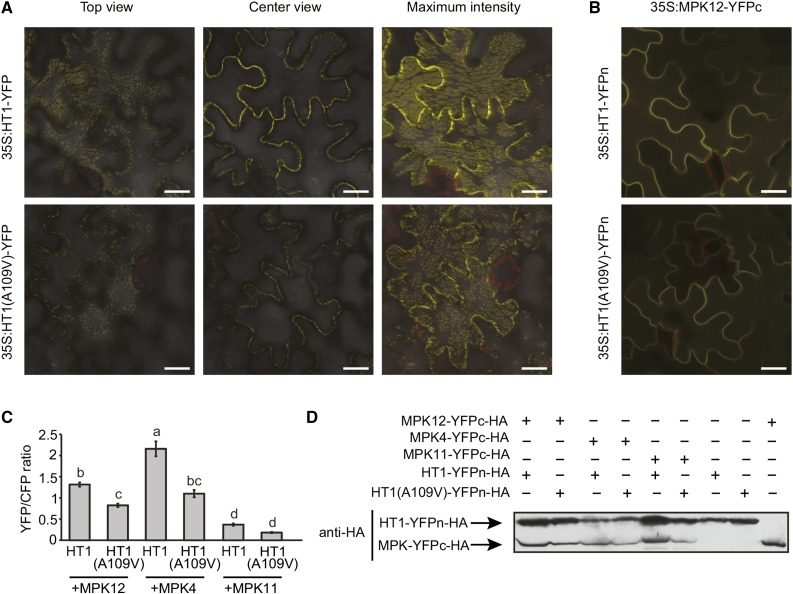
Protein subcellular localization can be connected with physiological function. Thus, we expressed and visualized YFP-tagged HT1 and HT1(A109V) in Nicotiana benthamiana to assess if the A109V mutation affected HT1 protein localization. In all of the studied cells, YFP fluorescence was detected in the cell periphery as distinct patches, which appeared as dashed lines along the plasma membrane in cross-sectional view (Figure 3A, center view). The size and shape of patches varied among experiments, possibly due to differences in the protein expression levels and physiological conditions of plants. Nevertheless, similar localization patterns were always observed for HT1 and HT1(A109V) within each experiment (Figure 3A). Therefore, it is unlikely that the phenotypes of plants with the dominant A109V mutation in HT1 are caused by the altered subcellular localization of the protein.
Figure 3.
HT1 and HT1(A109V) Localize to the Cell Periphery and Interact with MPK12.
(A) Subcellular localization of HT1 and HT1(A109V) YFP fusion proteins in N. benthamiana epidermal cells. Top view, center view, and maximum intensity projections are shown. Bars = 20 μm.
(B) BiFC assay with split-YFP-fused HT1 variants and MPK12. All the experiments were repeated at least three times, and representative BiFC images from the same leaf with identical confocal settings are shown. Bars = 20 μm.
(C) The average ± se (n = 5 for MPK4, 20 for MPK12, and 10 for MPK11) YFP/CFP signal intensity ratio measured via quantitative BiFC assay is shown. Statistically significantly different groups are denoted with different letters (ANOVA with Tukey unequal N HSD post hoc test, P < 0.05).
(D) Immunoblot showing the expression level of split-YFP fusion proteins in the quantitative BiFC experiment.
To further address the role of HT1 in CO2 signaling, we performed a split-ubiquitin yeast two-hybrid screen using HT1. Among several potential interactors, MPK4 was identified as a protein interacting with HT1 in yeast (Supplemental Figure 3A). In the yeast two-hybrid assay, the interaction of MPK4 with HT1 appeared to be stronger than the interaction of MPK4 with HT1(A109V). MPK4 is involved in CO2 signaling in Nicotiana tabacum (Marten et al., 2008). Because in Arabidopsis the mpk4 mutant is dwarfed, we analyzed stomatal responses to CO2 in mpk4 NahG plants (Petersen et al., 2000), as the NahG transgene rescues the size of the mpk4 mutant by degradation of salicylic acid. Plants expressing MPK4(D198G/E202A), a constitutively active version of MPK4 (Berriri et al., 2012), were also studied. Both the loss of function and the gain of function plants exhibited typical responses to changes in CO2 concentration (Supplemental Figures 4A to 4D), suggesting that either MPK4 is not needed for CO2 signaling or that there are other MPKs in Arabidopsis guard cells that can replace the function of MPK4 for CO2 signaling in mpk4 NahG plants.
In parallel projects, we demonstrated that the deletion of MPK12 and a specific point mutation in MPK12 lead to more open stomata and altered stomatal CO2 responses (Jakobson et al., 2016). As MPK4 and MPK12 both belong to subgroup B of the MPKs (Ichimura et al., 2002) and because MPK12 has been shown to be important in stomatal responses (Jammes et al., 2009; Des Marais et al., 2014), we analyzed whether HT1 or HT1(A109V) could also interact with MPK12 in a pairwise split-ubiquitin yeast two-hybrid assay. Wild-type HT1 interacted with both MPK12 and MPK4, but not with a related MAP kinase, MPK11, whereas the interaction was not observed in HT1(A109V) (Supplemental Figure 5). To further confirm this in planta, we performed bimolecular fluorescence complementation (BiFC) analyses with split-YFP-tagged MPK12 and HT1 or HT1(A109V) in N. benthamiana. Both versions of HT1 interacted with MPK12 in planta (Figure 3B). However, a ratiometric BiFC assay with SLAC1-CFP as the internal control revealed weakened interaction caused by the A109V substitution in the HT1. The interactions of HT1 and HT1(A109V) with MPK4 and MPK11 were also assayed in comparison. Similar to the case with MPK12, HT1(A109V) also showed a significantly lower YFP/CFP intensity ratio when tested with MPK4. For MPK11, the overall YFP/CFP signal intensity ratios were low, and there was no difference between HT1 and HT1(A109V), indicating no significant interaction between HT1 and MPK11 (Figure 3C; Supplemental Figure 6C). Expression of the investigated MPKs and versions of HT1 in the ratiometric BiFC experiments was verified by immunoblot (Figure 3D). These experiments suggested that the A109V substitution in HT1 partially impaired the interaction of HT1 with MPK4 and MPK12 and, as mpk4 NahG plants displayed normal CO2 responses, we were prompted to investigate the role of MPK12 in the regulation of HT1.
Efficient MPK12-Induced Inhibition of HT1 Activity Requires Ala-109 in HT1
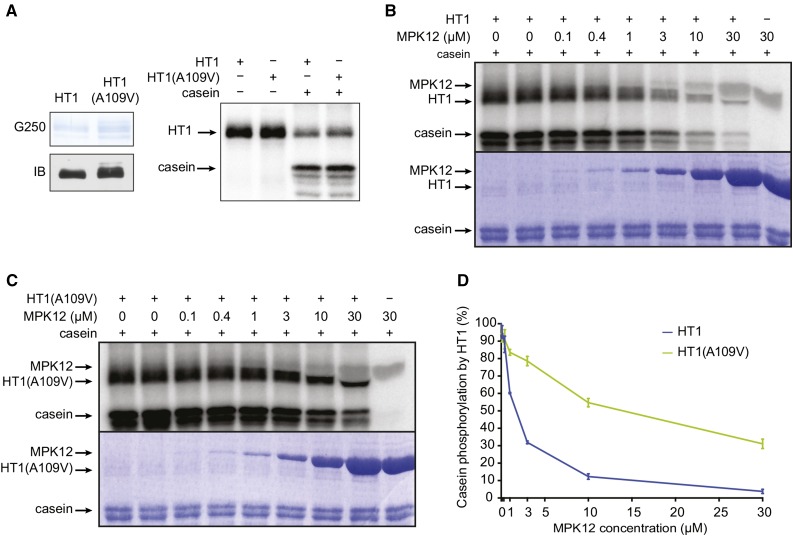
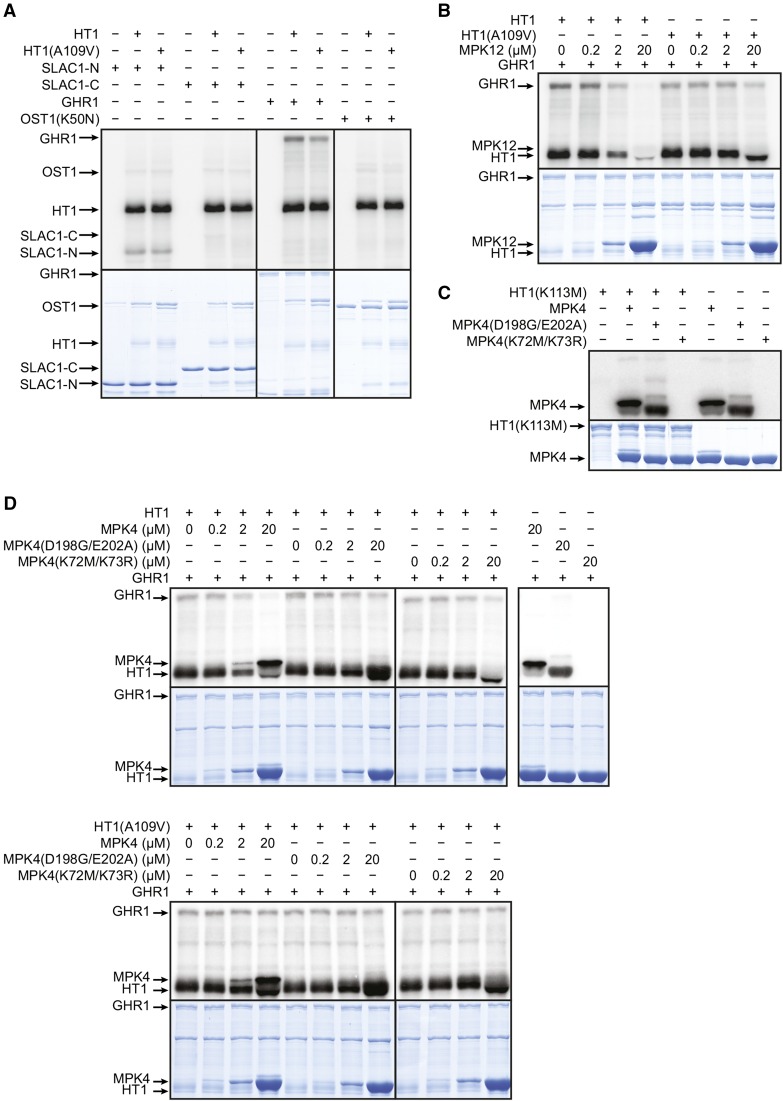
The A109V substitution is close to the ATP binding site in HT1 (Figure 1E), so we tested whether this mutation affects HT1 kinase activity. In vitro kinase assays demonstrated that HT1(A109V) autophosphorylates and phosphorylates the artificial substrate casein as efficiently as HT1 (Figure 4A). Thus, it is likely that the dominant stomatal phenotypes observed in plants carrying the A109V mutation in HT1 were not caused by major changes in HT1 kinase activity.
Figure 4.
HT1(A109V) Is an Active Kinase and HT1(A109V) Activity Is Not Inhibited by MPK12 as Efficiently as the Activity of HT1.
(A) HT1 and HT1(A109V) autophosphorylate and phosphorylate casein with similar activity. Coomassie Brilliant Blue G 250 staining (G250), immunoblot (IB), and autoradiograph are shown.
(B) and (C) HT1-induced autophosphorylation and casein phosphorylation are inhibited by MPK12, and the inhibition is decreased in HT1(A109V). Autoradiographs and Coomassie Brilliant Blue G 250 staining are shown.
(D) Quantification of MPK12-induced inhibition of HT1 and HT1(A109V) activity. Average of three experiments ± se is shown.
Because MPK12 interacted with HT1 in yeast and in planta, we performed in vitro kinase activity assays to address whether MPK12 affects HT1 activity. This experiment showed that MPK12 inhibited autophosphorylation of HT1 and phosphorylation of the artificial substrate casein by HT1 in vitro (Figure 4B). Importantly, this inhibition was markedly decreased in HT1(A109V) (Figure 4C), as illustrated by the quantification of the data (Figure 4D). The inhibition of HT1(A109V) by MPK12 was statistically significantly decreased compared with the inhibition of HT1 by MPK12 at MPK12 concentrations of 1 μM and above. These data suggest that MPK12 functions as an inhibitor of HT1 and that stomatal CO2 insensitivity of plants with the dominant A109V mutation in HT1 is caused by the decreased inhibition of HT1(A109V) by MPK12.
HT1 Inhibits SLAC1 Currents Induced by OST1 and GHR1 in Oocytes, and This Is Counteracted by MPK12
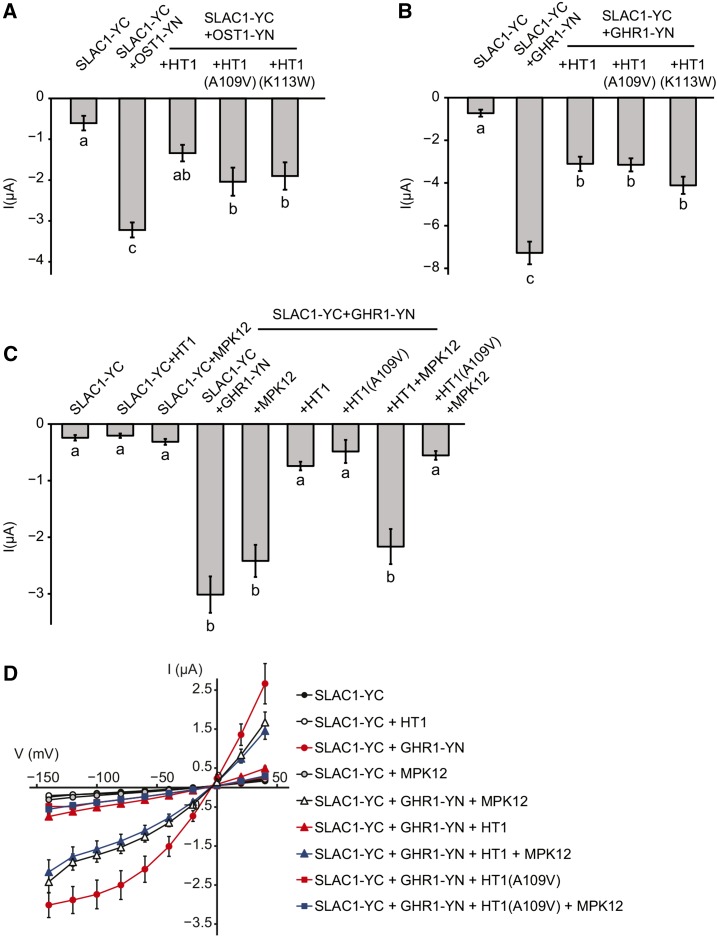
Ultimately, the molecular events leading to stomatal closure require the activation of guard cell anion channels. SLAC1 is the central guard cell S-type anion channel, and plants with deficient SLAC1 have impaired stomatal closure in response to elevated CO2 (Negi et al., 2008; Vahisalu et al., 2008). OST1 is an essential regulator of stomatal closure; it activates the SLAC1 anion channel via phosphorylation (Geiger et al., 2009; Lee et al., 2009) and has been shown to be involved in the regulation of CO2-induced stomatal closure (Xue et al., 2011; Merilo et al., 2013). Thus, we decided to test whether HT1 affects OST1-mediated SLAC1 activation. To address this question, we used a heterologous system and expressed SLAC1 fused with the C-terminal part of YFP (SLAC1-YC), OST1 fused with the N-terminal part of YFP (OST1-YN), and HT1 in X. laevis oocytes. Only background anion currents were observed when SLAC1-YC was expressed alone, whereas typical S-type anion currents were recorded when SLAC1-YC was expressed together with OST1-YN (Figure 5A). Expression of HT1 together with OST1-YN and SLAC1-YC inhibited S-type anion currents (Figure 5A; Supplemental Figure 7A). Similar inhibition always occurred in the presence of the kinase-inactive HT1(K113W) and also in the presence of HT1(A109V) in two experiments out of three. These experiments showed that the CO2-specific protein kinase HT1 could inhibit SLAC1 activation by OST1 in oocytes and that this inhibition was independent of HT1 kinase activity.
Figure 5.
HT1 Inhibits OST1- and GHR1-Activated SLAC1 Anion Currents in Oocytes and MPK12 Counteracts HT1 but Not HT1(A109V)-Induced Downregulation of SLAC1 Activity in Oocytes.
(A) HT1 inhibits OST1-induced SLAC1 activation in oocytes.
(B) HT1 inhibits GHR1-induced SLAC1 activation in oocytes.
(C) MPK12 releases the inhibition of GHR1-induced SLAC1 activation in oocytes caused by HT1, but not by HT1(A109V). In (A) to (C), average currents ± se at −140 mV are shown. Statistically significantly different groups are denoted with different letters (ANOVA + Tukey unequal N HSD post hoc test). Sample size was 10 to 17 in (A), 5 to 11 in (B), and 8 to 12 in (C).
(D) Current-voltage relationship curves for the oocytes presented in (C). Split YFP (BiFC) was used in all cases of SLAC1 and OST1 or SLAC1 and GHR1 coexpression and is indicated by -YN or-YC in the names of respective proteins. Replicates for the experiments are shown in Supplemental Figure 14.
The leucine-rich repeat receptor-like protein kinase GHR1 is centrally implicated in CO2-induced stomatal closure (M. Sierla and J. Kangasjärvi, personal communication). GHR1, first characterized by Hua et al. (2012), can also activate SLAC1 in oocytes. Therefore, we tested whether HT1 could affect SLAC1 anion currents induced by GHR1. Interestingly, HT1, but also HT1(A109V) and the kinase inactive HT1(K113W), inhibited SLAC1 anion currents in oocytes when expressed together with SLAC1-YC and GHR1-YN (Figure 5B; Supplemental Figure 7B). This suggested that neither HT1 kinase activity nor the alanine in position 109 in HT1 was required for the HT1-induced inhibition of SLAC1 activation by GHR1. Together, these experiments show that HT1 can inhibit SLAC1 anion currents triggered by either OST1 or GHR1 in oocytes and that HT1 kinase activity may not be required for the inhibition to occur.
Recently, HT1 kinase activity was suggested to be necessary for the inhibition of SLAC1 activation, as kinase-dead HT1(K113W) could not inhibit OST1-induced SLAC1 activation in oocytes (Tian et al., 2015). In these experiments, Tian et al. (2015) used a shorter version of HT1 that lacks the first 45 amino acids of the N terminus, as annotated in TAIR10, whereas we used the full-length HT1 as annotated in ARAPORT11 and as used previously by Hashimoto et al. (2006). To test whether these 45 amino acids play a role in HT1-induced inhibition of SLAC1 activation by OST1 and GHR1 in oocytes, we generated versions of HT1 with a shorter N terminus (referred to as HT1s) with mutations that correspond to A109V and K113W mutations in the full-length HT1 [HT1s(A64V) and HT1s(K68M), respectively]. We observed similar inhibition of SLAC1 activation induced by OST1 (Supplemental Figure 8A) or GHR1 (Supplemental Figure 8B) for all tested N-terminally shorter versions of HT1. Therefore, under the experimental conditions used here, both the full-length and N-terminally shorter HT1 could inhibit OST1- and GHR1-induced SLAC1 activation in oocytes. Furthermore, this inhibition did not require HT1 kinase activity or the alanine in position 64 (109) in HT1s (Supplemental Figures 8A and 8B).
The in vitro kinase assays showed that MPK12 could inhibit HT1 activity (Figure 4B); we thus tested whether MPK12 also functions as an inhibitor of HT1 in a heterologous system. To address this question, we expressed MPK12 and HT1 together with SLAC1-YC and GHR1-YN in oocytes. These experiments demonstrated that adding MPK12 restored the GHR1-mediated activation of SLAC1 anion currents in the presence of HT1 (Figures 5C and 5D; Supplemental Figure 7C), suggesting that MPK12 inhibited HT1 in oocytes and could prevent the inhibitory role of HT1 on SLAC1 activation by GHR1. As HT1(A109V) activity was inhibited to a lesser extent than that of HT1 by MPK12 in vitro (Figures 4C and 4D), we tested whether MPK12 could also restore GHR1-induced SLAC1 activation in oocytes in the presence of HT1(A109V). As expected, MPK12 could not rescue the inhibition of GHR1-induced SLAC1 anion channel activation by HT1(A109V) (Figures 5C and 5D; Supplemental Figure 7C). To clarify whether the HT1 N terminus is required for the inhibition by MPK12 in oocytes, we performed experiments with HT1 that had a shorter N terminus, which yielded similar results: MPK12 could restore GHR1-induced SLAC1 activation in the presence of short HT1 (Supplemental Figure 8C), suggesting that MPK12 could also inhibit the version of HT1 with a shorter N terminus. Together, these data further support a regulatory role for MPK12 in HT1-mediated CO2 signaling in guard cells and suggest that the phenotypes of ht1-8D are caused by the decreased inhibition of HT1(A109V) by MPK12.
HT1 Phosphorylates GHR1 and SLAC1 in Vitro
Because the experiments with oocytes showed that HT1 inhibited SLAC1 activation by OST1 and GHR1, we tested if HT1 could phosphorylate SLAC1, OST1, or GHR1 in vitro. Both HT1 and HT1(A109V) phosphorylated GHR1 and the SLAC1 N terminus (but not C terminus), whereas phosphorylation of inactive OST1(K50N) by HT1 did not increase above background levels (Figure 6A). We further aimed to test whether HT1 could inhibit SLAC1 phosphorylation by OST1 in vitro, as was recently described (Tian et al., 2015). However, under our conditions, HT1 did not inhibit OST1-induced phosphorylation of the SLAC1 N-terminal fragment in the in vitro assays (Supplemental Figure 9A). Because Tian et al. (2015) used a version of HT1 with a shorter N terminus, we also performed the inhibition assay with the short HT1 which also could not inhibit OST1-induced phosphorylation of the SLAC1 N-terminal fragment in the in vitro assays (Supplemental Figure 9B). These results disagree with the recent study suggesting that HT1 could inhibit the OST1-induced phosphorylation of SLAC1 (Tian et al., 2015).
Figure 6.
HT1 and HT1(A109V) Phosphorylate the SLAC1 N Terminus and GHR1.
MPK12 and MPK4 inhibit the phosphorylation of GHR1 by HT1(A109V) less than the phosphorylation of GHR1 by HT1. Autoradiographs and Coomassie Brilliant Blue G 250 staining are shown in (A) to (D).
(A) HT1 and HT1(A109V) phosphorylate GHR1 and SLAC1 N terminus but not OST1(K50N) or SLAC1 C terminus in tested conditions.
(B) HT1-induced autophosphorylation and phosphorylation of GHR1 are inhibited by MPK12, and the inhibition is decreased in HT1(A109V).
(C) MPK4, MPK4(D198G/E202A), and kinase-inactive MPK4(K72M/K73R) do not phosphorylate kinase-inactive HT1(K113M).
(D) MPK4 and MPK4(K72M/K73R) inhibit HT1-induced phosphorylation of GHR1, and the inhibition is decreased in HT1(A109V). MPK4(D198G/E202A) inhibits HT1 activity less than MPK4 and MPK4(K72M/K73R). MPK4, MPK4(D198G/E202A), and MPK4(K72M/K73R) do not phosphorylate GHR1.
MPK12 and MPK4 Can Inhibit the Phosphorylation of GHR1 by HT1
Because GHR1 was strongly phosphorylated by HT1 and because MPK12 inhibited HT1 activity toward the artificial substrate casein, we tested whether MPK12 could also inhibit the phosphorylation of GHR1 by HT1 in vitro. Indeed, MPK12 inhibited the phosphorylation of GHR1 by HT1, but less in the case of HT1(A109V) (Figure 6B). As MPK4 could also interact with HT1 (Figure 3C; Supplemental Figure 3), we tested whether MPK4 also affected HT1 activity in vitro. MPK4 could not phosphorylate HT1 (Figure 6C). However, similar to MPK12, in the presence of MPK4, the phosphorylation of GHR1 by HT1 was inhibited and the strength of inhibition was again dependent on the A109V substitution in HT1 (Figure 6D). When the kinase-inactive MPK4(K72M/K73R) was used, HT1 activity toward GHR1 was also inhibited, whereas in the presence of the constitutively active MPK4(D198G/E202A), the inhibition was less obvious (Figure 6D). These results suggest that the kinase activity of MPK4 may not be essential for the inhibition of HT1 activity; instead, the inhibition may be more dependent on protein structure. Together, these data support the role of MPK12 as an inhibitor of HT1 activity and suggest that MPK4 could also function in the regulation of HT1.
Lack of MPK4 and MPK12 in Guard Cells Abolishes Stomatal CO2 Responses, and HT1 Genetically Interacts with GHR1 in CO2-Induced Stomatal Movements
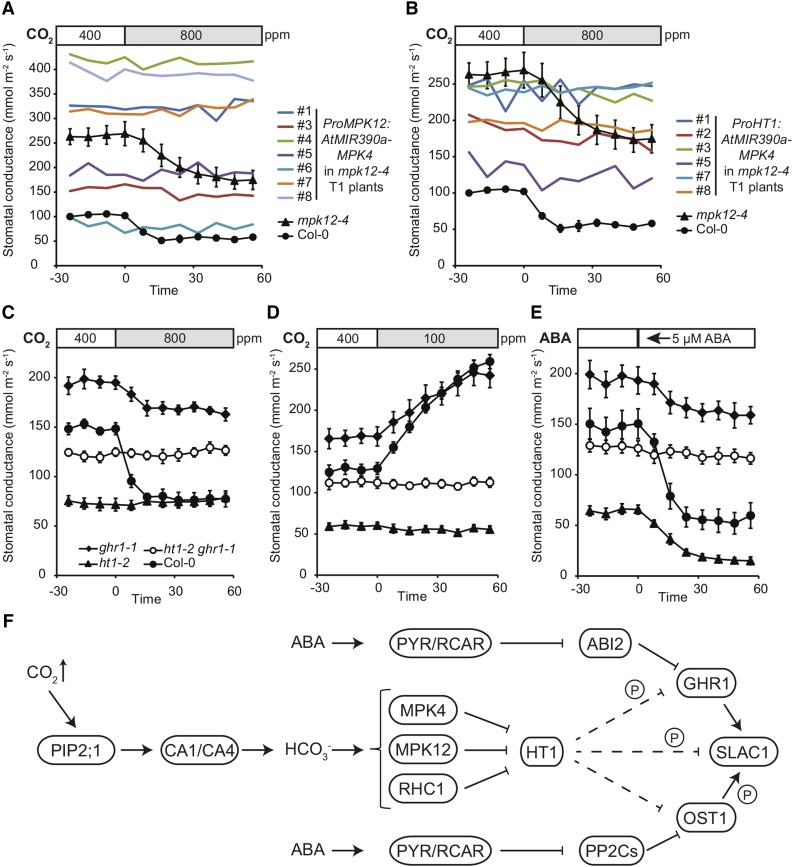
While the stomatal responses to CO2 were normal in mpk4 NahG plants (Supplemental Figures 4A and 4B), MPK4 interacted with HT1 and inhibited HT1 activity toward GHR1 in vitro (Figures 3C and 6D; Supplemental Figure 3). These experiments suggest that MPK4 may still play a role in guard cell CO2 signaling; however, its role may be less prominent than that of MPK12. As MPK12 and MPK4 are very similar kinases, it is conceivable that MPK12 could compensate for the lack of MPK4 in mpk4 NahG plants, resulting in functional stomatal CO2 signaling. To address the role of MPK4 in guard cell CO2 signaling, we generated transgenic plants in which a microRNA designed to specifically silence MPK4 was expressed in mpk12-4 plants, which completely lack MPK12 and display significant but partial inhibition of CO2-induced stomatal closure (Jakobson et al., 2016). To assess the function of MPK4 in guard cells and to avoid the dwarfism characteristic of mpk4 mutants, the expression of the silencing construct was driven by guard cell-preferential promoters, either ProMPK12 or ProHT1 (Hashimoto et al., 2006; Jammes et al., 2009). AtMIR390a-based technology was recently reported to induce efficient gene silencing by the T1 generation of transgenic plants (Carbonell et al., 2014). Expression of ProMPK12:AtMIR390a-MPK4 or ProHT1:AtMIR390a-MPK4 in mpk12-4 plants resulted in stunted growth or unusual leaf shape similar to mpk4 (Petersen et al., 2000) in some of the transgenic plants, whereas others displayed wild-type size and leaf shape (Supplemental Figure 10). This suggests that the guard cell specificity of the HT1 and MPK12 promoters is not complete, which is consistent with previous findings on ProHT1 expression (Hashimoto et al., 2006). Residual expression in other cell types combined with different transgene expression levels could amount to a different degree and varying cell-type specificity of MPK4 silencing in independent T1 plants. The plants with the mpk4-like dwarfed phenotype also displayed low stomatal conductance (Figures 7A and 7B). However, in all of the analyzed T1 mpk12-4 plants, expression of ProMPK12:AtMIR390a-MPK4 or ProHT1:AtMIR390a-MPK4 completely abolished stomatal closure in response to elevated CO2 (Figures 7A and 7B), as well as stomatal opening in response to low CO2 (Supplemental Figures 11A and 11B), similar to that observed in plants with mutations in HT1 (Figures 2A, 2B, 7C, and 7D). These data strongly suggest that MPK4, together with MPK12, contributes to CO2 signaling in guard cells.
Figure 7.
MPK4 Silencing in Guard Cells Abolishes CO2-Induced Stomatal Closure.
ht1-2 ghr1-1 double mutant is CO2 and ABA insensitive and has wild-type-like stomatal conductance.
(A) and (B) Stomatal response to increase in CO2 concentration (from 400 to 800 ppm) of intact mpk12-4 T1 plants transformed with ProMPK12:AtMIR390a-MPK4 or ProHT1:AtMIR390a-MPK4, respectively. Results from one experiment with several independent transgenic plants marked with numbers are shown. For Col-0 and mpk12-4, and error bars mark se (n = 4 and n = 6, respectively), and data from the same experimental series are presented in (A) and (B).
(C) to (E) Stomatal response of intact plants to increase in CO2 concentration (from 400 to 800 ppm), reduction in CO2 concentration (from 400 to 100 ppm), and 5 µM ABA, respectively. Stimulus was applied at time point zero, the experiment was repeated three times, and representative results from one experiment are shown. Error bars indicate se (n = 5).
(F) Model for the role of HT1, MPK12/MPK4, and GHR1 in the regulation of SLAC1 activation in CO2-induced stomatal closure. PIP2 aquaporins facilitate CO2 influx into guard cells. The PIP2;1-interacting carbonic anhydrase CA4 (Wang et al., 2016) together with CA1 accelerate CO2/bicarbonate signal transduction. MPK12/MPK4 and RHC1 form a double negative regulation chain with HT1, controlling GHR1 and then SLAC1 activation in response to CO2. CO2 activates MPK4/MPK12 and RHC1, which then inhibit HT1; thus, HT1 no longer inhibits SLAC1 activation by GHR1, resulting in stomatal closure in response to increased CO2 concentration. Direct HT1-induced inhibition of SLAC1 has not yet been excluded and its contribution would need to be characterized. HT1 can phosphorylate GHR1 and SLAC1. CO2 and ABA-signaling pathways converge at the level of GHR1 kinase, which can be regulated by ABI2, whereas OST1 is regulated by several PP2Cs (ABI, ABI2, PP2CA, and HAB1).
To characterize the genetic interaction of HT1 and GHR1 in planta, we generated an ht1-2 ghr1-1 double mutant and analyzed its stomatal responses. The double mutant displayed traits of both parents. As in ht1-2, the stomata of ht1-2 ghr1-1 plants were completely insensitive to elevated CO2-induced stomatal closure (Figure 7C) as well as low CO2-induced stomatal opening (Figure 7D). The double mutant also displayed ABA and darkness insensitivity similar to ghr1 (Figure 7E; Supplemental Figure 12). The stomatal conductance of ht1-2 ghr1-1 plants was intermediate when compared with that of ht1-2 or ghr1-1 plants (Figures 7C to 7E; Supplemental Figure 12), similar to that observed for ht1-2 ost1-3 plants (Matrosova et al., 2015), suggesting more efficient SLAC1 activation in double mutants compared with ghr1-1 and ost1-3 plants. Therefore, HT1 could act upstream of, and also in parallel with, GHR1 in stomatal closure responses.
Taken together, these data suggest that MPK12 and MPK4 play important roles in an ABA-independent branch of guard cell CO2 signaling by inhibiting the HT1 kinase. This releases SLAC1 from inhibition by HT1 and stomatal closure in response to elevated CO2 can occur (Figure 7F).
DISCUSSION
O3 can be used as a tool to identify genes involved in stomatal regulation, as plants with more open stomata or impaired stomatal closure form visible lesions upon O3 exposure due to higher O3 influx (Overmyer et al., 2000, 2008; Wrzaczek et al., 2009; Brosché et al., 2010). A good example is the characterization of an O3-sensitive mutant rcd3 that led to the identification of the SLAC1 anion channel (Vahisalu et al., 2008). Here, characterization of the suu mutant with dominant phenotypes from the same screen revealed an A109V mutation in HT1 that caused higher than wild-type stomatal conductance and a complete lack of CO2 responses (Figures 2A and 2B). Although both plants with the dominant HT1(A109V) and the recessive kinase-dead HT1 (ht1-2) were equally CO2-insensitive in stomatal responses, these mutations had opposite effects on stomatal conductance, which was reduced 2-fold for the kinase-dead allele and enhanced 2-fold for the dominant allele of HT1. This suggests a dual role for HT1 in CO2 signaling: one via kinase activity, which is impaired in ht1-2 plants and leads to a constitutive high CO2 phenotype accompanied with reduced stomatal conductance, and another via structure or interaction, which is impaired in plants with the A109V mutation in HT1 described in this study. Interestingly, another dominant mutation in HT1, R102K, was recently isolated (Hashimoto-Sugimoto et al., 2016). The ht1-3D plants with the R102K substitution in HT1 also exhibited constitutively higher stomatal conductance and lacked high CO2-induced stomatal closure and low CO2-induced stomatal opening, similar to ht1-8D isolated here. This suggests that the region of the HT1 protein harboring R102 and A109 is important for HT1 function in stomatal CO2 signaling, likely due to protein-protein interactions, as neither of these mutations disrupt HT1 kinase activity (Figure 4A; Hashimoto-Sugimoto et al., 2016).
We identified MPK12 (Jakobson et al., 2016) and MPK4 as negative regulators of HT1 kinase activity and show here that the A109V substitution renders HT1 less sensitive to inhibition by these MAP kinases in vitro (Figures 4D, 6B, and 6D). We also demonstrated that HT1 could inhibit activation of the guard cell anion channel SLAC1 by OST1 and GHR1 in oocytes, whereas adding MPK12 restored the GHR1-induced SLAC1-dependent anion currents in the presence of HT1, but not in the presence of HT1(A109V) (Figure 5). This experiment further supports MPK12 as an inhibitor of HT1 in the guard cells of Arabidopsis. These data also explain the absence of stomatal CO2 responses in plants with the dominant A109V mutation in HT1, as HT1(A109V) could not be inhibited by MPK4 and MPK12. Additionally, HT1(A109V) could still inhibit SLAC1 activation by GHR1 (Figure 5B), which would cause constitutive suppression of SLAC1 activation in plants with HT1(A109V). This would result in the lack of CO2-induced stomatal closure and high stomatal conductance in ht1-8D. Together, these experiments indicate an important role for MAP kinases in guard cell CO2 signal transduction via controlling the activity of HT1, which in turn can regulate activation of the guard cell anion channel SLAC1.
In Arabidopsis, MPK4 has been shown to be important in the regulation of salicylic and jasmonic acid signaling in defense responses (Petersen et al., 2000; Brodersen et al., 2006). However, MPK4 is also strongly expressed in guard cells (Petersen et al., 2000; Zhao et al., 2008; Rodriguez et al., 2010), suggesting that it could be involved in stomatal function in addition to its role in general defense responses. The complete lack of CO2 responses in the T1 transgenic plants with guard cell-specific silencing of MPK4 in an MPK12-deficient background (Figures 7A and 7B; Supplemental Figures 11A and 11B) indicates that MPK4, together with MPK12, plays a major role in stomatal CO2 signaling. In addition to MPK12 and MPK4, a potential role of other MPKs, such as MPK9 (Jammes et al., 2009), in the regulation of CO2-induced stomatal movements deserves more attention in future studies.
An important question in guard cell signaling during stomatal closure is the specificity and interaction of the response pathways to different stimuli that ultimately lead to the activation of SLAC1. Although plants with mutated OST1 display impaired stomatal CO2 responses (Xue et al., 2011; Merilo et al., 2013) and HT1 was recently reported to phosphorylate OST1 by Tian et al. (2015), we did not observe significant HT1-mediated OST1 phosphorylation or HT1-dependent inhibition of SLAC1 phosphorylation by OST1 in vitro (Figure 6A; Supplemental Figure 9A). Initially, we expected that this could be explained by the use of different versions of HT1 in these studies. Tian et al. (2015) used a version of HT1 missing 45 amino acids from the N terminus as annotated in TAIR10, whereas we used the previously published (Hashimoto et al., 2006) full-length version of HT1 as annotated in ARAPORT11. However, under our conditions, the short HT1 also could not inhibit the phosphorylation of SLAC1 by OST1 in vitro (Supplemental Figure 9B). We optimized the reaction conditions for both OST1 and HT1 kinases in vitro and identified that 20 mM MgCl2 is optimal for HT1 activity, whereas 5 mM MnCl2 is optimal for OST1 activity. Thus, the inhibition assay reported here was performed in a buffer containing both 20 mM MgCl2 and 5 mM MnCl2, whereas the inhibition assay conducted by Tian et al. (2015) contained only 10 mM MgCl2, which may be suboptimal for OST1 and HT1 and might explain the difference in the results. Moreover, the short HT1 and the versions of short HT1 with substitutions corresponding to K113W or A109V could all inhibit both OST1 and GHR1-induced SLAC1 activation in oocytes (Supplemental Figure 8). In Tian et al. (2015), the short HT1 with the K113W substitution designed to abolish the kinase activity of the protein was reported not to inhibit OST1-induced SLAC1 activation in oocytes. These differences may be explained by variance in experimental conditions across different laboratories. The similar behavior of the full-length HT1 and short HT1 in in vitro kinase assays, as well as in oocyte assays, suggests that the N-terminal 45 amino acids of HT1 may not be critical for its function in the regulation of SLAC1 activation in guard cell CO2 signaling.
Similar to MPK12, a MATE-type transporter protein RESISTANT TO HIGH CO2 (RHC1) has also been shown to inhibit HT1 activity in oocytes, but in a bicarbonate-dependent manner (Tian et al., 2015). Thus, RHC1 was proposed to act as a bicarbonate-sensing protein. RHC1 causes bicarbonate-insensitive ion currents when expressed in oocytes alone (Wang et al., 2016). It is conceivable that HT1 has different types of inhibitors in planta. As the experiments implicating RHC1 as a regulator of HT1 were performed with the version of HT1 lacking the 45 N-terminal amino acids (Tian et al., 2015), experiments with full-length HT1 and further characterization of the RHC1 protein would help to clarify the role of RHC1 in CO2 signaling and in the regulation of HT1. Because we observed strong phosphorylation of GHR1 by HT1, GHR1 could be an important target for HT1 in the regulation of CO2-induced stomatal closure. Previous studies demonstrated that GHR1 was regulated by the protein phosphatase ABSCISIC ACID INSENSITIVE2 (ABI2) but not by ABI1 (Hua et al., 2012), whereas OST1 is regulated by ABI1, ABI2, PROTEIN PHOSPHATASE 2CA (PP2CA), and HYPERSENSITIVE TO ABA1 (HAB1) (Fujii et al., 2009; Geiger et al., 2009; Lee et al., 2009; Umezawa et al., 2009; Vlad et al., 2009; Brandt et al., 2012). This, together with our earlier results showing a stronger impairment of the CO2 response in plants with a dominant mutation in ABI2 (abi2-1) than that of plants with a dominant mutation in ABI1 (abi1-1) (Merilo et al., 2013), suggests that the GHR1-dependent activation of the anion channel SLAC1 may contribute more to guard cell CO2 signaling than the activation of SLAC1 by OST1.
HT1 could inhibit SLAC1 currents induced by both OST1 and GHR1 in oocytes (Figures 5A and 5B). Experiments with both full-length and short HT1 suggest that HT1 kinase activity is not strictly necessary for this inhibition in oocytes. Whether the kinase activity of HT1 is required for the inhibition of SLAC1 activation in planta remains to be determined, and the molecular details of CO2 signal transduction downstream of HT1 require further investigation. HT1 could inhibit SLAC1 activation via affecting OST1 and GHR1, the latter of which is supported by the strong phosphorylation of GHR1 by HT1 in vitro (Figure 6A). Alternatively, HT1 could affect SLAC1 directly. The phosphorylation of the SLAC1 N terminus by HT1 in vitro (Figure 6A) supports the latter hypothesis. In light of the recent discovery of the importance of tyrosine residues in the SLAC1 transmembrane region (Y243 and Y462) in CO2-induced stomatal closure (Yamamoto et al., 2016), it would be interesting to modify these residues and to test the effect of HT1 on OST1/GHR1-induced activation of the modified SLAC1 in oocytes. Additionally, further analysis of the phosphorylation of full-length SLAC1 by HT1 and versions of SLAC1 with point mutations in potential target sites would be of interest.
In addition to SLAC1, another S-type anion channel, SLAC1 HOMOLOG3 (SLAH3), is expressed in the guard cells of Arabidopsis (Geiger et al., 2011) and could rescue stomatal responsiveness to elevated CO2 in slac1-2 (Negi et al., 2008). Thus, a potential role for SLAH3 in stomatal closure in response to CO2 merits further study. SLAH3 and the Ca2+-dependent protein kinase CPK21 were shown to colocalize with the membrane nanodomain marker protein AtRem1.3 (Demir et al., 2013). The observed pattern of the YFP signal was somewhat similar to the patchy pattern of HT1 localization shown here (Figure 3A). These data hint that similar to SLAH3, HT1 may be localized in specific membrane domains and future studies into the subcellular localization of HT1 as well as interaction partners of HT1 could bring valuable insight into the mechanisms by which HT1 regulates CO2 signaling.
Based on the results discussed here, we propose a model for the role of HT1, MPK12, and MPK4 in the regulation of SLAC1 in CO2-induced stomatal closure (Figure 7F). Briefly, HT1 acts as a negative regulator of CO2-induced stomatal closure by inhibiting the activation of SLAC1 by GHR1 and OST1. MPK12 and MPK4, and potentially also RHC1 (Tian et al., 2015), act as positive regulators by inhibiting HT1 in the presence of elevated CO2. This in turn releases SLAC1 from inhibition by HT1 and leads to the subsequent activation of SLAC1 in response to CO2 via a mechanism that is yet to be characterized. As the stomatal opening in response to low CO2 is also severely impaired both in loss-of-function ht1-2 (Hashimoto et al., 2006; Matrosova et al., 2015) and in the dominant ht1-8D allele identified in this study (Figure 2B), but is functional in plants defective in GHR1 (Figure 7D) and OST1 (Mustilli et al., 2002; Xue et al., 2011; Matrosova et al., 2015), it is likely that regulation of HT1 by MPKs also has a role in the stomatal opening pathway. This is further supported by the very high stomatal conductance of ht1-8D (Figure 2), which is higher than the stomatal conductance of plants with impaired SLAC1, GHR1, or OST1 (Figures 7C to 7E; Merilo et al., 2013). This suggests that MPKs and HT1 could also be linked to the low CO2-induced activation of plasma membrane H+-ATPases, which appears to be one of the most poorly understood branches of stomatal CO2 signaling, despite its ancient origin and central importance for plant growth (Kollist et al., 2014).
Taken together, the results presented here indicate an important role for MPK12 and MPK4 as negative regulators of HT1 and show that alanine 109 in HT1 is essential for this function. The data suggest that MPK12, MPK4, and HT1 control CO2-induced stomatal closure via the regulation of SLAC1 activation by OST1 and GHR1. These findings reveal important insights regarding the ABA-independent regulation of stomatal CO2 signaling and can be used for designing further research aimed to identify breeding targets that regulate plant water use efficiency for the shifting global climate.
METHODS
Mutant Isolation, Ozone Exposure, and Genetic Complementation
The mutant screen for O3 sensitivity in Arabidopsis thaliana has been described previously (Overmyer et al., 2000). The suu mutant was found segregating in the rcd7 mutant background. Gas exchange and O3-sensitivity analysis of multiple lines, including backcrossed plants, indicated that suu and rcd7 conferred their respective phenotypes independently. The suu mutation was isolated in a line lacking rcd7 from segregating backcrossed plants using CAPS markers indicated in Supplemental Table 1. The restriction enzymes used for digestion of PCR products were PleI for suu and HindIII for rcd7 mutation, respectively. The analysis of suu is presented here but rcd7 will be described elsewhere. The sequenced genome of rcd7, containing a heterozygous mutation in suu, was analyzed to identify heterozygous mutations from the suu candidate region defined by mapping (Supplemental Figure 1A). Briefly, SOLiD reads were mapped to reference genome (TAIR10) and single nucleotide polymorphisms (SNPs) were called using MAQ software. SNPs were analyzed by developing an in-house script in R (version 3.0.3) using Biostrings and Biomart packages. The script filtered the SNP list for the coordinates of the given window, selected SNPs with a quality score >20, mapped them to exons and introns using gene coordinates from TAIR10 (www.arabidopsis.org), and selected heterozygous SNPs. Due to the known role of HT1 in stomatal regulation, the ht1-8D mutation was targeted for complementation.
For genetic complementation, the HT1 locus, including the promoter and the 3′ untranslated region, was amplified with primers indicated in Supplemental Table 1 using genomic DNA extracted from the ht1-8D mutant as template. The primers included adapter regions to enable cloning the fragment into Gateway pDONR/Zeo vector (Thermo Fisher Scientific), which was followed by recombination of the fragment into pMDC100 destination vector (Curtis and Grossniklaus, 2003). Wild-type Col-0 plants were transformed with the construct and homozygous lines were selected by segregation analysis.
Three-week-old plants were exposed to O3 (350 ppb for 6 h). O3 damage was visually scored 1 and 2 d after exposure and photos of the plants were taken 1 d after exposure. For electrolyte leakage, rosettes were collected into 15 mL of MilliQ water 2 h after the end of exposure and conductance of the O3 treated and control samples was measured 2, 16, and 24 h following sample collection, with a Metler conductivity meter (Model FE30) and analyzed as described (Overmyer et al., 2000).
Silencing of MPK4 in mpk12-4
MPK4 silencing constructs were designed as described (Carbonell et al., 2014). Briefly, the oligonucleotides indicated in Supplemental Table 1 were annealed and ligated to vector pMDC32B-AtMIR390a-B/c where the 35S promoter had been replaced by ProHT1 (the 1865-bp sequence upstream of HT1 start codon) or ProMPK12 (the 546-bp sequence upstream of MPK12 start codon) using the primers listed in Supplemental Table 1. The resulting pProMPK12:AtMIR390a-MPK4 and pProHT1:AtMIR390a-MPK4 vectors were transformed to Agrobacterium tumefaciens strain C58GV3101, and Arabidopsis mpk12-4 plants were transformed with the floral dip method (Clough and Bent, 1998). T1 seeds were sown onto MS medium supplemented with 25 µg/mL hygromycin (PhytoTechnology Laboratories) and hygromycin-resistant individuals were transferred to pots for gas-exchange analysis after ∼1.5 weeks.
Split-Ubiquitin Yeast Two-Hybrid Assays
Initially, a partial MPK4 clone (with the first 18 amino acids missing) was isolated in a split-ubiquitin yeast two-hybrid library screen to identify candidate HT1-interacting proteins using the DUALhunter kit (Dualsystems Biotech). This partial MPK4 clone, in the pPR3-N vector, was isolated and its interaction with HT1 and HT1(A109V) was further verified by transforming yeast cells expressing either a HT1 or HT1(A109V) bait. A positive (pAI-Alg5 with the native NubI) and a negative (pDL2-Alg5 with the mutated NubG) prey controls were also used to ensure successful transformation. The transformation mixtures were split into half and plated on SD-LeuTrp (SD-2; for selection of both bait and prey constructs) and SD-LeuTrpHisAde (SD-4; for selection of interaction) plates. Interactions between HT1 variants and selected proteins were tested in pairwise split-ubiquitin yeast two-hybrid assays. The full-length coding sequences of each interaction pair were cloned into either pDHB1 (with the Cub-LexA-VP16 fusion) or pPR3-N (with a mutated NubG) vector at the SfiI site. The yeast strain NMY51 was cotransformed with bait and prey plasmids and grown on SD-2 plates. At least 10 colonies from each transformation were pooled and resuspended in water to OD600 of 0.5 to prepare for 10, 100, and 1000× serial dilutions and spotted on SD-2 and SD-4 plates. Plates were incubated at 30°C for 2 to 4 d and photographed.
Protein Localization and BiFC Assays
Binary constructs containing full-length or split YFPs were designed and generated for cloning genes of interest by the ligation-independent cloning (LIC) method. First, YFP, YFPn (amino acids 1 to 173 of eYFP), and YFPc (amino acids 155 to 279 of eYFP) were amplified by multi-PCR steps to incorporate sequences for LIC method and a HA tag at the 5′ and 3′ ends, respectively. The PCR products were digested by EcoRI and cloned into the modified p35S/pCAMBIA1390 (Wang et al., 2004) at the EcoRI/PmlI site to create 35S:YFPLIC, 35S:YFPn, and 35S:YFPc in the pCAMBIA1390 vector, respectively. Similarly, a 35s:CFPLIC construct was generated by a series of PCR and ligation with adaptors to incorporate sequences for LIC method at the 5′-end and a dual c-myc/6xHis tag at the 3′ end. The introduced sequences at the EcoRI/PmlI site of the p35S/pCAMBIA1390 are shown in the Supplemental Figure 13.
For subsequent cloning, each gene of interest was amplified by two consecutive PCRs: first with gene-specific primers and later with a pair of universal primers designed specifically for the LIC method. All primers used are listed in the Supplemental Table 1. To prepare vectors for LIC, plasmids of 35S:CFPLIC, 35S:YFPLIC, 35S:YFPn, and 35S:YFPc were linearized by PmlI digestion, followed by T4 DNA polymerase treatment with dGTP to create 15- to 16-nucleotide 5′-overhangs. For insert preparation, the final PCR products of target genes were incubated with T4 DNA polymerase in the presence of dCTP to create the complementary overhangs with the vectors. Both vector and insert were mixed at room temperature and transformed to Escherichia coli after 5 min. The final constructs were sequence verified and immobilized to the Agrobacterium GV3101 for agroinfiltration experiments.
For protein localization assays, Nicotiana benthamiana leaves were infiltrated with agrobacteria carrying either a 35S:HT1-YFP or 35S:HT1(A109V)-YFP construct, along with the silencing suppressor P19 in the infiltration buffer (10 mM MES, 10 mM MgCl2, and 200 μM acetosyringone). For the BiFC assays, four different agrobacterial clones each harboring a YFPn fusion, a YFPc fusion, an internal control (35S:SLAC1-CFP), or the gene silencing suppressor P19 were coinfiltrated to the leaves of N. benthamiana at an OD600 of 0.02 for each clone. Images were acquired at 3 dpi with a Zeiss LSM710 confocal microscope. The YFP signals were excited by a 514-nm laser, and emission between 518 and 564 nm was collected. The CFP signals were excited by a 405-nm laser, and emission at 460 to 530 nm was collected. Z-stack images of ∼15 μm thickness were collected and all images were acquired at the 16-bit depth for a higher dynamic range for the quantification assay. The fluorescence intensity was measured by the ImageJ software. The leaf samples used for imaging were collected and used for protein extraction followed by immunoblot analysis. N. benthamiana leaves infiltrated with agrobacteria harboring individual split YFP fusions and the P19 silencing suppressor were subjected to protein extraction and used as controls in the immunoblot analysis.
Immunoblot Analysis
The leaf samples (30 to 40 mg) were ground under liquid nitrogen and boiled 10 min in 100 μL of 6× Laemmli buffer. Twelve microliters of each sample was separated on 10% SDS polyacrylamide gel. After SDS-PAGE, proteins were transferred onto nitrocellulose membrane. Immunodetection of HA-tagged proteins was performed with monoclonal anti-HA antibody [16B12] (Biolegend; cat. no. 901502, lot no. B220767).
Gas-Exchange Measurements
Plants for gas-exchange measurements were sown into 4:2:3 peat:vermiculite:water mixture in glass-covered pots as described before (Kollist et al., 2007). Plants were grown in growth cabinets (AR-66LX; Percival Scientific; MCA1600, Snijders Scientific) with a 12-h-day (23°C) and 12-h-night (20°C) cycle at 70% relative humidity and 100 to 150 μmol m−2 s−1 light. Plants were watered once a week and 25- to 30-d-old plants were used for experiments.
Gas-exchange measurements were performed with a custom-built gas-exchange device (Kollist et al., 2007). Briefly, plants were inserted into experimental chambers where their stomatal conductance was recorded. When stomatal conductance had stabilized at ∼65 to 75% relative humidity and 100 μmol m−2 s−1 light, different stimuli were applied. Treatments included CO2 (increase from 400 ppm to 800 ppm or decrease from 400 ppm to 100 ppm), darkness, decrease in relative air humidity (from ∼75 to ∼35%), ozone (400 ppb for 3 min), and ABA. ABA-induced stomatal closure experiments were performed as described previously with 5 µM ABA (Merilo et al., 2015). In ABA-induced inhibition of light-induced opening experiments, plants were first allowed to stabilize in darkness; thereafter, plants were taken out from the experimental chamber, sprayed with 2.5 µM ABA, and immediately returned to chambers. Measurement of stomatal conductance was continued in light (100 μmol m−2 s−1).
Fresh Weight Loss Assay and Stomatal Density
Plants for measuring fresh weight loss and stomatal density were sown into 4:2:3 peat:vermiculite:water mixture and grown in growth room at 21°C with 12-h-light (100 μmol m−2 s−1) and 12-h-dark regime. Four-week-old plants were used for experiments.
For fresh weight loss assay, three leaves of each plant were excised and weighed. Leaves were left at room temperature abaxial side up for 2 h. Then leaves were reweighed and fresh weight loss was calculated as percentage leaf mass lost within the 2 h.
Stomatal density was measured via stomatal impressions as described before (Casson et al., 2009). Briefly, impression of one leaf per each plant was created via application of dental resin (Xantopren M mucosa; Heraeus). Nail varnish was applied to the impression and transferred to a microscope slide with transparent tape when dry. One area of ∼0.12 mm−2 in the central part of the leaf close to the central vein was imaged with a Zeiss SteREO Discovery.V20 stereomicroscope, stomata were counted using ImageJ 1.46r software (Schneider et al., 2012), and stomatal density per mm2 was calculated.
Protein Expression, Purification, and in Vitro Kinase Assays
For in vitro kinase assays, HT1, HT1(A109V), HT1(K113M), HT1s, HT1s(A64V), HT1s(K68M), MPK4, MPK4(K72M/K73R), MPK4(D198G/E202A), and MPK12 were cloned into the pET28a vector (Novagen) using primers with the indicated cloning sites listed in Supplemental Table 1. OST1, OST1(K50N), HT1(K113M), SLAC1 C terminus, and GHR1 were cloned into pGEX-4T-1 vector (GE Healthcare) using primers with the indicated cloning sites listed in Supplemental Table 1. Point mutations corresponding to K113M in HT1, K50N in OST1, and K72M/K73R in MPK4 were created with two-step PCR using primers listed in Supplemental Table 1. In the case of MPK4(D198G/E202A), cDNA from plants expressing MPK4(D198G/E202A) was used as the template for cloning (Berriri et al., 2012). The plasmid for the SLAC1 N-terminal coding region for amino acids 1 to 186 was established previously (Vahisalu et al., 2010).
6xHis-HT1, 6xHis-HT1(A109V), 6xHis-HT1(K113M), 6xHis-HT1s, 6xHis-HT1s(A64V), 6xHis-HT1s(K68M), 6xHis-MPK12, 6xHis-MPK4, 6xHis-MPK4(D198G/E202A), 6xHis-MPK4(K72M/K73R), 6xHis-SLAC-N, GST-HT1(K113M), GST-SLAC1-C, GST-GHR1, GST-OST1, and GST-OST1(K50N) were expressed in E. coli BL21(DE3) cells. The cultures were grown in 2xYT medium at 37°C to OD600 ∼0.6. Recombinant protein expression was induced with 0.3 mM IPTG at 16°C for 16 h. 6xHis-tagged proteins were purified by nickel-affinity chromatography. GST-tagged proteins were purified by glutathione-affinity chromatography. The presence of HT1 and HT1(A109V) was determined by immunoblot with anti-His antibody.
HT1 kinase activity assay was performed by incubating constant amounts of purified recombinant 6xHis-HT1 and 6xHis-HT1(A109V) with GST-GHR1 (0.3 μM), 6xHis-SLAC1-N (5 μM), GST-SLAC1-C (5 μM), GST-OST1(K50N) (2 μM), and casein (1 mg/mL) in a reaction buffer (50 mM Tris-HCl, pH7.4, 150 mM NaCl, 20 mM MgCl2, 500 μM ATP, 1 mM DTT, 0.2 mg/mL insulin, and 100 μCi/mL [γ-32P]ATP) at room temperature for 40 min.
The HT1 inhibition assay was performed by incubating constant amount of 6xHis-HT1 and 6xHis-HT1(A109V) with 6xHis-MPK12 or 6xHis-MPK4, 6xHis-MPK4(D198G/E202A) and 6xHis-MPK4(K72M/K73R) in a reaction buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 20 mM MgCl2, 1 mM DTT, and 0.2 mg/mL insulin) at room temperature for 10 min. Then GST-GHR1 (0.3 μM) or casein (1 mg/mL), 500 μM ATP, and 100 μCi/mL [γ-32P]ATP were added and reaction aliquots were taken at the 40-min time point. The final concentrations of MPK12 and MPK4 variants were 0 to 30 μM.
OST1 inhibition assay was performed by incubating GST-OST1 (1 μM) and GST-OST1(K50N) (1 μM) with constant amount of 6xHis-HT1, 6xHis-HT1(A109V), and 6xHis-HT1(K113M) or 6xHis-HT1s, 6xHis-HT1s(A64V), and 6xHis-HT1s(K68M) in a reaction buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 20 mM MgCl2, 500 μM ATP, 1 mM DTT, 0.2 mg/mL insulin, and 100 μCi/ml [γ-32P]ATP [only in control reactions]) at room temperature for 60 min. Then the reactions without [γ-32P]ATP were diluted (1/10 and 1/50) and 2.5 μM 6xHis-SLAC1-N, 2 mM MnCl2, and 100 μCi/mL [γ-32P]ATP were added. Reaction aliquots were taken at the 15-min time point.
All reactions were stopped by the addition of SDS loading buffer. Proteins were separated by 10% SDS-PAGE and visualized by Coomassie Brilliant Blue G 250 (Sigma-Aldrich) staining. HT1 and OST1 activity was determined by autoradiography.
Oocyte Electrophysiology
All constructs were cloned into the pNB1 oocyte expression vector using the USER method (Nour-Eldin et al., 2006). cRNAs were synthesized from 0.5 µg of linearized plasmid DNA template using the mMessage mMachine in vitro transcription kit (Ambion). Approximately 10 ng of each of the indicated cRNAs was injected into oocytes. Injected oocytes were then incubated in ND96 buffer at 16°C for 3 d before electrophysiological recording. The recording buffer contained 70 mM Na-gluconate, 24 mM NaCl, 10 mM MES/Tris, pH 7.4, 2 mM KCl, 1 mM CaCl2, and 1 mM MgCl2. Osmolality was adjusted to 220 mM using d-sorbitol. The presented results were obtained in at least three independent batches of oocytes. As expression levels can vary from one oocyte batch to another, data from one representative batch of oocytes are shown in the figures, with similar results, albeit at different absolute current magnitudes, having been obtained in independent oocyte batches. The replicates for the representative experiments are shown in Supplemental Figures 14 and 15. The numbers of oocytes investigated in each of the illustrated batches are given in the figures, with similar numbers of oocytes measured in each independent batch showing consistent data. Steady state currents were recorded from a holding potential of 0 mV and ranging from +40 to −160 mV in −20 mV decrement, followed by a −120 mV voltage “tail” pulse. Whole-cell ionic currents were recorded with a Cornerstone TEV-200 two-electrode voltage clamp amplifier (Dagan) and digitized using an Axon Instruments Digidata 1440A low-noise data acquisition system (Molecular Devices) controlled by pClamp 10 acquisition software (Molecular Devices). Microelectrodes were fabricated with a P-87 Flaming/Brown microelectrode micropipette puller (Sutter) from borosilicate glass (GC200TF-10; Warner Instruments).
Statistical Analyses
Statistical analyses were performed with Statistica, version 7.1 (StatSoft). ANOVA with Tukey or Tukey unequal N HSD post hoc test or Student’s t test were used as indicated in figure legends. All effects were considered significant at P < 0.05. The tables with the results of ANOVAs and post hoc tests are presented in the supplemental data.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: AT1G62400, AT2G46070, AT4G01370, AT4G20940, AT4G33950, and AT1G12480.
Supplemental Data
Supplemental Figure 1. HT1(A109V) is the underlying mutation of suu (ht1-8D) phenotypes.
Supplemental Figure 2. Stomatal responses of HT1(A109V)-expressing plants.
Supplemental Figure 3. MPK4 interacts with HT1 in a split-ubiquitin yeast two-hybrid assay.
Supplemental Figure 4. The CO2 responses are functional in mpk4 NahG mutants.
Supplemental Figure 5. HT1 but not HT1(A109V) interacts with MPK12 and MPK4, and neither HT1 nor HT1(A109V) interacts with MPK11 in the split-ubiquitin yeast two-hybrid assay.
Supplemental Figure 6. Representative images from quantitative BiFC assay.
Supplemental Figure 7. Representative ion current traces from single oocytes for experiments presented in Figure 5.
Supplemental Figure 8. HT1 with a deletion of the N-terminal 45 amino acids (HT1s) inhibits OST1- and GHR1-activated SLAC1 anion currents in oocytes and MPK12 counteracts HT1s-induced downregulation of SLAC1 activity in oocytes.
Supplemental Figure 9. Neither HT1 nor HT1 with a deletion of the N-terminal 45 amino acids (HT1s) inhibit OST1-induced SLAC1 phosphorylation in vitro.
Supplemental Figure 10. Phenotype of T1 transgenic mpk12-4 plants transformed with ProMPK12:AtMIR390a-MPK4 or ProHT1:AtMIR390a-MPK4.
Supplemental Figure 11. Response to low CO2 is absent in mpk12-4 plants with silenced MPK4.
Supplemental Figure 12. The ht1-2 ghr1-1 double mutant is darkness insensitive and has wild-type-like stomatal conductance.
Supplemental Figure 13. Diagram of ligation-independent cloning-compatible binary vectors.
Supplemental Figure 14. Replicate data for Figure 5: HT1 inhibits OST1- and GHR1-activated SLAC1 anion currents in oocytes and MPK12 counteracts HT1-induced downregulation of SLAC1 activity in oocytes.
Supplemental Figure 15. Replicate data for Supplemental Figure 8: HT1 with a deletion of the N-terminal 45 amino acids (HT1s) inhibits OST1- and GHR1-activated SLAC1 anion currents in oocytes and MPK12 counteracts HT1s-induced downregulation of SLAC1 activity in oocytes.
Supplemental Table 1. Primers used in this study.
Supplementary Material
Acknowledgments
We thank Leena Grönholm, Airi Lamminmäki, Tuomas Puukko, Irina Rasulova, Susanna Tarkiainen, and Elis Tiivoja for technical assistance. We thank Koh Iba for providing ht1-2 seeds, John Mundy for mpk4 NahG seeds, and Jean Colcombet for the seeds of mpk4-2 plant lines expressing MPK4(D198G/E202A) or wild-type MPK4. We thank Zhizhong Gong for providing ghr1-1 seeds. This work was supported by the Estonian Ministry of Science and Education (IUT2-21, H.K.; PUT-545, Y.-S.W.), the European Regional Development (Center of Excellence in Molecular Cell Engineering CEMCE; H.K.) and Social Fund (Mobilitas Top Researchers grant MTT9; M.B.), the European Research Council (Consolidator grant Phosphorocessors; M.L.), the U.S. National Science Foundation (MCB-1616236; J.I.S.), the U.S. National Institutes of Health (GM060396; J.I.S.), the University of Helsinki (K.O. and J.K.), the Academy of Finland Fellowship (Decisions 251397, 256073, and 283254; K.O.), the Academy of Finland Centre of Excellence programs (2006-11 and 2014-19; J.K.) and Research Grant (Decision 250336; J.K.), and the Ella and Georg Ehrnrooth Foundation (M.S.).
AUTHOR CONTRIBUTIONS
H.H., M.S., Y.-S.W., E.V., K.O., M.L., M.B., J.I.S., J.K., and H.K. designed the research. H.H., M.S., K.T., C.W., M.N., Y.-S.W., E.V., P.P., E.M., J.S., and K.O. performed the research. H.H., M.S., K.T., C.W., Y.-S.W., E.V., E.M., J.S., K.O., M.B., J.I.S., J.K., and H.K. analyzed the data. H.H. and H.K. wrote the article and all authors edited and commented on the manuscript.
Glossary
- ABA
abscisic acid
- BiFC
bimolecular fluorescence complementation
- SNP
single nucleotide polymorphism
- LIC
ligation-independent cloning
References
- Berriri S., Garcia A.V., Frei dit Frey N., Rozhon W., Pateyron S., Leonhardt N., Montillet J.L., Leung J., Hirt H., Colcombet J. (2012). Constitutively active mitogen-activated protein kinase versions reveal functions of Arabidopsis MPK4 in pathogen defense signaling. Plant Cell 24: 4281–4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B., Brodsky D.E., Xue S., Negi J., Iba K., Kangasjärvi J., Ghassemian M., Stephan A.B., Hu H., Schroeder J.I. (2012). Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc. Natl. Acad. Sci. USA 109: 10593–10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen P., Petersen M., Bjørn Nielsen H., Zhu S., Newman M.-A., Shokat K.M., Rietz S., Parker J., Mundy J. (2006). Arabidopsis MAP kinase 4 regulates salicylic acid- and jasmonic acid/ethylene-dependent responses via EDS1 and PAD4. Plant J. 47: 532–546. [DOI] [PubMed] [Google Scholar]
- Brosché M., Merilo E., Mayer F., Pechter P., Puzõrjova I., Brader G., Kangasjärvi J., Kollist H. (2010). Natural variation in ozone sensitivity among Arabidopsis thaliana accessions and its relation to stomatal conductance. Plant Cell Environ. 33: 914–925. [DOI] [PubMed] [Google Scholar]
- Carbonell A., Takeda A., Fahlgren N., Johnson S.C., Cuperus J.T., Carrington J.C. (2014). New generation of artificial MicroRNA and synthetic trans-acting small interfering RNA vectors for efficient gene silencing in Arabidopsis. Plant Physiol. 165: 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casson S.A., Franklin K.A., Gray J.E., Grierson C.S., Whitelam G.C., Hetherington A.M. (2009). phytochrome B and PIF4 regulate stomatal development in response to light quantity. Curr. Biol. 19: 229–234. [DOI] [PubMed] [Google Scholar]
- Chater C., et al. (2015). Elevated CO2-induced responses in stomata require ABA and ABA signaling. Curr. Biol. 25: 2709–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Curtis M.D., Grossniklaus U. (2003). A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 133: 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir F., Horntrich C., Blachutzik J.O., Scherzer S., Reinders Y., Kierszniowska S., Schulze W.X., Harms G.S., Hedrich R., Geiger D., Kreuzer I. (2013). Arabidopsis nanodomain-delimited ABA signaling pathway regulates the anion channel SLAH3. Proc. Natl. Acad. Sci. USA 110: 8296–8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Marais D.L., Auchincloss L.C., Sukamtoh E., McKay J.K., Logan T., Richards J.H., Juenger T.E. (2014). Variation in MPK12 affects water use efficiency in Arabidopsis and reveals a pleiotropic link between guard cell size and ABA response. Proc. Natl. Acad. Sci. USA 111: 2836–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H., Chinnusamy V., Rodrigues A., Rubio S., Antoni R., Park S.-Y., Cutler S.R., Sheen J., Rodriguez P.L., Zhu J.-K. (2009). In vitro reconstitution of an abscisic acid signalling pathway. Nature 462: 660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D., Maierhofer T., Al-Rasheid K.A., Scherzer S., Mumm P., Liese A., Ache P., Wellmann C., Marten I., Grill E., Romeis T., Hedrich R. (2011). Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Sci. Signal. 4: RA32–RA32. [DOI] [PubMed] [Google Scholar]
- Geiger D., Scherzer S., Mumm P., Marten I., Ache P., Matschi S., Liese A., Wellmann C., Al-Rasheid K.A., Grill E., Romeis T., Hedrich R. (2010). Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc. Natl. Acad. Sci. USA 107: 8023–8028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D., Scherzer S., Mumm P., Stange A., Marten I., Bauer H., Ache P., Matschi S., Liese A., Al-Rasheid K.A.S., Romeis T., Hedrich R. (2009). Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc. Natl. Acad. Sci. USA 106: 21425–21430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M., Negi J., Young J., Israelsson M., Schroeder J.I., Iba K. (2006). Arabidopsis HT1 kinase controls stomatal movements in response to CO2. Nat. Cell Biol. 8: 391–397. [DOI] [PubMed] [Google Scholar]
- Hashimoto-Sugimoto M., Negi J., Monda K., Higaki T., Isogai Y., Nakano T., Hasezawa S., Iba K. (2016). Dominant and recessive mutations in the Raf-like kinase HT1 gene completely disrupt stomatal responses to CO2 in Arabidopsis. J. Exp. Bot. 67: 3251–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Boisson-Dernier A., Israelsson-Nordström M., Böhmer M., Xue S., Ries A., Godoski J., Kuhn J.M., Schroeder J.I. (2010). Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nat. Cell Biol. 12: 87–93, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Rappel W.-J., Occhipinti R., Ries A., Böhmer M., You L., Xiao C., Engineer C.B., Boron W.F., Schroeder J.I. (2015). Distinct cellular locations of carbonic anhydrases mediate CO2 control of stomatal movements. Plant Physiol. 169: 1168–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua D., Wang C., He J., Liao H., Duan Y., Zhu Z., Guo Y., Chen Z., Gong Z. (2012). A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 24: 2546–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura K., et al. ; MAPK Group (2002). Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci. 7: 301–308. [DOI] [PubMed] [Google Scholar]
- Jakobson L., et al. (2016). Natural variation in Arabidopsis Cvi-0 accession uncovers regulation of guard cell CO2 signaling by MPK12. bioRxiv, http://dx.doi.org/10.1101/73015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jammes F., et al. (2009). MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc. Natl. Acad. Sci. USA 106: 20520–20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollist H., Nuhkat M., Roelfsema M.R.G. (2014). Closing gaps: linking elements that control stomatal movement. New Phytol. 203: 44–62. [DOI] [PubMed] [Google Scholar]
- Kollist T., Moldau H., Rasulov B., Oja V., Rämma H., Hüve K., Jaspers P., Kangasjärvi J., Kollist H. (2007). A novel device detects a rapid ozone-induced transient stomatal closure in intact Arabidopsis and its absence in abi2 mutant. Physiol. Plant. 129: 796–803. [Google Scholar]
- Lee S.C., Lan W., Buchanan B.B., Luan S. (2009). A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc. Natl. Acad. Sci. USA 106: 21419–21424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Szostkiewicz I., Korte A., Moes D., Yang Y., Christmann A., Grill E. (2009). Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324: 1064–1068. [DOI] [PubMed] [Google Scholar]
- Marten H., Hyun T., Gomi K., Seo S., Hedrich R., Roelfsema M.R.G. (2008). Silencing of NtMPK4 impairs CO2-induced stomatal closure, activation of anion channels and cytosolic Casignals in Nicotiana tabacum guard cells. Plant J. 55: 698–708. [DOI] [PubMed] [Google Scholar]
- Matrosova A., Bogireddi H., Mateo-Peñas A., Hashimoto-Sugimoto M., Iba K., Schroeder J.I., Israelsson-Nordström M. (2015). The HT1 protein kinase is essential for red light-induced stomatal opening and genetically interacts with OST1 in red light and CO2-induced stomatal movement responses. New Phytol. 208: 1126–1137. [DOI] [PubMed] [Google Scholar]
- Merilo E., Jalakas P., Kollist H., Brosché M. (2015). The role of ABA recycling and transporter proteins in rapid stomatal responses to reduced air humidity, elevated CO2, and exogenous ABA. Mol. Plant 8: 657–659. [DOI] [PubMed] [Google Scholar]
- Merilo E., Laanemets K., Hu H., Xue S., Jakobson L., Tulva I., Gonzalez-Guzman M., Rodriguez P.L., Schroeder J.I., Brosche M., Kollist H. (2013). PYR/RCAR receptors contribute to ozone-, reduced air humidity-, darkness-, and CO2-induced stomatal regulation. Plant Physiol. 162: 1652–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustilli A.-C., Merlot S., Vavasseur A., Fenzi F., Giraudat J. (2002). Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14: 3089–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi J., Matsuda O., Nagasawa T., Oba Y., Takahashi H., Kawai-Yamada M., Uchimiya H., Hashimoto M., Iba K. (2008). CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452: 483–486. [DOI] [PubMed] [Google Scholar]
- Nour-Eldin H.H., Hansen B.G., Nørholm M.H.H., Jensen J.K., Halkier B.A. (2006). Advancing uracil-excision based cloning towards an ideal technique for cloning PCR fragments. Nucleic Acids Res. 34: e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmyer K., Kollist H., Tuominen H., Betz C., Langebartels C., Wingsle G., Kangasjärvi S., Brader G., Mullineaux P., Kangasjärvi J. (2008). Complex phenotypic profiles leading to ozone sensitivity in Arabidopsis thaliana mutants. Plant Cell Environ. 31: 1237–1249. [DOI] [PubMed] [Google Scholar]
- Overmyer K., Tuominen H., Kettunen R., Betz C., Langebartels C., Sandermann H. Jr., Kangasjärvi J. (2000). Ozone-sensitive arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. Plant Cell 12: 1849–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.-Y., et al. (2009). Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M., et al. (2000). Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell 103: 1111–1120. [DOI] [PubMed] [Google Scholar]
- Rodriguez M.C., Petersen M., Mundy J. (2010). Mitogen-activated protein kinase signaling in plants. Annu. Rev. Plant Biol. 61: 621–649. [DOI] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W., et al. (2015). A molecular pathway for CO2 response in Arabidopsis guard cells. Nat. Commun. 6: 6057. [DOI] [PubMed] [Google Scholar]
- Umezawa T., Sugiyama N., Mizoguchi M., Hayashi S., Myouga F., Yamaguchi-Shinozaki K., Ishihama Y., Hirayama T., Shinozaki K. (2009). Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc. Natl. Acad. Sci. USA 106: 17588–17593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T., et al. (2010). Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. Plant J. 62: 442–453. [DOI] [PubMed] [Google Scholar]
- Vahisalu T., Kollist H., Wang Y.-F., Nishimura N., Chan W.-Y., Valerio G., Lamminmäki A., Brosché M., Moldau H., Desikan R., Schroeder J.I., Kangasjärvi J. (2008). SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452: 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlad F., Rubio S., Rodrigues A., Sirichandra C., Belin C., Robert N., Leung J., Rodriguez P.L., Laurière C., Merlot S. (2009). Protein phosphatases 2C regulate the activation of the Snf1-related kinase OST1 by abscisic acid in Arabidopsis. Plant Cell 21: 3170–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Hu H., Qin X., Zeise B., Xu D., Rappel W.-J., Boron W.F., Schroeder J.I. (2016). Reconstitution of CO2 regulation of SLAC1 anion channel and function of CO2-permeable PIP2;1 aquaporin as carbonic anhydrase 4 interactor. Plant Cell 28: 568–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.-S., Motes C.M., Mohamalawari D.R., Blancaflor E.B. (2004). Green fluorescent protein fusions to Arabidopsis fimbrin 1 for spatio-temporal imaging of F-actin dynamics in roots. Cell Motil. Cytoskeleton 59: 79–93. [DOI] [PubMed] [Google Scholar]
- Wrzaczek M., Brosché M., Kollist H., Kangasjärvi J. (2009). Arabidopsis GRI is involved in the regulation of cell death induced by extracellular ROS. Proc. Natl. Acad. Sci. USA 106: 5412–5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S., Hu H., Ries A., Merilo E., Kollist H., Schroeder J.I. (2011). Central functions of bicarbonate in S-type anion channel activation and OST1 protein kinase in CO2 signal transduction in guard cell. EMBO J. 30: 1645–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Negi J., Wang C., Isogai Y., Schroeder J.I., Iba K. (2016). The transmembrane region of guard cell SLAC1 channels perceives CO2 signals via an ABA-independent pathway in Arabidopsis. Plant Cell 28: 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Zhang W., Stanley B.A., Assmann S.M. (2008). Functional proteomics of Arabidopsis thaliana guard cells uncovers new stomatal signaling pathways. Plant Cell 20: 3210–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.