Abstract
Objective:
To evaluate the evidence for EEG theta/beta power ratio for diagnosing, or helping to diagnose, attention-deficit/hyperactivity disorder (ADHD).
Methods:
We identified relevant studies and classified them using American Academy of Neurology criteria.
Results:
Two Class I studies assessing the ability of EEG theta/beta power ratio and EEG frontal beta power to identify patients with ADHD correctly identified 166 of 185 participants. Both studies evaluated theta/beta power ratio and frontal beta power in suspected ADHD or in syndromes typically included in an ADHD differential diagnosis. A bivariate model combining the diagnostic studies shows that the combination of EEG frontal beta power and theta/beta power ratio has relatively high sensitivity and specificity but is insufficiently accurate.
Conclusions:
It is unknown whether a combination of standard clinical examination and EEG theta/beta power ratio increases diagnostic certainty of ADHD compared with clinical examination alone.
Recommendations:
Level B: Clinicians should inform patients with suspected ADHD and their families that the combination of EEG theta/beta power ratio and frontal beta power should not replace a standard clinical evaluation. There is a risk for significant harm to patients from ADHD misdiagnosis because of the unacceptably high false-positive diagnostic rate of EEG theta/beta power ratio and frontal beta power. Level R: Clinicians should inform patients with suspected ADHD and their families that the EEG theta/beta power ratio should not be used to confirm an ADHD diagnosis or to support further testing after a clinical evaluation, unless such diagnostic assessments occur in a research setting.
In 2013, the US Food and Drug Administration (FDA) approved the Neuropsychiatric EEG-Based ADHD Assessment Aid (NEBA), stating that the NEBA “uses the theta/beta ratio of the EEG measured at electrode CZ on a patient 6–17 years of age combined with a clinician's evaluation to aid in the diagnosis of ADHD” and “should only be used by a clinician as confirmatory support for a completed clinical evaluation or as support for the clinician's decision to pursue further testing following a clinical evaluation.”1 The FDA further stated that “(t)he device is NOT to be used as a stand-alone in the evaluation or diagnosis of ADHD.” The NEBA calculates the ratio of the power of the EEG theta and beta bands at Cz, which is the EEG electrode halfway between the inion and the nasion.
Individuals with attention-deficit/hyperactivity disorder (ADHD) demonstrate a persistent pattern of inattention or hyperactivity/impulsivity, or both, that interferes with functioning and is inappropriate for developmental age. ADHD is typically diagnosed with a clinical examination. Appendix e-1 at Neurology.org presents the DSM-5 clinical criteria used for ADHD diagnosis.2 The American Academy of Pediatrics clinical practice guideline for the diagnosis, evaluation, and treatment of ADHD in children and adolescents provides a strong recommendation that when assessing a patient for ADHD, the primary care physician should determine that DSM criteria have been met, including documentation of impairment in more than one major setting.3 The use of validated ADHD instruments completed by parents, teachers, and adolescents is encouraged, as these instruments can be helpful in obtaining the information required to make a diagnosis of ADHD. For example, the Conners 3 ADHD Index has parent-, teacher-, and youth-completed measures that are based on a large normative sample and can discriminate children with ADHD from nonclinical populations with a sensitivity of 92% and a specificity of 94% (for the parent-completed measure).4 The use of information from multiple sources and multiple informants, particularly teachers, can enhance diagnostic certainty.5 Other than a comprehensive physical and neurologic examination, no additional laboratory or diagnostic tests are routinely performed.
Recent reviews on this topic suggested that more research is needed before the NEBA can be used as a clinical diagnostic tool.6,7 The purpose of this practice advisory is to examine the published evidence to determine whether quantitative EEG measures have utility in the diagnosis of ADHD and to make practice recommendations based on the evidence.
Therefore, we sought to answer the following questions:
For patients with ADHD, does the combination of a clinical examination and an examination of the EEG theta/beta power ratio increase diagnostic certainty compared with clinical examination alone?
For patients with a possible but uncertain diagnosis of ADHD, how accurately does the EEG theta/beta power ratio identify patients with ADHD compared with a clinical examination?
DESCRIPTION OF THE ANALYTIC PROCESS
In October 2013, the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology (AAN) convened a panel of experts to develop this practice advisory (appendices e-2 and e-3). The panel followed the methods described in the 2011 edition of the AAN's guideline development process manual, as amended, including the process for developing practice advisories.8
We performed a comprehensive literature search of the MEDLINE, Embase, and Science Citation Index databases, without time constraints, using the keywords “ADHD,” “EEG,” “theta/beta ratio,” and their associated variants. Appendix e-4 presents the complete search strategy. The search yielded 959 abstracts, and each abstract was reviewed for relevance by 2 panel members working independently of each other. Articles were considered for inclusion if they (1) examined the theta/beta power ratio in patients with ADHD and (2) could address either of the clinical questions. Articles were excluded if they (1) enrolled fewer than 10 participants, which would have resulted in too great a risk of bias; (2) were rated as Class IV by AAN criteria (including reviews and meta-analyses; see appendix e-5 for classification of evidence scheme for diagnostic articles); or (3) studied ADHD as determined by clinical examination criteria other than those2 from the DSM-5 and its earlier variants. An additional criterion for exclusion was information not published in the peer-reviewed literature, with one exception. Data were provided to the FDA for the NEBA, a portion of which we obtained in self-published form.9 Additional information about the trial was found in the de novo FDA application.1 In this practice advisory, we include in our assessment both the published data and the data from the de novo FDA application.10
After reviewing the selected full-text articles, we classified each according to the AAN's evidence-based methodology (appendix e-5). Our confidence in the evidence was determined by factors derived from the AAN's modified Grading of Recommendations Assessment, Development and Evaluation criteria (see appendices e-6 and e-7) approach.8 We based the strength of the recommendations on results from a modified Delphi process to determine the weight of several factors, including the evidence rating, cost considerations, risks, and feasibility (appendix e-8).
ANALYSIS OF EVIDENCE
Question.
For patients with ADHD, does the combination of a clinical examination and an examination of the EEG theta/beta power ratio increase diagnostic certainty compared with clinical examination alone?
Evidence.
A single Class III study addressed this question.10 The study was rated Class III because it made clear in only 77% of study cases that diagnostic testing was performed. To be included in the study, participants had to stop taking all psychiatric medications or all pharmacologic and nonpharmacologic psychoactive medications, or both psychiatric and psychoactive medications, in order to be evaluated for ADHD. The comparative intervention was clinical examination by a multidisciplinary team of experts, with data available from 4 clinical visits. At the first visit, a clinician judged the probability of a diagnosis of ADHD. The EEG theta/beta power ratio was used to augment the clinician's judgment when the clinician did not rule out the diagnosis of ADHD. The accuracy of a clinician's judgment and the EEG theta/beta power ratio was 88% (95% confidence interval 84%–91%).
Conclusion.
It is unknown whether a combination of standard clinical examination and EEG theta/beta power ratio increases the diagnostic certainty of ADHD compared with clinical examination alone (1 Class III study).
Clinical context.
A single Class III study results in the ranking of very low confidence in the evidence. Such a ranking leads to a U or R recommendation level after the modified Delphi process is applied, because the data resulted from too high a risk of bias, regardless of whether the study in question is positive or negative.
Question.
For patients with a possible but uncertain diagnosis of ADHD, how accurately does the EEG theta/beta power ratio identify patients with ADHD compared with a clinical evaluation?
Evidence.
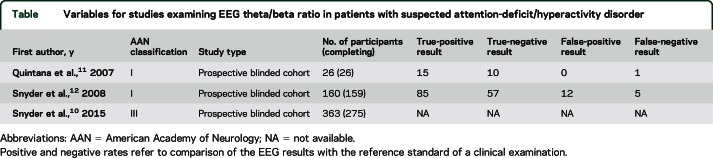
A total of 32 articles addressed this question; 30 were rated as Class IV and 2 were rated as Class I (table).11,12 The first Class I study was a prospective blinded cohort study examining 26 children and adolescents who presented with a suspected diagnosis of ADHD.11 The study appropriately enrolled people whose conditions could be mistaken diagnostically for ADHD, and thus avoided spectrum bias. Two quantitative EEG criteria were used for determining an ADHD diagnosis: frontal beta power and theta/beta power ratio. Frontal beta power was calculated from 6 EEG sites, using a diagnostic cutoff of 2 SDs. The theta/beta power ratio was calculated at Cz using a definition of theta as the frequency 4–7.5 Hz and beta as 13–20.5 Hz, using a diagnostic cutoff of 1.5 SDs. This cutoff was established a priori in normal controls in previous work. Twenty-five of the 26 were correctly classified according to the EEG compared with the psychiatric evaluation; 1 child who had ADHD according to the clinical evaluation was not classified correctly. This child had comorbid conduct disorder. Of note, none of the EEG ADHD diagnoses was made with the frontal beta power; all were made on the basis of excessive theta/beta power ratio.
Table.
Variables for studies examining EEG theta/beta ratio in patients with suspected attention-deficit/hyperactivity disorder
The other Class I prospective blinded cohort study used a similar methodology. This study enrolled 160 participants with a possible ADHD diagnosis, of which 159 participants were evaluated.12 One participant was excluded because data for the psychiatric evaluation were incomplete. Of the sample of 159, 97 were diagnosed with ADHD by clinical evaluation; of these, 84 were diagnosed with ADHD by theta/beta power ratio and 1 by frontal beta power. Although not indicated in the article, the same numbers for false-positive EEG evaluations can be calculated from the overall accuracy of 89%: of the 62 who were not diagnosed with ADHD by clinical examination, 5 were incorrectly given an ADHD diagnosis by EEG, yielding a false-positive rate of 8%.
The 2 Class I studies were combined using a bivariate model for diagnostic studies and RevMan 5.3 software (Cochrane Collaboration, Copenhagen, Denmark). See appendix e-6 for the combined effects.
Conclusion.
Because of the combined estimate of medical errors in the United States of 5%, which was considered an unacceptably high rate of errors,13 the false-positive rate of >5% was considered an unacceptably high false-positive rate. EEG frontal power and theta/beta ratio is not an effective diagnostic test for ADHD because of this unacceptably high false-positive rate (2 Class I studies).11,12
Clinical context.
In examining these 2 studies in aggregate, one risks identifying participants as having ADHD when they do not have the diagnosis. In the studies, 19 of the 185 participants were misidentified. The accuracy rate for this test is too low for it to supplant the standard clinical evaluation.2,3 Because of a positive cutoff of the theta/beta power ratio of 1.5 SDs above the mean, it seems unlikely that this test will achieve a higher accuracy rate without a different approach.
Theta activity is increased by drowsiness and medication effects and is increased in many neurologic disorders. Theta power is known to be a highly nonspecific feature of EEGs. Likewise, there are many reasons (other than ADHD) why frontal beta power values may be higher or lower than average in certain individuals. These values also change with the patient's state of awareness, so values may differ when a patient is retested just minutes after the previous testing.
There is low diagnostic certainty for replacing a standard clinical evaluation with a measurement of EEG theta/beta power ratio because of the lack of generalizability of the 2 Class I studies.11,12 One study used a medication washout period of more than 3 days,12 a timeframe that may have mixed acceptance in a clinical setting. The studies also excluded participants on more than one medication and failed to assess for sleep deprivation. Without data to establish the EEG theta/beta power ratios for conditions that could be confused with ADHD, it is impossible to distinguish patients with ADHD from those with conditions that mimic ADHD.
PRACTICE RECOMMENDATIONS
Rationale.
Diagnosis with clinical examination and EEG testing.
The evidence for the utility of EEG theta/beta power ratio to augment a clinician's judgment when he or she is diagnosing possible ADHD is not strong enough to make a recommendation. A test must have a demonstrated advantage over the existing common clinical practice to supersede that practice. A research study is the proper setting in which to demonstrate that the current clinical practice of using a clinical examination in evaluation for ADHD can be improved on.
Recommendation.
Clinicians should inform patients with suspected ADHD and their families that the EEG theta/beta power should not be used to confirm an ADHD diagnosis or to support further testing after a clinical evaluation, unless such diagnostic assessments take place within the limits of a research study (Level R).
Note: Level R recommendations are ones that “the guideline authors assert should be applied only in research settings.”8
Rationale.
Accuracy of EEG theta/beta power ratio.
We downgraded our confidence in the evidence to moderate because of significant problems with generalizability (see appendix e-6). Physicians pledge to do no harm when they take the Hippocratic Oath. There is a risk for significant harm to people misdiagnosed with ADHD because of an unacceptably high false-positive EEG result. Because of this risk of harm, the combination of theta/beta power ratio and frontal beta power should not be used in place of a standard clinical examination.
Recommendations.
Clinicians should inform patients with suspected ADHD and their families that the combination of EEG theta/beta power ratio and frontal beta power should not replace a standard clinical evaluation (Level B). There is a risk for significant harm to patients of being misdiagnosed with ADHD because of the unacceptably high false-positive diagnostic rate of EEG theta/beta power ratio and frontal beta power (Level B).
RECOMMENDATIONS FOR FUTURE RESEARCH
With regard to the current data, there does not seem to be a clinical use for EEG theta/beta power or EEG frontal power in the evaluation for ADHD; EEG theta/beta power or EEG frontal power should not supplant the clinical examination, nor should it be used to augment the clinical examination. The use of the EEG theta/beta power ratio or EEG frontal beta power to diagnose ADHD requires greater knowledge of these features in the other disorders in the differential diagnosis of ADHD, the test–retest reliability of the measurements, the effects of active reference electrodes, and medication effects, among other factors.
Additional research is required to understand why children with ADHD have high theta/beta power values at Cz. One prior report, which has not been replicated, identified positive spikes as a normal variant in children with ADHD,14 which may explain the presence of increased theta/beta power ratios in these children.
The parent of a child with some ADHD symptoms presenting for evaluation sometimes comes in with the clinical question “Does my child have ADHD?” However, the more frequently encountered clinical question may be “What disorder does my child have?” The differential diagnosis may include depression, anxiety, learning disabilities, and behavioral disorders such as oppositional defiant disorder.15–17 It is not known whether these disorders are also associated with theta/beta power ratios that mimic the reported findings in children with ADHD. The authors of the Class III study relevant to our first clinical question did not provide information about the final diagnosis of the children who were not diagnosed with ADHD, and thus it is not apparent whether all the possible parts of the differential diagnosis were included in the study.10 This makes the issue of generalizability an even greater concern. In the future, a well-designed study could address this issue.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Gary Gronseth, MD, for discussions about this practice advisory.
GLOSSARY
- AAN
American Academy of Neurology
- ADHD
attention-deficit/hyperactivity disorder
- DSM-5
Diagnostic and Statistical Manual of Mental Disorders, 5th edition
- FDA
Food and Drug Administration
- NEBA
Neuropsychiatric EEG-Based ADHD Assessment Aid
Footnotes
Supplemental data at Neurology.org
Editorial, page 2288
AUTHOR CONTRIBUTIONS
Dr. David Gloss: study concept and design, acquisition of data, analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content, study supervision. Dr. Jay K. Varma: acquisition of data, analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. Dr. Tamara Pringsheim: interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. Dr. Marc R. Nuwer: analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content.
STUDY FUNDING
This practice advisory was developed with financial support from the American Academy of Neurology. Authors who serve as AAN subcommittee members or methodologists (D.G., T.P.) were reimbursed by the AAN for expenses related to travel to subcommittee meetings where drafts of manuscripts were reviewed.
DISCLOSURE
D. Gloss is an evidence-based medicine consultant for the American Academy of Neurology. J. Varma reports no disclosures relevant to the manuscript. T. Pringsheim has received a research grant from Shire Canada. M. Nuwer has served as an expert in legal proceedings related to QEEG. Go to Neurology.org for full disclosures.
DISCLAIMER
Clinical practice guidelines, practice advisories, systematic reviews, and other guidance published by the American Academy of Neurology and its affiliates are assessments of current scientific and clinical information provided as an educational service. The information: (1) should not be considered inclusive of all proper treatments, methods of care, or as a statement of the standard of care; (2) is not continually updated and may not reflect the most recent evidence (new evidence may emerge between the time information is developed and when it is published or read); (3) addresses only the question(s) specifically identified; (4) does not mandate any particular course of medical care; and (5) is not intended to substitute for the independent professional judgment of the treating provider, as the information does not account for individual variation among patients. In all cases, the selected course of action should be considered by the treating provider in the context of treating the individual patient. Use of the information is voluntary. AAN provides this information on an “as is” basis, and makes no warranty, expressed or implied, regarding the information. AAN specifically disclaims any warranties of merchantability or fitness for a particular use or purpose. AAN assumes no responsibility for any injury or damage to persons or property arising out of or related to any use of this information or for any errors or omissions.
CONFLICT OF INTEREST
The American Academy of Neurology is committed to producing independent, critical, and truthful practice advisories. Significant efforts are made to minimize the potential for conflicts of interest to influence the recommendations of this practice advisory. To the extent possible, the AAN keeps separate those who have a financial stake in the success or failure of the products appraised in the practice advisories and the developers of these documents. Conflict of interest forms were obtained from all authors and reviewed by an oversight committee prior to project initiation. AAN limits the participation of authors with substantial conflicts of interest. The AAN forbids commercial participation in, or funding of, practice advisory projects. Drafts of the practice advisory have been reviewed by at least 3 AAN committees, a network of neurologists, Neurology® peer reviewers, and representatives from related fields. The AAN Guideline Author Conflict of Interest Policy can be viewed at aan.com. For complete information on this process, access the 2011 AAN process manual.8
REFERENCES
- 1.US Department of Health and Human Services. FDA approval letter for NEBA System. Available at: accessdata.fda.gov/cdrh_docs/pdf11/K112711.pdf. Accessed November 20, 2014.
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 3.Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 2011;128:1007–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collett BR, Ohan JL, Myers KM. Ten-year review of rating scales: V: scales assessing attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2003;42:1015–1037. [DOI] [PubMed] [Google Scholar]
- 5.Tripp G, Schaughency EA, Clarke B. Parent and teacher rating scales in the evaluation of attention-deficit hyperactivity disorder: contribution to diagnosis and differential diagnosis in clinically referred children. J Dev Behav Pediatr 2006;27:209–218. [DOI] [PubMed] [Google Scholar]
- 6.Loo SK, Makeig S. Clinical utility of EEG in attention-deficit/hyperactivity disorder: a research update. Neurotherapeutics 2012;9:569–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arns M, Loo SK, Sterman MB, et al. . Editorial Perspective: how should child psychologists and psychiatrists interpret FDA device approval? Caveat emptor. J Child Psychol Psychiatr 2016;57:656–658. [DOI] [PubMed] [Google Scholar]
- 8.American Academy of Neurology. Clinical Practice Guideline Process Manual. 2011 ed. St. Paul, MN: The American Academy of Neurology. Available at: aan.com/Guidelines/Home/Development. Accessed August 15, 2013. [Google Scholar]
- 9.Summary of NEBA Clinical Investigation: Key Results. Available at: nebahealth.com/content/MRK0027_form_for_clinician%20-%20key%20results%20summary_20130722__(2).pdf. Accessed October 21, 2013.
- 10.Snyder SM, Rugino TA, Hornig M, Stein MA. Integration of an EEG biomarker with a clinician's ADHD evaluation. Brain Behav 2015;5:e00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quintana H, Snyder SM, Purnell W, Aponte C, Sita J. Comparison of a standard psychiatric evaluation to rating scales and EEG in the differential diagnosis of attention-deficit/hyperactivity disorder. Psychiatry Res 2007;152:211–222. [DOI] [PubMed] [Google Scholar]
- 12.Snyder SM, Quintana H, Sexson SB, Knott P, Haque AF, Reynolds DA. Blinded, multi-center validation of EEG and rating scales in identifying ADHD within a clinical sample. Psychiatry Res 2008;159:346–358. [DOI] [PubMed] [Google Scholar]
- 13.Singh H, Meyer AND, Thomas EJ. The frequency of diagnostic errors in outpatient care: estimations from three large observational studies involving US adult populations. BMJ Qual Saf 2014;23:727–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes JR, DeLeo AJ, Melyn MA. The electroencephalogram in attention deficit-hyperactivity disorder: emphasis on epileptiform discharges. Epilepsy Behav 2000;1:271–277. [DOI] [PubMed] [Google Scholar]
- 15.Kernberg OF, Yeomans FE. Borderline personality disorder, bipolar disorder, depression, attention deficit/hyperactivity disorder, and narcissistic personality disorder: practical differential diagnosis. Bull Menninger Clin 2013;77:1–22. [DOI] [PubMed] [Google Scholar]
- 16.Connor DF, Doerfler LA. ADHD with comorbid oppositional defiant disorder or conduct disorder: discrete or nondistinct disruptive behavior disorders? J Atten Disord 2008;12:126–134. [DOI] [PubMed] [Google Scholar]
- 17.American Academy of Pediatrics. Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. Pediatrics 2000;105:1158–1170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.