Genomic, transcriptomic, genetic, and biochemical evidence implicates Arabidopsis TH2 in dephosphorylation of thiamin monophosphate, a key step in biosynthesis of thiamin diphosphate, an essential cofactor for central metabolic enzymes.
Abstract
To synthesize the cofactor thiamin diphosphate (ThDP), plants must first hydrolyze thiamin monophosphate (ThMP) to thiamin, but dedicated enzymes for this hydrolysis step were unknown and widely doubted to exist. The classical thiamin-requiring th2-1 mutation in Arabidopsis thaliana was shown to reduce ThDP levels by half and to increase ThMP levels 5-fold, implying that the THIAMIN REQUIRING2 (TH2) gene product could be a dedicated ThMP phosphatase. Genomic and transcriptomic data indicated that TH2 corresponds to At5g32470, encoding a HAD (haloacid dehalogenase) family phosphatase fused to a TenA (thiamin salvage) family protein. Like the th2-1 mutant, an insertional mutant of At5g32470 accumulated ThMP, and the thiamin requirement of the th2-1 mutant was complemented by wild-type At5g32470. Complementation tests in Escherichia coli and enzyme assays with recombinant proteins confirmed that At5g32470 and its maize (Zea mays) orthologs GRMZM2G148896 and GRMZM2G078283 are ThMP-selective phosphatases whose activity resides in the HAD domain and that the At5g32470 TenA domain has the expected thiamin salvage activity. In vitro and in vivo experiments showed that alternative translation start sites direct the At5g32470 protein to the cytosol and potentially also to mitochondria. Our findings establish that plants have a dedicated ThMP phosphatase and indicate that modest (50%) ThDP depletion can produce severe deficiency symptoms.
INTRODUCTION
In all forms of life, thiamin diphosphate (ThDP) is an essential cofactor for central metabolic enzymes such as α-keto acid dehydrogenases, transketolase, pyruvate decarboxylase, and acetolactate synthase (Goyer, 2010). ThDP is synthesized de novo by prokaryotes, fungi, and plants but not by humans and other animals, which can only convert preformed thiamin (vitamin B1) to ThDP (Jurgenson et al., 2009). Plants are the main source of thiamin in human diets, and there is consequently active interest in metabolic engineering of the plant pathway to thiamin and ThDP to combat thiamin deficiency among populations whose diet consists largely of low-thiamin foods (Pourcel et al., 2013; Dong et al., 2015). A second driver for interest in engineering thiamin synthesis is its potential to increase plant resistance to biotic and abiotic stresses (Tunc-Ozdemir et al., 2009; Dong et al., 2015).
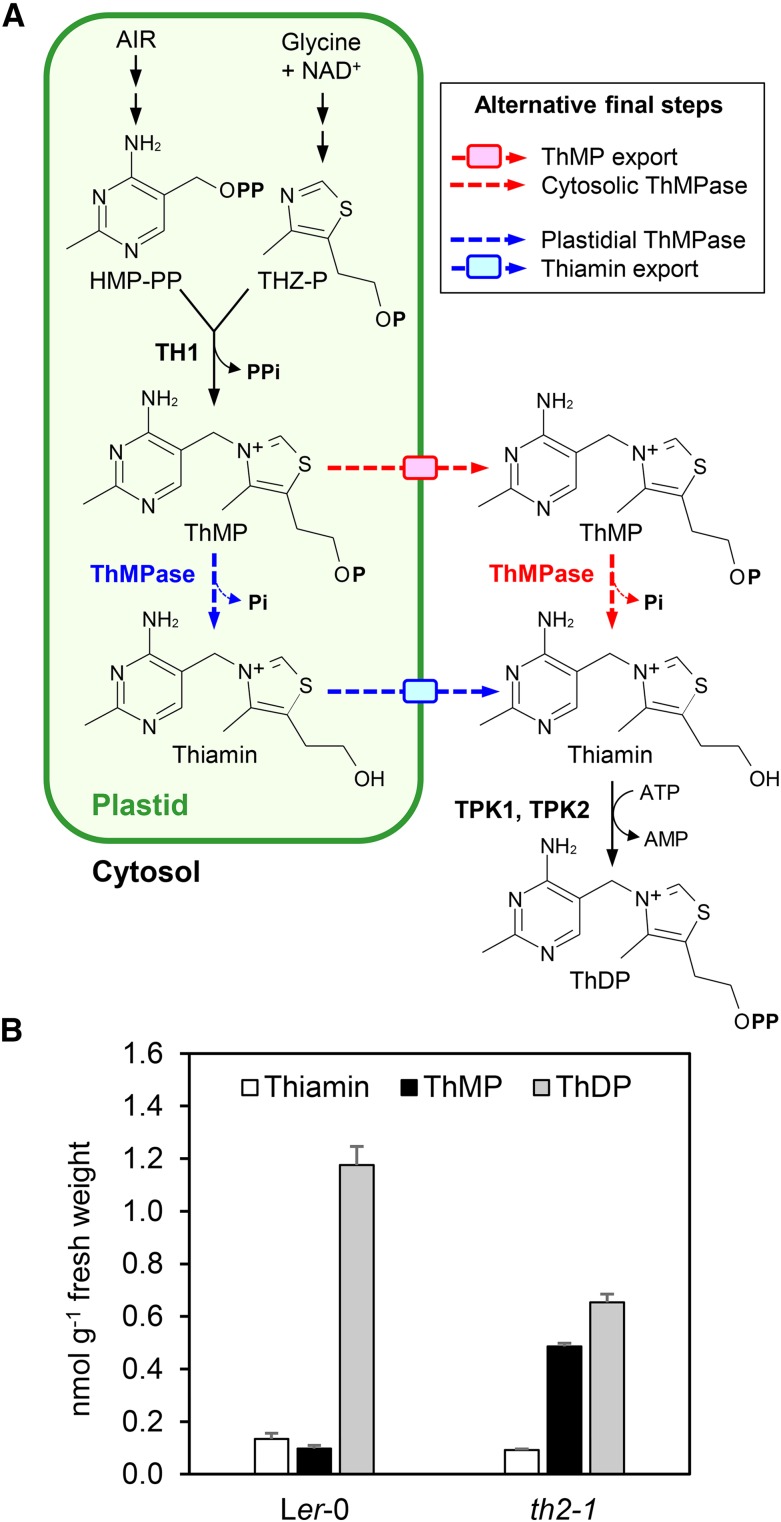
Rational metabolic engineering of the thiamin pathway using transgenic technology or natural variation rests on thorough knowledge of the steps in the pathway and of the corresponding genes and enzymes (Martin, 2013). Fortunately, the steps in the plant thiamin pathway and their subcellular locations are now fairly well understood (Figure 1A) (Gerdes et al., 2012; Pourcel et al., 2013; Dong et al., 2015). In brief, the pyrimidine and thiazole moieties of thiamin are made separately and coupled together in plastids by thiamin-phosphate diphosphorylase (TH1) to form thiamin monophosphate (ThMP). ThMP is then dephosphorylated to give thiamin, either in the plastid or after export to the cytosol. Finally, thiamin is converted to ThDP by cytosolic thiamin diphosphokinases TPK1 and 2. Genes and enzymes are known for all the steps in the plant pathway except for a deadenylation step in thiazole synthesis and for ThMP dephosphorylation (Gerdes et al., 2012). This is also basically the case for the yeast pathway (Nosaka, 2006; Begley et al., 2012; Kuznetsova et al., 2015).
Figure 1.
The Arabidopsis ThDP Synthesis Pathway and the Thiamin Phenotype of the th2-1 Mutant.
(A) ThDP synthesis pathway. The pyrimidine (2-methyl-4-amino-5-hydroxymethylpyrimidine diphosphate [HMP-PP]) and thiazole [4-methyl-5-(2-phosphoethyl)-thiazole (THZ-P)] moieties of thiamin are made in plastids and coupled together by TH1 to give ThMP. ThMP is then hydrolyzed to thiamin, which is converted to ThDP in the cytosol by thiamin diphosphokinases TPK1 and 2. It is not known whether ThMP is exported from plastids and dephosphorylated in the cytosol or dephosphorylated in plastids followed by thiamin export. These alternatives are shown as red and blue dashed arrows, respectively. AIR, aminoimidazole ribotide; P, phosphate group; ThMPase, ThMP phosphatase.
(B) Levels of thiamin and its phosphates in wild-type and th2-1 seedlings. Wild-type Ler-0 and th2-1 homozygote plants were cultured for 3 weeks on agar medium. Whole plants were harvested for thiamin analysis. Data are means and se for three biological replicates, each from different seedlings.
That ThMP phosphatase is still an “orphan” enzyme and gene (i.e., without known sequences; Hanson et al., 2009) has led to the general belief that ThMP is dephosphorylated by nonspecific hydrolases such as the broad-spectrum acid phosphatase isolated from maize (Zea mays) (Rapala-Kozik et al., 2009; Goyer, 2010; Pourcel et al., 2013). However, this belief (that no dedicated ThMP phosphatase exists) has recently been overturned, for bacteria at least (Hasnain et al., 2016). Bacteria that synthesize ThDP via a plant-type pathway were found to have genes encoding HAD (haloacid dehalogenase) family phosphatases that occur in predicted operons with, or as fusions to, thiamin synthesis genes, and these HAD enzymes were found to strongly prefer ThMP to a wide range of other phosphoesters. The same study also identified a homologous enzyme in Arabidopsis thaliana (At4g29530) that showed a similarly strong preference for ThMP. However, knocking out the At4g29530 gene did not affect growth or the levels of thiamin, ThMP, or ThDP, indicating that At4g29530 is not the major Arabidopsis ThMP phosphatase (Hasnain et al., 2016).
A possible candidate for the missing major ThMP phosphatase in Arabidopsis is the product of the classical genetic locus TH2 on chromosome 5; this locus is one of several thiamin biosynthesis loci for which auxotrophic mutations can be recovered (Li and Rédei, 1969; Meinke et al., 2009), which contrasts with other vitamin biosynthesis pathways in plants (Li et al., 1967). Mutants at this locus can be rescued by supplying thiamin but not its thiazole or pyrimidine precursors, which places th2 lesions late in the thiamin pathway (Li and Rédei, 1969), potentially at the ThMP dephosphorylation step (Figure 1A). Accordingly, in this study we first measured the levels of thiamin and its phosphates in th2-1 mutants. As this analysis revealed ThMP accumulation, we used genomic, biochemical, and cell biology approaches to clone the TH2 gene, to demonstrate that its product is the main ThMP phosphatase and to determine where this enzyme localizes within cells. To confirm that our findings apply to other plant species, we biochemically characterized maize TH2 orthologs. We also found that the th2-1 mutant sheds light on a little studied area: thiamin deficiency in plants.
RESULTS
The Arabidopsis th2-1 Mutant Accumulates ThMP
The th2-1 mutant plants grow slowly and have variegated yellow-green leaves unless given small doses of thiamin (Langridge, 1965; Li and Rédei, 1969). To probe the nature of the th2-1 lesion, we compared the thiamin, ThMP, and ThDP levels in seedlings of the mutant and the corresponding wild type (Ler-0) when cultured axenically on sucrose-containing medium. Relative to the wild type, ThDP levels in th2-1 seedlings fell by half (from 1.18 to 0.65 nmol g−1 fresh weight) and ThMP levels rose 5-fold (Figure 1B). This mutant phenotype could be accounted for by a defect in ThMP export from plastids or by a defect in ThMP dephosphorylation in plastids or cytosol (Figure 1A).
The th2-1 Mutation Maps to the At5g32470 Gene
The ThMP-ThDP metabolic phenotype of the th2-1 mutant led us to map the mutation by combining genome sequencing and transcriptomic data. The th2 locus in the centromere region of chromosome 5 is defined by a single allele induced by x-ray mutagenesis (Langridge, 1955) of a wild-type strain identified as ‘Estland’ (Est) (Langridge, 1965). The th2-1 mutant stock distributed by the Arabidopsis Resource Center is the product of introgression into a Landsberg erecta (Ler) background (Stock CS80; www.arabidopsis.org). Given the wealth of haplotype data available for hundreds of Arabidopsis ecotypes (Cao et al., 2011; Schmitz et al., 2013), we reasoned that in principle candidates for TH2 could be identified using a whole-genome sequencing (WGS) approach. To this end, we generated 12.2 Gb of WGS data from th2-1 using the Illumina platform (150-base unpaired reads). In parallel, we performed a replicated RNA-seq analysis of aerial organs of Ler and th2-1 seedlings to identify genes that are expressed differentially between these genotypes.
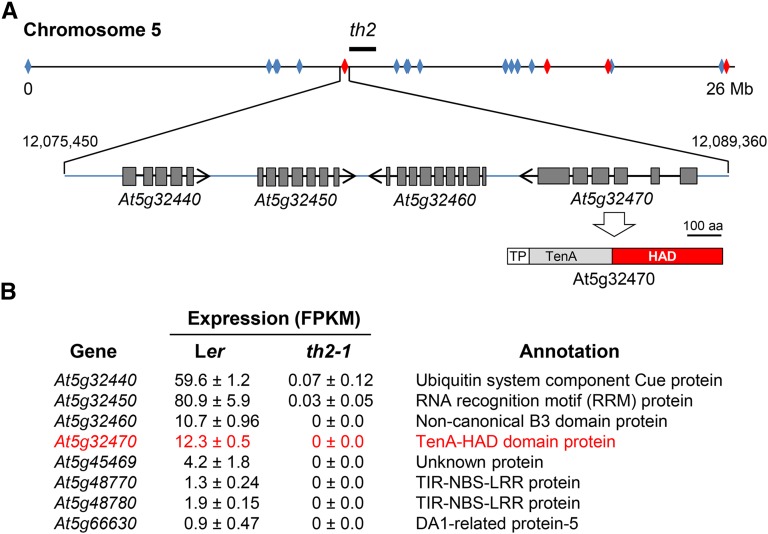
To identify candidate genes, we used a bottom-up approach based on k-mer frequency analysis to identify genes on chromosome 5 of the Col reference genome that were polymorphic (i.e., contained at least one single nucleotide polymorphism) in th2-1. JELLYFISH (Marçais and Kingsford, 2011) was used to compare frequencies of all 22-mer sequences included in coding sequences of the representative gene set for chromosome 5 in the Col reference genome (TAIR10) and the th2-1 WGS data set. Overall, the average frequency of single-copy Col k-mers in the th2-1 data set was 91 counts, consistent with the expected genome coverage of the WGS data. We then analyzed the distribution of Col single copy k-mers that were absent (<5 counts) in th2-1 WGS data to identify genes that were either polymorphic or missing from the th2-1 genome. A single nucleotide polymorphism in a single-copy gene sequence typically eliminates counts for 22 consecutive k-mers; by this measure, at least 2375 of 6414 genes in the representative gene set for chromosome 5 were polymorphic. This set included 29 Col genes for which >85% of k-mers were absent in the th2-1 data set (Figure 2A). The latter were of particular interest because the x-ray-induced th2-1 mutation (Langridge, 1955) had a strong likelihood of being a deletion.
Figure 2.
The th2-1 Mutation Is Linked to a 13.9-kb Deletion on Chromosome 5.
(A) Physical map positions of 29 missing reference genes (diamonds) identified by profiling frequencies of 22-mers in WGS data from th2-1. Locations of eight genes in this set that also showed a qualitative expression difference between Ler and th2-1 (B) are colored red. The bold line above the chromosome shows the approximate physical position of the th2 locus determined by integration of physical and classical genetic maps (Meinke et al., 2009). A 13.9-kb deletion (triangle) in the vicinity of the th2 locus includes four gene models. Nucleotide coordinates of the deletion breakpoints are indicated on the left and right of the triangle. The TenA and HAD domains of the protein encoded by At5g32470 are shown below the gene model. TP, predicted N-terminal targeting peptide.
(B) RNA-seq data for eight genes in the set of 29 missing genes (red diamonds in [A]) that showed significant expression in wild-type Ler plants, but not in the th2-1 mutant. The differentially expressed genes include the four deleted genes diagrammed in (A). The At5g32470 candidate gene is in red font.
To further narrow the list of candidates for TH2, the k-mer profiles were overlaid with the RNA-seq data to identify polymorphic genes that also showed a qualitative difference in expression in th2-1 seedlings compared with the wild type. Eight of the 29 putative missing genes had significant FPKM values in triplicate wild-type samples but no detectable expression in th2-1 (Figure 2B). This set included four genes (At5g32440, At5g32450, At5g32460, and At5g32470) in a contiguous block located near the expected position of th2 based on the classical genetic map (Meinke et al., 2009). Further analysis of single-copy k-mers from the surrounding region of the Est genome sequence (Schmitz et al., 2013) resolved a 13.9-kb deletion in th2-1 between nucleotides 12,075,450 and 12,089,360 of the chromosome 5 reference sequence. Notably, one of the four genes in this interval, At5g32470, encodes a protein that has previously been implicated in thiamin metabolism (Zallot et al., 2014); this protein, which is conserved throughout land plants, comprises an N-terminal TenA domain and a C-terminal HAD domain (Figure 2A). By contrast, the annotations of At5g32440, At5g32450, and At5g32460 (Figure 2B) did not suggest obvious connections to thiamin.
Complementation and Insertional Mutation Confirm At5g32470 as the TH2 Locus
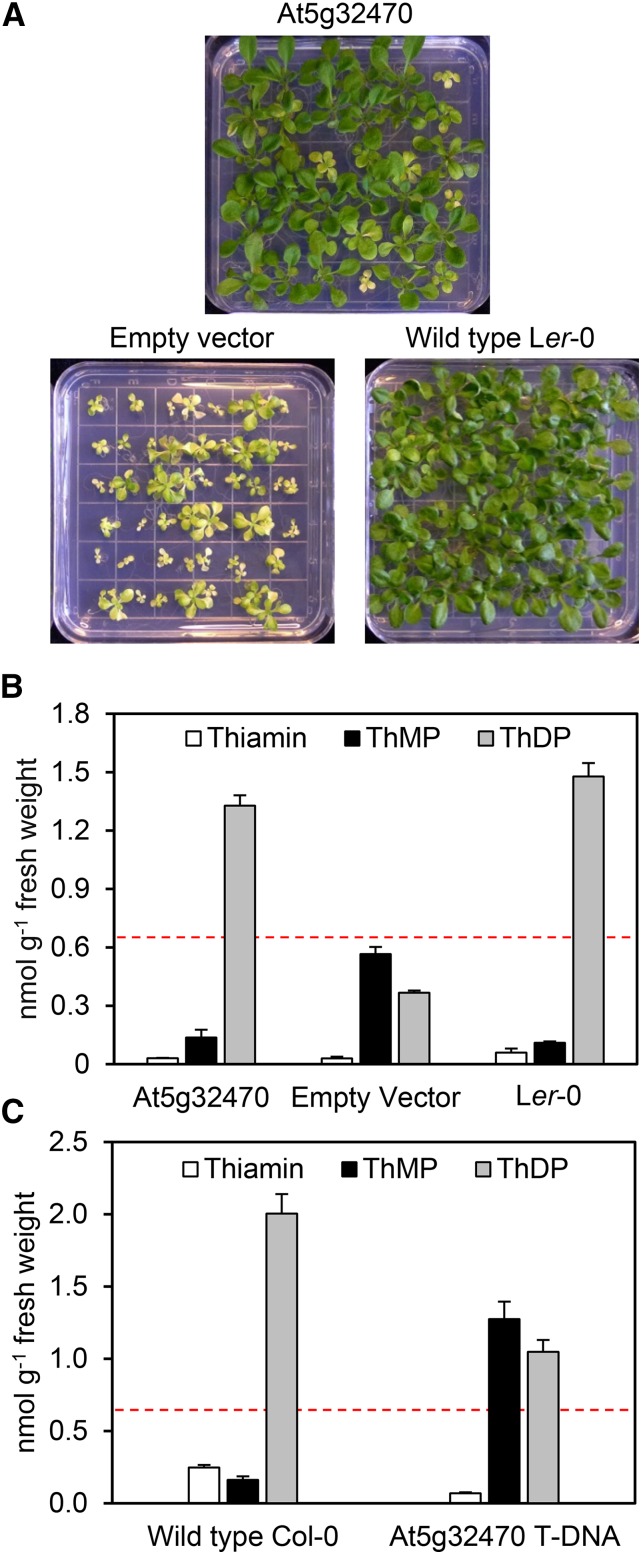
We first used complementation to confirm that deletion of At5g32470 is indeed responsible for the th2 phenotype. An 8-kb fragment of Ler-0 genomic DNA containing the At5g32470 coding sequence and 2.2-kb of the 5′ sequence was cloned into the promoterless pCAMBIA1300 vector and introduced into the homozygous th2-1 mutant. Primary transformants were supplemented with thiamin and allowed to self-pollinate, and three independent T1 progenies were cultured without thiamin, along with empty vector and wild-type controls (Figure 3A). Each T1 progeny segregated 3:1 for the wild-type phenotype versus the th2-1 phenotype, indicating that the wild-type At5g32470 gene was able to complement the lesion in the th2-1 mutant. Consistent with complementation, the phenotypically normal transformant progeny had wild-type levels of ThMP and ThDP (Figure 3B), whereas empty-vector control plants showed the expected ThMP accumulation and ThDP depletion to below the level (0.65 nmol g−1 fresh weight) associated with severe symptoms (Figure 1B). The wild-type At5g32470 gene is thus sufficient to fully revert the growth and vitamin phenotypes of the th2-1 mutant.
Figure 3.
Biochemical-Genetic Evidence That the At5g32470 Gene Corresponds to the TH2 Locus.
(A) Visual phenotypes of the T1 progeny of th2-1 mutant plants transformed with the Ler-0 At5g32470 gene or with empty vector; wild-type Ler-0 plants are included for comparison. Plants were cultured on thiamin-free medium for 2 weeks. Note the presence of approximately one-quarter transgene-free segregants, as expected.
(B) Levels of thiamin and thiamin phosphates in the T1 progeny of the th2-1 mutant transformed with At5g32470 or empty vector. Only th2-1 transformants with a normal phenotype were analyzed. Wild-type Ler-0 plants were included for comparison. Aerial parts were analyzed. Data are means and se for three biological replicates, each from different seedlings, which for T1 plants represented independent transformation events. The dashed red line shows the ThDP level in the mutant th2-1 plants of Figure 1B.
(C) Levels of thiamin and its phosphates in leaves of wild-type Col-0 and the homozygous At5g32470 T-DNA mutant. Plants were cultured on thiamin-free medium for 3 weeks. Data are means and se for three biological replicates, each from different seedlings. The dashed red line is the same as above.
Further support for correspondence between At5g32470 and theTH2 locus was obtained from a T-DNA mutant with an insertion 32 codons after the first potential start codon of the At5g32470 coding sequence (Supplemental Figures 1A and 1B). The position of the insert made this mutant a priori likely to be leaky and, consistent with leakiness, homozygotes had detectable levels of a truncated but potentially functional At5g32470 transcript (Supplemental Figure 1C) and showed modest reductions in root length (37%) and shoot growth (17%) (Supplemental Figure 2). Analysis of thiamin and its phosphates in the T-DNA mutant showed a pattern of ThMP accumulation and ThDP depletion like that in the th2-1 mutant (Figure 1B), but less drastic inasmuch as the ThDP level was well above the 0.65 nmol g−1 level associated with severe symptoms (Figure 3C). Partial insertional inactivation of At5g32470 thus results in a ThDP synthesis lesion similar to the th2-1 mutation but less severe.
Inactivating HAD Phosphatase At4g29530 Does Not Affect the Phenotype of the th2-1 Mutant
Although the At5g32470 gene is deleted in the th2-1 mutant, this mutant clearly retains some ThMP phosphatase activity because ThDP synthesis is not completely eliminated (Figure 1B). Our previous work raised the possibility that the At4g29530 HAD enzyme makes a minor contribution to total ThMP phosphatase activity in vivo (Hasnain et al., 2016). We therefore checked whether At4g29530 activity is a source of the residual ThMP phosphatase in th2-1 mutant plants by crossing the At4g29530 knockout (Hasnain et al., 2016) with th2-1 plants to obtain the double homozygous mutant. Double mutant individuals did not show a more serious growth defect than th2-1 single mutants (Supplemental Figure 3), indicating that At4g29530 does not contribute significantly to the residual ThMP phosphatase activity in the th2-1 mutant.
The At5g32470 HAD Domain and Its Maize Orthologs Are ThMP-Selective Phosphatases
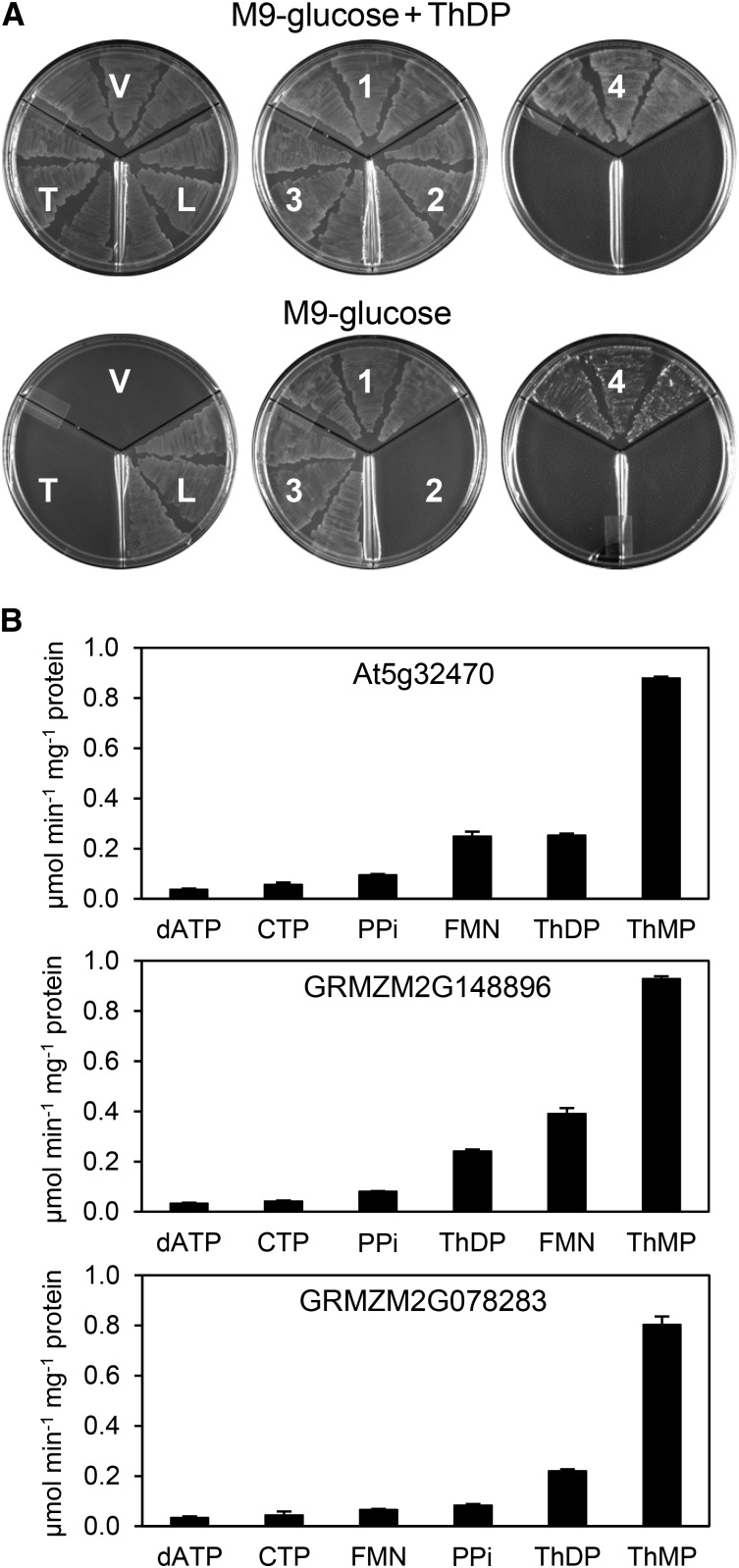
To corroborate the genetic evidence that the At5g32470 gene product is a ThMP phosphatase, and to confirm that this is also true of orthologous proteins in divergent plant species, we turned first to functional complementation of an Escherichia coli strain engineered to require an active ThMP phosphatase for growth by deleting thiL and expressing mouse thiamin diphosphokinase from a plasmid (Hasnain et al., 2016). Expression in this strain of the At5g32470 protein or either of its maize orthologs (GRMZM2G148896 and GRMZM2G078283) enabled growth on minimal medium (Figure 4A), indicating that these proteins act as efficient ThMP phosphatases in E. coli. Consistent with ThMP phosphatase activity, E. coli cells expressing these proteins had smaller ThMP pools and up to 20-fold larger thiamin pools than empty vector controls (Supplemental Figure 4). The complementation assay was also used to show that the ThMP phosphatase activity resides in the HAD domain of the At5g32470 protein: Substituting alanine for the catalytically essential active site aspartate nucleophile (D317) of the HAD domain (Burroughs et al., 2006) abolished complementing activity (Figure 4A). Consistent with complete activity loss, cells overexpressing the D317A mutant protein had normal ThMP and thiamin levels (Supplemental Figure 4).
Figure 4.
Phosphatase Activities of the At5g32470 TenA-HAD Protein and Its Maize Orthologs.
(A) Evidence that the TenA-HAD proteins have in vivo ThMP phosphatase activity in E. coli and that the activity resides in the HAD domain. Three independent isolates of an E. coli ΔthiL strain transformed with vector alone (V) or harboring mouse TPK only (T), or mouse TPK plus native At5g32470 (1), the At5g32470 D317A mutant (2), maize GRMZM2G148896 (3), or maize GRMZM078283 (4) were plated on M9-glucose medium with or without 3.5 µM ThDP. The ΔthiL strain transformed with E. coli thiL (L) was included as a positive control. Plates contained 50 µg/mL chloramphenicol and 1 µg/mL anhydrotetracycline inducer. Images were captured after incubation for 24 h at 37°C. The predicted targeting sequences were removed from all the TenA-HAD proteins.
(B) Substrate profiles of recombinant TenA-HAD proteins, truncated to remove predicted targeting sequences. The panel of 95 substrates screened, and their abbreviations, are listed in Supplemental Table 1. Assays contained 0.25 mM substrate and 10 mM Mg2+. Data are means and se for four independent replicate assays. Activities against substrates not shown were <4% of that against ThMP for each enzyme.
To obtain direct evidence that the At5g32470 protein and its maize orthologs are ThMP phosphatases, they were expressed in E. coli in histidine-tagged form and purified by Ni2+ affinity chromatography followed by gel filtration (Supplemental Figure 5A), which indicated that they exist as monomers (Supplemental Figure 5B). The purified proteins were then screened for activity against a panel of 95 diverse phosphate esters (Supplemental Table 1). All three proteins showed their highest activity against ThMP; they also showed substantial activity against flavin mononucleotide and ThDP, as well as somewhat less against inorganic pyrophosphate, CTP, and dATP (Figure 4B). The activities against ThDP, pyrophosphate, and nucleoside triphosphates, i.e., phosphoanhydride hydrolase activities, are quite unusual for HAD phosphatases, most of which act only on monophosphates (Burroughs et al., 2006; Kuznetsova et al., 2006, 2015). For all three enzymes, activities against the other 89 substrates tested were very low (<4% of that with ThMP) or undetectable. Kinetic characterization of all three enzymes with ThMP as substrate gave Km values in 1.9 to 3.6 μM range, and kcat values of 8.2 to 16.9 s−1 (Table 1). These Km values are two to three orders of magnitude lower than those for recombinant bacterial ThMP phosphatases (0.58 to 1.38 mM) or Arabidopsis At4g29530 (5.1 mM) (Hasnain et al., 2016). Taken with the complementation data, these findings confirm that the HAD domains of At5g32470 and its orthologs are ThMP-selective phosphatases and are highly catalytically efficient (Bar-Even et al., 2011).
Table 1. Kinetic Parameters of the At5g32470, GRMZM2G148896, and GRMZM2G078283 TenA-HAD Proteins with ThMP as Substrate.
| Enzyme | Km (μM) | kcat (s−1) | kcat/Km (s−1 M−1) |
|---|---|---|---|
| At5g32470 | 1.86 ± 0.37 | 13.5 ± 0.8 | 7.25 × 106 |
| GRMZM2G148896 | 3.61 ± 0.68 | 16.9 ± 1.3 | 4.68 × 106 |
| GRMZM2G078283 | 3.20 ± 1.04 | 8.2 ± 1.0 | 2.57 × 106 |
All proteins were truncated to remove predicted targeting sequences. Assays contained 50 mM Tris-HCl buffer, pH 7.5, 10 mM MgCl2, 10% glycerol, and 1 mg/mL BSA. Data are means and se for three independent determinations.
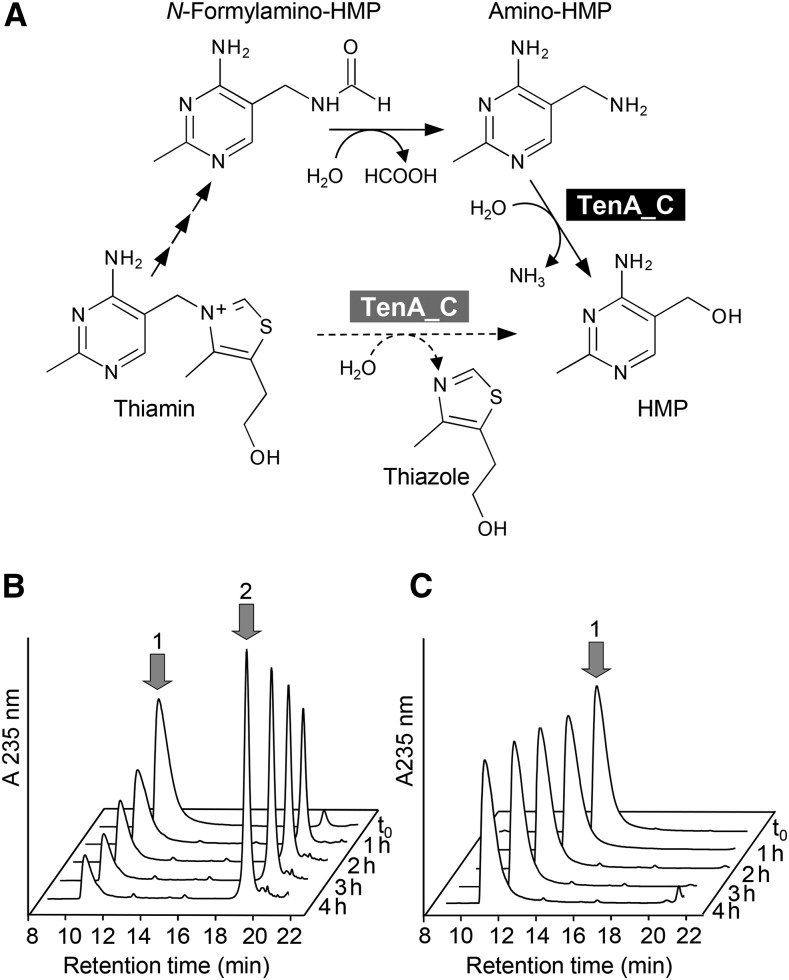
The At5g32470 TenA Domain Has Thiamin Salvage Hydrolase Activity
The TenA domains of At5g32470 and its maize orthologs belong to the subfamily of TenA proteins (TenA_C) that have an active-site cysteine (Zallot et al., 2014). TenA_C proteins from yeast (Onozuka et al., 2008) and bacteria (Jenkins et al., 2007; Barison et al., 2009) participate in salvage of the thiamin pyrimidine moiety; they hydrolyze the breakdown product 4-amino-5-aminomethyl-2-methylpyrimidine (amino-HMP) to the thiamin precursor 4-amino-5-hydroxymethyl-2-methylpyrimidine (HMP) (Figure 5A) and can in some cases also slowly hydrolyze thiamin itself. TenA_C proteins lack activity against the N-formyl derivative of amino-HMP (another thiamin breakdown product; Figure 5A) (Jenkins et al., 2007; Zallot et al., 2014). We therefore tested the At5g32470 protein for activity against the breakdown products amino-HMP, N-formylamino-HMP, and desthiothiamin, as well as thiamin, ThMP, and ThDP. We detected activity only against amino-HMP (Figure 5B). This activity was quite low, 5.4 nmol min−1 mg−1 protein, i.e., one to two orders of magnitude less than standalone bacterial TenA_C proteins (Jenkins et al., 2007; Barison et al., 2009). Mutating the active-site cysteine (Cys-213) to alanine abolished the activity (Figure 5C), confirming that it resides in the TenA domain.
Figure 5.
Amino-HMP Aminohydrolase Activity of the At5g32470 TenA-HAD Protein.
(A) Thiamin breakdown and salvage reactions, showing the two salvage reactions that are mediated by typical TenA_C proteins, i.e., the major amino-HMP aminohydrolase activity (solid arrow, black highlighting) and the minor thiaminase II activity (dashed arrow, gray highlighting).
(B) Chromatographic evidence that recombinant Arabidopsis At5g32470 protein converts amino-HMP (1) to HMP (2). Reactions (50 µL) containing 1 mM amino-HMP and 60 µg of protein were incubated at 30°C for the times indicated and analyzed by HPLC with UV detection. The small amount of HMP present in the zero time (t0) reaction was formed during sample processing.
(C) Elimination of the aminohydrolase activity of Arabidopsis At5g32470 by the Cys213Ala mutation. Assay conditions were as in (B).
The At5g32470 Protein Localizes to the Cytosol and Potentially to Mitochondria
The At5g32470, GRMZM2G148896, and GRMZM2G078283 proteins and their orthologs from other plants have N-terminal extensions that TargetP (Emanuelsson et al., 2000) and Predotar (Small et al., 2004) predict to be mitochondrial matrix targeting peptides (Supplemental Figure 6). However, a proteomic study (Ito et al., 2011) found the At5g32470 protein in a cytosolic fraction. Prompted by this latter finding, we discovered that At5g32470 and all of the orthologous sequences examined include a methionine residue near the end of the targeting peptide (residue 47 in At5g32470) that could potentially serve as an alternative translation start site (Supplemental Figure 6), as reported for other plant proteins (Daras et al., 2014; Niehaus et al., 2014; Bohrer et al., 2015; Ellens et al., 2015). If used, this second translation start site would eliminate the putative mitochondrial targeting peptide.
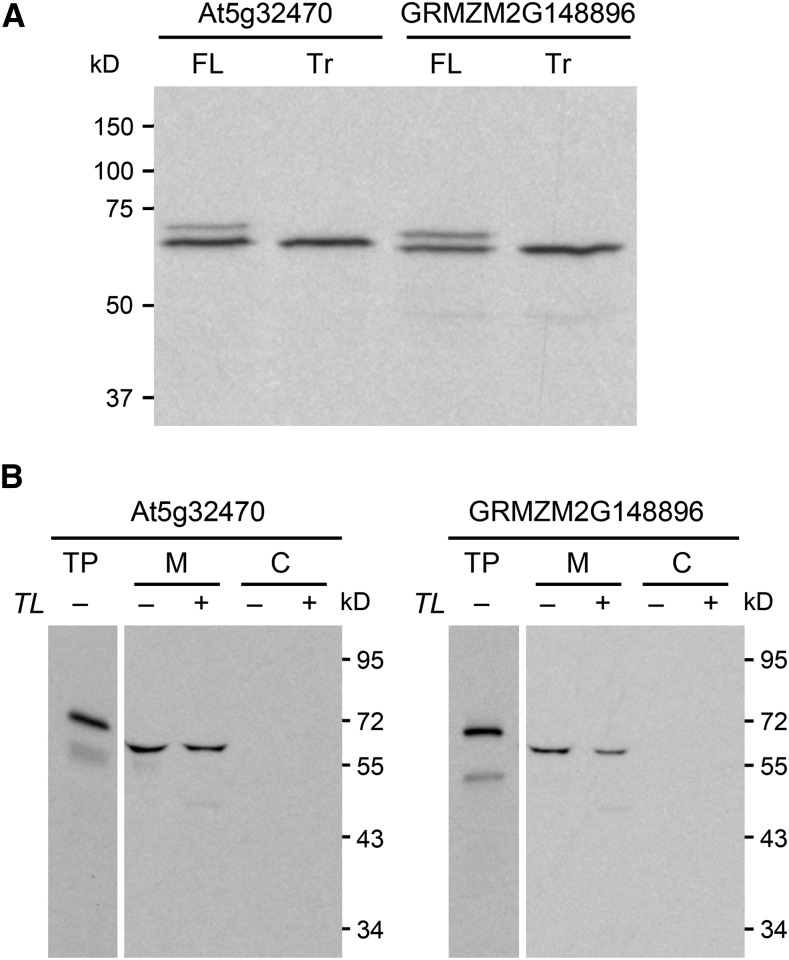
We therefore used a coupled transcription-wheat germ translation system to test which translation start sites in the At5g32470 and GRMZM2G148896 sequences are functional in vitro. When preceded by a strong Kozak translation initiation sequence (ACCATG…) (Kozak, 1981), both full-length sequences yielded two protein products, whereas sequences truncated up to the second start site yielded only the smaller product (Figure 6A). The products’ masses, as estimated from the gel (∼70 and ∼65 kD), agree with the calculated masses of the proteins initiated from the first and second start sites, and for both Arabidopsis and maize the smaller product predominates (Figure 6A). These results establish that the TenA-HAD proteins have alternative translation start sites and that the second site is preferred in vitro. Dual import assays with pea (Pisum sativum) chloroplasts and mitochondria (Rudhe et al., 2002) showed that translation from the first start site in the Arabidopsis or maize sequence generates a protein that is targeted to and processed by mitochondria, but not chloroplasts (Figure 6B). A rabbit reticulocyte system was used in these experiments as it privileges the first start site (Figure 6B) and is the standard system for dual import assays (Rudhe et al., 2002).
Figure 6.
In Vitro Evidence for Dual Translational Start Sites and Potential Mitochondrial Targeting of Arabidopsis and Maize TenA-HAD Proteins.
(A) In vitro-coupled transcription-translation identifies the first and second methionine codons as alternative start sites. Full-length (FL) At5g32470 and GRMZM2G148896 cDNAs and corresponding truncated (Tr) cDNAs starting at the second methionine codon (codon 47 and codon 44, respectively; Supplemental Figure 6) were transcribed and translated (in a wheat germ system) in the presence of [3H]leucine. Translation products were resolved by SDS-PAGE; radioactive bands were visualized by fluorography. Positions of molecular mass markers are indicated. All sequences were preceded by a strong Kozak translation initiation sequence (ACCATG…).
(B) The full-length At5g32470 and GRMZM2G148896 proteins are imported into and processed by mitochondria, but not chloroplasts. At5g32470 and GRMZM2G148896 full-length cDNAs preceded by native Kozak sequences were transcribed in vitro and translated (in a rabbit reticulocyte system) in the presence of [3H]leucine. The translation products (TP) were incubated in the light with mixed pea mitochondria (M) and chloroplasts (C). The organelles were mock treated (−) or thermolysin (TL) treated (+) to remove adsorbed proteins and then reisolated on a Percoll gradient. Proteins were separated by SDS-PAGE and visualized by fluorography. Samples were loaded on the basis of equal chlorophyll or mitochondrial protein content next to an aliquot of the translation product. Exposure times were adjusted to give comparable band intensities in all tracks. Positions of molecular mass markers (in kD) are shown. Note that the reticulocyte translation system, unlike the wheat germ system (Figure 6A), privileges the first translation start site, does not recognize the second, and apparently recognizes one or more minor, artifactual start sites within the sequence of the mature protein.
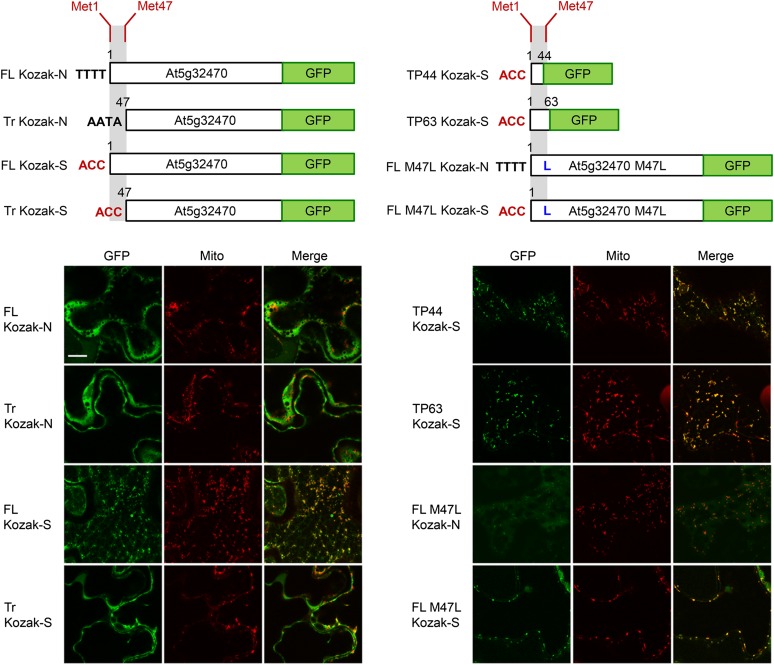
We next assessed intracellular localization in vivo by transiently expressing various At5g32470-C-terminal GFP fusions in Agrobacterium tumefaciens-transformed tobacco leaf epidermal cells, a well-characterized system for studying protein localization (Sparkes et al., 2006). In total, eight fusion constructs were examined with respect to their intracellular localization based on differences in translational initiation from the two potential start sites and the predicted N-terminal mitochondrial targeting peptide (see schematic illustrations in Figure 7 and the corresponding legend for details). When the first translation start site in At5g32470 was preceded by its native Kozak translation initiation sequence, the full-length native fusion construct (FL Kozak-N) appeared to localize exclusively to the cytosol, as judged by its diffuse fluorescence pattern distributed throughout the cell (Figure 7, row 1, left). When preceded by the strong Kozak sequence, however, the full-length construct (FL Kozak-S) exhibited a distinct punctate fluorescence pattern that coincided with that attributable to the mitochondrial marker protein, Cherry-At1g55450 (Marty et al., 2014) coexpressed in the same cell (Figure 7, row 3, left). Similarly, the constructs in which the predicted N-terminal targeting peptide (residues 1 to 44 or 1 to 63), preceded by the strong Kozak sequence, was fused directly to GFP, also localized to Cherry-At1g55450-containing mitochondria (TP44 Kozak-S and TP63 Kozak-S; Figure 7, rows 1 and 2, right). Two N-terminal peptides were tested because the cleavage site of the processing peptidase could not be precisely predicted (Supplemental Figure 6). Mitochondrial localization was also observed for both full-length M47L fusion constructs, wherein the second methionine codon (codon 47) was changed to leucine (M47L) and preceded by the native Kozak sequence (FL M47L Kozak-N) or a strong Kozak sequence (FL M47L Kozak-S) (Figure 7, rows 3 and 4, right). Finally, as expected, the N-terminal-truncated constructs lacking the predicted targeting peptide (Tr Kozak-N and Tr Kozak-S) both localized solely to the cytosol (Figure 7, rows 2 and 4, left).
Figure 7.
In Vivo Evidence for Targeting of the Arabidopsis TH2 Protein to Cytosol and Mitochondria.
Representative confocal laser scanning micrographs of tobacco leaf epidermal cells transiently expressing various At5g32470-C-terminal-GFP fusions, including, as shown in schematic illustrations at the top, GFP fused to either the full-length At5g32470 sequence (FL) beginning at methionine codon 1 (Met1) (which can potentially be translated to yield proteins with or without the predicted N-terminal mitochondrial targeting peptide); the truncated At5g32470 sequence (Tr) beginning at methionine codon 47 (Met-47) (which yields a protein that lacks the predicted N-terminal targeting peptide); the full-length At5g32470 sequence with methionine codon 47 changed to leucine (FL M47L) (which yields a protein that contains the N-terminal targeting peptide); or the predicted N-terminal mitochondrial targeting peptide in At5g32470 corresponding to either first 44 residues (TP44) or 63 residues (TP63) of the protein (refer to Supplemental Figure 6). All coding sequences were preceded either by the native Kozak sequence (Kozak-N) immediately upstream of the first translation start site in At5g32470 (TTTTATG… for full-length sequences; AATAATG… for truncated sequences) or by a strong Kozak translation initiation sequence (Kozak-S) (ACCATG…). Cells shown were coagroinfiltrated with binary plasmids encoding each fusion construct (diagrammed above the images) along with a Cherry-tagged mitochondrial marker protein (the mitochondrial outer membrane protein At1g55450; Marty et al., 2014). Each row of images corresponds to the fluorescence attributable to (as indicated by labels) the candidate fusion protein (GFP) and the mitochondrial marker (Mito) (green and red, respectively) and the corresponding merged image. Note that the FL M47L Kozak-N construct displayed an atypically low transformation efficiency and weak fluorescence signal relative to the other constructs, suggesting it was poorly expressed. Bar = 20 μm.
Overall, these results indicate that, at least in tobacco leaf epidermal cells, the native At5g32470 sequence is translated primarily from the second potential start site, resulting in the protein residing predominantly in the cytosol. However, when preceded by a strong Kozak sequence, the first start codon in At5g32470 can also be used in vivo, as it can in vitro (Figure 6A), so that the protein is localized predominantly to mitochondria. Whether or not the latter results are a result of capturing a normal process exaggerated by overexpression, it is clear that the At5g32470 protein has the potential for dual targeting to the cytosol and mitochondria.
DISCUSSION
Identification of the TH2 Gene Product as a ThMP-Selective HAD Phosphatase
Our results provide genomic, genetic, and biochemical evidence establishing that the classical Arabidopsis TH2 locus (Li and Rédei, 1969) corresponds to the At5g32470 gene encoding ThMP phosphatase, a hitherto “orphan” enzyme of ThDP biosynthesis whose existence as a specific enzyme was in doubt. With the identification of the ThMP phosphatase, the set of plant thiamin and ThDP synthesis enzymes and genes is now almost complete; only the enzyme mediating a deadenylation step in thiazole synthesis remains unidentified in plants and other organisms (Gerdes et al., 2012). The way is thus now clear to engineering very nearly all the enzymatic steps in the thiamin pathway.
That the ThMP phosphatases encoded by the TH2/At5g32470 gene and its maize orthologs belong to the HAD family conforms to an established pattern, for this family includes other pathway-specific phosphatases as well as nonspecific phosphatases (Burroughs et al., 2006). The Arabidopsis and maize proteins are highly selective for ThMP and show low Km and high kcat values for this substrate, which is consistent with their functioning in vivo as ThMP phosphatases.
Our genome sequence data demonstrate that the th2-1 mutation is a full deletion. That this mutation is not lethal (Li and Rédei, 1969) implies that another phosphatase or phosphatases can partly substitute for At5g32470 when ThMP builds up to abnormal levels. The At4g29530 phosphatase (Hasnain et al., 2016) most likely does not play such a substitutionary role because the phenotype of the th2-1 At4g29530 double mutant is no more severe than that of the th2-1 single mutant. More generally, the partial redundancy of ThMP phosphatase contrasts with the nonredundancy of other biosynthetic phosphatases in plants, notably those acting on phosphoserine (Cascales-Miñana et al., 2013) and histidinol (Petersen et al., 2010). However, there are cases of partial redundancy of biosynthetic phosphatases in bacteria (Klaus et al., 2005; Lu et al., 2011).
The TenA Domain of TH2 Proteins
The TH2/At5g32470 protein and its orthologs in other plants are fusions between the HAD phosphatase domain and a domain belonging to a family (TenA) that is associated with thiamin metabolism (Jenkins et al., 2007; French et al., 2011; Hasnain et al., 2016). The TenA domain belongs to the TenA_C subfamily, other members of which have amino-HMP aminohydrolase activity (Figure 5A) (Jenkins et al., 2007; French et al., 2011). We demonstrated that the At5g32470 TenA domain likewise has amino-HMP aminohydrolase activity. That this activity was low could be due to instability during isolation because activity appeared not to survive beyond the Ni2+-affinity step in purification. The low activity could also be because amino-HMP is not the physiological substrate, this substrate being a breakdown product upstream of amino-HMP (Figure 5A), as proposed for Helicobacter pylori TenA_C (Barison et al., 2009). If so, the TenA-HAD fusion could be rationalized as follows. Amino-HMP is the last in a series of ill-characterized thiamin or ThDP breakdown products containing successively less of the thiazole moiety (Jenkins et al., 2007). At5g32470 could attack an early breakdown product that retains the thiazole side chain with an attached diphosphate group. This group blocks the action of other TenA_C proteins (Murata, 1982; Müller et al., 2009). The HAD domain of At5g32470 and its orthologs, via their di- and monophosphatase activities, could remove both phosphate groups from ThDP breakdown products, allowing the TenA_C domain to act.
Intracellular Localization of ThMP Phosphatase and Its Implications
We obtained strong bioinformatic and experimental evidence that, as proteome data (Ito et al., 2011), suggest the At5g32470 protein is localized to the cytosol as a consequence of translational initiation from the second of two potential start sites and that the same likely applies to an orthologous maize protein. That ThMP phosphatase is cytosolic disambiguates the substrate specificity of the still missing transporter that exports a thiamin moiety from plastids (Gerdes et al., 2012), i.e., if ThMP hydrolysis occurs in the cytosol, the plastidial transporter must act on ThMP, not thiamin (Figure 1A).
Given the presence of a conserved upstream start site (Supplemental Figure 6) whose use (when directed by a strong Kozak sequence) results in the At5g32470 protein being targeted to mitochondria both in vitro (Figure 6B) and in vivo (Figure 7), this protein clearly has the potential for dual localization to cytosol and mitochondria. However, whether this potential is realized and, if so, what its functional significance may be remain to be investigated. Nevertheless, one possible function for a mitochondrially located TenA-HAD protein relates to the two-step salvage role proposed above. Three major ThDP-dependent enzymes (the pyruvate, 2-oxoglutarate, and branched-chain 2-oxoacid dehydrogenase complexes) are in mitochondria, which are hotspots for reactive oxygen species damage (Noctor et al., 2007). The thiazole moiety of ThDP is highly vulnerable to such damage (Sümegi and Alkonyi, 1983; Bunik et al., 2007). A mitochondrial TenA-HAD protein might therefore mediate HMP salvage from oxidatively damaged ThDP, while its cytosolic counterpart functions mainly in ThDP synthesis.
Thiamin Deficiency in Plants
A final inference from our data is that a severe deficiency syndrome sets in when the ThDP level in Arabidopsis falls from the normal range (1.2 to 2.0 nmol g−1) to 0.65 nmol g−1 or below, i.e., by ∼50 to 70%. This inference is broadly consistent with results for Arabidopsis tpk1 (thiamin diphosphokinase) mutant seedlings, which grew normally with modestly depleted ThDP levels (Ajjawi et al., 2007). The threshold for severe ThDP deficiency may be higher in maize, in which the thi2-blk thiamin synthesis mutant showed grossly defective leaf blade development and meristem maintenance although its ThDP level was only ∼30% less than the wild type (Woodward et al., 2010). However, caution is needed in comparing thresholds in this way (1) because ThDP levels in bulk tissue (as measured in all these cases) may not reflect levels in the dividing and expanding cells that drive growth, and (2) because, as just noted, the “normal” level is a range, not a constant reference value that can be set at 100%.
The ThMP that accumulates in the th2-1 mutant can be discounted as a cause of its severe chlorosis and growth symptoms because (1) ThMP levels are similarly elevated in the At5g32470 insertional mutant, which does not display severe symptoms, and (2) ThMP is not known to substantially inhibit any plant enzyme (Chang et al., 2015).
The consequences of particular levels of ThDP deficiency in plants cannot be compared directly with those in animals. However, data for young rats (Rains et al., 1997) indicate that weight gain totally ceased when liver ThDP level fell by ∼60%, implying that smaller ThDP losses must substantially impact growth. Thus, there is some indication that animals and plants alike develop severe deficiency symptoms when ThDP levels drop by about half, i.e., that neither group has a large margin of safety.
If plants normally operate quite close to ThDP deficiency, this could help explain two sets of classical observations. First, it could help account for the large number of reported thiamin mutants (Boynton, 1966; Li et al., 1967; Kumar and Sharma, 1986); high susceptibility to ThDP deficiency would make deficiency easy to attain via hypomorphic as well as null alleles of biosynthesis genes. Second, normal operation not far above the threshold of ThDP deficiency could explain reports that plants grown in stress conditions benefit from thiamin supplementation, whereas controls in nonstressed conditions do not (Bonner, 1943; Tunc-Ozdemir et al., 2009). Perhaps stress-related decreases in ThDP production or increases in its breakdown drive ThDP levels down to the deficiency threshold.
METHODS
Chemicals
Amino-HMP and HMP were from Toronto Research Chemicals. N-Formyl-4-amino-5-aminomethyl-2-methylpyrimidine (formylamino-HMP) was prepared from amino-HMP as described (Jenkins et al., 2007; Zallot et al., 2014). Thiamin hydrochloride, thiamin monophosphate chloride dihydrate, thiamin diphosphate, and other chemicals were obtained from Sigma-Aldrich. Desthiothiamin was prepared as described (Kurata et al., 1968). Briefly, thiamin-HCl (15.5 mmol) and NaOH (31.1 mmol) were dissolved in 10 mL water, and glycine (15.5 mmol) and NaOH (15.5 mmol) were dissolved in 7.5 mL water. The two solutions were mixed and incubated for 3 d at 22°C with continuous stirring. Desthiothiamin crystals were harvested by filtration and washed with ethanol. Oligonucleotide primers were from Eurofins MWG Operon and are listed in Supplemental Table 2.
Plant Material and Growth Conditions
Seeds of the Arabidopsis thaliana th2-1 (CS80), Ler-0 (CS20), and Col-0 (CS60000) lines, the At4g29530 SALK_081194.31.30.x T-DNA knockout line, and the At5g32470 SAIL_572_G11 (CS824383) T-DNA knockout line were from the Arabidopsis Biological Resource Center. Seeds were surface-sterilized and plated on half-strength MS medium containing 1% (w/v) sucrose, 0.1% (w/v) MES, pH 5.7, and 0.6% (w/v) Phytagel; for th2-1, 10 µM thiamin was added to the medium. Plates were held at 4°C for 3 d before being placed under fluorescent lights (Sylvania F40/CWP 40W cool-white plus, photosynthetic photon flux 100 to 150 µmol photons m−2 s−1) on a 12-h-light/12-h-dark cycle at 22°C. For thiamin vitamer profiling experiments, 36 seeds were evenly spaced on 9-cm square plates containing medium with the indicated concentration of thiamin; aerial portions of the plants were harvested, immediately frozen in liquid N2, and stored at −80°C. To generate seeds, plants were transferred to soil 2 to 3 weeks after germination; th2-1 plants were watered with 100 µM thiamin until seeds were harvested. Note that this procedure did not supply a controlled thiamin dose because plants were watered as needed (i.e., at irregular intervals based on water use) and thiamin is unstable in soil. Thus, the amount of thiamin available to developing siliques most likely varied between plants and with time, causing differences in thiamin endowments among seeds. Such differences could account for the observed heterogeneity in the growth of th2-1 plants transformed with empty vector when cultured without thiamin (Figure 3A).
Genomic DNA was isolated from young leaves of the At5g32470 SAIL T-DNA line and from wild-type Col-0 plants; PCR reactions were performed with primer pairs for the wild-type or T-DNA insertion alleles (1 and 2, or 3 and 2, respectively; Supplemental Figure 1). Amplicons were analyzed by agarose-gel electrophoresis. The amplicon obtained with the T-DNA insertion allele primers was T/A cloned into pGEM-T Easy (Promega) and sequenced to confirm the insertion site. Total RNA was isolated from homozygous plants using the RNeasy Plant Mini Kit (Qiagen) with on-column DNase treatment. First-strand cDNA was created with the Superscript III first-strand synthesis system (Life Technologies). To detect transcripts, PCR reactions were performed with primers designed to amplify the full-length coding sequence of the At5g32470 transcript (4 and 5), the full-length coding sequence starting from the second potential start codon (5 and 6), or a fragment of the actin-2 transcript (7 and 8); amplicons were analyzed by agarose-gel electrophoresis. Genotyping the At4g29530 SALK T-DNA knockout line was previously described (Hasnain et al., 2016).
Nicotiana benthamiana (tobacco) plants used for Agrobacterium tumefaciens-mediated transient expression experiments were grown in a growth chamber in soil at 28°C under a mixture of fluorescent (Sylvania 60W cool-white plus) and incandescent (Philips T8 bulbs [F96T8/TL841/H0/Plus/Alto) lighting (100 to 150 µmol photons m−2 s−1) with a 16-h-light/8-h-dark cycle. Leaves of 4-week-old plants were infiltrated with Agrobacterium (strain LBA4404) carrying selected binary vectors (see Subcellular Localization section below for details on vector construction). Agrobacterium transformed with the tomato bushy stunt virus gene P19 was included in all infiltrations to enhance transgene expression (Petrie et al., 2010). Procedures for Agrobacterium growth, transformation, infiltration, and processing of leaf material for microscopy (see below) have been described elsewhere (McCartney et al., 2005; Cai et al., 2015).
Genome and Transcriptome Analysis
For transcriptome analysis, th2-1 and Ler-0 seeds were plated on medium lacking thiamin (36 seeds evenly spaced on 9-cm square plates) and grown for 17 d after germination. Aerial portions of plants were collected, frozen at once in liquid N2, and stored at −80°C. Total RNA was isolated from plants collected from three independent plates using the RNeasy Plant Mini Kit with on-column DNase treatment. RNA-seq libraries were prepared using the TruSeq Stranded Total RNA Sample Prep Kit (Illumina) and sequenced with the Illumina NextSeq500 platform. RNA-seq data were analyzed using the Galaxy Web-based platform provided by University of Florida Research Computing. Briefly, the short reads from RNA-seq were mapped to the Arabidopsis TAIR10 genome using TopHat2. Cufflinks was used to assemble transcripts and estimate their abundance on TAIR10 genome annotation downloaded from the Ensemble Plants website (http://plants.ensembl.org). Cuffdiff was then used to find genes differentially expressed in th2-1 and Ler-0.
For WGS analysis, th2-1 seeds were plated on medium supplemented with 10 µM thiamin and grown for 23 d after germination. Entire plants were collected and DNA was isolated with the DNeasy Plant Mini Kit (Qiagen). A WGS library was constructed with the NEXTflex DNA sequencing kit following the manual. A unidirectional, mid-scale run on the Illumina NextSeq500 instrument yielded 14 Gb of raw sequence. To detect the polymorphisms in the th2-1 genome, k-mer frequency analysis was performed with JELLYFISH software (Marçais and Kingsford, 2011) using a k-mer length of 22 bases. Frequencies of k-mers derived from genes located on chromosome 5 and included in the TAIR10 representative gene set (http://plants.ensembl.org) were profiled in the th2-1 WGS data set to identify genes that were either absent or highly polymorphic in the th2-1 genome (genes for which a high percentage of k-mers had zero frequency in the WGS data). The average frequency of k-mers that are single copy in the Columbia genome was ∼90, indicating 90× genome coverage overall. The analysis yielded a set of 29 genes from chromosome 5 for which >85% of k-mers were absent from the WGS data. The RNA-seq analysis identified two genes (At5g32460 and At5g32470) in this set that were also significantly downregulated in th2-1 plants.
Functional Complementation Tests of TH2 Genes
For functional complementation tests, the Escherichia coli BW25113 ΔthiL strain and plasmid pBY279.1 carrying the mouse mTPK1 cDNA were used, with the E. coli thiL gene as positive control (Hasnain et al., 2016). To test the ThMPase activity of TH2 proteins, artificial operons were constructed in pBY279.1 with the At5g32570, GRMZM2G148896, or GRMZM2G078283 sequences, starting at the second methionine (Supplemental Figure 6), placed downstream from mTPK1. Amplifications used PfuTurbo DNA polymerase (Stratagene) and primers with restriction sites for cloning. Site-directed mutagenesis was performed on the pBY279.1-TPK/At5g32570 construct using the Agilent QuikChange II Kit to change aspartate codon 317 to alanine (GAT to GCT); the mutated construct was sequence-verified. The ΔthiL strain was transformed with pBY279.1 alone or containing E. coli thiL, mTPK1, or mouse TPK1 plus At5g32570, At5g32570-D317A, GRMZM2G148896, or GRMZM2G078283. Transformants were selected on LB medium containing 50 µg/mL kanamycin, 50 µg/mL chloramphenicol, and 3.5 μM ThDP. Three transformants per construct were then streaked on M9-glucose medium containing 50 µg/mL kanamycin, 50 µg/mL chloramphenicol, and 1 µM anhydrotetracycline, plus or minus 3.5 µM ThDP. Plates were incubated at 37°C for 24 h.
Genetic Experiments
For functional complementation tests, an 8-kb Ler-0 genomic region including the At5g32470 coding sequence and 2.2-kb promoter was cloned into the pCAMBIA1300 vector. PCR was performed using primers containing 16 bases homologous to sequences in the pCAMBIA1300 vector (Supplemental Table 2). pCAMBIA1300 was cut with XbaI and SalI, and the 8-kb genomic fragment was inserted using the In-Fusion HD Cloning Kit (Clontech). The resulting plasmid was transformed into Agrobacterium strain GV3101, which was used to transform the th2-1 homozygous mutant (T0 generation) by the floral dip method (Zhang et al., 2006). Seeds were collected from T0 generation plants and T1 plants were identified by hygromycin-resistant and thiamin-independent growth phenotypes. Progeny of T1 plants (T2 generation) were grown for 2 weeks on the half-strength MS medium described above without thiamin and used for thiamin vitamer analysis. To cross the At4g29530 SALK_081194.31.30.x T-DNA knockout line and th2-1, plants were grown in soil (th2-1 plants were watered with 10 µM thiamin) and crossed using standard procedures. Briefly, pollen-producing anthers from SALK_081194.31.30.x plants were used to pollinate the stigmas of prepared pistils from th2-1 plants. Recovered F1 seeds were germinated on plates and transferred to soil, genomic DNA was isolated from young leaves, and PCR reactions were performed with wild-type allele primers for At4g29530 or T-DNA insertion allele primers (Supplemental Table 2); amplicons were analyzed by agarose-gel electrophoresis. F2 seeds were collected from plants shown to be heterozygous for the T-DNA insertion in At4g29530 and germinated on plates. About one-quarter of the F2 plants showed the th2-1 phenotype; these plants were genotyped as described above.
Expression and Isolation of Recombinant Proteins
mRNA was extracted from Arabidopsis and maize (Zea mays) tissues using the RNeasy Plant Mini Kit (Qiagen). First-strand cDNA was synthesized using 1 μg of total RNA, oligo(dT) primer, and SuperScript III reverse transcriptase (Invitrogen). The At5g32470 and GRMZM2G148896 coding sequences were amplified by PCR from the leaf cDNAs using Phusion DNA polymerase (New England Biolabs). The clones ZM_BFb0076H17 and ZM_BFc0019H05 carrying GRMZM2G078283 cDNAs were ordered from the Arizona Genomics Institute and used as templates for further cloning. All constructs were verified by DNA sequencing. The At5g32570, GRMZM2G148896, or GRMZM2G078283 cDNAs (starting at the second methionine) were cloned into pET28b (Novagen), adding the C-terminal His tag encoded by the vector. Sequence-verified constructs were introduced into E. coli BL21-CodonPlus (DE3)-RIPL cells. Cultures (1 liter) were grown at 37°C in Terrific Broth containing 50 μg/mL kanamycin and 100 µM ThDP. When OD600 reached 0.8, IPTG was added (final concentration 1 mM) and incubation was continued for 24 h at 16°C. Cells were harvested by centrifugation, washed with PBS, and frozen in liquid N2. Subsequent steps were at 4°C. Bacterial pellets were suspended in 50 mM NaH2PO4, 300 mM NaCl, and 10 mM imidazole, pH 8.0, and sonicated. The extract was clarified by centrifugation (10,000g, 30 min, 4°C), applied to a 0.5-mL Ni2+-nitrilotriacetic acid agarose column (Qiagen) equilibrated with the above buffer, and allowed to drain. After washing with 16 mL 50 mM NaH2PO4, 300 mM NaCl, and 20 mM imidazole, pH 8.0, proteins were eluted with 2.5 mL of this buffer containing 250 mM imidazole, desalted on PD-10 columns (GE Healthcare) equilibrated in 50 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 10% (v/v) glycerol, and concentrated to ∼1 mg/mL in Amicon Ultra-0.5 mL 10K units. Proteins were further purified using an FLPC gel filtration step. A Superdex 200 (GE Healthcare Life Sciences) was equilibrated with 50 mM Tris-HCl, pH 7.0, and 150 mM NaCl. After sample injection, proteins were eluted using 1 column volume (24 mL) of equilibration buffer with a constant flow rate of 0.5 mL/min, and 1-mL fractions were collected. Proteins were detected by absorption at 280 nm. Native molecular masses were estimated using a calibration curve with a Sigma-Aldrich gel filtration molecular weight marker kit (12,000 to 200,000 D). Fractions were analyzed by SDS-PAGE and Coomassie Brilliant Blue staining. Purified proteins were desalted and concentrated as above, frozen in liquid N2, and stored at −80°C. For TenA activity assays, proteins were purified by Ni2+-affinity chromatography only, as described (Zallot et al., 2014). The Agilent QuikChange II Kit was used as above to make a Cys213Ala mutation. Protein concentrations were estimated by dye binding (Bradford, 1976) with BSA as standard.
Enzyme Assays
Purified TH2 proteins were screened for phosphatase activity against a panel of 95 phosphorylated metabolites (Supplemental Table 1) using the Malachite Green reagent as previously described (Kuznetsova et al., 2006). The screening assays were run for 15 min at 37°C; they contained 50 mM HEPES-KOH, pH 7.5, 10 mM Mg2+, 0.25 mM substrate, and 1 μg of enzyme. Kinetic studies of ThMPase activity used triplicate 100-μL reaction mixtures containing 50 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 10% (v/v) glycerol, 1 mg/mL BSA, the specified concentrations of ThMP, and 2 ng of enzyme. Assays were run at 37°C for 2 min and stopped and derivatized by adding 100 μL of oxidation reagent (3.35 M NaOH containing 12.14 mM potassium ferricyanide), which converts thiamin vitamers to thiochrome derivatives. Standards were diluted in assay buffer and oxidized to thiochromes by adding 100 μL of oxidation reagent. Samples and standards were then neutralized with 100 μL of 3 M phosphoric acid, followed by the addition of 15 μL methanol and deproteinization using Amicon Ultra 0.5-mL 10K units. Aliquots (typically 30 to 150 µL) of the flow-through were separated on an Altima HP C18 amide column (150 × 4.6 mm; 5 μm; 190Å; Alltech). The thiochromes were detected fluorometrically (excitation 367 nm, emission 435 nm). The mobile phase (1 mL/min) consisted of a gradient of potassium phosphate (140 mM, pH 7.00)/12% methanol (A) to 70% methanol in water (B). Runs began with 100% A; the proportion of B was increased linearly to 50% during the first 10 min, then from 50 to 70% during the next 2 min, and finally from 70 to 100% during 5 min. Values of kcat and Km were estimated by nonlinear fitting using GraphPad Prism version 7.00.
Assays for hydrolysis of amino-HMP, N-formylamino-HMP, desthiothiamin, thiamin, and ThDP were made in 50-μL reaction mixtures containing 50 mM Tris-HCl, pH 7.5, 1 mM DTT, 500 mM glycine betaine, 1 mM substrate, and 60 μg of enzyme protein. Assays were run at 30°C for 1 to 4 h, stopped on ice, mixed with 250 μL water, and then deproteinized at 4°C using Amicon Ultra 0.5-mL 10K units. Samples (typically 100 μL) of the flow-through were analyzed by HPLC with UV detection as described (Zallot et al., 2014) except that the elution buffer was 10 mM potassium phosphate, pH 6.6. The detection limit in these assays was 6 pmol min−1 mg−1 protein.
Thiamin Vitamer Analysis
Plant samples (∼300 mg) were ground to a fine powder in liquid N2. E. coli BL21-CodonPlus (DE3)-RIPL cells (Agilent) harboring pET28b alone or encoding At5g32570, At5g32570-D317A, GRMZM2G148896, or GRMZM2G078283, His-tagged and minus their predicted targeting sequences as above, were cultured in 10 mL of LB medium containing 50 µg/mL kanamycin at 37°C, with continuous shaking at 220 rpm. When OD600 reached 0.4, IPTG (final concentration 0.5 mM) was added and incubation was continued until OD600 reached 1.0. Cells were then harvested, washed with PBS, and frozen in liquid N2. To analyze thiamin and its phosphates, plant and bacterial samples were extracted in 500 μL of 7.2% perchloric acid by sonicating for 30 min in a bath and holding on ice for 15 min with periodic vortex-mixing, then cleared by centrifugation (14,000g, 10 min, 22°C) and converted to thiochrome derivatives followed by HPLC with fluorometric detection (Fraccascia et al., 2007; Hasnain et al., 2016).
Subcellular Localization
Full-length or N-terminally truncated At5g32470 and GRMZM2G148896 cDNAs were cloned into pGEM4Z (Promega), and the constructs were verified by DNA sequencing. In vitro transcription-translation was performed using a TnT SP6 Coupled Wheat Germ Extract System (Promega), according to the manufacturer's instructions, with 1 μg of plasmid and 35-40 μCi [3H]leucine (115.8 Ci/mmol; Perkin-Elmer) as label. Samples were resolved by SDS-PAGE (10% gel), and the gels were impregnated with 2,5-diphenyloxazole before the bands were visualized by fluorography.
Full-length or N-terminally truncated At5g32470 cDNA, or the predicted mitochondrial targeting sequence, were cloned into the NcoI site of pUC18/NcoI-mGFP, immediately 5′ of the open reading frame for monomeric GFP (Clark et al., 2009) to create peptide-mGFP fusions. The targeting regions that were removed, or fused directly to GFP, are shown in Figure 7. The Agilent QuikChange II Kit was used as above to change the ATG codon corresponding to the second methionine to TTG (leucine). The generated plasmids containing the peptide-GFP fusions obtained were used as templates for PCR amplification followed by cloning of the corresponding sequences into Gateway plasmid pDONR207 (Life Technologies) using Gateway BP Clonase II Enzyme Mix (Life Technologies). The constructs were then transferred to the destination vector pMDC32, harboring a dual 35S CaMV promoter and a NOS terminator (Curtis and Grossniklaus, 2003), using Gateway LR Clonase II enzyme mix. Construction of the pMDC32-based binary plasmid encoding Cherry-At1g55450, which encodes the monomeric Cherry fluorescent protein fused to the N terminus of At1g55450, an S-adenosyl-l-methionine-dependent methyltransferase protein that is localized in the outer membrane of mitochondria, has been described previously (Marty et al., 2014). Primers used for each construct are given in Supplemental Table 2.
Agrobacterium-infiltrated tobacco leaves were processed for confocal laser scanning microscopy imaging as described previously (Cai et al., 2015). Microscopy images of transiently cotransformed tobacco leaves were acquired using a Leica DM RBE microscope with a Leica 63× Plan Apochromat oil-immersion objective, a Leica TCS SP2 scanning head, and the Leica TCS NT software package (Leica). GFP and Cherry were excited with a 488-nm and a 543-nm laser, respectively, and emission fluorescence signals were collected at 500 to 540 nm for GFP and 590 to 640 nm for Cherry. All fluorophore emissions were collected sequentially and single-labeling experiments revealed no detectable crossover at the settings used for data collection. Images were acquired as individual single optical sections and all fluorescence images of cells shown are representative of at least two separate experiments, including at least 15 independent transformations. All figure compositions were generated using Adobe Photoshop CS and Illustrator CS2 (Adobe Systems).
Accession Numbers
Arabidopsis Genome Initiative locus identifiers for the genes mentioned in this article are as follows: TH2, At5g32470; and HAD phosphatase, At4g29530.
Supplemental Data
Supplemental Figure 1. Characterization of an At5g32470 T-DNA Insertional Mutant.
Supplemental Figure 2. The At5g32470 T-DNA Insertional Mutant Has Mild Growth Defects.
Supplemental Figure 3. The Phenotype of the At4g29530 th2-1 Double Mutant.
Supplemental Figure 4. Levels of Thiamin and Its Phosphates in E. coli Cells Overexpressing Wild-Type or D317A Mutant At5g32470, or Maize Orthologs GRMZM2G148896 or GRMZM078283.
Supplemental Figure 5. Isolation of Recombinant At5g32470, GRMZM2G148896, and GRMZM2G078283 Proteins and Estimation of Native Molecular Mass.
Supplemental Figure 6. Alignment of the N-Terminal Regions of the At5g32470, GRMZM2G148896, and GRMZM078283 Proteins and Their Orthologs, and Mitochondrial Targeting Prediction Scores.
Supplemental Table 1. The Panel of 95 Phosphoesters Used in Activity Screens.
Supplemental Table 2. Oligonucleotide Primers Used in This Study.
Supplementary Material
Acknowledgments
This research was supported by U.S. National Science Foundation Award IOS-1444202 (to A.D.H.), by National Sciences and Engineering Research Council of Canada Award 217291 (to R.T.M.), by NSERC Strategic Network Grant IBN (to A.F.Y.), and by an endowment from the C.V. Griffin Sr. Foundation. R.T.M. holds a University of Guelph Research Chair. We thank K.N. Allen for advice on HAD enzyme catalysis, J. Pillet for help in vector construction, and M. Ziemak for technical support.
AUTHOR CONTRIBUTIONS
D.R.M. and A.D.H. designed research. M.M., R.Z., T.D.N., G.H., V.d.C.-L., G.B., A.F.Y., J.F.G., D.R.M., and A.D.H. performed biochemical and genetic research. G.B. and A.F.Y. ran phosphatase activity screening assays. S.K.G., T.N.D.N., E.M.A., and R.T.M. carried out in vivo protein localization studies. All authors analyzed data. D.R.M., R.T.M, and A.D.H. wrote the article.
Glossary
- ThDP
thiamin diphosphate
- ThMP
thiamin monophosphate
- WGS
whole-genome sequencing
Footnotes
Articles can be viewed without a subscription.
References
- Ajjawi I., Rodriguez Milla M.A., Cushman J., Shintani D.K. (2007). Thiamin pyrophosphokinase is required for thiamin cofactor activation in Arabidopsis. Plant Mol. Biol. 65: 151–162. [DOI] [PubMed] [Google Scholar]
- Bar-Even A., Noor E., Savir Y., Liebermeister W., Davidi D., Tawfik D.S., Milo R. (2011). The moderately efficient enzyme: evolutionary and physicochemical trends shaping enzyme parameters. Biochemistry 50: 4402–4410. [DOI] [PubMed] [Google Scholar]
- Barison N., Cendron L., Trento A., Angelini A., Zanotti G. (2009). Structural and mutational analysis of TenA protein (HP1287) from the Helicobacter pylori thiamin salvage pathway: evidence of a different substrate specificity. FEBS J. 276: 6227–6235. [DOI] [PubMed] [Google Scholar]
- Begley T.P., Ealick S.E., McLafferty F.W. (2012). Thiamin biosynthesis: still yielding fascinating biological chemistry. Biochem. Soc. Trans. 40: 555–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohrer A.S., Yoshimoto N., Sekiguchi A., Rykulski N., Saito K., Takahashi H. (2015). Alternative translational initiation of ATP sulfurylase underlying dual localization of sulfate assimilation pathways in plastids and cytosol in Arabidopsis thaliana. Front. Plant Sci. 5: 750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J. (1943). Effects of application of thiamin to Cosmos. Bot. Gaz. 104: 475–479. [Google Scholar]
- Boynton J.E. (1966). Chlorophyll-deficient mutants in tomato requiring vitamin B1. I. Genetics and physiology. Hereditas 56: 171–199. [Google Scholar]
- Bradford M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Bunik V.I., Schloss J.V., Pinto J.T., Gibson G.E., Cooper A.J. (2007). Enzyme-catalyzed side reactions with molecular oxygen may contribute to cell signaling and neurodegenerative diseases. Neurochem. Res. 32: 871–891. [DOI] [PubMed] [Google Scholar]
- Burroughs A.M., Allen K.N., Dunaway-Mariano D., Aravind L. (2006). Evolutionary genomics of the HAD superfamily: understanding the structural adaptations and catalytic diversity in a superfamily of phosphoesterases and allied enzymes. J. Mol. Biol. 361: 1003–1034. [DOI] [PubMed] [Google Scholar]
- Cai Y., Goodman J.M., Pyc M., Mullen R.T., Dyer J.M., Chapman K.D. (2015). Arabidopsis SEIPIN proteins modulate triacylglycerol accumulation and influence lipid droplet proliferation. Plant Cell 27: 2616–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., et al. (2011). Whole-genome sequencing of multiple Arabidopsis thaliana populations. Nat. Genet. 43: 956–963. [DOI] [PubMed] [Google Scholar]
- Cascales-Miñana B., Muñoz-Bertomeu J., Flores-Tornero M., Anoman A.D., Pertusa J., Alaiz M., Osorio S., Fernie A.R., Segura J., Ros R. (2013). The phosphorylated pathway of serine biosynthesis is essential both for male gametophyte and embryo development and for root growth in Arabidopsis. Plant Cell 25: 2084–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A., Schomburg I., Placzek S., Jeske L., Ulbrich M., Xiao M., Sensen C.W., Schomburg D. (2015). BRENDA in 2015: exciting developments in its 25th year of existence. Nucleic Acids Res. 43: D439–D446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S.M., Di Leo R., Dhanoa P.K., Van Cauwenberghe O.R., Mullen R.T., Shelp B.J. (2009). Biochemical characterization, mitochondrial localization, expression, and potential functions for an Arabidopsis γ-aminobutyrate transaminase that utilizes both pyruvate and glyoxylate. J. Exp. Bot. 60: 1743–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis M.D., Grossniklaus U. (2003). A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 133: 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daras G., Rigas S., Tsitsekian D., Zur H., Tuller T., Hatzopoulos P. (2014). Alternative transcription initiation and the AUG context configuration control dual-organellar targeting and functional competence of Arabidopsis Lon1 protease. Mol. Plant 7: 989–1005. [DOI] [PubMed] [Google Scholar]
- Dong W., Stockwell V.O., Goyer A. (2015). Enhancement of thiamin content in Arabidopsis thaliana by metabolic engineering. Plant Cell Physiol. 56: 2285–2296. [DOI] [PubMed] [Google Scholar]
- Ellens K.W., Richardson L.G., Frelin O., Collins J., Ribeiro C.L., Hsieh Y.F., Mullen R.T., Hanson A.D. (2015). Evidence that glutamine transaminase and omega-amidase potentially act in tandem to close the methionine salvage cycle in bacteria and plants. Phytochemistry 113: 160–169. [DOI] [PubMed] [Google Scholar]
- Emanuelsson O., Nielsen H., Brunak S., von Heijne G. (2000). Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J. Mol. Biol. 300: 1005–1016. [DOI] [PubMed] [Google Scholar]
- Fraccascia P., Sniekers M., Casteels M., Van Veldhoven P.P. (2007). Presence of thiamine pyrophosphate in mammalian peroxisomes. BMC Biochem. 8: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French J.B., Begley T.P., Ealick S.E. (2011). Structure of trifunctional THI20 from yeast. Acta Crystallogr. D Biol. Crystallogr. 67: 784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes S., Lerma-Ortiz C., Frelin O., Seaver S.M., Henry C.S., de Crécy-Lagard V., Hanson A.D. (2012). Plant B vitamin pathways and their compartmentation: a guide for the perplexed. J. Exp. Bot. 63: 5379–5395. [DOI] [PubMed] [Google Scholar]
- Goyer A. (2010). Thiamine in plants: aspects of its metabolism and functions. Phytochemistry 71: 1615–1624. [DOI] [PubMed] [Google Scholar]
- Hanson A.D., Pribat A., Waller J.C., de Crécy-Lagard V. (2009). ‘Unknown’ proteins and ‘orphan’ enzymes: the missing half of the engineering parts list--and how to find it. Biochem. J. 425: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasnain G., Roje S., Sa N., Zallot R., Ziemak M.J., de Crécy-Lagard V., Gregory J.F. III, Hanson A.D. (2016). Bacterial and plant HAD enzymes catalyse a missing phosphatase step in thiamin diphosphate biosynthesis. Biochem. J. 473: 157–166. [DOI] [PubMed] [Google Scholar]
- Ito J., Batth T.S., Petzold C.J., Redding-Johanson A.M., Mukhopadhyay A., Verboom R., Meyer E.H., Millar A.H., Heazlewood J.L. (2011). Analysis of the Arabidopsis cytosolic proteome highlights subcellular partitioning of central plant metabolism. J. Proteome Res. 10: 1571–1582. [DOI] [PubMed] [Google Scholar]
- Jenkins A.H., Schyns G., Potot S., Sun G., Begley T.P. (2007). A new thiamin salvage pathway. Nat. Chem. Biol. 3: 492–497. [DOI] [PubMed] [Google Scholar]
- Jurgenson C.T., Begley T.P., Ealick S.E. (2009). The structural and biochemical foundations of thiamin biosynthesis. Annu. Rev. Biochem. 78: 569–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus S.M., Wegkamp A., Sybesma W., Hugenholtz J., Gregory J.F. III, Hanson A.D. (2005). A nudix enzyme removes pyrophosphate from dihydroneopterin triphosphate in the folate synthesis pathway of bacteria and plants. J. Biol. Chem. 280: 5274–5280. [DOI] [PubMed] [Google Scholar]
- Kozak M. (1981). Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 9: 5233–5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Sharma S.B. (1986). Mutations in three of the genes determining thiamine biosynthesis in Pisum sativum. Mol. Gen. Genet. 204: 473–476. [Google Scholar]
- Kurata G., Sakai T., Miyara T. (1968). Studies on antagonists of thiamine: XVIII. Reaction condition in formation of desthiothiamine from alkaline thiamine solution with amino acids. Vitamins (Kyoto) 37: 398–402. [Google Scholar]
- Kuznetsova E., et al. (2015). Functional diversity of haloacid dehalogenase superfamily phosphatases from Saccharomyces cerevisiae: biochemical, structural, and evolutionary insights. J. Biol. Chem. 290: 18678–18698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova E., et al. (2006). Genome-wide analysis of substrate specificities of the Escherichia coli haloacid dehalogenase-like phosphatase family. J. Biol. Chem. 281: 36149–36161. [DOI] [PubMed] [Google Scholar]
- Langridge J. (1955). Biochemical mutations in the crucifer Arabidopsis thaliana (L.) Heynh. Nature 176: 260–261. [DOI] [PubMed] [Google Scholar]
- Langridge J. (1965). Temperature-sensitive, vitamin-requiring mutants of Arabidopsis thaliana. Aust. J. Biol. Sci. 18: 311–321. [Google Scholar]
- Li S.L., Rédei G.P. (1969). Thiamine mutants of the crucifer, Arabidopsis. Biochem. Genet. 3: 163–170. [DOI] [PubMed] [Google Scholar]
- Li S.L., Rédei G.P., Gowans C.S. (1967). A phylogenetic comparison of mutation spectra. Mol. Gen. Genet. 100: 77–83. [DOI] [PubMed] [Google Scholar]
- Lu Y.H., Guan Z., Zhao J., Raetz C.R. (2011). Three phosphatidylglycerol-phosphate phosphatases in the inner membrane of Escherichia coli. J. Biol. Chem. 286: 5506–5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marçais G., Kingsford C. (2011). A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics 27: 764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C. (2013). The interface between plant metabolic engineering and human health. Curr. Opin. Biotechnol. 24: 344–353. [DOI] [PubMed] [Google Scholar]
- Marty N.J., Teresinski H.J., Hwang Y.T., Clendening E.A., Gidda S.K., Sliwinska E., Zhang D., Miernyk J.A., Brito G.C., Andrews D.W., Dyer J.M., Mullen R.T. (2014). New insights into the targeting of a subset of tail-anchored proteins to the outer mitochondrial membrane. Front. Plant Sci. 5: 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney A.W., Greenwood J.S., Fabian M.R., White K.A., Mullen R.T. (2005). Localization of the tomato bushy stunt virus replication protein p33 reveals a peroxisome-to-endoplasmic reticulum sorting pathway. Plant Cell 17: 3513–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinke D., Sweeney C., Muralla R. (2009). Integrating the genetic and physical maps of Arabidopsis thaliana: identification of mapped alleles of cloned essential (EMB) genes. PLoS One 4: e7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller I.B., Bergmann B., Groves M.R., Couto I., Amaral L., Begley T.P., Walter R.D., Wrenger C. (2009). The vitamin B1 metabolism of Staphylococcus aureus is controlled at enzymatic and transcriptional levels. PLoS One 4: e7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K. (1982). Actions of two types of thiaminase on thiamin and its analogues. Ann. N. Y. Acad. Sci. 378: 146–156. [DOI] [PubMed] [Google Scholar]
- Niehaus T.D., Richardson L.G., Gidda S.K., ElBadawi-Sidhu M., Meissen J.K., Mullen R.T., Fiehn O., Hanson A.D. (2014). Plants utilize a highly conserved system for repair of NADH and NADPH hydrates. Plant Physiol. 165: 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G., De Paepe R., Foyer C.H. (2007). Mitochondrial redox biology and homeostasis in plants. Trends Plant Sci. 12: 125–134. [DOI] [PubMed] [Google Scholar]
- Nosaka K. (2006). Recent progress in understanding thiamin biosynthesis and its genetic regulation in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 72: 30–40. [DOI] [PubMed] [Google Scholar]
- Onozuka M., Konno H., Kawasaki Y., Akaji K., Nosaka K. (2008). Involvement of thiaminase II encoded by the THI20 gene in thiamin salvage of Saccharomyces cerevisiae. FEMS Yeast Res. 8: 266–275. [DOI] [PubMed] [Google Scholar]
- Petersen L.N., Marineo S., Mandalà S., Davids F., Sewell B.T., Ingle R.A. (2010). The missing link in plant histidine biosynthesis: Arabidopsis myoinositol monophosphatase-like2 encodes a functional histidinol-phosphate phosphatase. Plant Physiol. 152: 1186–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie J.R., Shrestha P., Liu Q., Mansour M.P., Wood C.C., Zhou X.R., Nichols P.D., Green A.G., Singh S.P. (2010). Rapid expression of transgenes driven by seed-specific constructs in leaf tissue: DHA production. Plant Methods 6: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourcel L., Moulin M., Fitzpatrick T.B. (2013). Examining strategies to facilitate vitamin B1 biofortification of plants by genetic engineering. Front. Plant Sci. 4: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rains T.M., Emmert J.L., Baker D.H., Shay N.F. (1997). Minimum thiamin requirement of weanling Sprague-Dawley outbred rats. J. Nutr. 127: 167–170. [DOI] [PubMed] [Google Scholar]
- Rapala-Kozik M., Gołda A., Kujda M. (2009). Enzymes that control the thiamine diphosphate pool in plant tissues. Properties of thiamine pyrophosphokinase and thiamine-(di)phosphate phosphatase purified from Zea mays seedlings. Plant Physiol. Biochem. 47: 237–242. [DOI] [PubMed] [Google Scholar]
- Rudhe C., Chew O., Whelan J., Glaser E. (2002). A novel in vitro system for simultaneous import of precursor proteins into mitochondria and chloroplasts. Plant J. 30: 213–220. [DOI] [PubMed] [Google Scholar]
- Schmitz R.J., Schultz M.D., Urich M.A., Nery J.R., Pelizzola M., Libiger O., Alix A., McCosh R.B., Chen H., Schork N.J., Ecker J.R. (2013). Patterns of population epigenomic diversity. Nature 495: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small I., Peeters N., Legeai F., Lurin C. (2004). Predotar: A tool for rapidly screening proteomes for N-terminal targeting sequences. Proteomics 4: 1581–1590. [DOI] [PubMed] [Google Scholar]
- Sparkes I.A., Runions J., Kearns A., Hawes C. (2006). Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 1: 2019–2025. [DOI] [PubMed] [Google Scholar]
- Sümegi B., Alkonyi I. (1983). Paracatalytic inactivation of pig heart pyruvate dehydrogenase complex. Arch. Biochem. Biophys. 223: 417–424. [DOI] [PubMed] [Google Scholar]
- Tunc-Ozdemir M., Miller G., Song L., Kim J., Sodek A., Koussevitzky S., Misra A.N., Mittler R., Shintani D. (2009). Thiamin confers enhanced tolerance to oxidative stress in Arabidopsis. Plant Physiol. 151: 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward J.B., Abeydeera N.D., Paul D., Phillips K., Rapala-Kozik M., Freeling M., Begley T.P., Ealick S.E., McSteen P., Scanlon M.J. (2010). A maize thiamine auxotroph is defective in shoot meristem maintenance. Plant Cell 22: 3305–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallot R., et al. (2014). Salvage of the thiamin pyrimidine moiety by plant TenA proteins lacking an active-site cysteine. Biochem. J. 463: 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Henriques R., Lin S.S., Niu Q.W., Chua N.-H. (2006). Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 1: 641–646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.