This work describes a model for the transcriptional control of fatty acid composition in endosperm tissue.
Abstract
In angiosperms, double fertilization of the embryo sac initiates the development of the embryo and the endosperm. In Arabidopsis thaliana, an exalbuminous species, the endosperm is reduced to one cell layer during seed maturation and reserves such as oil are massively deposited in the enlarging embryo. Here, we consider the strikingly different fatty acid (FA) compositions of the oils stored in the two zygotic tissues. Endosperm oil is enriched in ω-7 monounsaturated FAs, that represent more than 20 mol% of total FAs, whereas these molecular species are 10-fold less abundant in the embryo. Two closely related transcription factors, MYB118 and MYB115, are transcriptionally induced at the onset of the maturation phase in the endosperm and share a set of transcriptional targets. Interestingly, the endosperm oil of myb115 myb118 double mutants lacks ω-7 FAs. The identification of two Δ9 palmitoyl-ACP desaturases responsible for ω-7 FA biosynthesis, which are activated by MYB115 and MYB118 in the endosperm, allows us to propose a model for the transcriptional control of oil FA composition in this tissue. In addition, an initial characterization of the structure-function relationship for these desaturases reveals that their particular substrate specificity is conferred by amino acid residues lining their substrate pocket that distinguish them from the archetype Δ9 stearoyl-ACP desaturase.
INTRODUCTION
In spermatophyta, also known as seed plants, the double fertilization of the embryo sac initiates the development of zygotic tissues, namely, the embryo and the endosperm. They are protected by the seed coat, which comprises several cell layers of maternal origin derived from the ovular integuments. Seed formation therefore requires the coordinated growth of tissues of distinct origins that undergo two successive developmental phases: morphogenesis and maturation (Vicente-Carbajosa and Carbonero, 2005). Maturing seeds accumulate reserve compounds that are remobilized to fuel postgerminative seedling establishment. Depending on the species considered, the nature, relative proportion, and tissue localization of these reserve components vary greatly. Exalbuminous seeds of Arabidopsis thaliana store approximately equivalent amounts of oil (triacylglycerols) and storage proteins (2S albumins and 12S globulins), these compounds being mostly deposited in a large embryo structure acquired at the expense of the endosperm (Baud et al., 2002). In mature dry seeds, the residual endosperm consists of a thin peripheral cell layer that contains no more than 10% of total seed reserves (Li et al., 2006). The fine biochemical characterization of the endosperm has revealed a reserve composition clearly different from that of the embryo, with a strongly decreased abundance of globulins (Barthole et al., 2014) and a unique oil fatty acid (FA) composition (Penfield et al., 2004). If all the FAs detected in the endosperm are also present in the embryo, the former contains 10-fold higher proportions of ω-7 monounsaturated FAs, like vaccenic acid (cis-ω-7 C18:1) and paullinic acid (cis-ω-7 C20:1), that account for more than 50% of the total ω-7 FAs present in the whole seed.
In plants, de novo synthesis of FAs occurs in plastids (Harwood, 1996). Production of 16- or 18-carbon saturated FAs is catalyzed by the type II fatty acid synthase. Stromal ∆9 acyl-ACP desaturases (AADs) can introduce a carbon-carbon double bond (also called unsaturation) within these saturated acyl chains to form cis-monoenes (Lindqvist et al., 1996). AAD isoforms with different substrate specificities catalyze the formation of distinct monoenes differing by the position of the unsaturation within their aliphatic chains (referred to as ω-x). For instance, ∆9 stearoyl-ACP desaturases (SADs) efficiently desaturate C18:0 to form cis-ω-9 C18:1 (oleic acid). SADs represent the predominant AAD isoforms in most seed plants. Accordingly, the majority of the FAs found in embryo oil of Arabidopsis consist of oleic acid and of its derivatives. However, other AAD isoforms prefer C16:0 instead of C18:0 as a substrate. These ∆9 palmitoyl-ACP desaturases (PADs) catalyze the formation of cis-ω-7 C16:1 (palmitoleic acid), which can be further elongated to cis-ω-7 C18:1 (vaccenic acid), then to cis-ω-7 C20:1 (paullinic acid). Monoenes of the ω-7 FA series occur infrequently in most seed plants, with the noticeable exception of a few plant species that produce unusual oils enriched in these ω-7 monounsaturated FAs, such as cat’s claw vine (Doxantha unguis-cati) or sea buckthorn (Hippophae rhamnoides) (Bondaruk et al., 2007; Fatima et al., 2012).
The genome of the model plant Arabidopsis contains seven closely related genes coding for AADs (Kachroo et al., 2007). FAB2, the best-characterized member of the family, encodes a SAD (Lightner et al., 1994), whereas the other members of the family have been poorly characterized. The PADs responsible for the production ω-7 monounsaturated FAs that accumulate at high levels in the endosperm oil of Arabidopsis seeds remain to be identified.
Over the last decade, our knowledge on the regulation of storage compound metabolism in maturing seeds has increased tremendously. This knowledge has arisen mostly from genetic analyses performed in Arabidopsis. Transcriptional regulators ensuring that maturation-related programs, such as oil biosynthesis, are correctly deployed during the transition phase between embryogenesis and seed maturation have been identified (Santos-Mendoza et al., 2008). These transcription factors (TFs) participate in a complex network essential for completion of seed filling (Roscoe et al., 2015). Master regulators of the maturation program include members of the ABSCISIC ACID INSENSITIVE3/FUSCA3/LEAFY COTYLEDON2 network. These TFs belong to the B3 domain superfamily of DNA binding proteins and cooperate with LEAFY COTYLEDON1 (LEC1), a protein homologous to the HAP3 subunit of CCAAT-box binding proteins (Lotan et al., 1998; Suzuki and McCarty, 2008). Next to these master regulators, other TFs like basic leucine zippers bZIP53 or bZIP67 confer correct expression patterns to maturation genes (Mendes et al., 2013). Several genes encoding storage proteins or actors involved in triacylglycerol assembly and storage were shown to be direct targets of the above-mentioned TFs. By contrast, transcriptional activation of many glycolytic and FA biosynthetic genes, which is essential to support sustained rates of oil production, is indirectly mediated by WRINKLED1 (WRI1), a TF of the AP2-EREBP family (Cernac and Benning, 2004; Baud and Lepiniec, 2010). Together, these studies have led to a significant breakthrough in our understanding of the activation of reserve compound synthesis in the embryo, while the regulation of endosperm metabolism has scarcely been investigated in Arabidopsis. The recent characterization of MYB118, a TF transcriptionally induced in the maturing endosperm and repressing storage compound accumulation in this seed compartment, has shed some new light on the differential regulation of reserve partitioning between the embryo and endosperm (Barthole et al., 2014). However, the regulatory mechanisms explaining the contrasting compositions of these reserves remain completely unknown.
To isolate new regulators of endosperm maturation and elucidate the peculiar composition of endosperm reserves, new screening procedures have been undertaken. Here, we report the functional characterization of MYB115 (At5g40360), a close homolog of MYB118 also induced in the endosperm at the onset of seed maturation. We provide evidence that the master regulator LEC2 positively regulates the two genes. This regulation and the negative feedback exerted by MYB118 on LEC2 expression suggest a partial compensation of the myb118 mutation by an overexpression of MYB115 in this genetic background. This hypothesis was confirmed by the thorough characterization of maturing myb115 myb118 mutant seeds demonstrating that the two MYBs are positive regulators of ω-7 monounsaturated FA synthesis in the endosperm. We finally describe the identification of two targets of MYB115 and MYB118 belonging to the AAD multigene family. We show that these targets, namely, AAD2 and AAD3, encode two ∆9 PADs responsible for the biosynthesis of ω-7 FAs in the maturing endosperm. Taken together, these results allow us to establish a model for a transcriptional activation cascade participating in the control of oil FA composition within the maturing endosperm of Arabidopsis seeds.
RESULTS
MYB115 Is Induced in the Endosperm of Maturing Seeds
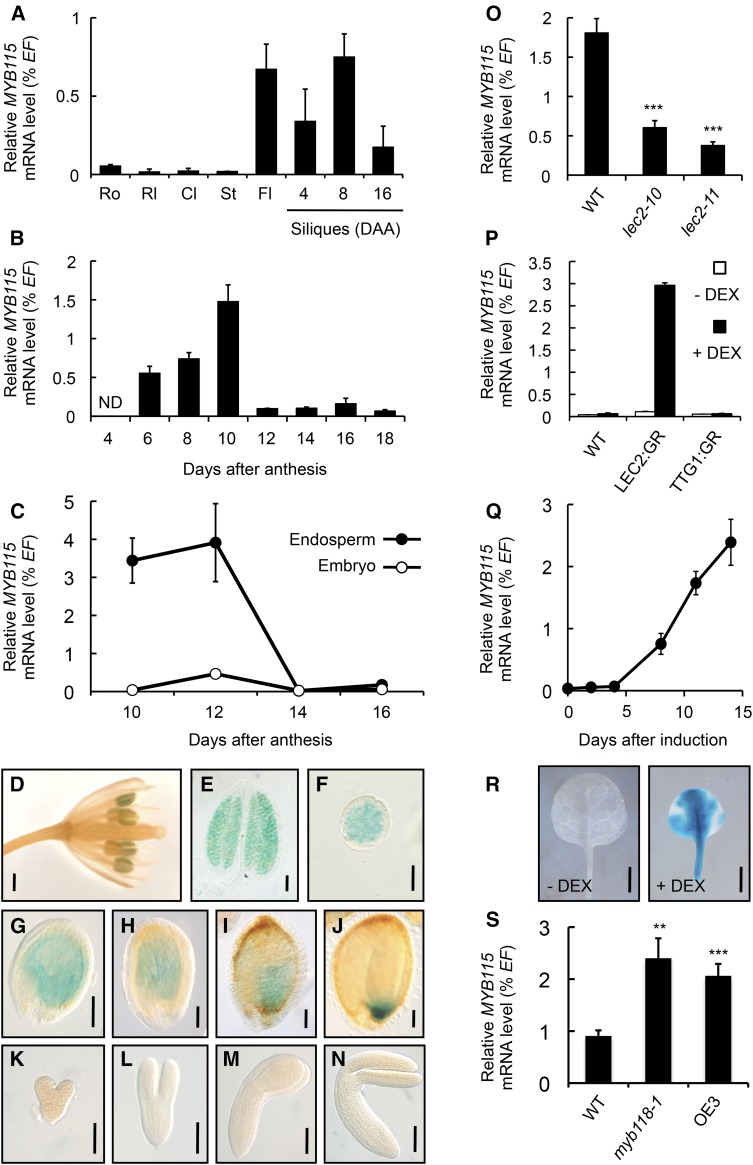
Functional redundancy between related TFs has been previously documented in Arabidopsis and recent studies suggest that MYB118 and MYB115, two close relatives of the MYB family (Wang et al., 2009; Zhang et al., 2009; Dubos et al., 2010), share transcriptional targets involved in glucosinolate biosynthesis (Zhang et al., 2015). To determine whether overlaps in function between MYB118 and close homologs occur in the maturing endosperm, we first examined the expression patterns of the three closest paralogs of MYB118, namely, MYB115, MYB22, and MYB100 (Dubos et al., 2010), by RT-qPCR on a set of cDNAs prepared from a range of plant organs of the wild-type accession Columbia-0 (Col-0). In all analyzed tissues, the accumulation of MYB22 and MYB100 transcripts was below detectable levels; this was consistent with previously published transcriptomic analyses (Schmid et al., 2005; Le et al., 2010). By contrast, MYB115 appeared to be expressed at very low levels in vegetative organs and induced in reproductive organs (flowers and developing siliques) (Figure 1A). To further characterize the expression pattern of MYB115, a time-course analysis of MYB115 mRNA abundance was performed in developing seeds excised from siliques, which revealed a peak of transcript accumulation at the onset of seed maturation (Figure 1B). Maturing seeds were then dissected and the two fractions obtained, namely, embryo and endosperm fraction (comprising the endosperm and the seed coat; see Methods), were independently analyzed. MYB115 mRNA abundance was high in the endosperm fraction during early maturation and hardly detected in the embryo (Figure 1C).
Figure 1.
Expression Pattern and Transcriptional Regulation of MYB115.
(A) to (C) Analysis of relative mRNA accumulation of MYB115 was performed in different plant organs (A), in developing seeds (B), and in developmental series of endosperm and embryo fractions (C). The results obtained are standardized to the EF1αA4 (EF) gene expression level. Values are the means and se of three to six replicates performed on cDNA dilutions obtained from three independent mRNA extractions. Cl, cauline leaves; Fl, flowers; ND, not detected; Rl, rosette leaves; Ro, roots; St, stems.
(D) to (N) Pattern of activity of the ProMYB115:uidA cassette in flowers (D), in stamens (E), in pollen grains (F), in developing seeds harvested 6 (G), 8 (H), 10 (I), or 12 (J) DAA, and in early maturing embryos harvested 6 (K), 8 (L), 10 (M), or 12 (N) DAA. For histochemical detection of GUS activity, tissues were incubated overnight in a buffer containing 0.2 mM each of potassium ferrocyanide and potassium ferricyanide. The results for GUS activity were observed on whole-mounted inflorescences; microscopy observations of stamens, pollen grains, seeds, and excised embryos were performed using Nomarski optics. Bars = 1 mm in (D), 50 μm in (E), 20 μm in (F), 100 μm in (G) to (J), and 50 μm in (K) to (N).
(O) Accumulation of MYB115 mRNA in lec2 mutant seeds was quantified 10 DAA by RT-qPCR and presented as the percentage of the EF1αA4 (EF) gene expression. Values are the means and se of three to six replicates performed on three independent cDNA preparations obtained from batches of seeds dissected from four to five siliques. The three silique sets were harvested on distinct individuals. Asterisks indicate significant difference from the wild type according to t test, ***P < 0.001.
(P) Accumulation of MYB115 mRNA in leaves of transgenic Pro35S:LEC2:GR, Pro35S:TTG1:GR (negative control), or wild-type 10-d-old plants cultured in vitro on a medium with (+ DEX) or without (− DEX) 10−5 M DEX for two additional weeks, quantified by RT-qPCR, and presented as percentage of the EF1αA4 (EF) gene expression. Values are the means and se of three to four replicates performed on three independent cDNA preparations.
(Q) Time-course analysis of MYB115 mRNA accumulation in leaves of 10-d-old Pro35S:LEC2:GR plants transferred to a growth medium containing 10−5 M DEX (induction) and grown for 2 weeks in vitro. Accumulation of mRNA was determined by RT-qPCR and presented as the percentage of the EF1αA4 (EF) gene expression. Values are the means and se of three to six replicates performed on three independent cDNA preparations.
(R) Transgenic ProMYB115:uidA × Pro35S:LEC2:GR seedlings were transferred 10 d after germination on a DEX-containing medium (10−5 M; + DEX). Rosette leaves were analyzed 2 weeks after induction. For histochemical detection of GUS activity, tissues were incubated overnight in a buffer containing 0.2 mM each of potassium ferrocyanide and potassium ferricyanide. Bars = 0.2 cm.
(S) Accumulation of MYB115 mRNA in myb118 mutant seeds (myb118-1 and OE3) was quantified 10 DAA by RT-qPCR and presented as the percentage of the EF1αA4 (EF) gene expression. Values are the means and se of three replicates performed on cDNA dilutions obtained from three independent mRNA extractions. OE3, Pro35Sdual:MYB118 transgenic line exhibiting a strong repression of MYB118 expression (Barthole et al., 2014); WT, wild type (Col-0). Asterisks indicate significant difference from the wild type according to t test at ***P < 0.001 and **P < 0.01, respectively.
To gain complementary information about the expression pattern of MYB115, the spatiotemporal activity of the MYB115 promoter was investigated. A 1-kb promoter fragment was transcriptionally fused to the uidA reporter gene. The corresponding construct was assayed for the resulting uidA expression pattern in transgenic Arabidopsis lines (Figures 1D to 1N). GUS activity was observed in pollen grains (Figures 1D and 1E) and in seeds. A closer examination of developing seeds showed that the endosperm was stained (Figures 1G to 1J), whereas the seed coat and the embryo (Figures 1K to 1N) were not. The intense staining observed in the chalazal endosperm 12 d after anthesis (DAA; Figure 1J) was consistent with previous results of laser-capture microdissection of maturing seeds followed by mRNA quantification using stringent analyses of Affymetrix ATH1 GeneChip hybridization data (Le et al., 2010). MYB115 therefore appears to be coexpressed with MYB118 in the endosperm of early-maturing seeds (Barthole et al., 2014).
Transcriptional Activation of MYB115 by LEC2
The master regulator LEC2 plays a key role in the transcriptional activation of MYB118 (Barthole et al., 2014). To test whether LEC2 also influences the transcriptional activation of MYB115, analysis of MYB115 mRNA abundance was first performed in lec2 mutants. MYB115 transcripts were analyzed by RT-qPCR on cDNA prepared from seeds 10 DAA. MYB115 transcript steady state levels were significantly reduced in lec2 alleles, suggesting the downregulation of MYB115 expression in the absence of LEC2 (Figure 1O). To test the transcriptional activation of MYB115 by LEC2, we used a dexamethasone (DEX; a synthetic glucocorticoid that activates the rat glucocorticoid receptor [GR]) inducible system (Santos Mendoza et al., 2005). The relative expression level of MYB115 was quantified by RT-qPCR in leaves of Pro35S:LEC2:GR plants. In transgenic plants treated for 2 weeks with DEX, a specific and significant accumulation of MYB115 mRNA was observed (Figure 1P). A time-course analysis of MYB115 mRNA accumulation in rosette leaves treated with DEX revealed a marked increase of MYB115 mRNA levels from 4 d after induction onwards (Figure 1Q). Finally, the ProMYB115:uidA construct was introduced into transgenic Pro35S:LEC2:GR lines. The seedlings obtained were grown for 14 d on DEX-containing medium and were then assayed for the resulting uidA expression pattern (Figure 1R). GUS staining was detected in rosette leaves of these seedlings, confirming the ability of the LEC2:GR fusion protein to trigger MYB115 transcription.
Transcriptional Repression of MYB115 by MYB118 Is LEC2 Dependent
MYB115 transcripts were analyzed by RT-qPCR on cDNA prepared from 10-DAA myb118 mutant seeds. MYB115 transcript steady state levels were significantly increased both in myb118-1 seeds and in the OE3 line (a Pro35Sdual:MYB118 transgenic line exhibiting a strong repression of MYB118 expression; Barthole et al., 2014), suggesting the upregulation of MYB115 expression in the absence of MYB118 (Figure 1S). To further evaluate the effect of MYB118 on MYB115 promoter activity, the ProMYB115:uidA construct was introduced into the myb118-1 mutant background and the resulting uidA expression pattern assayed in seeds aged 10 and 12 DAA (Supplemental Figure 1). The proportion of stained seeds and the intensity of GUS staining were drastically increased in myb118-1 seeds at both developmental stages, showing the importance of MYB118 for repressing MYB115 promoter activity in maturing seeds.
Considering the antagonistic regulation exerted by LEC2 and MYB118 on MYB115 expression, we tested whether these regulations were independent of each other (Supplemental Figure 2). For this purpose, MYB115 transcript levels were quantified by RT-qPCR on cDNA prepared from 10-DAA lec2 myb118-1 seeds and compared with that of single mutants. MYB115 transcripts levels in seeds of the double mutants were not significantly different from that measured in lec2 single mutants, demonstrating that the derepression of MYB115 observed in response to the myb118-1 mutation is LEC2 dependent.
MYB115 and MYB118 Are Transcriptional Regulators That Share Common Targets
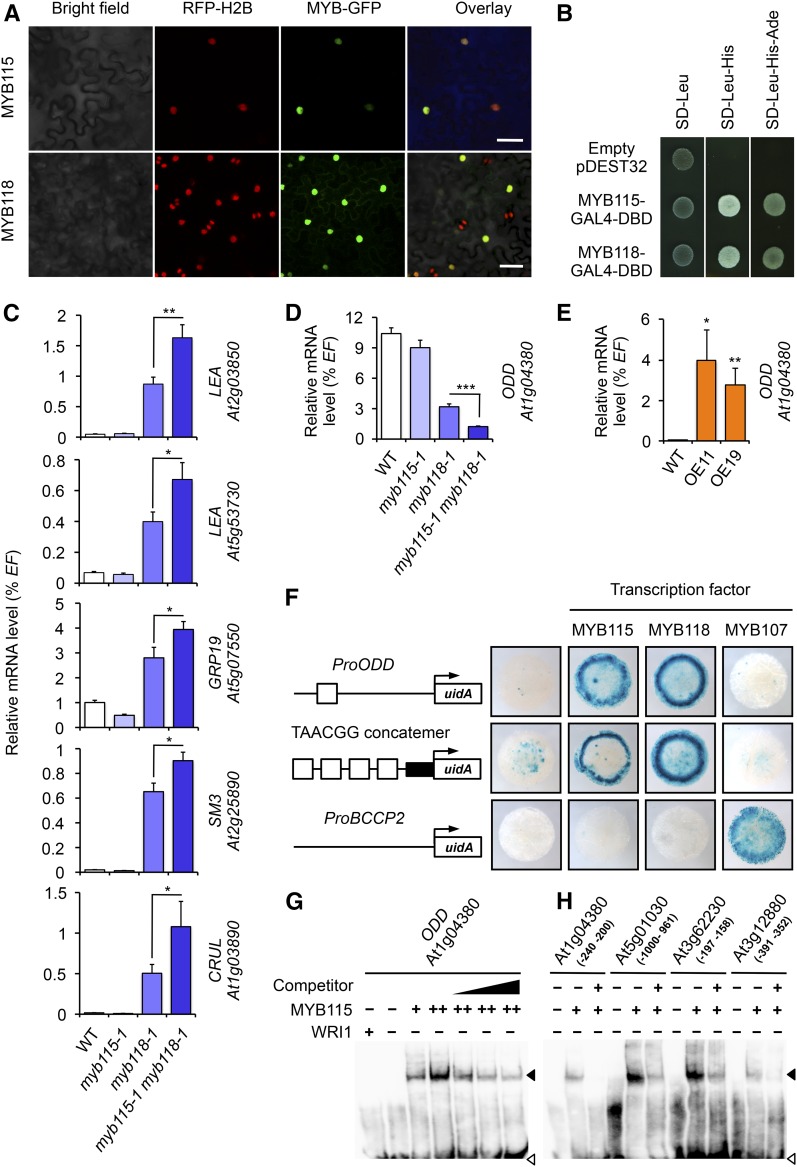
To firmly establish that MYB115 is a functional TF, we first investigated the in vivo subcellular localization of the protein with the aid of GFP. A derivative of GFP, mGFP6, was fused to MYB115 cDNA and placed under the control of the CaMV dual35S promoter for ubiquitous and high expression. The Pro35S:MYB115:GFP construct was transfected into leaves of transgenic Nicotiana benthamiana stably expressing RFP fused to histone 2B (RFP-H2B, used as nuclear marker; Martin et al., 2009). The Pro35S:MYB118:GFP construct was used as a positive control. Confocal imaging of transfected cells showed a colocalization of the GFP and RFP signals, demonstrating the nuclear targeting of MYB-GFP fusions (Figure 2A).
Figure 2.
Functional Characterization of MYB118 and MYB115.
(A) Confocal micrographs showing localization of MYB115:GFP and MYB118:GFP fusion proteins in transgenic N. benthamiana plants expressing RFP:H2B (Martin et al., 2009). Plants were coinfiltrated with the Pro35Sdual:MYB:GFP construct and a vector allowing the expression of the p19 protein of tomato bushy stunt virus (TBSV) that prevents the onset of posttranscriptional gene silencing (Shamloul et al., 2014). GFP was observed 4 d after infiltration. Bar = 50 µm.
(B) Transcriptional activity of MYB115 and MYB118. MYB115 and MYB118 coding sequences were cloned in frame with the GAL4 DNA binding domain (DBD). The fusion constructs were introduced into reporter yeast containing the HIS3 and ADE2 reporter genes, before being plated on appropriate media to maintain the expression of the vectors (SD-Leu) and to test the activation of the HIS3 (SD-Leu-His) or HIS3 and ADE2 reporter genes (SD-Leu-His-Ade). Data presented are representative from the results obtained for eight independent colonies. SD, synthetic dropout medium.
(C) and (D) RT-qPCR analysis of transcript abundance for negatively (C) and positively (D) regulated targets of MYB118 in cDNA prepared from wild-type (Col-0) and mutant seeds harvested 10 DAA. Values are the means and se of 12 replicates performed on cDNA dilutions obtained from three independent mRNA extractions. Asterisks indicate significant difference from the wild type according to t test at ***P < 0.001, ** P < 0.01, and * P < 0.05, respectively.
(E) RT-qPCR analysis of transcript abundance in cDNA prepared from rosette leaves of Pro35Sdual:MYB115 lines (OE11 and OE19). Values are the means and se of nine replicates performed on cDNA dilutions obtained from three independent mRNA extractions. Asterisks indicate significant difference from the wild type according to t test at **P < 0.01 and *P < 0.05, respectively.
(F) Transactivation assay in leaves of N. benthamiana. Schematic representations of the reporter constructs used are presented. Open boxes indicate TAACGG elements, and the closed box represents the 35S cauliflower mosaic virus minimal promoter. Pro:uidA reporter constructs alone or in combination with a vector allowing the expression of MYB115, MYB118, or MYB107 (negative control) were coinfiltrated in young leaves of N. benthamiana with a vector allowing the expression of the p19 protein. Leaf discs were assayed for GUS activity 3 d after infiltration. Tissues were incubated 17 h in a buffer containing 2 mM each of potassium ferrocyanide and potassium ferricyanide. Representative discs (diameter = 0.8 cm) are presented. TAACGG concatemer, promoter sequence made of a concatemer of TAACGG elements separated by 10 nucleotides (Barthole et al., 2014).
(G) Binding of MYB115 to the proximal upstream region of ODD. EMSA of a probe covering a region from −240 to −200 bp upstream from the ATG codon of ODD with increasing amounts of MYB115 (+ = 0.5 µg; ++ = 1.5 µg). WRI1 was used as a negative control. Competition of MYB115 binding was performed in the presence of 75-, 100-, and 200-fold amounts of the unlabeled ProODD (−240 to −200 bp) fragment. Position of free probe (open arrowhead) and the shifted bands (closed arrowhead) are indicated.
(H) Binding of MYB115 to the proximal upstream regions of targets of MYB118, namely, At5g01030, At3g62230, and At3g12880. The promoter sequence of the ODD gene (At1g04380) was used as a positive control. For each gene considered, the promoter region covered by the probe is indicated between parentheses.
In order to determine whether MYB115, like MYB118, possesses transcriptional activity, coding regions of the TFs were individually cloned in frame with the GAL4 DNA binding domain (GAL4-DBD). The constructs thus obtained were introduced into the yeast strain AH109, which carries the HIS3 and ADE2 reporter genes under the control of heterologous GAL4-responsive upstream activating sequences and promoter elements. The expression of these two reporters could be activated in the presence of MYB115 or MYB118 fused to GAL4-DBD, thus establishing their ability to activate transcription (Figure 2B).
The predicted DNA binding domains of MYB115 and MYB118 share 73% amino acid identity. In order to test whether MYB115 and MYB118 share common transcriptional targets, myb115-1, myb118-1, and myb115-1 myb118-1 lines were grown together with wild-type controls and the expression level of target genes of MYB118 (Barthole et al., 2014) was measured by RT-qPCR on cDNA prepared from 10-DAA seeds of each line. Repressed targets of MYB118 were first considered (Figure 2C). Whereas no derepression of these genes could be detected in myb115-1, a significant overaccumulation of corresponding cDNA was measured in the double mutant compared with the myb118-1 single mutant, showing that MYB115 and MYB118 redundantly repress this set of genes. Conversely, the mRNA level of the 2-OXOGLUTARATE-DEPENDENT DIOXYGENASE (ODD) gene, a direct inducible target of MYB118, was further decreased in the double mutant as compared with myb118-1, suggesting that inducible targets of MYB118 can also be shared by the two TFs (Figure 2D). To validate the response of the ODD gene to MYB115 deregulation, Pro35Sdual:MYB115 transgenic lines were generated (OE11 and OE19; Supplemental Figure 3). However, these lines exhibited altered vegetative development and were partially sterile, preventing us from analyzing the effect of MYB115 overexpression in seeds. Measurements were consequently performed on rosette leaves that demonstrated the ability of MYB115 to ectopically activate ODD (Figure 2E).
To test the ability of MYB115 to directly activate ODD expression, we used a ProODD:uidA reporter construct in transactivation assays in N. benthamiana (Figure 2F). The ProBCCP2:uidA construct was used as a negative control. Reporter constructs were infiltrated alone or in combination with a vector allowing the expression of MYB115, MYB118 (positive control), or MYB107 (negative control) in young leaves of N. benthamiana. MYB115, like MYB118, was able to specifically activate the ProODD:uidA reporter construct, showing a strong increase in GUS activity compared with the reporter alone or the reporter cotransfected with MYB107. A similar result was obtained with a reporter construct made of four repeats of the TAACGG element fused to the 35S cauliflower mosaic virus minimal promoter upstream of the uidA reporter gene. This element was proposed to be the cis-regulatory element bound by MYB118 in the promoter sequence of ODD (Barthole et al., 2014). Together, these results establish the ability of MYB115 and MYB118 to activate common target genes, possibly through the same cis-regulatory element.
The binding of MYB115 to the ODD promoter sequence was examined in vitro by electrophoretic mobility shift assay (EMSA). Purified recombinant MYB115 was incubated with a 40-bp promoter fragment containing the TAACGG element and binding was determined using a biotin-labeled DNA probe. Addition of MYB115 resulted in the formation of shifted bands (Figure 2G). The signal intensity increased with the concentration of MYB115 in the assay, indicating that the protein binds to the DNA fragment. The binding was specific since addition of the recombinant WRI1 TF did not result in the appearance of shifted bands. Furthermore, in competition experiments, addition of increasing amounts of unlabeled promoter fragments decreased the binding of MYB115 to the labeled probe. Using EMSA, we finally demonstrated the binding of MYB115 to three additional promoter fragments containing the TAACGG element and previously shown to be bound by MYB118 (At5g01030, At3g62230, and At3g12880 promoters; Figure 2H). These results confirm that the two TFs bind common cis-regulatory elements.
Impact of myb115 and myb118 Mutations on ω-7 Monoene Accumulation in Seeds
To investigate the function of MYB115 during endosperm maturation and to test its redundancy with MYB118, the myb115-1, myb118-1, and myb115-1 myb118-1 mutants were grown under controlled conditions. Vegetative development of the mutants was unaffected. Whole-mount clearing of developing seeds was performed during embryo morphogenesis and early maturation. The structure and early development of the three tissues composing the seed were unaffected in the various mutant backgrounds considered (Supplemental Figure 4). Likewise, observation of 14-DAA peeled endosperms suggested that the organization of the monolayer of endosperm cells was unmodified in the mutant lines. During the course of seed maturation, a slight delay could be observed in the elongation and enlargement of myb115-1 myb118-1 embryos that was associated with a moderate decrease of mature seed dry weight with respect to the other genotypes (Supplemental Figure 4).
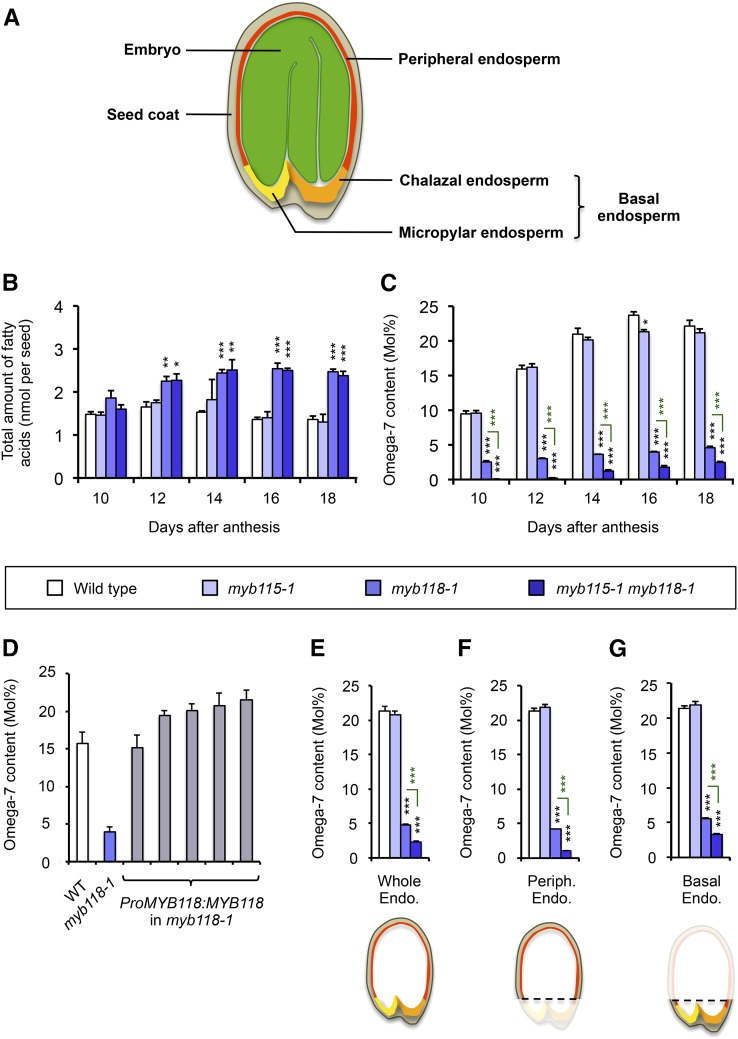
Since MYB115 and MYB118 are strongly induced in the maturing endosperm, we then evaluated the effects of their mutations on endosperm filling (Figure 3). Dissected endosperm and embryo fractions were collected separately during the course of seed maturation and total FAs were quantified by gas chromatography. Whereas the myb118 mutation led to a significantly increased FA content in the endosperm fraction from 12 DAA onward (Figure 3B; Barthole et al., 2014) compensated by an equivalent decrease of the embryo FA content (Supplemental Figure 5A), no effect of the myb115 mutation on the overall amount of FAs stored in the endosperm could be detected.
Figure 3.
Impact of myb115 and myb118 Mutations on the Accumulation of ω-7 Fatty Acids in the Endosperm Fraction of Developing Seeds.
(A) Schematic representation of mature Arabidopsis seeds.
(B) and (C) Time-course analysis of total FA content (B) and relative proportions of ω-7 FAs (cis-ω-7 C18:1 and cis-ω-7 C20:1) (C) in whole endosperm fractions dissected from wild-type and mutant seeds. Values are the means and se of five replicates performed on batches of 20 to 40 seeds from five distinct plants. Asterisks indicate significant difference according to t test at ***P < 0.001, **P < 0.01, and *P < 0.05, respectively.
(D) ω-7 FA (cis-ω-7 C18:1 and cis-ω-7 C20:1) content of whole endosperm fractions dissected from wild-type, myb118-1, or complemented mature dry seeds. Values are the means and se of five replicates performed on batches of 20 seeds from five distinct plants.
(E) to (G) ω-7 Fatty acid content (cis-ω-7 C18:1 and cis-ω-7 C20:1) of whole endosperm fractions (E), peripheral endosperm fractions (F), and basal endosperm fractions (G) dissected from mature seeds. Values are the means and se of five replicates performed on batches of 40 seeds from five distinct plants. Asterisks indicate significant difference according to t test at ***P < 0.001.
The effect of the myb mutations on the FA composition of the oil stored in the endosperm fraction was then examined. A focus was put on monoenes of the ω-7 series that were previously reported to be highly abundant in the endosperm oil of mature Arabidopsis seeds (Penfield et al., 2004; Li et al., 2006). Before addressing the role of the MYB TFs in the control of oil FA composition, we first characterized the accumulation of ω-7 FAs in the two zygotic tissues of wild-type seeds (Supplemental Figure 6). In the endosperm fraction, ω-7 FAs were massively deposited between 9 and 14 DAA. In mature seeds, they accounted for more than 20 mol% of total FAs, with paullinic acid representing the more abundant species of the ω-7 series of FAs. The pattern of ω-7 FA accumulation was strikingly different in the embryo, with a later storage of ω-7 FA species, a decreased abundance of these monoenes (they represent only 2 mol% of total FAs in dry embryos), and a predominance of vaccenic acid over paullinic acid. We then evaluated the effect of the myb mutations on the accumulation of ω-7 FAs in the endosperm (Figure 3C). The myb118 mutation yielded a sharp decrease in the proportion of ω-7 FAs stored in this compartment. Reversion of this phenotype could be obtained by introgression of a wild-type copy of the MYB118 gene into the mutant background (Figure 3D). Whereas the single myb115 mutation did not affect the accumulation of ω-7 FAs, an aggravated phenotype could be detected in the double myb115-1 myb118-1 mutant with respect to the myb118-1 background, denoting partially redundant functions of MYB115 and MYB118 in the control of ω-7 FA biosynthesis in the endosperm (Figure 3C). The endosperm tissue comprises different territories and the expression patterns of MYB115 and MYB118 were not similar in this tissue. MYB118 exhibited a high and homogeneous induction throughout the endosperm (Barthole et al., 2014), whereas the induction level of MYB115 was weaker and less homogeneous, the promoter activity of MYB115 being particularly intense in the chalazal endosperm (see above). We therefore measured the ω-7 FA contents of subfractions of the endosperm compartment in mature dry seeds. The peripheral endosperm was separated from the basal endosperm (comprising both the chalazal and micropylar endosperms) and the collected subfractions were analyzed separately by gas chromatography (Figures 3E to 3G). The results obtained suggest that MYB115 and MYB118 redundantly control ω-7 FA production in all endosperm territories, with the action of MYB118 predominating over that of MYB115 everywhere. A slight but reproducible negative effect of the myb mutations on ω-7 FA synthesis was also observed in the embryo (Supplemental Figure 5).
Identification of Two Acyl-ACP Desaturases Transcriptionally Activated by MYB115 and MYB118
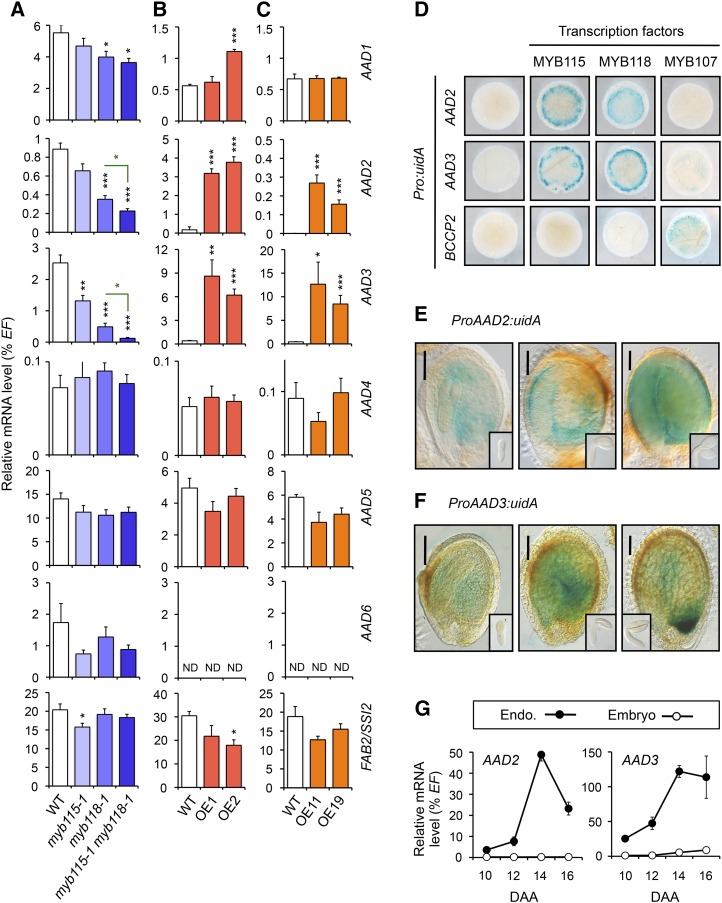
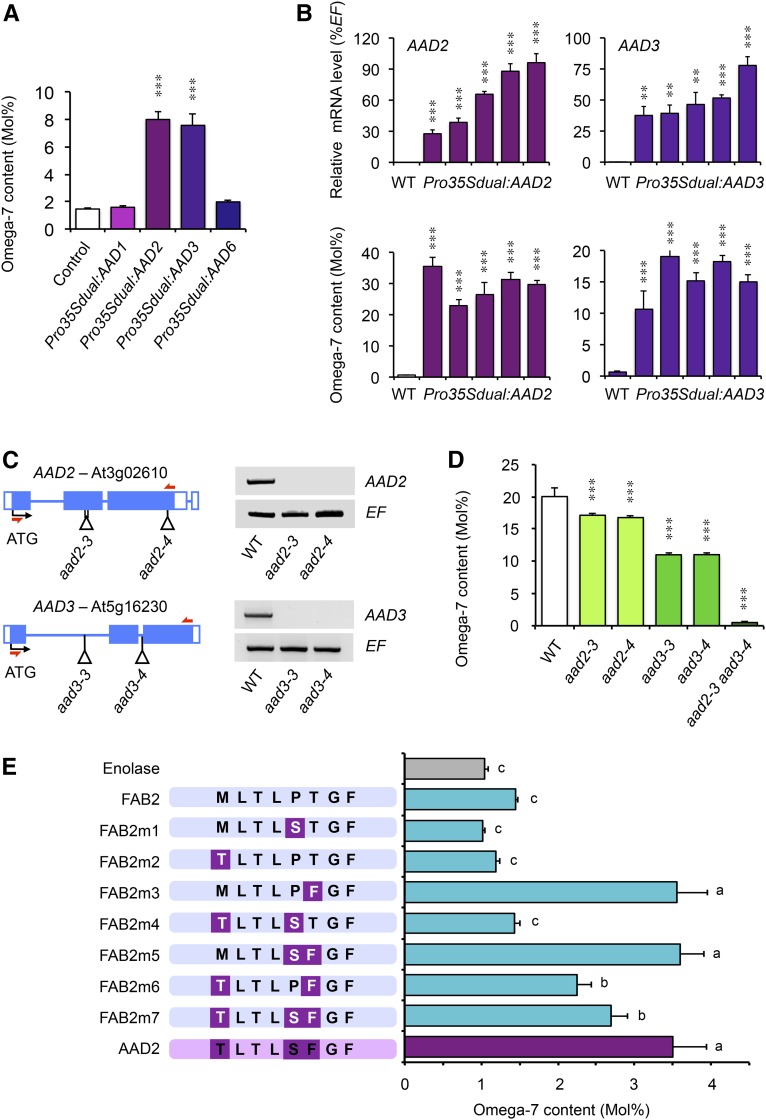
In order to identify the Δ9 PADs responsible for ω-7 FA biosynthesis in seeds of Arabidopsis, we used an RT-qPCR approach and examined the expression profiles of the seven Arabidopsis genes predicted to encode AADs (named AAD1/SAD1 to AAD6/SAD6, plus FAB2) in the search for positively regulated targets of MYB115 and MYB118. Target mRNAs were quantified in maturing myb mutant seeds (10 DAA) and in rosette leaves of transgenic lines overexpressing MYB118 (lines OE1 and OE2) or MYB115 (lines OE11 and OE19). Two AAD genes, namely, AAD2 and AAD3, were strongly downregulated in myb mutant seeds with corresponding mRNA levels correlating with the reduced ω-7 FA contents in these seeds (Figure 4A). Conversely, MYB115 and MYB118 were able to ectopically activate these two putative target genes (Figures 4B and 4C).
Figure 4.
AAD Genes Induced by MYB115 and MYB118.
(A) to (C) RT-qPCR analysis of transcript abundance in cDNA prepared from myb115-1, myb118-1, and myb115-1 myb118-1 mutant seeds harvested 10 DAA (A), from rosette leaves of Pro35Sdual:MYB118 lines (OE1 and OE2) (B), or from rosette leaves of Pro35Sdual:MYB115 lines (OE11 and OE19) (C). Values are the means and se of three to nine replicates performed on cDNA dilutions obtained from three ([B] and [C]) or four (A) independent mRNA extractions. Asterisks indicate significant difference from the wild type according to t test at ***P < 0.001, **P < 0.01, and *P < 0.05, respectively.
(D) Transactivation assay in leaves of N. benthamiana. Pro:uidA reporter constructs alone or in combination with a vector allowing the expression of MYB115, MYB118, or MYB107 (negative control) were coinfiltrated in young leaves of N. benthamiana with a vector allowing the expression of the p19 protein. Leaf discs were assayed for GUS activity 3 d after infiltration. Tissues were incubated 4 h in a buffer containing 2 mM each of potassium ferrocyanide and potassium ferricyanide. Representative discs (diameter = 0.8 cm) are presented.
(E) and (F) Pattern of activity of the ProAAD2:uidA (E) and ProAAD3:uidA (F) cassettes in developing seeds harvested 8, 10, or 12 DAA (main pictures, from left to right) and in early maturing embryos harvested 8, 10, or 12 DAA (secondary small pictures, from left to right). For histochemical detection of GUS activity, tissues were incubated overnight in a buffer containing 2 mM each of potassium ferrocyanide and potassium ferricyanide. Microscopy observations of seeds and excised embryos were performed using Nomarski optics. Bars = 100 µm.
(G) Analysis of relative mRNA accumulation of AAD2 and AAD3 was performed in developmental series of endosperm and embryo fractions. The results obtained are standardized to the EF1αA4 (EF) gene expression level. Values are the means and se of three to six replicates performed on cDNA dilutions obtained from three independent mRNA extractions. Endo., endosperm fraction.
To confirm the ability of the two MYBs to directly activate AAD2 and AAD3 expression, we used reporter ProAAD2:uidA and ProAAD3:uidA constructs in transactivation assays in N. benthamiana leaves. The ProBCCP2:uidA construct was used as a negative control. Reporter constructs were infiltrated alone or in combination with vectors allowing the expression of MYB115, MYB118, or MYB107 (negative control) in young leaves (Figure 4D). MYB115 and MYB118 were able to specifically activate ProAAD2:uidA and ProAAD3:uidA reporter constructs, showing a strong increase in GUS activity compared with the reporters alone or the reporters cotransfected with MYB107.
We finally characterized the expression patterns of AAD2 and AAD3 in developing seeds. The reporter constructs previously used for transient activation assays in N. benthamiana were stably introduced in Arabidopsis and resulting uidA expression patterns were assayed. For each construct tested, GUS staining was specifically observed in the maturing endosperm (Figures 4E and 4F). Then, RT-qPCR experiments performed with cDNA prepared from dissected seed fractions confirmed the tissue specificity of AAD2 and AAD3 expression, with corresponding mRNA levels peaking 14 DAA in the endosperm fraction (Figure 4G). Expression patterns of AAD2 and AAD3 were consistent with the positive transcriptional regulation exerted both by MYB115 and MYB118 on these genes.
Role of AAD2 and AAD3 in the Biosynthesis of ω-7 Monoenes in Seeds
In order to demonstrate that transcriptional activation of AAD2 and AAD3 is sufficient to trigger the biosynthesis of ω-7 monoenes, we transiently overexpressed these desaturases in leaves of N. benthamiana. FA composition was analyzed in transformed leaves 5 d after infiltration and revealed a significant enrichment in ω-7 monoenes in this material (Figure 5A). This ectopic stimulation of ω-7 FA synthesis was specific since overexpression of other Arabidopsis AAD isoforms (AAD1 or AAD6) had no effect on the biosynthesis of these FA species. To confirm these results, Pro35Sdual:AAD2 and Pro35Sdual:AAD3 constructs were stably introduced in Arabidopsis transgenic lines. First, RT-qPCR experiments performed with cDNAs prepared from rosette leaves demonstrated the efficient overexpression of the transgenes in the selected lines (Figure 5B). Then, total FA analyses performed with leaf material confirmed the ability of AAD2 and AAD3 to ectopically induce ω-7 FA biosynthesis.
Figure 5.
Functional Characterization of AAD2 and AAD3.
(A) Transient expression assay in leaves of N. benthamiana. Pro35Sdual:AAD constructs were coinfiltrated in young leaves of N. benthamiana with a vector allowing the expression of the p19 protein. Leaf discs harvested 5 d after infiltration were subjected to total FA analyses to determine the relative proportion of ω-7 FAs (cis-ω-7 C18:1) in this material. Values are the means and se of 10 replicates performed on batches of two disks from two to three distinct plants. Asterisks indicate significant difference from control according to t test at ***P < 0.001.
(B) Stable overexpression of AAD2 and AAD3 in transgenic Arabidopsis lines. RT-qPCR analysis of transcript abundance in cDNA prepared from rosette leaves of the transgenic lines were first performed to assess efficient overexpression of the transgenes (upper panel). Values are the means and se of three replicates performed on cDNA dilutions obtained from three independent mRNA extractions. Rosette leaves were then subjected to total FA analyses to determine the relative proportion of ω-7 FAs (cis-ω-7 C18:1) in this material (lower panel). Values are the means and se of 10 replicates performed on batches of two leaf tips from ten distinct plants. Asterisks indicate significant difference from the wild type according to t test at ***P < 0.001 and **P < 0.01, respectively.
(C) Molecular characterization of aad2 and aad3 mutants. Structure of the AAD2 and AAD3 genes showing the position of T-DNA insertions in aad2-3, aad2-4, aad3-3, and aad3-4 are presented. For each T-DNA insertion considered, confirmed flanking sequence tag(s) are anchored in the gene structure and represented by vertical bar(s). Closed boxes represent exons and open boxes untranslated regions. Accumulation of AAD2 and AAD3 mRNA in wild-type and corresponding mutant backgrounds was studied by RT-PCR reaction on developing seeds harvested 16 DAA. EF1αA4 (EF) gene expression was used as a constitutive control. Primers used for this study are indicated by arrows (Supplemental Table 2).
(D) Relative proportion of ω-7 FAs (cis-ω-7 C18:1 and cis-ω-7 C20:1) in endosperm fractions dissected from wild-type and aad mature dry seeds. Values are the means and se of five replicates performed on batches of 20 seeds from five distinct plants. Asterisks indicate significant difference from the wild type according to t test at ***P < 0.001.
(E) Site-directed mutagenesis experiments followed by transient expression assays in leaves of N. benthamiana. Constructs allowing the expression of the plastidial enolase (At1g74030; negative control), FAB2, AAD2, and mutagenized versions of FAB2 (FAB2m1-7) were coinfiltrated in young leaves of N. benthamiana with a vector allowing the expression of the p19 protein. Leaf discs harvested 5 d after infiltration were subjected to total FA analyses to determine the relative proportion of ω-7 FAs (cis-ω-7 C18:1) in this material. Values are the means and se of 20 replicates performed on batches of two disks from four distinct plants. Statistical analyses of the data were performed using ANOVA, followed by a comparison of means using the Newman-Keuls (SNK) test (P < 0.05). A schematic view of the eight residues lining the bottom part of the substrate pockets of the desaturases assayed is presented (see also Supplemental Figure 8).
To firmly establish that transcriptional activation of AAD2 and AAD3 is responsible for ω-7 FA biosynthesis in the endosperm of Arabidopsis seeds, a collection of aad2 and aad3 T-DNA insertion alleles (all in Col-0 background) was obtained and characterized at the molecular level (Figure 5C). The mutants were grown under controlled conditions. Their mature dry seeds were dissected and seed fractions were subjected to biochemical analyses. Determinations of total FA compositions demonstrated that the aad mutations negatively affected ω-7 FA accumulation in the endosperm fraction of seeds (Figure 5D). For each pair of mutants considered, an allelism test was performed. No complementation could be observed in the F1 hybrid seeds (Supplemental Figure 7), demonstrating that the mutated aad alleles of each pair were allelic and ascertaining the link between the T-DNA insertions and the oil phenotype characterized. Ultimately, the aad2-3 aad3-3 double mutant was obtained. Only traces of ω-7 FAs could be detected in the endosperm fraction of the double mutant (Figure 5D), demonstrating that AAD2 and AAD3 are the major contributors to ω-7 FA biosynthesis in this tissue, the relative contributions of the two isoforms being proportional to their respective induction levels (Figure 4G).
Molecular Determination of Substrate Specificity of AAD2 and AAD3
Previous studies have established the importance of the side chains of the eight amino acid residues lining the bottom part of the substrate channel of AADs for determining the substrate specificity of the enzymes (Cahoon et al., 1997). The channel of the archetype ∆9 SAD is deep enough to accommodate C18:0 substrates, thus forming ω-9 monoenes (Supplemental Figure 8). A shorter channel is more adapted to C16:0 substrates, yielding a ∆9 PAD activity and the production of ω-7 monoenes. These eight amino acid residues were first identified thanks to the crystal structure of a ∆9 SAD from Ricinus communis (Lindqvist et al., 1996). They appear to be well conserved among the SAD isoforms characterized so far in higher plants, as in the Arabidopsis FAB2 protein (Supplemental Figure 8). Alignments of amino acid sequences and 3D structure modeling using the SWISS-MODEL server (http://swissmodel.expasy.org/) (Arnold et al., 2006; Bordoli et al., 2009) and the SAD from R. communis (PDB code 1AFR; Lindqvist et al., 1996) as a template allowed identification of the corresponding amino acid residues in the AAD2 and AAD3 sequences and a proposed model for the substrate channel of these enzymes (Supplemental Figure 8). These analyses revealed three divergent residues with respect to the SAD archetype. To test the importance of these residues in determining the specific PAD activity of AAD2 and AAD3, amino acid substitutions were realized by site-directed mutagenesis in the sequence of FAB2 so as to introduce these residues, alone or in combination, in a SAD sequence. Modified versions of FAB2 were then transiently expressed in leaves of N. benthamiana and the production of ω-7 FAs was monitored in order to detect PAD activities. Plasmids allowing the expression of the Arabidopsis plastidial enolase or that of AAD2 were used as negative and positive controls, respectively. Whereas ectopic overexpression of FAB2 was not sufficient to trigger a significant accumulation of ω-7 FAs in transformed leaves, mutagenized versions of FAB2 harboring a T219F mutation, alone or in combination with other substitutions, significantly stimulated the biosynthesis of ω-7 FAs, denoting a PAD activity (Figure 5E). These results strongly suggested that the Phe residue lining the bottom part of the substrate channel of AAD2 and AAD3 (at position 226 or 216, respectively) plays a key role in determining the substrate specificity of these isoforms, most probably by reducing the depth of this channel thanks to its bulky lateral chain.
DISCUSSION
Reserve accumulation in maturing seeds is finely regulated. Developmental regulators ensure that the corresponding biosynthetic pathways are activated during the transition phase between embryogenesis and seed maturation, then shut down in late maturing and germinating seeds. Superimposed on this temporal pattern of regulation, spatial control elements regulate the differential partitioning of reserves between seed compartments. Arabidopsis seeds indeed consist of two compartments accumulating storage compounds, namely, the endosperm and the embryo, the latter accounting for ∼90% of total seed reserves. Early during seed formation, developmental programs establish the embryo as the preponderant tissue within these exalbuminous seeds. Then, transcriptional repressors of the maturation program expressed in the endosperm during the maturation phase further reinforce a differential partitioning of reserves between zygotic tissues (Barthole et al., 2014). Beyond this differential partitioning, the fine characterization of Arabidopsis seeds has revealed the strikingly different compositions of the reserves stored in the two compartments, with endosperm oil exhibiting for instance dramatically increased proportions of the economically important ω-7 monounsaturated FAs (Penfield et al., 2004). Here, we demonstrate that the two closely related MYB115 and MYB118 TFs, which are transcriptionally induced by LEC2 in the endosperm at the onset of seed maturation, activate the transcription of two ∆9 PAD genes, namely, AAD2 and AAD3, in this tissue. Transcriptional activation of these two isoforms is necessary and sufficient to promote ω-7 FA biosynthesis in the endosperm. Taken together, these data allow a better understanding of how FA metabolism is developmentally regulated at the spatiotemporal level, adding another level of complexity to the regulatory network controlling reserve accumulation in maturing Arabidopsis seeds.
Transcriptional Activation of MYB115 in the Maturing Endosperm
Detailed characterization of the MYB115 expression pattern based on complementary approaches like RT-qPCR and promoter:GUS analyses established the endosperm-specific induction of this gene in early maturing seeds. These data are fully consistent with the results of transcriptome analyses of developmental series of seeds and seed tissues microdissected by laser capture (Le et al., 2010; Day et al., 2008; Belmonte et al., 2013). They suggest that the closely related MYB115 and MYB118 genes, both induced in the maturing endosperm, may have similar cis-regulatory elements in their promoter sequences. Their respective expression patterns are not strictly identical though. If accumulation of the two mRNA populations dramatically increases at the onset of seed maturation, before decreasing during the course of seed maturation, induction level of MYB115 is much weaker. Then, expression of MYB115 is not restricted to seeds since the activity of the MYB115 promoter is also detected in pollen grains. These observations reflect the ongoing divergent evolution of the promoter sequences of the two paralogs.
The pattern of MYB115 promoter activity matches MYB115 mRNA accumulation both at the spatial and temporal levels; therefore, MYB115 tissue-specific expression is probably largely controlled at the transcriptional level. Interestingly, the data presented in this study establish that MYB115, like MYB118, is a target of the master regulator LEC2. This common transcriptional activation of the two TFs by LEC2, together with the negative feedback regulation exerted by MYB118 on LEC2 expression (Barthole et al., 2014) provides an interesting mechanism explaining how the myb118 mutation can be partially compensated by overexpression of MYB115 in the myb118 mutant background (Figure 6). At the temporal level, the examination of the expression profiles of LEC2, MYB118, and MYB115 is consistent with a transcriptional activation of the MYB TFs by the master regulator. At the spatial level, however, the lack of induction of the two MYBs in the embryo is striking considering that LEC2 is expressed in the embryo too (Kroj et al., 2003; Barthole et al., 2014). To reconcile these apparent discrepancies, one has to postulate the existence of endosperm-specific factors directing the expression of MYB115 and MYB118 in this tissue or that of embryo specific repressors counteracting LEC2 in the embryo. Endosperm development appears to be predominantly under epigenetic control (Berger, 2003; Sun et al., 2010); therefore, it would be interesting to further investigate how these controls affect oil metabolism in the maturing endosperm (Fatihi et al., 2013), possibly through dedicated transcriptional regulators such as MYB115 and MYB118.
Figure 6.
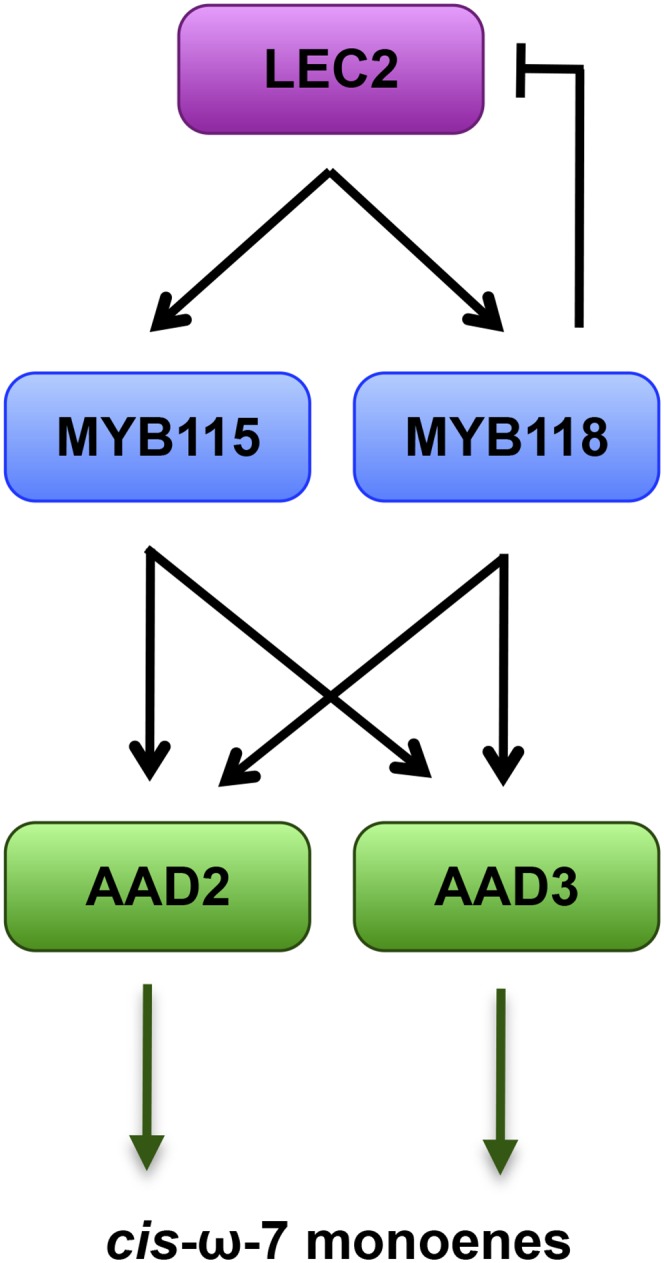
Model for the Regulation of ω-7 Monoene Fatty Acid Production in Arabidopsis Seeds.
MYB115 and MYB118 are induced in the endosperm at the onset of the maturation phase. The master regulator LEC2 activates their transcription, and MYB118 exerts negative feedback regulation on LEC2. MYB115 and MYB118 coordinately trigger the transcription of two soluble ∆9 palmitoyl-ACP desaturases, namely, AAD2 and AAD3. These enzymes catalyze the synthesis of ω-7 monounsaturated fatty acids, which are accumulated at high levels in the oil stored within the endosperm. The transcriptional negative regulatory loop involving LEC2 and MYB118 was previously described by Barthole et al. (2014).
MYB115 and MYB118 Have Common Transcriptional Targets
MYB118 was previously shown to antagonistically regulate distinct gene networks. Whereas several maturation-related genes are repressed by the TF in the maturing endosperm, MYB118 directly triggers the expression of several endosperm-induced genes (Barthole et al., 2014). The data presented in this study demonstrate that sets of target genes are shared by MYB115 and MYB118 in each of the two subcircuits (e.g., GRP19, SM3, and CRUL, or ODD, AAD2, and AAD3). These observations are consistent with a recent report by Zhang et al. (2015) depicting how the two conserved TFs co-control expression of genes encoding enzymes of the benzoyloxy glucosinolate pathway in seeds. Expression analyses supported by yeast one-hybrid assays have shown that several actors (AOP3, BZO1, and SCPL17) of this newly evolved biosynthetic pathway are transcriptionally repressed by the two MYBs. Interestingly, the TAACGG element present as a part of the in vivo MYB118 binding site, that may consequently belong to type 1 MYB binding sites (pAACnG, where p indicates T or C, and n indicates any nucleotide; Romero et al., 1998; Prouse and Campbell, 2012) was identified both in positively and in negatively regulated targets of the MYB TFs under study (Barthole et al., 2014; Zhang et al., 2015). These observations raise the question of (1) the molecular mechanisms specifying the type of regulation exerted by the TFs and of (2) the identity of the unknown actors participating in these regulations. In the same line, a detailed structure-function study using in vitro techniques like SELEX or Biacore, or in vivo chromatin immunoprecipitation experiments, would be useful to fully characterize the respective DNA binding matrixes of MYB115 and MYB118 in order to (1) better characterize the spectrum of putative targets of the TFs and (2) determine whether these matrixes are identical or not. The amino acid identity shared by the predicted DNA binding domains of MYB115 and MYB118 (73%) falls within the range of values obtained for other MYBs previously shown to act redundantly. For instance, DNA binding domains of TT2/MYB123 (At5g35550) and MYB5 (At3g13540) share 72% amino acid identity (Xu et al., 2014). Some of the data presented in this article (e.g., induction levels of target genes in stable Pro35Sdual:MYB lines) indicate that the two MYBs exhibit different behaviors in the presence of certain targets, implying that their binding specificity may have started diverging. This could explain why the effects of the myb115 and myb118 mutations are additive for some phenotypes (e.g., production of ω-7 monounsaturated FAs or some glucosinolates) and not for some others (e.g., total amount of oil stored in the endosperm). However, diverging binding specificities are not the only driving force for differentiating sets of targets between related TFs: Evolution of protein-protein interactions between protein partners of a transcriptional complex can also play a key role. In this regard, complementary studies would now be required to characterize the transcriptional complexes involving MYB115 and MYB118.
Transcriptional Control of ω-7 Monounsaturated FA Biosynthesis
The most common monoenes in land plants are of the ω-9 series, as oleic acid. Unlike the ω-9, ω-7 monoenes like palmitoleic acid and its elongation products vaccenic acid and paullinic acid are relatively rare. Plant oils containing ω-7 monoenes, though uncommon, are enriched in seeds or fruits of non-crop species like cat’s claw vine, macadamia, or sea buckthorn (Bondaruk et al., 2007; Fatima et al., 2012). Oils enriched in ω-7 FA were also described in seeds of the Brassicaceae family, but this enrichment solely concerned the endosperm (Penfield et al., 2004; Li et al., 2006). The transcriptional activation cascade described in this study (Figure 6) allows us to understand how two ∆9 PADs specifically induced in the maturing endosperm of Arabidopsis seeds, namely, AAD2 and AAD3, confer its peculiar FA composition to the oil stored in this tissue. Further work will be required to fully elucidate the molecular mechanisms underpinning the transcriptional activation of these two desaturases by MYB115 and MYB118 and to precisely identify the cis-elements required for this activation.
Interestingly, MYB118 exerts antagonistic control on different actors of oil metabolism: While repressing the overall amount of oil stored in the endosperm, this TF promotes the biosynthesis of ω-7 monoenes over that of ω-9, raising the question of the biological function of these molecular species in the endosperm. As for the ω-7 FAs present at low levels in the embryo, their biosynthesis may result from a very limited, though detectable, activation of the above-mentioned transcriptional regulatory cascade in this tissue.
The position of the unsaturation within the aliphatic chains of monounsaturated FAs contributes to the physicochemical properties of acyl lipids derived from these FAs. The specific functions of ω-7 containing lipids in the plant cell, if any, remain unknown. Under standard growth conditions, development of the aad2 aad3 double mutant is unaffected, but the remnant ω-7 FAs present in this genetic background may impair the detection of phenotypes. Complementary studies, possibly requiring the preparation of new multiple mutants for the AAD genes, will be essential to address this question. Regardless of their function in plants, these ω-7 have uses for a number of industrial applications. Biodiesel produced from plant oils with high ω-7 content have superior functional properties (Wu et al., 2012). The ω-7 FAs also have considerable potential as a feedstock for the production of 1-octene by metathesis chemistry (Meier, 2009), 1-octene representing a high-demand feedstock mainly used to make linear low-density polyethylene. Vegetable oils enriched in ω-7 FAs have finally been ascribed a number of beneficial health properties. In animals, adipose tissues use lipokines such as palmitoleic acid to communicate with distant organs and regulate systemic metabolic homeostasis (Cao et al., 2008). There is growing evidence that palmitoleic acid plays a key role in the pathophysiology of insulin resistance in humans, increasing muscle response to insulin (Stefan et al., 2010). Palmitoleic acid is also a candidate antimelanogenic agent (Yoon et al., 2010). Aside from above-mentioned nutritional functions, palmitoleic acid also has antioxidant, antimicrobial, and antiaging properties (Wu et al., 2012). Pharmaceutical companies have already developed foods and nutraceuticals for health purposes enriched in ω-7 FAs, often sourced from species exhibiting low yields and poor agronomic properties such as sea buckthorn. In recent years, biotechnical approaches have been implemented to develop specialized high-yielding platforms through the metabolic engineering of oilseed crops (Bondaruk et al., 2007; Nguyen et al., 2015). Findings regarding the control of ω-7 FA biosynthesis in the plant cell may provide new interesting tools for the development of new strategies for ω-7 FA production.
AAD2 and AAD3 Are ∆9 Palmitoyl-ACP Desaturases
The Arabidopsis genome contains seven related genes coding for predicted acyl-ACP desaturases, previously named FAB2 and SAD1 to SAD6 (Kachroo et al., 2007). FAB2, the best characterized member of the family, encodes a ∆9 SAD (producing ω-9 FAs) essential for plant development and defense signaling (Lightner et al., 1994; Kachroo et al., 2001). Despite recent advances in the characterization of this multigene family (Klinkenberg et al., 2014), the functions of most members of the family have long remained elusive. In the light of the research presented in this article, it appears that two isoforms have diverged from the archetype SAD and exhibit a ∆9 PAD activity responsible for the biosynthesis of ω-7 monoenes. We therefore propose to adopt a new nomenclature and to rename the already published SAD1-6 genes AAD1-6, this general designation encompassing the variety of in planta activities exhibited by the corresponding isoforms. The ∆9 PAD activities of AAD2 and AAD3 are responsible for the production of ω-7 monoenes stored in the endosperm of Arabidopsis seeds. Using a quantitative trait loci approach followed by genetic analyses, Bryant et al. (2016) have also identified AAD2 and AAD3 as the major desaturases synthesizing ω-7 monoenes in the endosperm. These observations are consistent with previous results from Kachroo et al. (2007), who assayed the enzymatic activities and substrate specificities of several Arabidopsis ∆9 AADs produced in Escherichia coli. These in vitro data already pointed out the preference of DES3/AAD3 for C16:0 substrates, yielding a PAD activity. The enzymatic characterization of AAD from Arabidopsis (Kachroo et al., 2007) and other plant species (Cahoon et al., 1998; Rodríguez et al., 2015) then unraveled that, beyond marked preferences of the desaturases for substrates of a given chain length, the enzymes can also desaturate slightly shorter or longer chains, although with a reduced affinity. The traces of ω-7 FAs detected in aad2 aad3 seeds may consequently originate from the activity of SADs like FAB2, which is also expressed in maturing seeds.
A group of eight residues lining the bottom part of the substrate channels of AADs was previously shown to set constraints on the chain lengths of FA substrates, thus determining the substrate specificity of the enzymes (Cahoon et al., 1997, 1998). Despite the functional divergence of some of its founding members, the Arabidopsis AADs still share a high degree of amino acid sequence similarity and a common structural fold (Kachroo et al., 2007). Interestingly, three of the eight amino acid residues determining substrate specificity distinguish AAD2 and AAD3 from the archetype SAD structure. The results of our site-directed mutagenesis experiments point out the importance of one of the divergent amino acids identified, namely, Phe-226 in AAD2 or -216 in AAD3, for conferring a PAD activity to the desaturases. The bulky lateral chain of this Phe residue may reduce the depth of the substrate pocket, thus favoring the binding of shorter C16:0-ACP substrate. Beyond the predominant role played by this residue, the two other divergent residues identified in AAD2 and AAD3 may also contribute to create the substrate profile displayed by these isoforms. A ∆9 PAD from cat’s claw vine was previously cloned and characterized (Cahoon et al., 1998). Despite similar enzymatic activities, the ∆9 PAD from Arabidopsis and cat’s claw vine do not have identical substrate channels. In the case of cat’s claw vine, only one residue diverged from the archetype SAD sequence: Replacement of a Leu by a Trp residue at the extremity of the channel was presented as the major determinant of substrate specificity for this isoform (Cahoon et al., 1998). During the evolution of AADs, the diversification of which was probably favored by the emergence of multigene families within the genome of higher plants, ∆9 PAD activities may therefore have appeared independently in different species. The prevalence of structural rules (e.g., presence of a hydrophobic residue with a bulky lateral chain lining the bottom part of the substrate channel) over highly conserved consensus sequences for determining the substrate specificity of PAD enzymes was further established by metabolic engineering based on combinatorial saturation mutagenesis and logical redesign of desaturases (Whittle and Shanklin, 2001; Cahoon et al., 1997; Cahoon and Shanklin, 2000). These approaches have allowed tailoring several original PAD enzymes by reducing the ability of the substrate pocket of SAD enzymes to accommodate the longer 18:0-ACP substrate. Most of the combinations of amino acid substitutions yielding such a result involved the replacement of a hydrophobic residue lining the pocket by a bulkier and still hydrophobic residue.
In conclusion, these results exemplify how transcriptional regulations significantly contribute to the differential regulation of FA and oil metabolism in the two zygotic tissues comprising maturing seeds of Arabidopsis, leading to different oil compositions in these adjacent compartments. In the maturing endosperm, two closely related MYB TFs activated by LEC2, namely, MYB118 and MYB115, trigger in turn the expression of the AAD2 and AAD3 genes, that code for ∆9 PADs responsible for the biosynthesis of the ω-7 monounsaturated FAs stored at high levels in the endosperm oil. Specialization of oil metabolism in the endosperm arose both from the emergence of an original structure of the substrate channel of one of the members of the AAD family and from the concomitant set up of a complex transcriptional regulatory cascade able to precisely control the spatiotemporal expression of this desaturase. Further work will be required to decipher the upstream molecular mechanisms that allow this cascade to be specifically activated in the endosperm.
METHODS
Plant Material and Growth Conditions
Arabidopsis thaliana seeds of the Col-0 accession were obtained from the Arabidopsis thaliana resource center for genomics at the Institut Jean-Pierre Bourgin (http://www-ijpb.versailles.inra.fr/), and T-DNA mutant lines (aad2-3, N670942; aad2-4, N584160; aad3-3, N567280; aad3-4, N825777) were ordered from the NASC (http://arabidopsis.info). Lec2-10, lec2-11, myb115-1, and myb118-1 T-DNA mutant lines were described previously by Barthole et al. (2014). Plants were cultured as described by Baud et al. (2007a). DEX induction experiments using the Pro35S:LEC2:GR construct were performed as described by Santos Mendoza et al. (2005). To sample embryo and endosperm fractions, seeds excised from siliques were dissected using a scalpel and dissecting tweezers under an optical glass binocular magnifier. Material used for RNA extraction was frozen in liquid nitrogen immediately after harvest and then stored at −80°C.
Molecular Characterization of T-DNA Mutants
Plant genomic DNA flanking the T-DNA border of the mutants were amplified by PCR (Supplemental Table 1) and sequenced to confirm the flanking sequence tags identified. Homozygous lines were then isolated for further characterization. RT-PCR analyses were ultimately performed to analyze gene expression in mutant backgrounds (Supplemental Table 2).
Constructs and Plant Transformation
The sequences of primers used for DNA amplification are indicated in Supplemental Table 3.
For construction of the ProMYB115:uidA transgene, region −998 to −1 bp relative to the MYB115 translational start codon was amplified with the proofreading Pfu Ultra DNA polymerase (Stratagene) from Col-0 genomic DNA. The PCR product was introduced by BP recombination into the pDONR207 entry vector (Invitrogen) and transferred into the destination vector pBI101-R1R2-GUS (Baud et al., 2007b) by LR recombination. The resulting binary vector was electroporated into Agrobacterium tumefaciens C58C1 strain and used for agroinfiltration of flower buds of Arabidopsis (Bechtold et al., 1993). Primary transformants were selected on Murashige and Skoog medium containing kanamycin (50 mg⋅L−1) and transferred to soil for further characterization: 23 independent transgenic lines were analyzed.
For construction of the ProAAD3:uidA transgene, a similar procedure was adopted. Region −2022 to −1 bp relative to the AAD3 translational start codon was cloned; 12 independent transgenic lines were analyzed.
For construction of the ProAAD2:uidA transgene, a similar procedure was adopted. Region −1000 to −1 bp relative to the AAD2 translational start codon was cloned into the pGWB3 vector (Nakagawa et al., 2007); 19 independent transgenic lines were analyzed.
For construction of the Pro35Sdual:MYB115, Pro35Sdual:AAD, and Pro35Sdual:FAB2 transgenes, the procedure adopted was similar to that described for the construction of Pro35Sdual:MYB107 and Pro35Sdual:MYB118 transgenes (Barthole et al., 2014).
For construction of the Pro35Sdual:FAB2m1-7 transgenes, mutations on the FAB2 cDNA cloned in pDONR207 were performed with the QuickChange site-directed mutagenesis kit (Agilent) according to the manufacturer’s instructions. Primers used are presented in Supplemental Table 4. Mutagenized cDNAs were then transferred in pMDC32 as described above.
For construction of the ProADH1:GAL4-DBD:MYB115 and ProADH1:GAL4-DBD:MYB118 transgenes, MYB115 and MYB118 cDNAs previously cloned into the pDONR207 entry vector were transferred into a modified pDEST32 (carrying a kanamycin resistance gene) for GAL4-DBD fusion (Invitrogen).
For construction of the Pro35Sdual:MYB115:mGFP6 and Pro35Sdual:MYB118:mGFP6 transgenes, cDNAs without STOP codon were amplified with the proofreading Pfu Ultra DNA polymerase (Stratagene) from a mixture of seed cDNA (Col-0 accession). The PCR products were introduced by BP recombination into the pDONR207 entry vector (Invitrogen) and transferred into the destination vector pMDC83 (Curtis and Grossniklaus, 2003) by LR recombination.
Construction of the ProMYB118:MYB118 transgene was previously described by Barthole et al. (2014).
RNA Analyses
RNA extraction, reverse transcription, RT-PCR, and real-time RT quantitative PCR were performed as previously described (Baud et al., 2004). The sequences of primers used for RT-PCR and real-time RT-qPCR are indicated in Supplemental Tables 2 and 5. Purity of the different seed fractions sampled was assessed as described by Barthole et al. (2014). Briefly, marker genes for each of the fractions sampled, namely, ZHOUPI (endosperm-specific) and At2g23230 (embryo-specific), were quantified on cDNA prepared from these fractions, thus confirming that no significant contamination occurred between fractions.
Lipid Analyses
Total FA analyses were performed as previously described (Li et al., 2006) on leaf disks from agroinfiltrated Nicotiana benthamiana or on pools of Arabidopsis seeds or seed fractions. The endosperm tissue was analyzed with the seed coat attached as described by Penfield et al. (2004). However, this procedure did not bias our evaluation of the endosperm oil content since the integuments of the seed do not accumulate storage compounds and in fact undergo programmed cell death early during maturation (Beeckman et al., 2000; Li et al., 2006).
EMSAs
The expression plasmid was constructed by transferring MYB115 cDNA from the pDONR207 to the expression vector pETG10A (http://www.embl-hamburg.de/). The resulting vector was electroporated into Escherichia coli RosettaBlue(DE3)pLysS strain (Novagen) for expression. After induction by 1 mM IPTG in Luria-Bertani buffer, cells were grown overnight at 20°C. Cell lysis and protein purification were performed as previously described (Baud et al., 2009). To prepare DNA probes, complementary biotin-labeled (at the 5′ end) oligonucleotides (Eurofins MWG Operon) were annealed. For DNA binding assays, MYB115 recombinant protein was incubated with 30 fmol probe in binding buffer [20 mM Tris-HCl, pH 8, 250 mM NaCl, 2 mM MgCl2, 1% glycerol (v/v), 1 mg⋅mL−1 BSA, 1 mM DTT, 85 ng µL−1 poly(dI-dC)]. For competition assays, the unbiotinylated competitor was incubated briefly with the recombinant protein before the biotinylated probe was added. After addition of the biotinylated probe, reactions were incubated 30 min at room temperature, then fractionated at 4°C by 6% PAGE. Electrophoretic transfer to nylon membrane and detection of the biotin-labeled DNA were performed according to the manufacturer’s instructions (Chemiluminescent Nucleic Acid Detection Module; Pierce) using an ImageQuant LAS 4000 system (GE Healthcare).
Microscopy
Histochemical detection of GUS activity and bright-field microscopy observations were performed as described by Baud et al. (2007a). Leaves of N. benthamiana were imaged with a Zeiss LSM710 confocal microscope as described by Miart et al. (2014).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: AAD1/SAD1, At5g16240; AAD2/SAD2, At3g02610; AAD3/SAD3, At5g16230; AAD4/SAD4, At3g02620; AAD5/SAD5, At3g02630; AAD6/SAD6, At1g43800; BCCP2, At5g15530; CRUL, At1g03890; EF1αA4, At5g60390; FAB2/SSI2, At2g43710; GRP19, At5g07550; LEC2, At1g28300; MYB107, At3g02940; MYB115, At5g40360; MYB118, At3g27785; ODD, At1g04380; and SM3, At2g25890.
Supplemental Data
Supplemental Figure 1. Characterization of the regulation of MYB115 by MYB118.
Supplemental Figure 2. Characterization of the LEC2-dependent derepression of MYB115 in myb118-1.
Supplemental Figure 3. Characterization of MYB115-overexpressing lines.
Supplemental Figure 4. Characterization of myb115-1, myb118-1, and myb115-1 myb118-1 seed development.
Supplemental Figure 5. Impact of myb115 and myb118 mutations on ω-7 fatty acid accumulation in the embryo of developing seeds.
Supplemental Figure 6. Time-course analysis of ω-7 monounsaturated fatty acid accumulation in Arabidopsis seeds.
Supplemental Figure 7. Complementation tests among the aad mutants.
Supplemental Figure 8. Complementary information for the sequences, structures, and functions of acyl-ACP desaturases in Arabidopsis.
Supplemental Table 1. Primers used for molecular characterization of T-DNA insertions.
Supplemental Table 2. Primers used for characterizing gene expression by RT-PCR (as displayed in Figure 5C).
Supplemental Table 3. Primers used for construct preparation.
Supplemental Table 4. Primers used for site-directed mutagenesis experiments.
Supplemental Table 5. Primers used for quantitative RT-PCR.
Supplementary Material
Acknowledgments
We thank D. Kosma for critical reading of the article and D. de Vos, C. Boulard, A. Wilch, and O. Grandjean (Observatoire du Végétal, INRA-IJPB) for their technical assistance. This work was supported by the French National Research Agency (SOLAR, Grant ANR-10-GENM-009) and by the Research Executive Agency (TRIANON, Grant PIEF-GA-2013-625204). The IJPB benefits from the support of the Labex Saclay Plant Sciences-SPS (ANR-10-LABX-0040-SPS). The confocal equipment used in this study was partly financed by the Ile-de-France Region.
AUTHOR CONTRIBUTIONS
A.T., G.T., and M.M. performed the research and analyzed the data. L.L. designed the research and analyzed the data. M.A.T.-P., G.B., and S.B. designed and performed the research, analyzed the data, and wrote the article.
Glossary
- FA
fatty acid
- AAD
∆9 acyl-ACP desaturase
- SAD
∆9 stearoyl-ACP desaturase
- PAD
∆9 palmitoyl-ACP desaturase
- TF
transcription factor
- DAA
days after anthesis
- DEX
dexamethasone
- EMSA
electrophoretic mobility shift assay
References
- Arnold K., Bordoli L., Kopp J., Schwede T. (2006). The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22: 195–201. [DOI] [PubMed] [Google Scholar]
- Barthole G., To A., Marchive C., Brunaud V., Soubigou-Taconnat L., Berger N., Dubreucq B., Lepiniec L., Baud S. (2014). MYB118 represses endosperm maturation in seeds of Arabidopsis. Plant Cell 26: 3519–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud S., Boutin J.P., Miquel M., Lepiniec L., Rochat C. (2002). An integrated overview of seed development in Arabidopsis thaliana ecotype WS. Plant Physiol. Biochem. 40: 151–160. [Google Scholar]
- Baud S., Vaultier M.-N., Rochat C. (2004). Structure and expression profile of the sucrose synthase multigene family in Arabidopsis. J. Exp. Bot. 55: 397–409. [DOI] [PubMed] [Google Scholar]
- Baud S., Wuillème S., Dubreucq B., de Almeida A., Vuagnat C., Lepiniec L., Miquel M., Rochat C. (2007a). Function of plastidial pyruvate kinases in seeds of Arabidopsis thaliana. Plant J. 52: 405–419. [DOI] [PubMed] [Google Scholar]
- Baud S., Mendoza M.S., To A., Harscoët E., Lepiniec L., Dubreucq B. (2007b). WRINKLED1 specifies the regulatory action of LEAFY COTYLEDON2 towards fatty acid metabolism during seed maturation in Arabidopsis. Plant J. 50: 825–838. [DOI] [PubMed] [Google Scholar]
- Baud S., Wuillème S., To A., Rochat C., Lepiniec L. (2009). Role of WRINKLED1 in the transcriptional regulation of glycolytic and fatty acid biosynthetic genes in Arabidopsis. Plant J. 60: 933–947. [DOI] [PubMed] [Google Scholar]
- Baud S., Lepiniec L. (2010). Physiological and developmental regulation of seed oil production. Prog. Lipid Res. 49: 235–249. [DOI] [PubMed] [Google Scholar]
- Bechtold N., Ellis J., Pelletier G. (1993). In planta infiltration of adult Arabidopsis plants. C. R. Acad. Sci. Paris Life Sci. 316: 1194–1199. [Google Scholar]
- Beeckman T., De Rycke R., Viane R., Inze D. (2000). Histological study of seed coat development in Arabidopsis thaliana. J. Plant Res. 113: 139–148. [Google Scholar]
- Belmonte M.F., et al. (2013). Comprehensive developmental profiles of gene activity in regions and subregions of the Arabidopsis seed. Proc. Natl. Acad. Sci. USA 110: E435–E444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant F.M., Munoz-Azcarate O., Kelly A.A., Beaudoin F., Kurup S., Eastmond P.J. (2016). ACYL-ACYL CARRIER PROTEIN DESATURASE2 and 3 are responsible for making omega-7 fatty acids in the Arabidopsis aleurone. Plant Physiol. 172: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger F. (2003). Endosperm: the crossroad of seed development. Curr. Opin. Plant Biol. 6: 42–50. [DOI] [PubMed] [Google Scholar]
- Bondaruk M., Johnson S., Degafu A., Boora P., Bilodeau P., Morris J., Wiehler W., Foroud N., Weselake R., Shah S. (2007). Expression of a cDNA encoding palmitoyl-acyl carrier protein desaturase from cat’s claw (Doxantha unguis-cati L.) in Arabidopsis thaliana and Brassica napus leads to accumulation of unusual unsaturated fatty acids and increased stearic acid content in the seed oil. Plant Breed. 126: 186–194. [Google Scholar]
- Bordoli L., Kiefer F., Arnold K., Benkert P., Battey J., Schwede T. (2009). Protein structure homology modeling using SWISS-MODEL workspace. Nat. Protoc. 4: 1–13. [DOI] [PubMed] [Google Scholar]
- Cahoon E.B., Lindqvist Y., Schneider G., Shanklin J. (1997). Redesign of soluble fatty acid desaturases from plants for altered substrate specificity and double bond position. Proc. Natl. Acad. Sci. USA 94: 4872–4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoon E.B., Shah S., Shanklin J., Browse J. (1998). A determinant of substrate specificity predicted from the acyl-acyl carrier protein desaturase of developing cat’s claw seed. Plant Physiol. 117: 593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoon E.B., Shanklin J. (2000). Substrate-dependent mutant complementation to select fatty acid desaturase variants for metabolic engineering of plant seed oils. Proc. Natl. Acad. Sci. USA 97: 12350–12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Gerhold K., Mayers J.R., Wiest M.M., Watkins S.M., Hotamisligil G.S. (2008). Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 134: 933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernac A., Benning C. (2004). WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. Plant J. 40: 575–585. [DOI] [PubMed] [Google Scholar]
- Curtis M.D., Grossniklaus U. (2003). A Gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 133: 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day R.C., Herridge R.P., Ambrose B.A., Macknight R.C. (2008). Transcriptome analysis of proliferating Arabidopsis endosperm reveals biological implications for the control of syncytial division, cytokinin signaling, and gene expression regulation. Plant Physiol. 148: 1964–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubos C., Stracke R., Grotewold E., Weisshaar B., Martin C., Lepiniec L. (2010). MYB transcription factors in Arabidopsis. Trends Plant Sci. 15: 573–581. [DOI] [PubMed] [Google Scholar]
- Fatihi A., Zbierzak A.M., Dörmann P. (2013). Alterations in seed development gene expression affect size and oil content of Arabidopsis seeds. Plant Physiol. 163: 973–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima T., Snyder C.L., Schroeder W.R., Cram D., Datla R., Wishart D., Weselake R.J., Krishna P. (2012). Fatty acid composition of developing sea buckthorn (Hippophae rhamnoides L.) berry and the transcriptome of the mature seed. PLoS One 7: e34099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood J.L. (1996). Recent advances in the biosynthesis of plant fatty acids. Biochim. Biophys. Acta 1301: 7–56. [DOI] [PubMed] [Google Scholar]
- Kachroo P., Shanklin J., Shah J., Whittle E.J., Klessig D.F. (2001). A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proc. Natl. Acad. Sci. USA 98: 9448–9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo A., Shanklin J., Whittle E., Lapchyk L., Hildebrand D., Kachroo P. (2007). The Arabidopsis stearoyl-acyl carrier protein-desaturase family and the contribution of leaf isoforms to oleic acid synthesis. Plant Mol. Biol. 63: 257–271. [DOI] [PubMed] [Google Scholar]
- Klinkenberg J., Faist H., Saupe S., Lambertz S., Krischke M., Stingl N., Fekete A., Mueller M.J., Feussner I., Hedrich R., Deeken R. (2014). Two fatty acid desaturases, STEAROYL-ACYL CARRIER PROTEIN Δ9-DESATURASE6 and FATTY ACID DESATURASE3, are involved in drought and hypoxia stress signaling in Arabidopsis crown galls. Plant Physiol. 164: 570–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroj T., Savino G., Valon C., Giraudat J., Parcy F. (2003). Regulation of storage protein gene expression in Arabidopsis. Development 130: 6065–6073. [DOI] [PubMed] [Google Scholar]
- Le B.H., et al. (2010). Global analysis of gene activity during Arabidopsis seed development and identification of seed-specific transcription factors. Proc. Natl. Acad. Sci. USA 107: 8063–8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Beisson F., Pollard M., Ohlrogge J. (2006). Oil content of Arabidopsis seeds: the influence of seed anatomy, light and plant-to-plant variation. Phytochemistry 67: 904–915. [DOI] [PubMed] [Google Scholar]
- Lightner J., Wu J., Browse J. (1994). A mutant of Arabidopsis with increased levels of stearic acid. Plant Physiol. 106: 1443–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist Y., Huang W., Schneider G., Shanklin J. (1996). Crystal structure of delta9 stearoyl-acyl carrier protein desaturase from castor seed and its relationship to other di-iron proteins. EMBO J. 15: 4081–4092. [PMC free article] [PubMed] [Google Scholar]
- Lotan T., Ohto M., Yee K.M., West M.A., Lo R., Kwong R.W., Yamagishi K., Fischer R.L., Goldberg R.B., Harada J.J. (1998). Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell 93: 1195–1205. [DOI] [PubMed] [Google Scholar]
- Martin K., Kopperud K., Chakrabarty R., Banerjee R., Brooks R., Goodin M.M. (2009). Transient expression in Nicotiana benthamiana fluorescent marker lines provides enhanced definition of protein localization, movement and interactions in planta. Plant J. 59: 150–162. [DOI] [PubMed] [Google Scholar]
- Meier M.A.R. (2009). Metathesis with oleochemicals: new approaches for the utilization of plant oils as renewable resources in polymer science. Macromol. Chem. Phys. 210: 1073–1079. [Google Scholar]
- Mendes A., Kelly A.A., van Erp H., Shaw E., Powers S.J., Kurup S., Eastmond P.J. (2013). bZIP67 regulates the omega-3 fatty acid content of Arabidopsis seed oil by activating fatty acid desaturase3. Plant Cell 25: 3104–3116.23995083 [Google Scholar]
- Miart F., Desprez T., Biot E., Morin H., Belcram K., Höfte H., Gonneau M., Vernhettes S. (2014). Spatio-temporal analysis of cellulose synthesis during cell plate formation in Arabidopsis. Plant J. 77: 71–84. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Kurose T., Hino T., Tanaka K., Kawamukai M., Niwa Y., Toyooka K., Matsuoka K., Jinbo T., Kimura T. (2007). Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 104: 34–41. [DOI] [PubMed] [Google Scholar]
- Nguyen H.T., Park H., Koster K.L., Cahoon R.E., Nguyen H.T.M., Shanklin J., Clemente T.E., Cahoon E.B. (2015). Redirection of metabolic flux for high levels of omega-7 monounsaturated fatty acid accumulation in camelina seeds. Plant Biotechnol. J. 13: 38–50. [DOI] [PubMed] [Google Scholar]
- Penfield S., Rylott E.L., Gilday A.D., Graham S., Larson T.R., Graham I.A. (2004). Reserve mobilization in the Arabidopsis endosperm fuels hypocotyl elongation in the dark, is independent of abscisic acid, and requires PHOSPHOENOLPYRUVATE CARBOXYKINASE1. Plant Cell 16: 2705–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prouse M.B., Campbell M.M. (2012). The interaction between MYB proteins and their target DNA binding sites. Biochim. Biophys. Acta 1819: 67–77. [DOI] [PubMed] [Google Scholar]
- Rodríguez M.F., Sánchez-García A., Salas J.J., Garcés R., Martínez-Force E. (2015). Characterization of soluble acyl-ACP desaturases from Camelina sativa, Macadamia tetraphylla and Dolichandra unguis-cati. J. Plant Physiol. 178: 35–42. [DOI] [PubMed] [Google Scholar]
- Romero I., Fuertes A., Benito M.J., Malpica J.M., Leyva A., Paz-Ares J. (1998). More than 80R2R3-MYB regulatory genes in the genome of Arabidopsis thaliana. Plant J. 14: 273–284. [DOI] [PubMed] [Google Scholar]
- Roscoe T.T., Guilleminot J., Bessoule J.-J., Berger F., Devic M. (2015). Complementation of seed maturation phenotypes by ectopic expression of ABSCISIC ACID INSENSITIVE3, FUSCA3 and LEAFY COTYLEDON2 in Arabidopsis. Plant Cell Physiol. 56: 1215–1228. [DOI] [PubMed] [Google Scholar]
- Santos Mendoza M., Dubreucq B., Miquel M., Caboche M., Lepiniec L. (2005). LEAFY COTYLEDON 2 activation is sufficient to trigger the accumulation of oil and seed specific mRNAs in Arabidopsis leaves. FEBS Lett. 579: 4666–4670. [DOI] [PubMed] [Google Scholar]
- Santos-Mendoza M., Dubreucq B., Baud S., Parcy F., Caboche M., Lepiniec L. (2008). Deciphering gene regulatory networks that control seed development and maturation in Arabidopsis. Plant J. 54: 608–620. [DOI] [PubMed] [Google Scholar]
- Schmid M., Davison T.S., Henz S.R., Pape U.J., Demar M., Vingron M., Schölkopf B., Weigel D., Lohmann J.U. (2005). A gene expression map of Arabidopsis thaliana development. Nat. Genet. 37: 501–506. [DOI] [PubMed] [Google Scholar]
- Shamloul M., Trusa J., Mett V., Yusibov V. (2014). Optimization and utilization of Agrobacterium-mediated transient protein production in Nicotiana. J. Vis. Exp. 86: 51204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan N., Kantartzis K., Celebi N., Staiger H., Machann J., Schick F., Cegan A., Elcnerova M., Schleicher E., Fritsche A., Häring H.U. (2010). Circulating palmitoleate strongly and independently predicts insulin sensitivity in humans. Diabetes Care 33: 405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Shantharaj D., Kang X., Ni M. (2010). Transcriptional and hormonal signaling control of Arabidopsis seed development. Curr. Opin. Plant Biol. 13: 611–620. [DOI] [PubMed] [Google Scholar]
- Suzuki M., McCarty D.R. (2008). Functional symmetry of the B3 network controlling seed development. Curr. Opin. Plant Biol. 11: 548–553. [DOI] [PubMed] [Google Scholar]
- Vicente-Carbajosa J., Carbonero P. (2005). Seed maturation: developing an intrusive phase to accomplish a quiescent state. Int. J. Dev. Biol. 49: 645–651. [DOI] [PubMed] [Google Scholar]
- Wang X., Niu Q.-W., Teng C., Li C., Mu J., Chua N.-H., Zuo J. (2009). Overexpression of PGA37/MYB118 and MYB115 promotes vegetative-to-embryonic transition in Arabidopsis. Cell Res. 19: 224–235. [DOI] [PubMed] [Google Scholar]
- Whittle E., Shanklin J. (2001). Engineering delta 9-16:0-acyl carrier protein (ACP) desaturase specificity based on combinatorial saturation mutagenesis and logical redesign of the castor delta 9-18:0-ACP desaturase. J. Biol. Chem. 276: 21500–21505. [DOI] [PubMed] [Google Scholar]
- Wu Y., Li R., Hildebrand D.F. (2012). Biosynthesis and metabolic engineering of palmitoleate production, an important contributor to human health and sustainable industry. Prog. Lipid Res. 51: 340–349. [DOI] [PubMed] [Google Scholar]
- Xu W., Grain D., Bobet S., Le Gourrierec J., Thévenin J., Kelemen Z., Lepiniec L., Dubos C. (2014). Complexity and robustness of the flavonoid transcriptional regulatory network revealed by comprehensive analyses of MYB-bHLH-WDR complexes and their targets in Arabidopsis seed. New Phytol. 202: 132–144. [DOI] [PubMed] [Google Scholar]
- Yoon W.J., Kim M.J., Moon J.Y., Kang H.J., Kim G.O., Lee N.H., Hyun C.G. (2010). Effect of palmitoleic acid on melanogenic protein expression in murine b16 melanoma. J. Oleo Sci. 59: 315–319. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Cao G., Qu L.-J., Gu H. (2009). Involvement of an R2R3-MYB transcription factor gene AtMYB118 in embryogenesis in Arabidopsis. Plant Cell Rep. 28: 337–346. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li B., Huai D., Zhou Y., Kliebenstein D.J. (2015). The conserved transcription factors, MYB115 and MYB118, control expression of the newly evolved benzoyloxy glucosinolate pathway in Arabidopsis thaliana. Front. Plant Sci. 6: 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.