Abstract
Background
Robust data on patient-reported outcome measures comparing treatments for clinically localized prostate cancer are lacking. We investigated the effects of active monitoring, radical prostatectomy, and radical radiotherapy with hormones on patient-reported outcomes.
Methods
We compared patient-reported outcomes among 1643 men in the Prostate Testing for Cancer and Treatment (ProtecT) trial who completed questionnaires before diagnosis, at 6 and 12 months after randomization, and annually thereafter. Patients completed validated measures that assessed urinary, bowel, and sexual function and specific effects on quality of life, anxiety and depression, and general health. Cancer-related quality of life was assessed at 5 years. Complete 6-year data were analyzed according to the intention-to-treat principle.
Results
The rate of questionnaire completion during follow-up was higher than 85% for most measures. Of the three treatments, prostatectomy had the greatest negative effect on sexual function and urinary continence, and although there was some recovery, these outcomes remained worse in the prostatectomy group than in the other groups throughout the trial. The negative effect of radiotherapy on sexual function was greatest at 6 months, but sexual function then recovered somewhat and was stable thereafter; radiotherapy had little effect on urinary continence. Sexual and urinary function declined gradually in the active-monitoring group. Bowel function was worse in the radiotherapy group at 6 months than in the other groups but then recovered somewhat, except for the increasing frequency of bloody stools; bowel function was unchanged in the other groups. Urinary voiding and nocturia were worse in the radiotherapy group at 6 months but then mostly recovered and were similar to the other groups after 12 months. Effects on quality of life mirrored the reported changes in function. No significant differences were observed among the groups in measures of anxiety, depression, or general health-related or cancer-related quality of life.
Conclusions
In this analysis of patient-reported outcomes after treatment for localized prostate cancer, patterns of severity, recovery, and decline in urinary, bowel, and sexual function and associated quality of life differed among the three groups. (Funded by the U.K. National Institute for Health Research Health Technology Assessment Program; ProtecT Current Controlled Trials number, ISRCTN20141297; ClinicalTrials.gov number, NCT02044172.)
As reported in a companion article in the Journal, the U.K. National Institute for Health Research–supported Prostate Testing for Cancer and Treatment (ProtecT) trial has shown no significant difference in prostate cancer–specific mortality or all-cause mortality among men with prostate cancer detected by prostate-specific antigen (PSA) testing who were randomly assigned to radical prostatectomy, active monitoring (a surveillance strategy), or radical conformal radiotherapy with neoadjuvant hormonal therapy, at a median of 10 years of follow-up; however, the ProtecT trial has shown higher rates of metastases and disease progression among men in the active-monitoring group than among men in the radical-treatment groups.1 In this article, we focus on the prospective assessments by the participants of the effects of treatments on urinary, sexual, and bowel function and specific and general aspects of quality of life; validated measures were completed regularly by the participants to assess these outcomes.
Systematic reviews2–5 and studies involving large, prospective cohorts6,7 have shown particular effects on urinary, bowel, and sexual function and little effect on general quality of life after radical treatments, but clear comparisons among contemporary treatments have been hindered by differences in outcome definitions, limited use of validated outcome measures, mostly short-term follow-up, and sparse data on radiotherapy or active surveillance programs.8 Randomized clinical trials have not included the full range of validated patient-reported outcome measures. Using a questionnaire specific to the study, the investigators in the Scandinavian Prostate Cancer Group-4 (SPCG-4) trial showed that prostatectomy had a greater effect on sexual and urinary function and quality of life than did watchful waiting among men who had clinically identified prostate cancer.9,10 Using three single symptoms items, the investigators in the Prostate Cancer Intervention versus Observation Trial (PIVOT) reported worse urinary incontinence and erectile dysfunction after prostatectomy than after observation, and similar bowel function, among men with PSA-detected prostate cancer.11 Here we present a comprehensive set of patient-reported outcomes from the ProtecT trial over 6 years of follow-up.
Methods
Protect Trial Participants
Details of the recruitment methods of the ProtecT trial and the baseline data have been published previously (see also Table S1A in the Supplementary Appendix, available with the full text of this article at NEJM.org).12 In brief, after population-based PSA testing and standardized diagnostic procedures had been performed between 1999 and 2009, a total of 2896 men received a diagnosis of prostate cancer, including 2664 men with clinically localized disease. A total of 1643 of these men (62%) underwent randomization; 545 were assigned to active monitoring (regular PSA testing with clinical review to enable change to radical treatment if disease progressed), 553 to radical prostatectomy (most of the operations involved an open retropubic, nerve-sparing approach), and 545 to radiotherapy (external-beam three-dimensional conformal radiotherapy delivered at a total dose of 74 Gy in 37 fractions, along with neoadjuvant androgen deprivation therapy). The prespecified primary outcome was prostate-cancer mortality at a median of 10 years of follow-up, with prostate-cancer–related deaths defined as deaths that were definitely or probably due to prostate cancer or its treatment.13
Trial Design and Oversight
The authors vouch for the accuracy and completeness of the data and analyses and for the fidelity of the study to the protocol, available at NEJM.org. The ProtecT trial was approved by the East Midlands (formerly Trent) Multicenter Research Ethics Committee in the United Kingdom (reference number 01/4/025). The ProtecT trial followed the Consolidated Standards of Reporting of Trials (CONSORT) guidelines for patient-reported outcomes.14
Patient-Reported Outcome Measures
Patient-reported outcomes were prespecified secondary outcomes that were assessed with the use of validated measures in four key domains15 (Table 1). Domain A comprised urinary function, including urinary incontinence and lower urinary tract symptoms, and the effect of urinary function on quality of life; outcomes were assessed with the use of the International Consultation on Incontinence Questionnaire (ICIQ),16 the Expanded Prostate Cancer Index Composite (EPIC) instrument,17 and the International Continence Society Male Short-Form (ICSmaleSF) questionnaire.18 Domain B comprised sexual function, including erectile function, and the effect of sexual function on quality of life; outcomes were assessed with the use of the EPIC instrument.17 Domain C comprised bowel function, including the occurrence of loose and bloody stools and incontinence, and the effect of bowel function on quality of life; outcomes were assessed with the use of the EPIC instrument.17 Domain D comprised measures of health-related quality of life, which included general health status (as assessed with the use of the Medical Outcomes Study 12-Item Short-Form General Health Survey [SF-12]19), anxiety and depression (as assessed with the use of the Hospital Anxiety and Depression Scale [HADS]),20 and cancer-related quality of life (as assessed with the use of the European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire–Core 30 module (EORTC QLQ-C30).21
Table 1. Patient-Reported Outcome Measure Domains, Scores, and Items.*.
| Domain A: Urinary function and effect on quality of life |
| Incontinence |
| Assessment score: ICIQ16 score |
| Key item: EPIC17 pad-use item |
| Effect on quality of life: ICIQ interference with quality of life item |
| Lower urinary tract symptoms |
| Assessment scores: ICSmaleSF18 voiding score, EPIC urinary summary score |
| Key item: ICSmaleSF nocturia |
| Effect on quality of life: ICSmaleSF effect of urinary symptoms on quality of life item |
| Domain B: Sexual function and effect on quality of life |
| Erectile dysfunction |
| Key item: EPIC item on erections firm enough for intercourse |
| Effect on quality of life: EPIC problem with erectile dysfunction item |
| Overall sexual function |
| Assessment scores: EPIC sexual function subscale score, EPIC sexual bother subscale score |
| Effect on quality of life: EPIC impact of sexual dysfunction item |
| Domain C: Bowel function and effect on quality of life |
| Assessment scores: EPIC bowel function subscale score, EPIC bowel bother subscale score |
| Key items: EPIC items on loose stools, fecal incontinence, bloody stools |
| Effect on quality of life: EPIC impact of bowel habits item |
| Domain D: Health-related quality of life |
| General health status: SF-12 physical health and mental health19 |
| HADS percentage of potentially significant clinical cases of anxiety and depression20 |
| Cancer-related quality of life: EORTC QLQ-C3021 |
Table S2 in the Supplementary Appendix provides patient-reported outcomes for EPIC (Expanded Prostate Cancer Index Composite) urinary incontinence subscale score, urinary bother subscale score, urinary obstruction/irritation subscale score, sexual summary score, and bowel summary score; ICSmaleSF (International Continence Society Male Short-Form) questionnaire urinary incontinence score and daytime urine frequency score; HADS (Hospital Anxiety and Depression Scale) mean anxiety subscale and depression subscale score; and EORTC QLQ-C30 (European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire–Core 30 module) global health status score, five functional scales, and nine symptom scales. ICIQ denotes International Consultation on Incontinence Questionnaire, and SF-12 Medical Outcomes Study 12-Item Short-Form General Health Survey.
Study questionnaires were completed at baseline (i.e., at the time of biopsy, before the diagnosis was known), at 6 and 12 months after randomization, and annually thereafter. The ICSmaleSF questionnaire, the SF-12, and the HADS were included in the study during the entire course of the ProtecT trial; the ICIQ was included starting in 2001, and the EPIC instrument was included starting in 2005. Because the EORTC QLQ-C30 concerns cancer-related quality of life, this questionnaire was included at year 5 only. Patient-reported outcome measures were scored and analyzed as recommended by the authors of the assessments, with key items identified to aid in the interpretation of clinical relevance (Table 1). Men received therapies as required for side effects of treatments in accordance with guidelines,22–25 and their questionnaire responses include influences of the effects of these therapies.
Statistical Analysis
Analyses were performed according to the intention-to-treat principle, and summary statistics and 95% confidence intervals are reported according to randomization group. For each outcome measure in turn, all available data after randomization for each man were compared between the treatment groups; a likelihood-ratio test evaluated the evidence against a null hypothesis of equal mean response over 6 years of follow-up across the three groups. Two-level random-effects models were used to accommodate the correlation between the repeated assessments for each man. Two-level linear models (also known as variance component models) were used for continuous measures, and two-level logistic models were used for binary measures; normal random-effects distributions were used in both the linear and logistic models. All models included as covariates the variables that were used for stratification or minimization in the randomization process: age and PSA level at baseline (continuous variables) and Gleason score and study center (dummy variables). Although we had planned to include baseline measures as covariates, we did not include them because the EPIC instrument and the ICIQ were not available for men who were recruited early in the trial. No meaningful differences in patient-reported outcome measures across treatment groups were observed at baseline.15
Missing data were not imputed; all data from men with at least one measure available after randomization were included in the analysis. The random-effects models used here provided unbiased estimates of treatment comparisons, under the assumption that any systematic determinant of data being missing was predictable from the covariates that were included in the model, such as the treatment group or earlier measures of the outcome (i.e., data were missing at random).26 All analyses were performed with the use of Stata software, version 14.1 (StataCorp).
Results
Response Rates
The response rates during follow-up were higher than 85% for most measures, including sexual function, and did not decline over time (Table S1B in the Supplementary Appendix). A total of 55 men (3.3%) stopped completing questionnaires, and some men did not complete all the questionnaires at every time point. Outcomes in the four domains are presented in this section, and selected scores and items are shown in Fig. 1, Fig. 2, Fig. 3, and Fig. 4 (details of all patient-reported outcomes are provided in Table S2 in the Supplementary Appendix).
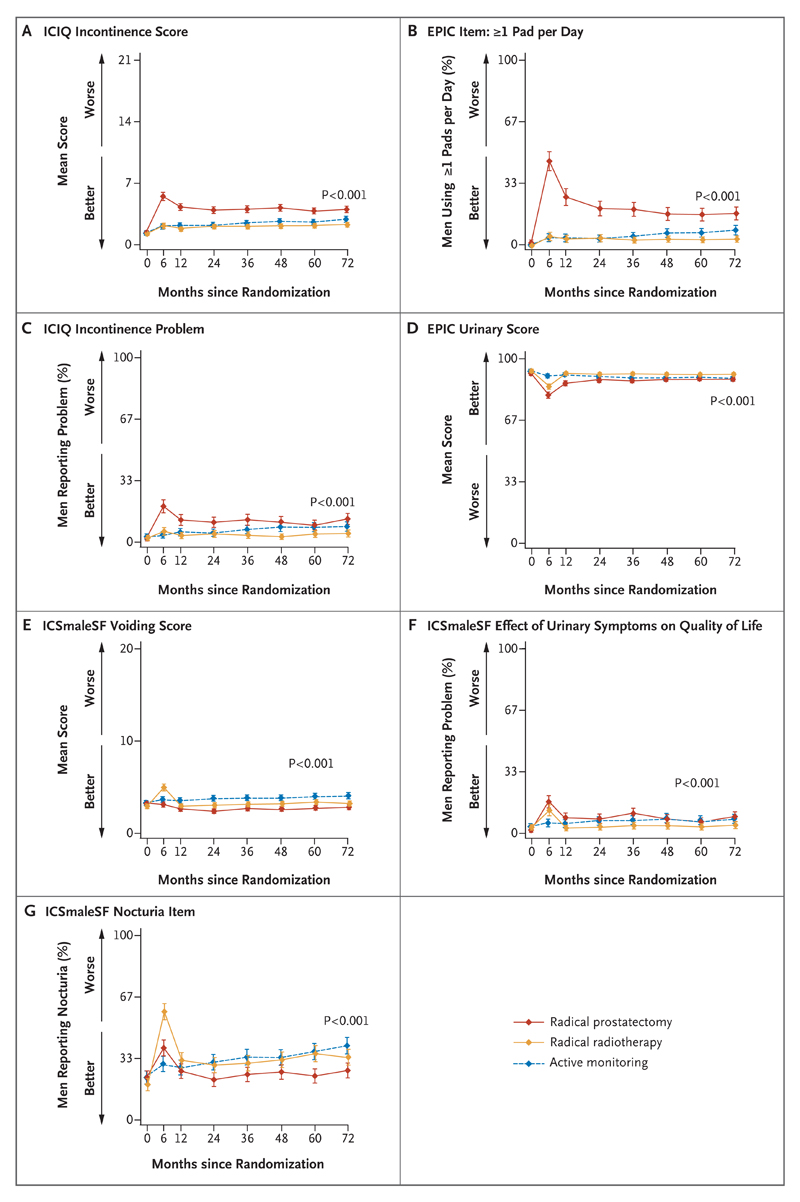
Figure 1. Outcomes for Urinary Function and Effect on Quality of Life.
Shown are the effects of the treatments on urinary function (including urinary incontinence) and quality of life. The International Consultation on Incontinence Questionnaire (ICIQ) incontinence scores, shown in Panel A, range from 0 to 21. Panel B shows the percentage of men who used one or more absorbent pads per day for urinary incontinence, as assessed by the Expanded Prostate Cancer Index Composite (EPIC) instrument. In Panel C, the percentages shown are for men who reported a moderate-to-severe incontinence problem, as assessed by the ICIQ. The EPIC urinary scores, shown in Panel D, comprise several urinary symptoms, including incontinence; scores are formed by linear transformation of raw scores and range from 0 to 100. The International Continence Society Male Short-Form (ICSmaleSF) voiding scores, shown in Panel E, range from 0 to 20. Panel F shows the percentage of men reporting that urinary symptoms affected their quality of life somewhat to a lot, and Panel G, the percentage of men reporting nocturia at least two times per night — both as assessed by the ICSmaleSF. The P values show the strength of evidence for a difference in mean response over 6 years of follow-up across the three groups, with P values of 0.01 or lower indicating strong evidence of a difference. I bars represent 95% confidence intervals.
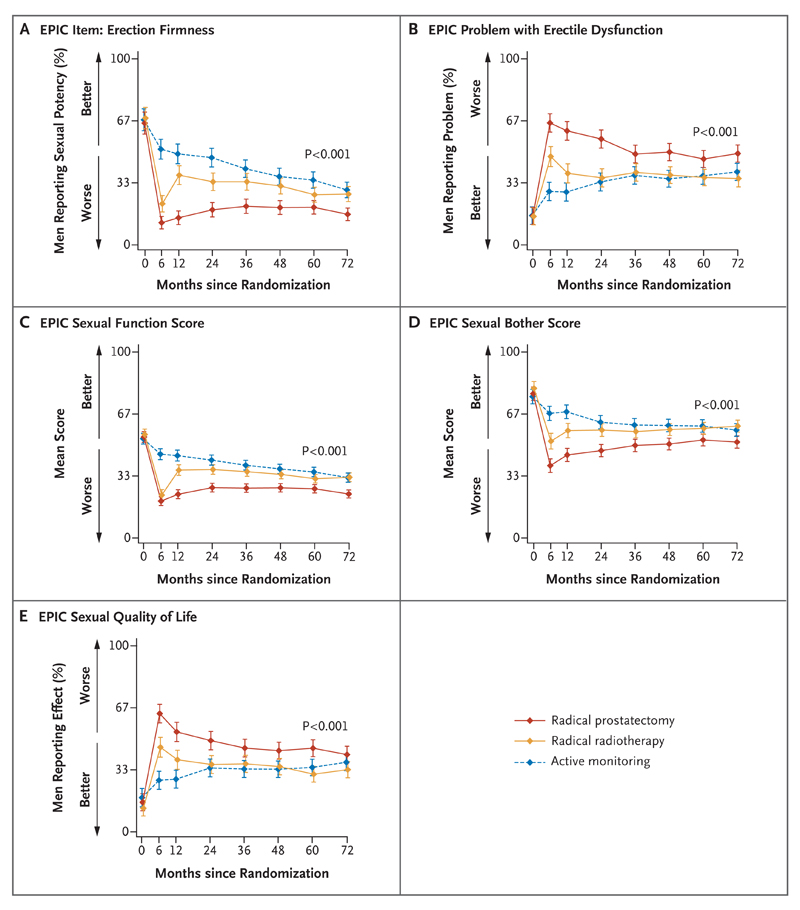
Figure 2. Outcomes for Sexual Function and Effect on Quality of Life.
Shown are the effects of the treatments on sexual function (including erectile dysfunction) and quality of life. Panel A shows the percentage of men reporting erections firm enough for intercourse. In Panel B, the percentages are for men who reported a moderate-to-severe problem with erectile dysfunction. The EPIC sexual function scores, shown in Panel C, range from 0 to 100. The EPIC sexual bother scores, shown in Panel D, range from 0 to 100. In Panel E, the percentages are for men who reported a moderate-to-severe effect on sexual quality of life. The P values show the strength of evidence for a difference in mean response over 6 years of follow-up across the three groups, with P values of 0.01 or lower indicating strong evidence of a difference. I bars represent 95% confidence intervals.
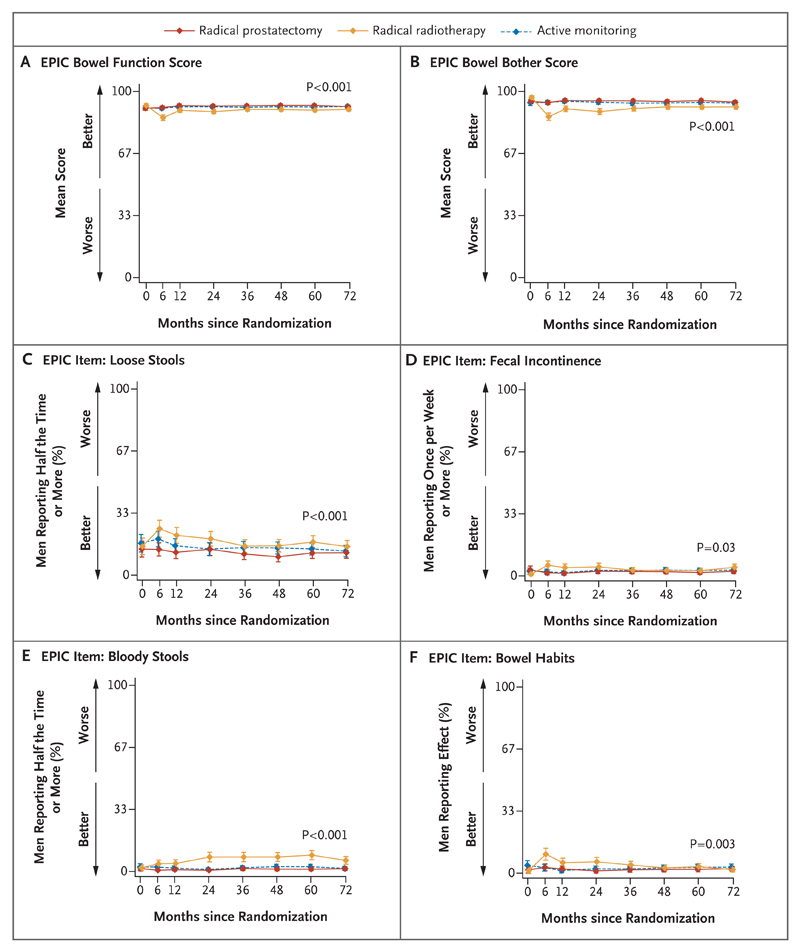
Figure 3. Outcomes for Bowel Function and Effect on Quality of Life.
Shown are the effects of the treatments on bowel function and quality of life. In Panel A, the EPIC bowel function scores range from 0 to 100. In Panel B, the EPIC bowel bother scores range from 0 to 100. In Panel C, the percentages are for men who reported having loose stools half the time or more. In Panel D, the percentages are for men who reported having fecal incontinence at least once per week. In Panel E, the percentages are for men who reported having bloody stools half the time or more. In Panel F, the percentages are for men who reported a moderate-to-severe negative effect on bowel habits. The P values show the strength of evidence for a difference in mean response over 6 years of follow-up across the three groups, with P values of 0.01 or lower indicating strong evidence of a difference. I bars represent 95% confidence intervals.
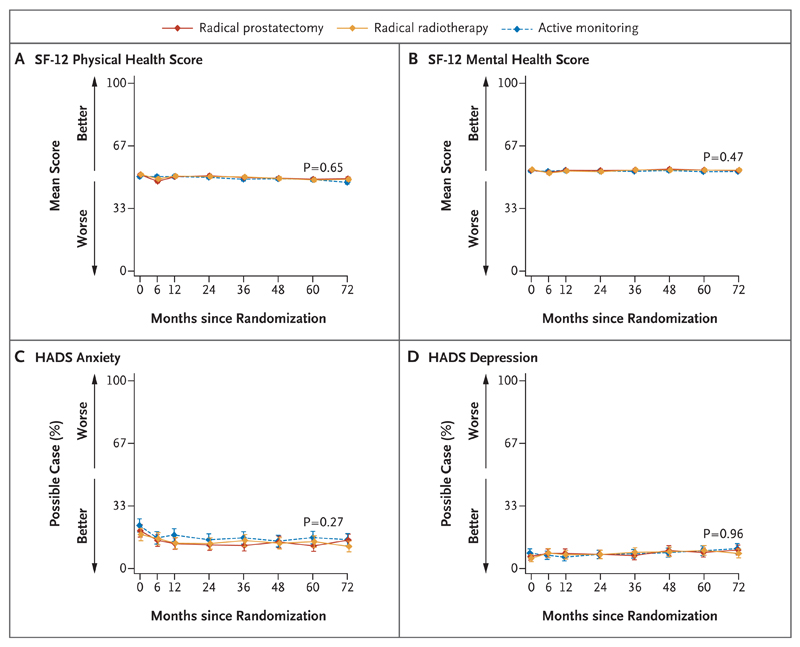
Figure 4. Outcomes for Health-Related Quality of Life.
Shown are the effects of the treatments on health-related quality of life. Medical Outcomes Study 12-Item Short-Form General Health Survey (SF-12) physical health scores (Panel A) and mental health scores (Panel B) range from 0 to 100. “Possible case” indicates the percentages of patients, who were assessed with the use of the Hospital Anxiety and Depression Scale (HADS), with scores suggesting clinically significant cases of anxiety (Panel C) and depression (Panel D). The P values show the strength of evidence for a difference in mean response over 6 years of follow-up across the three groups, with P values of 0.01 or lower indicating strong evidence of a difference. I bars represent 95% confidence intervals.
Domain A: Urinary Function and Effect on Quality of Life
Prostatectomy had the greatest negative effect on urinary continence at 6 months, and although there was some recovery, urinary incontinence remained worse in the prostatectomy group than in the radiotherapy group and active-monitoring group at all time points (P<0.001 for each measure) (Fig. 1A and 1B, and Table S2A in the Supplementary Appendix). Radiotherapy and active monitoring had little effect on urinary continence; the rates of urinary incontinence were similar in the two treatment groups, although the rate rose slightly in the active-monitoring group over time. The rate of use of absorbent pads increased from 1% at baseline to 46% at 6 months in the prostatectomy group, as compared with 4% at 6 months in the active-monitoring group and 5% at 6 months in the radiotherapy group. By year 6, 17% of men in the prostatectomy group were using pads, as compared with 8% in the active-monitoring group and 4% in the radiotherapy group (Fig. 1B). The effect of urinary incontinence on quality of life was worse in the prostatectomy group for 2 years, but then became somewhat similar to that reported in the other groups (Fig. 1C). A similar pattern was shown for scores that combined lower urinary tract symptoms and incontinence (Fig. 1D and 1F). Scores for voiding symptoms were a little worse in the radiotherapy group than in the other treatment groups at 6 months but then returned close to baseline levels and were similar to the scores in the prostatectomy group and the active-monitoring group (Fig. 1E). Urinary frequency remained similar across the treatment groups (Table S2A in the Supplementary Appendix). The percentage of men reporting nocturia increased in all treatment groups; the increase occurred particularly in the radiotherapy group at 6 months, but this percentage then decreased to become similar to that in the active-monitoring group. The percentage of men reporting nocturia returned closest to the baseline level in the prostatectomy group (Fig. 1G).
Domain B: Sexual Function and Effect on Quality of Life
Erectile function was reduced from baseline to 6 months in all the men, with clear differences among the treatment groups (P<0.001) (Fig. 2A). At baseline, 67% of men reported erections firm enough for intercourse, but by 6 months this rate fell to 52% in the active-monitoring group, to 22% in the radiotherapy group, and to 12% in the prostatectomy group. Erectile function remained worse in the prostatectomy group at all time points, and although there was some recovery to 21% with erections firm enough for inter-course at 36 months, this rate had declined again to 17% at 6 years. In the radiotherapy group, the percentage of men reporting erections firm enough for intercourse increased between 6 months and 12 months and then declined again to 27% at 6 years, and in the active-monitoring group, the percentage declined year to year, with 41% of men reporting this outcome at year 3 and 30% at year 6. Very similar patterns across the treatment groups and over time were observed for the other measures of overall sexual function, bother (the level of the problem experienced), and effect on quality of life (Fig. 2B through 2E, and Table S2B in the Supplementary Appendix).
Domain C: Bowel Function and Effect on Quality of Life
Bowel function and bother scores and the effect of bowel habits on quality of life were unchanged in the prostatectomy group and active-monitoring group, but scores for these outcomes were worse in the radiotherapy group, particularly at 6 months (Fig. 3A, 3B, and 3F, and Table S2C in the Supplementary Appendix). The percentage of men reporting fecal incontinence and loose stools was similar across the treatment groups (Fig. 3C and 3D), but the percentage of men reporting bloody stools from year 2 onward was higher in the radiotherapy group than in the other treatment groups (P<0.001) (Fig. 3E). The scores on the “bowel bother” assessment and the effect on quality of life were also a little worse in the radiotherapy group than in the other treatment groups (Table S2C in the Supplementary Appendix).
Domain D: Health-Related Quality of Life
The comparisons of health-related quality of life revealed no significant differences among the treatment groups in the physical and mental health subscores of the SF-12 general health measure, in scores on the HADS, or in any of the symptom or function scale scores of the EORTC QLQ-C30 at year 5 (Fig. 4, and Table S2D in the Supplementary Appendix).
Discussion
The ProtecT trial has shown that all three treatment groups had similar, very high rates of survival after treatment, but higher rates of metastases and disease progression were observed in the active-monitoring group than in the two radical-treatment groups.1 In this context, understanding the effects of the treatments and how the treatments affect men’s lives becomes crucial for decision making. The patient-reported outcome measures in the ProtecT trial included key domains that were recommended by international groups,4,27,28 and we followed reporting guidelines14 to provide unbiased comparisons of the effects of standardized prostatectomy, radiotherapy, and active-monitoring management strategies for PSA-detected clinically localized prostate cancer. The findings of the ProtecT trial have clarified the distinct effects of prostate-cancer treatments on urinary, sexual, and bowel function and condition-specific quality of life. The negative effect of prostatectomy on urinary continence and sexual function, particularly erectile function, was greatest at 6 months, and although there was some recovery, the effect was worse than in the other treatment groups over 6 years; however, prostatectomy was associated with no change in bowel function. At 6 months, the negative effect of radiotherapy with neoadjuvant androgen deprivation therapy on sexual function, particularly erectile function, was only a little less than that of prostatectomy, and bowel function, urinary voiding, and nocturia were worse in the radiotherapy group than in the other groups. However, there was then considerable recovery in the radiotherapy group for these measures, apart from more frequent bloody stools. In the active-monitoring group, sexual (including erectile) function and urinary continence and function were affected much less than in the radical-treatment groups initially but worsened gradually over time, as increasing numbers of men received radical treatments and age-related changes occurred (Table S3B in the Supplementary Appendix); bowel function was unchanged.
With respect to numbers needed to treat, we estimated that treating 4 men with prostatectomy or 8 men with radiotherapy rather than active monitoring would cause one additional case of erectile dysfunction at 2 years; treating 5 men with prostatectomy or 143 men with radiotherapy rather than active monitoring would cause one additional case of urinary incontinence at 2 years. By the end of follow-up at 6 years, urinary and sexual function had stabilized in the radiotherapy group after improving for 2 or 3 years, and with the steady decline that was evident in the active-monitoring group, the outcomes became similar in the active-monitoring group and the radiotherapy group but remained worse in the prostatectomy group. These profiles of the effects of treatments on function were mirrored in outcomes reported for the sexual, urinary, and bowel quality-of-life items, with some evidence of accommodation to changes over time. No effects were observed with respect to general health status (mental or physical) or anxiety or depression in any treatment group at any time or in cancer-related quality of life at 5 years.
The paucity of published data, lack of consistency in definitions of outcomes, and variability in timing of assessment severely constrain our ability to compare ProtecT findings directly with those of other randomized trials or major cohort studies of treatments.3,5 Table 2 presents the findings for two specific items that we could compare — erectile function and the use of pads for urinary incontinence. The findings in the ProtecT trial were similar to those in the SPCG-4 trial and PIVOT with respect to erectile function after prostatectomy and active monitoring (or watchful waiting).9,11,30 The slightly worse results in observational cohorts6,7,29 could be related to age or selection biases. The percentage of patients who required the use of pads after prostatectomy or active monitoring was considerably lower in the ProtecT trial than in the SPCG-4 trial and was similar to that in PIVOT; the results regarding pad use after radiotherapy were similar in the three observational studies at all time points (Table 2). Broadly similar results were also found with respect to bowel function and urinary symptoms after radiotherapy4,6 and for urinary voiding after prostatectomy.6 The EPIC scores in the ProtecT trial were similar to those in other studies.31,32 Other studies also reported similar results for assessments of general health-related or psychological aspects of quality of life.3,9,33
Table 2. Comparisons of Key ProtecT Trial Outcomes with Those Found in Other Trials and Cohorts.*.
| Variable | Treatment |
|||
|---|---|---|---|---|
| Watchful Waiting | Active Monitoring or Active Surveillance | Radical Prostatectomy | Radical Radiotherapy | |
| percentage of participants | ||||
| Erection not firm enough for intercourse | ||||
| At 12-mo follow-up | ||||
| ProtecT | — | 51 | 85 | 62 |
| SPCG-49 | 45 | — | 80 | — |
| Sanda et al.6 | — | — | 75 | 64 |
| At 24-mo follow-up | ||||
| ProtecT | — | 53 | 81 | 66 |
| PIVOT11 | 44 | — | 81 | — |
| Resnick et al.29 | — | — | 79 | 61 |
| At 36-mo follow-up | ||||
| ProtecT | — | 59 | 79 | 66 |
| Smith et al.7 | — | 54 | 68 and 87† | 68 |
| At 60-mo follow-up: Resnick et al.29 | — | — | 76 | 72 |
| At 72-mo follow-up: ProtecT | — | 70 | 83 | 73 |
| At 144-mo follow-up: SPCG-430 | 80 | — | 84 | — |
| Incontinence: any use of absorbent pads | ||||
| At 12-mo follow-up | ||||
| ProtecT | — | 4 | 26 | 4 |
| SPCG-49 | 16 | — | 71 | — |
| Sanda et al.6 | — | — | 24 | 3 |
| At 24-mo follow-up | ||||
| ProtecT | — | 4 | 21 | 4 |
| PIVOT11‡ | 6 | — | 17 | — |
| Resnick et al.29 | — | — | 27 | 2 |
| At 36-mo follow-up | ||||
| ProtecT | — | 5 | 20 | 3 |
| Smith et al.7 | — | 3 | 9 and 15§ | 3 |
| At 60-mo follow-up: Resnick et al.29 | — | — | 28 | 4 |
| At 72-mo follow-up: ProtecT | — | 8 | 17 | 4 |
| At 144-mo follow-up: SPCG-430 | 25 | — | 54 | — |
Dashes indicate not applicable. The median age of the participants in the Prostate Testing for Cancer and Treatment (ProtecT) trial (current study) was 62 years. The mean age of the participants in Scandinavian Prostate Cancer Group Study Number 4 (SPCG-4)9 was 64 years. In the study by Sanda et al.,6 the median age of the participants who received radical prostatectomy was 59 years, and of those who received radiotherapy, 69 years. The mean age of the participants in Prostate Cancer Intervention versus Observation Trial (PIVOT)11 was 67 years. In the study by Resnick et al.,29 the median age of the participants who received radical prostatectomy was 64 years, and of those who received radiotherapy, 69 years. In the study by Smith et al.,7 the mean age of the participants who received active surveillance was 66 years; of those who received radical prostatectomy, 60 years; and of those who received radiotherapy, 64 years.
Erection not firm enough for intercourse at 36 months was reported by 68% of the patients who received nerve-sparing prostatectomy and by 87% of the patients who received non–nerve-sparing prostatectomy.
Patient reports of “have a lot of problems with urinary dribbling,” “lose larger amounts of urine than dribbling but not all day,” “have no control over urine,” or “have an indwelling catheter” were used to define incontinence instead of “any use of pads.”
Any use of pads was reported by 9% of the patients who received nerve-sparing prostatectomy and by 15% of the patients who received non–nerve-sparing prostatectomy.
The primary analysis of patient-reported outcome measures according to treatment group is essential for policy development, but the interpretation of the overall scores for decision making by an individual patient or clinician is difficult because factors related to the design and analysis of the ProtecT trial and its treatment policies will have affected some scores. The receipt of therapies to ameliorate the side effects of treatments will also have affected some scores. These issues are considered further in section S3 in the Supplementary Appendix. Determining the clinical significance of outcome measures is also challenging; minimal clinically important differences were proposed to be half the baseline standard deviation or 10 points on some scores but were not defined for other scores.15 We have provided figures for key outcomes according to treatment group (Fig. 1, Fig. 2, Fig. 3, and Fig. 4), as well as a table containing all summary statistics, with P values that were not adjusted for multiple testing (Table S2 in the Supplementary Appendix), to enable readers to make their own judgments.
The interventions in the ProtecT trial remain the three most common contemporary methods of treatment, but there have been developments since the study began. In the ProtecT trial, among the men in the prostatectomy group, 324 received open retropubic procedures, 23 received laparoscopic procedures, and 25 received robot-assisted procedures (the specific procedure was not specified in the case of 19), and most of the prostatectomies were nerve sparing (205 bilateral, 53 unilateral, and 12 unspecified). Observational studies suggest that minimally invasive procedures result in a shorter length of hospital stay and fewer adverse events than do open procedures.34 However, a recent trial has shown that the functional outcomes 12 weeks after a robot-assisted procedure were similar to those after an open retropubic approach,35 and another study showed levels of erectile dysfunction (88%) and urinary incontinence (31%) among men receiving robot-assisted procedures that were very similar to those in the prostatectomy group in the ProtecT trial at 12 months36 The radiotherapy protocol in the ProtecT trial conforms with contemporary guidelines,37 but other techniques such as brachytherapy and intensity modulation have been introduced. Although many active-surveillance programs were developed during the ProtecT trial period, there remains little consensus on inclusion criteria or monitoring and intervention strategies.38 The active-monitoring policy in the ProtecT trial had less selective inclusion criteria than do many active-surveillance programs, and follow-up did not include scheduled repeat biopsies or magnetic resonance imaging; however, the rate of men in the active-monitoring group in the ProtecT trial who changed treatment strategies was similar to that in other studies.
There are strengths and limitations in the design and conduct of the ProtecT trial. Key strengths are the inclusion of radiotherapy, the use of validated patient-reported outcome measures, well-balanced baseline data, high response rates, and concordance between measures across the range of domains affected by treatments for localized prostate cancer. A high rate of eligible participants underwent randomization (62%).39,40 The generalizability of the ProtecT trial is enhanced by its inclusion in a larger trial evaluating prostate cancer screening. In the Cluster Randomized Trial of PSA Testing for Prostate Cancer (CAP), general practices were randomly assigned to form the intervention group or the control group (the intervention group enrolled participants in the ProtecT trial and the control group followed usual care, which did not include an organized program of PSA testing).41 The diagnosis of prostate cancer in the ProtecT trial participants was made after population-based PSA testing and standardized diagnostic procedures.12 An important limitation in the current trial was that only a small number of men of nonwhite race were included, although this reflected the population in the recruitment areas.15 Other limitations are related to changes in diagnostic and treatment strategies since the inception of the trial and the low levels of previous PSA testing in the population42; however, as confirmed on biopsy, the ProtecT trial involved numbers of men who had stage T1 disease (76%) and disease with a Gleason score of 6 (on a scale of 2 to 10, with higher scores indicating a worse prognosis) (77%) that were similar to or higher than the numbers in other treatment or screening trials in the era of PSA testing.11,43,44
This primary analysis has provided data on patient-reported outcomes over 6 years after treatment assignment in the ProtecT trial. These data, combined with the findings of the companion article,1 can be used by policymakers who are developing guidelines and by patients and clinicians who are making decisions about treatments for newly diagnosed localized prostate cancer or who are contemplating PSA testing. However, follow-up for an additional 5 to 10 years is required to fully inform decisions involving the tradeoff between the shorter-term effects of the management strategies shown here and the longer course of progression and treatment of prostate cancer in the context of the onset of other life-threatening conditions.
Supplementary Material
Acknowledgments
The views and opinions expressed in this article are those of the authors and do not necessarily reflect those of the U.K. Department of Health.
Supported by the United Kingdom National Institute for Health Research (NIHR) Health Technology Assessment Program (projects 96/20/06 and 96/20/99, with the University of Oxford as sponsor; www.nets.nihr.ac.uk/projects/hta/962099). Drs. Donovan, Hamdy, Peters, and Neal are NIHR senior investigators. Dr. Donovan is also supported by the NIHR Collaboration for Leadership in Applied Health Research and Care West, hosted by University Hospitals Bristol NHS Foundation Trust. Dr. Hamdy is supported by the Oxford NIHR Biomedical Research Centre Surgical Innovation and Evaluation Theme and the Cancer Research United Kingdom Oxford Center.
Appendix
The authors’ full names and academic degrees are as follows: Jenny L. Donovan, Ph.D., F.Med.Sci., Freddie C. Hamdy, F.R.C.S.(Urol.), F.Med.Sci., J. Athene Lane, Ph.D., Malcolm Mason, M.D., Chris Metcalfe, Ph.D., Eleanor Walsh, M.Sc., Jane M. Blazeby, Ph.D., F.R.C.S., Tim J. Peters, Ph.D., F.Med.Sci., Peter Holding, R.G.N., Susan Bonnington, R.G.N., Teresa Lennon, R.G.N., Lynne Bradshaw, R.G.N., Deborah Cooper, R.G.N., Phillipa Herbert, R.G.N., Joanne Howson, R.G.N., Amanda Jones, R.G.N., Norma Lyons, R.G.N., Elizabeth Salter, R.G.N., Pauline Thompson, R.G.N., Sarah Tidball, R.G.N., Jan Blaikie, R.G.N., Catherine Gray, R.G.N., Prasad Bollina, M.B., B.S., F.R.C.S.(Urol.), James Catto, Ph.D., F.R.C.S.(Urol.), Andrew Doble, M.S., F.R.C.S.(Urol.), Alan Doherty, F.R.C.S.(Urol.), David Gillatt, M.S., F.R.C.S.(Urol.), Roger Kockelbergh, D.M., F.R.C.S.(Urol.), Howard Kynaston, M.D., F.R.C.S.(Urol.), Alan Paul, M.D., F.R.C.S.(Urol.), Philip Powell, M.D., F.R.C.S.(Urol.), Stephen Prescott, M.D., F.R.C.S.(Urol.), Derek J. Rosario, M.D., F.R.C.S.(Urol.), Edward Rowe, M.D., F.R.C.S.(Urol.), Michael Davis, M.Sc., Emma L. Turner, Ph.D., Richard M. Martin, Ph.D., and David E. Neal, F.R.C.S., F.Med.Sci.
The authors’ affiliations are as follows: the School of Social and Community Medicine (J.L.D., J.A.L., C.M., E.W., J.M.B., M.D., E.L.T., R.M.M.), Bristol Randomised Trials Collaboration (J.A.L., C.M.), and the School of Clinical Sciences (T.J.P.), University of Bristol, National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care West at University Hospitals Bristol NHS Foundation Trust (J.L.D.), and Bristol Urological Institute, North Bristol NHS Trust (L.B., E.S., D.G., E.R.), Bristol, Nuffield Department of Surgical Sciences, University of Oxford, Oxford (F.C.H., P. Holding, D.E.N.), the School of Medicine, University of Cardiff (M.M.), and the Department of Urology, Cardiff and Vale University Health Board (A.J., S.T., H.K.), Cardiff, the Department of Urology, University Hospitals Leicester, Leicester (S.B., R.K.), the Department of Urology, Freeman Hospital, Newcastle-upon-Tyne (T.L., P.P.), the Department of Urology, Leeds Teaching Hospitals NHS Trust, Leeds (D.C., C.G., A.P., S.P.), the Department of Urology, Addenbrooke’s Hospital (P. Herbert, A. Doble), and the Academic Urology Group, University of Cambridge (D.E.N.), Cambridge, the Academic Urology Unit, University of Sheffield, Sheffield (J.H., J.C., D.J.R.), the Department of Urology and Surgery, Western General Hospital, Edinburgh (N.L., J.B., P.B.), and the Department of Urology, Queen Elizabeth Hospital, Birmingham (P.T., A. Doherty) — all in the United Kingdom.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank Ms. Joanna Penny for her help in the preparation of an earlier version of the manuscript and all the ProtecT trial participants and researchers for their contributions.
References
- 1.Hamdy FC, Donovan JL, Lane JA, et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–24. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 2.Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148:435–48. doi: 10.7326/0003-4819-148-6-200803180-00209. [DOI] [PubMed] [Google Scholar]
- 3.Chou R, Croswell JM, Dana T, et al. Screening for prostate cancer: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:762–71. doi: 10.7326/0003-4819-155-11-201112060-00375. [DOI] [PubMed] [Google Scholar]
- 4.Chen RC, Chang P, Vetter RJ, et al. Recommended patient-reported core set of symptoms to measure in prostate cancer treatment trials. J Natl Cancer Ins. 2014;106:1–7. doi: 10.1093/jnci/dju132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whiting PF, Moore TH, Jameson CM, et al. Symptomatic and quality-of-life outcomes after treatment for clinically localised prostate cancer: a systematic review. BJU Int. 2016;118:193–204. doi: 10.1111/bju.13499. [DOI] [PubMed] [Google Scholar]
- 6.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 7.Smith DP, King MT, Egger S, et al. Quality of life three years after diagnosis of localised prostate cancer: population based cohort study. BMJ. 2009;339:b4817. doi: 10.1136/bmj.b4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellardita L, Valdagni R, van den Bergh R, et al. How does active surveillance for prostate cancer affect quality of life? A systematic review. Eur Urol. 2015;67:637–45. doi: 10.1016/j.eururo.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Steineck G, Helgesen F, Adolfsson J, et al. Quality of life after radical prostatectomy or watchful waiting. N Engl J Med. 2002;347:790–6. doi: 10.1056/NEJMoa021483. [DOI] [PubMed] [Google Scholar]
- 10.Johansson E, Steineck G, Holmberg L, et al. Long-term quality-of-life outcomes after radical prostatectomy or watchful waiting: the Scandinavian Prostate Cancer Group-4 randomised trial. Lancet Oncol. 2011;12:891–9. doi: 10.1016/S1470-2045(11)70162-0. [DOI] [PubMed] [Google Scholar]
- 11.Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–13. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lane JA, Donovan JL, Davis M, et al. Active monitoring, radical prostatectomy, or radiotherapy for localised prostate cancer: study design and diagnostic and baseline results of the ProtecT randomised phase 3 trial. Lancet Oncol. 2014;15:1109–18. doi: 10.1016/S1470-2045(14)70361-4. [DOI] [PubMed] [Google Scholar]
- 13.Metcalfe C, Peters TJ, Hamdy FC. Prostate Testing for Cancer and Treatment (ProtecT) Study: statistical analysis plan, version 1.0. Bristol, United Kingdom: University of Bristol; 2015. pp. 1–22. [Google Scholar]
- 14.Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA. 2013;309:814–22. doi: 10.1001/jama.2013.879. [DOI] [PubMed] [Google Scholar]
- 15.Lane A, Metcalfe C, Young GJ, et al. Patient-reported outcomes in the ProtecT randomized trial of clinically localized prostate cancer treatments: study design, and baseline urinary, bowel and sexual function and quality of life. BJU Int. 2016 Aug 17; doi: 10.1111/bju.13582. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn. 2004;23:322–30. doi: 10.1002/nau.20041. [DOI] [PubMed] [Google Scholar]
- 17.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the Expanded Prostate Cancer Index Composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 18.Donovan JL, Peters TJ, Abrams P, Brookes ST, de aa Rosette JJ, Schäfer W. Scoring the short form ICSmaleSF questionnaire. J Urol. 2000;164:1948–55. [PubMed] [Google Scholar]
- 19.Gandek B, Ware JE, Aaronson NK, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51:1171–8. doi: 10.1016/s0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 20.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 21.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 22.Male sexual dysfunction. Arnheim, the Netherlands: European Association of Urology; https://uroweb.org/guideline/male-sexual-dysfunction/ [Google Scholar]
- 23.Prostate cancer: diagnosis and management. London: National Institute for Health and Care Excellence; ( https://www.nice.org.uk/guidance/cg175/resources/prostate-cancer-diagnosis-and-management-35109753913285) [PubMed] [Google Scholar]
- 24.Urinary incontinence. Arnheim, the Netherlands: European Association of Urology; ( https://uroweb.org/guideline/urinary-incontinence/#4) [Google Scholar]
- 25.Erectile dysfunction. Linthicum, MD: American Urological Association; ( https://www.auanet.org/education/guidelines/erectile-dysfunction.cfm) [Google Scholar]
- 26.Rubin DB. Inference and missing data. Biometrika. 1976;63:581–92. [Google Scholar]
- 27.Martin NE, Massey L, Stowell C, et al. Defining a standard set of patient-centered outcomes for men with localized prostate cancer. Eur Urol. 2015;67:460–7. doi: 10.1016/j.eururo.2014.08.075. [DOI] [PubMed] [Google Scholar]
- 28.MacLennan S, Bekema HJ, Williamson PR, et al. A core outcome set for localised prostate cancer effectiveness trials: protocol for a systematic review of the literature and stakeholder involvement through interviews and a Delphi survey. Trials. 2015;16:76. doi: 10.1186/s13063-015-0598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Resnick MJ, Koyama T, Fan K-H, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368:436–45. doi: 10.1056/NEJMoa1209978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bill-Axelson A, Garmo H, Holmberg L, et al. Long-term distress after radical prostatectomy versus watchful waiting in prostate cancer: a longitudinal study from the Scandinavian Prostate Cancer Group-4 randomized clinical trial. Eur Urol. 2013;64:920–8. doi: 10.1016/j.eururo.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 31.Wei JT, Dunn RL, Sandler HM, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002;20:557–66. doi: 10.1200/JCO.2002.20.2.557. [DOI] [PubMed] [Google Scholar]
- 32.Parker PA, Davis JW, Latini DM, et al. Relationship between illness uncertainty, anxiety, fear of progression and quality of life in men with favourable-risk prostate cancer undergoing active surveillance. BJU Int. 2016;117:469–77. doi: 10.1111/bju.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buckley BS, Lapitan MC, Glazener CM. The effect of urinary incontinence on health utility and health-related quality of life in men following prostate surgery. Neurourol Urodyn. 2012;31:465–9. doi: 10.1002/nau.21231. [DOI] [PubMed] [Google Scholar]
- 34.Tewari A, Sooriakumaran P, Bloch DA, Seshadri-Kreaden U, Hebert AE, Wiklund P. Positive surgical margin and peri-operative complication rates of primary surgical treatments for prostate cancer: a systematic review and meta-analysis comparing retropubic, laparoscopic, and robotic prostatectomy. Eur Urol. 2012;62:1–15. doi: 10.1016/j.eururo.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 35.Yaxley JW, Coughlin GD, Chambers SK, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet. 2016;388:1057–66. doi: 10.1016/S0140-6736(16)30592-X. [DOI] [PubMed] [Google Scholar]
- 36.Barry MJ, Gallagher PM, Skinner JS, Fowler FJ., Jr Adverse effects of robotic-assisted laparoscopic versus open retropubic radical prostatectomy among a nationwide random sample of Medicare-age men. J Clin Oncol. 2012;30:513–8. doi: 10.1200/JCO.2011.36.8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prostate cancer: diagnosis and management. London: National Institute for Health and Care Excellence; 2014. ( https://www.nice.org.uk/guidance/cg175) [PubMed] [Google Scholar]
- 38.Simpkin AJ, Tilling K, Martin RM, et al. Systematic review and meta-analysis of factors determining change to radical treatment in active surveillance for localized prostate cancer. Eur Urol. 2015;67:993–1005. doi: 10.1016/j.eururo.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Donovan JL, Lane JA, Peters TJ, et al. Development of a complex intervention improved randomization and informed consent in a randomized controlled trial. J Clin Epidemiol. 2009;62:29–36. doi: 10.1016/j.jclinepi.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Lane JA, Wade J, Down L, et al. A Peer Review Intervention for Monitoring and Evaluating sites (PRIME) that improved randomized controlled trial conduct and performance. J Clin Epidemiol. 2011;64:628–36. doi: 10.1016/j.jclinepi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Turner EL, Metcalfe C, Donovan JL, et al. Design and preliminary recruitment results of the Cluster randomised triAl of PSA testing for Prostate cancer (CAP) Br J Cancer. 2014;110:2829–36. doi: 10.1038/bjc.2014.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams N, Hughes LJ, Turner EL, et al. Prostate-specific antigen testing rates remain low in UK general practice: a cross-sectional study in six English cities. BJU Int. 2011;108:1402–8. doi: 10.1111/j.1464-410X.2011.10163.x. [DOI] [PubMed] [Google Scholar]
- 43.Andriole GL, Crawford ED, Grubb RL, III, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.