Abstract
Objective:
To determine the independent effects of aphasia on outcomes during acute stroke admission, controlling for total NIH Stroke Scale (NIHSS) scores and loss of consciousness.
Methods:
Data from the Tulane Stroke Registry were used from July 2008 to December 2014 for patient demographics, NIHSS scores, length of stay (LOS), complications (sepsis, deep vein thrombosis), and discharge modified Rankin Scale (mRS) score. Aphasia was defined as a score >1 on question 9 on the NIHSS on admission and hemiparesis as >1 on questions 5 or 6.
Results:
Among 1,847 patients, 866 (46%) had aphasia on admission. Adjusting for NIHSS score and inpatient complications, those with aphasia had a 1.22 day longer LOS than those without aphasia, whereas those with hemiparesis (n = 1,225) did not have any increased LOS compared to those without hemiparesis. Those with aphasia had greater odds of having a complication (odds ratio [OR] 1.44, confidence interval [CI] 1.07–1.93, p = 0.0174) than those without aphasia, which was equivalent to those having hemiparesis (OR 1.47, CI 1.09–1.99, p = 0.0137). Controlling for NIHSS scores, aphasia patients had higher odds of discharge mRS 3–6 (OR 1.42 vs 1.15).
Conclusion:
Aphasia is independently associated with increased LOS and complications during the acute stroke admission, adding $2.16 billion annually to US acute stroke care. The presence of aphasia was more likely to produce a poor functional outcome than hemiparesis. These data suggest that further research is necessary to determine whether establishing adaptive communication skills can mitigate its consequences in the acute stroke setting.
Stroke is the leading cause of disability in the United States.1 Among its most devastating manifestations is aphasia, affecting 21%–38% of acute stroke patients,2 of which 80% arise from an ischemic event.3 Poststroke aphasia has higher attributable costs of care after discharge compared to stroke without aphasia,4 and results in a higher rate of stroke recurrence, believed to be related to failure to understand treatment regimens or to communicate symptoms.5–7 Little is known, however, about how aphasia affects outcomes during the acute stroke admission. The purpose of this study was to determine the association of aphasia on outcomes during the acute stroke admission, derived from a large database from a comprehensive stroke center. We hypothesized that aphasia would have an independent effect on acute stroke outcomes, controlling for NIH Stroke Scale (NIHSS) severity and vascular risk factors. We also sought to compare the effect of aphasia with that of hemiparesis during this clinical period.
METHODS
Study population.
A retrospective analysis of prospectively collected data of all patients with acute ischemic stroke or intracerebral hemorrhage (ICH) who presented to a single TJC Comprehensive Stroke Center between July 2008 and December 2014 was performed using previously described methods.8 The prospectively collected registry includes demographic variables, baseline clinical, laboratory, medication, and imaging variables, as well as follow-up clinical, laboratory, medication, and imaging variables on all patients admitted with a stroke.8 Patients with a TIA or a subarachnoid hemorrhage were excluded.
Standard protocol approvals, registrations, and patient consents.
The Tulane University Medical Center Institutional Review Board approved this study, with waiver of informed consent.
Exposure and outcome definitions.
The NIHSS9 examinations were administered to each patient on admission (baseline) by a certified NIHSS examiner.10 Aphasia was defined as having a score of 1 or greater on admission NIHSS question 9. Hemiparesis was defined as having a score of 1 or greater on admission NIHSS question 5 or 6. Consciousness was derived from question 1a. Outcomes of interest included inpatient complications, length of stay (LOS), and poor functional outcome at discharge. Inpatient complications were defined as the patient having a recurrent stroke, myocardial infarction, pulmonary embolus, deep vein thrombosis, sepsis, hospital-acquired infection, systemic bleeding, ventriculitis, or neuroworsening. Poor functional outcome at the time of discharge was defined as discharge modified Rankin Scale (mRS) score 3–6. mRS was documented by a vascular neurologist certified in mRS.11
Statistical analysis.
We compared admission information between patients who had aphasia on admission and those who did not have aphasia on admission. Pearson χ2 (or Fisher exact test where appropriate) was used to compare proportions. The Wilcoxon rank sum test was used to compare medians of non-normally distributed continuous data, while the t test was used to compare normally distributed continuous data. Crude and adjusted nonparametric linear regression was used to investigate the relationship between aphasia and LOS. Crude and adjusted logistic regression models were used to estimate the odds ratios (OR) and 95% confidence intervals (CI) for the relationship between aphasia and poor functional outcome at discharge. A subanalysis was conducted assessing the relationship among dose response of the aphasia score, hemiparesis score, and outcomes.
A mediation analysis using the Sobel test was performed to assess the complex relationships among aphasia on admission, development of an inpatient complication, LOS, and poor functional outcome at discharge. Mediation occurred when (1) the independent variable (aphasia on admission) significantly affected the mediator (inpatient complication), (2) the independent variable (aphasia on admission) significantly affected the dependent variable (LOS or poor functional outcome at discharge) in the absence of the mediator (inpatient complication), (3) the mediator (inpatient complication) had a significant unique effect on the dependent variable (LOS or poor functional outcome at discharge), and (4) the independent variable (aphasia on admission) remained associated with the dependent variable (LOS or poor functional outcome at discharge) when the mediator was added to the model.12,13 The Sobel test, which is capable of handling dichotomous mediators and outcomes, was used to test whether the indirect effect of ICH score on poor functional outcome at discharge through the mediator (inpatient complication) was significantly different from zero. An α of 0.05 was used as the level of significance.
RESULTS
Baseline demographics.
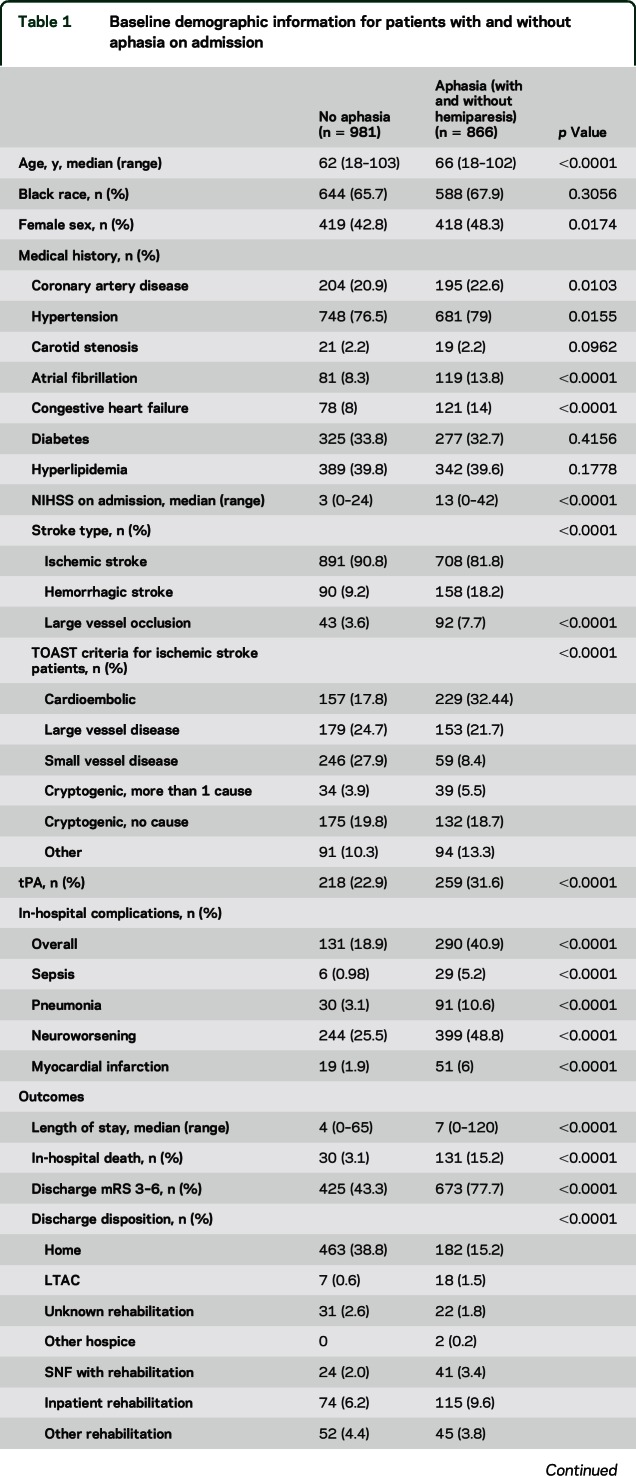
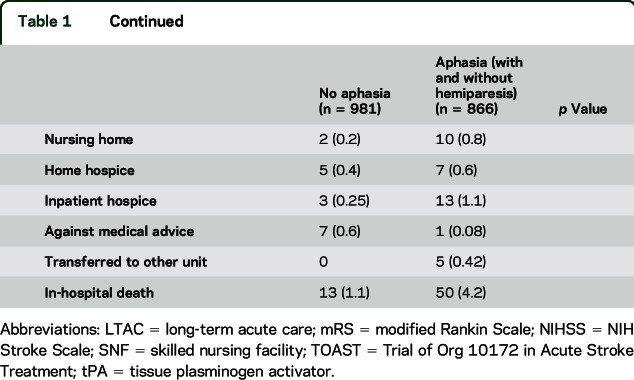
Among 1,847 patients included in this study, 866 (46%) had aphasia on admission and 1,225 (64.2%) had hemiparesis on admission. Of these patients, 526 (28.5%) had only hemiparesis, 168 (9.1%) had only aphasia, and 698 (37.8%) had both aphasia and hemiparesis. A total of 708 (44.3%) ischemic stroke patients presented with aphasia on admission, while 158 ICH patients presented with aphasia on admission (63.7%). Those with aphasia had a median age of 66 (18–102), compared to those without aphasia, with a median age of 62 (18–100). A total of 48.3% of those with aphasia were female, while 42.8% of those without aphasia were female (p = 0.0174). The median admitting NIHSS score of those with aphasia only was 5 (range 1–18), the median admitting NIHSS of those with only hemiparesis was 6 (range 1–22), the median admitting NIHSS of those with both hemiparesis and aphasia was 16 (1–42), while the median admitting NIHSS without aphasia or hemiparesis was 2 (range 0–11) (p < 0.0001). Patients with aphasia had a higher proportion of congestive heart failure and atrial fibrillation (table 1). Of those patients who presented with aphasia, 80% also had hemiparesis on admission.
Table 1.
Baseline demographic information for patients with and without aphasia on admission
Inpatient complications.
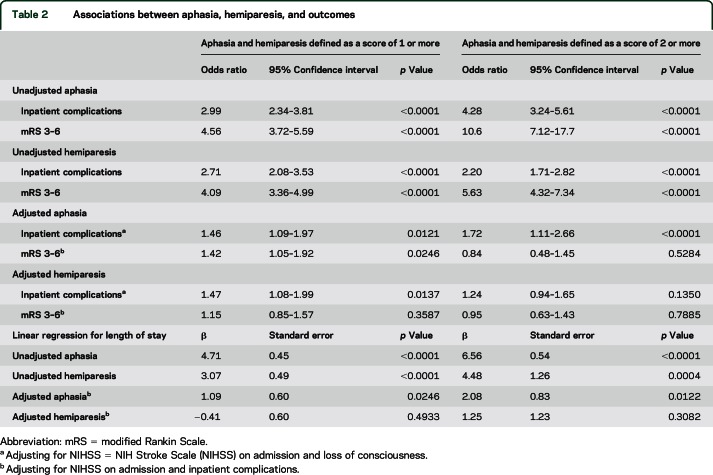
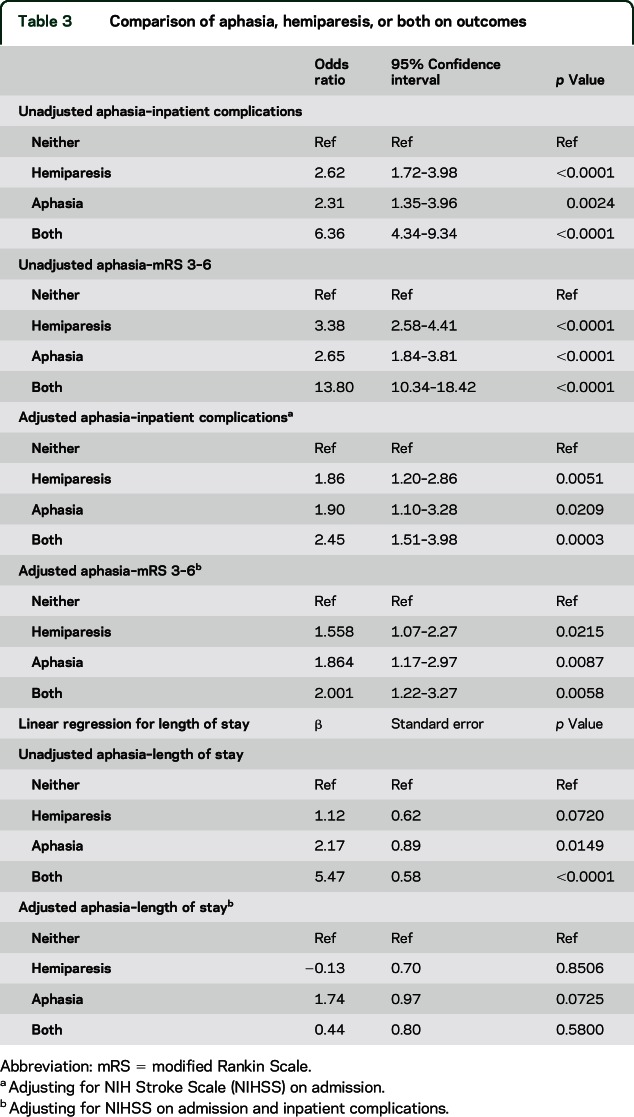
As shown in table 2, patients with aphasia had a higher odds of inpatient complications (OR 2.99, 95% CI 2.34–3.81, p < 0.0001), and this association remained after adjusting for admission NIHSS and loss of consciousness (OR 1.46, 95% CI 1.09–1.97, p = 0.0121) when aphasia is defined as a score of 1 or more. When adjusting for coronary artery disease, female sex, atrial fibrillation, hypertension, and congestive heart failure in the mediation model, 53% of the relationship between aphasia and discharge mRS 3–6 is explained through inpatient complications, indicating that regardless of these prior comorbidities, aphasia is strongly linked to outcomes through inpatient complications. The relationship between aphasia and inpatient complications is similar to the relationship between hemiparesis and inpatient complications in both the unadjusted and adjusted models (adjusted OR 1.47, 95% CI 1.08–1.99, p = 0.0137) when hemiparesis is defined as a score of 1 or more. These relationships were the same regardless of defining aphasia or hemiparesis as a score of 1 or more or a score of 2 or more (table 2). We looked at the relationship between aphasia, hemiparesis, or both with inpatient complications (table 3); after adjustment, patients with both aphasia and hemiparesis have a higher odds of inpatient complications (OR 2.45, 95% CI 1.51–3.98, p = 0.0003). Patients with hemiparesis (OR 1.86, 95% CI 1.20–2.86, p = 0.0051) or aphasia (OR 1.90, 95% CI 1.10–3.28, p = 0.0209) have similarly increased odds of inpatient complications in adjusted models.
Table 2.
Associations between aphasia, hemiparesis, and outcomes
Table 3.
Comparison of aphasia, hemiparesis, or both on outcomes
Length of stay.
In unadjusted analyses, aphasia and hemiparesis are both statistically significantly related to LOS (table 2). Accounting for NIHSS at baseline, inpatient complications, and loss of consciousness, patients with aphasia (defined as a score of 1 or more) stayed an average of 1.22 days longer than those without aphasia (β = 1.22, SE 0.61, p = 0.0450). In a mediation analysis among aphasia (defined as a score of 1 or more), inpatient complications, and LOS, 77% of the relationship between aphasia and LOS is explained through inpatient complications (Sobel 2.36, p = 0.018). Controlling for NIHSS score at baseline and inpatient complications, patients with hemiparesis (defined as a score of 1 or more) did not stay longer than those without hemiparesis (β = −0.41, SE 0.598, p = 0.4933). These relationships were the same regardless of defining aphasia or hemiparesis as a score of 1 or more or a score of 2 or more (table 2).
Looking at the relationship between aphasia, hemiparesis, or both with LOS (table 3), we found in the unadjusted analyses that patients with both aphasia and hemiparesis have increased LOS (β 5.47, SE 0.58, p < 0.0001). Hemiparesis alone, however, was not related to LOS in the unadjusted model (β 1.12, SE 0.62, p = 0.0702), while patients with aphasia alone have an increased LOS in the unadjusted models (β 2.17, SE 0.89, p = 0.0149). In the adjusted models, the relationship between hemiparesis and LOS was no longer significant.
Discharge disposition.
Both aphasia and hemiparesis were related to discharge mRS in the unadjusted analyses with a higher odds of poor functional outcome (table 2). Accounting for NIHSS score at baseline and inpatient complications, patients with aphasia (defined as a score of 1 or more) were at higher odds of discharge mRS 3–6 than those without aphasia (OR 1.42, 95% CI 1.05–1.92, p = 0.0246). In a mediation analysis among aphasia (defined as a score of 1 or more), inpatient complications, and discharge mRS 3–6, 57% of the relationship between aphasia and discharge mRS 3–6 is explained through inpatient complications (Sobel 2.28, p = 0.022). Accounting for NIHSS score at baseline and inpatient complications, patients with hemiparesis (defined as a score of 1 or more) were not at higher odds for discharge mRS 3–6 (OR 1.15, 95% CI 0.85–1.57, p = 0.3587). These relationships were the same regardless of defining aphasia or hemiparesis as a score of 1 or more or a score of 2 or more (table 2).
Table 3 shows the relationship between aphasia, hemiparesis, or both with poor functional outcome (table 3); after adjustment, patients with both aphasia and hemiparesis have higher odds of inpatient complications (OR 2.00, 95% CI 1.22–3.27, p = 0.0583). Patients with hemiparesis (OR 1.56, 95% CI 1.07–2.27, p = 0.0215) or aphasia (OR 1.86, 95% CI 1.17–2.97, p = 0.0087) have a similarly increased odds of inpatient complications in adjusted models with aphasia patients being at slightly higher odds.
Subanalysis of aphasia score.
The majority of aphasia patients had a score of 1 on item 9 of the NIHSS scale (418, 22.6% of all patients), followed by a score of 2 (285, 15.4% of all patients), with the smallest proportion having a score of 3 (163, 8.8% of all patients). In unadjusted analyses, there appeared to be a dose response of aphasia score with outcomes, in which the odds of inpatient complications, poor functional outcome, and LOS increases as the aphasia score increases (table e-1 at Neurology.org). However, once the models are adjusted for covariates of interest, the dose response no longer remains (table e-1).
The NIHSS items evaluating aphasia and hemiparesis are not comparable in the assignment of severity ratings, so that a hemiparesis score of 1 might be a mild drift while aphasia score 1 corresponds to a mild to moderate aphasia, making aphasia look worse than hemiparesis in its predictive value. To address this discrepancy, we also examined the dose response for hemiparesis severity on outcomes. Table e-1 shows that in adjusted analyses, the odds of inpatient complications did not reach significance until an NIHSS score of 3 (no effort against gravity), and that no degree of hemiparetic severity on the NIHSS was predictive of increased LOS or mRS 3–6.
DISCUSSION
We found that aphasia is independently associated with increased complications and LOS during the acute stroke admission, with an effect comparable to hemiparesis, and sometimes greater. These data demonstrate the underrecognized consequences of a communication disorder during the acute stroke hospitalization.
An important finding was that aphasia was an independent factor associated with 1.22 more days of hospitalization than in patients without aphasia, even after controlling for NIHSS score, whereas the presence vs absence of hemiparesis was not related to LOS. Others have reported increased LOS among those with aphasia, but one study used billing codes and a nonstandardized and a nonvalidated proxy for aphasia, which likely resulted in underreporting (12%) of cases.4 In another study, patients who were discharged within 7 days after admission with aphasia were excluded.14 One potential reason for the longer stay lies in the requirement for admission to an acute rehabilitation unit in the United States under Medicare. Among the determinants for medical necessity is the requirement that a patient must need either physical or occupational therapy.15 Thus, a patient with a dense Wernicke aphasia but few physical abnormalities has difficulty getting a rehabilitation placement. In our cohort, however, 80% of the patients with aphasia also had hemiparesis.
Our mediation analysis suggests that complications are one driving force behind the need to stay longer in the hospital. The overall complication rate was more than double among patients with aphasia than those without aphasia, and held for each of the 4 specific complications in our analysis (sepsis, pneumonia, neuroworsening, myocardial infarction). Patients with aphasia in our cohort were older and therefore more likely to have comorbidities, as has been shown in prior studies.4 In our group, they had higher rates of atrial fibrillation and congestive heart disease, which are among the most common etiologies of embolic stroke,16 and increasing the likelihood of higher cortical syndromes such as aphasia17 and unilateral neglect.18 When adjusting for medical issues, however, the mediation model showed that regardless of prior comorbidities, aphasia is strongly linked to outcomes through inpatient complications. It has been shown that difficulty in cognition impedes poststroke recovery in the longer term,7 and so it is plausible that an inability to communicate complaints such as the presence or worsening of physical symptoms and to understand instructions could contribute to increased rates of complications.
In contrast to the 21%–38% aphasia rates most commonly reported among stroke admissions, 46% of our cases were found have aphasia based on the administration of the NIHSS and excluding any patients with an alteration of consciousness. The embolic stroke in this population has been reported as high. The reasons for our higher aphasia rates are not clear, and clearly exceed the rates identified by comparable instruments, such the Scandinavian Stroke Scale in other studies.19 The basis for diagnosing aphasia has varied across studies, ranging from the use of Medicare charge codes to the administration of fine-grained instruments, such as a non-English adaptation of the Western Aphasia Battery.20 It has also been found in other disease contexts that using NIHSS-certified examiners, rather than non-neurologists who were not so certified, has resulted in much higher rates of detection of neurologic events than previously reported.21 Nevertheless, our cohort had comparable rates of higher incidence of aphasia among older patients, women, and those with systemic conditions that produce cardioembolic stroke.
There were several limitations to this study. First, the presence and severity of aphasia were based on the administration of the NIHSS. We did not have a delineation of aphasia subtypes, which would have been determined with a more refined aphasia assessment instrument. We therefore could not ascertain the extent to which disorders of receptive or expressive language contributed to our overall findings. It has been shown, for example, that patients with deficits in auditory and reading comprehension have increased odds of being discharged to a setting other than home,22 and are associated with more severe basal neurologic and functional status at admission to a rehabilitation setting.23 One advantage of using a comprehensive neurologic tool is that it becomes possible to control for confounding factors, such as consciousness in our case, and other stroke manifestations that could result in longer LOS. A limitation of comparing aphasia and hemiparesis on the NIHSS, or any other scale, is that ratings of severity (e.g., 1–3) may not be equivalent in terms of impairment and disability. In terms of NIHSS score, a score of 3 on the aphasia item results in a score of 2 on item 10 (articulation) when patients have no speech or a score of 2 on item 1b when comprehension is poor, inflating the overall score when aphasia is severe. To account for the lack of comparability between the aphasia and hemiparesis items, we controlled for severity score. When investigating the dose response of hemiparesis and aphasia on inpatient complications, functional outcome at discharge, and LOS, it becomes apparent that a patient needed to have a more severe hemiparesis to have an independent effect on these outcomes after adjusting for covariates, whereas a milder aphasia has a stronger independent effect on these outcomes after adjusting for covariates. We also do not have information on whether patients received any aphasia therapy during their acute stroke hospitalization, although it is not currently the standard care in the United States, nor is there a recommendation for an aphasia assessment in current American Heart Association/American Stroke Association guidelines for acute stroke evaluation and treatment.24 The costs associated with the increased LOS were based on US-based estimates, which may not be representative for other countries. We also did not have mRS scores on patients before admission prior to 2011. The strengths of this study included one of the largest sample sizes from a single institution to date, and NIHSS-certified neurologists with maintained credentialing who made the aphasia assessment.
Overall, our study showed that the presence (vs absence) of aphasia is associated with more complications and increased LOS, which significantly add to the burden of the health care system. The disability arising from acute aphasia is comparable to that of hemiparesis. A major implication of the extended stay lies in the increased cost of care. Assuming a 30% aphasia rate among the estimated 795,000 new US stroke cases each year,1 and the $9,100 cost per day of an ischemic stroke admission (not accounting for the additional cost of hemorrhagic stroke),25 then the presence of aphasia adds $2.16 billion annually to the care of these patients. Although addressing underlying disease remains the major objective during the acute stroke admission, the data suggest that greater attention is needed on developing adaptive communication skills during the acute stroke admission that could improve patient outcomes.
Supplementary Material
GLOSSARY
- CI
confidence interval
- LOS
length of stay
- mRS
modified Rankin Scale
- NIHSS
NIH Stroke Scale
- OR
odds ratio
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Amelia Boehme conducted the data analysis, interpreted the data, and wrote a portion of the manuscript. Sheryl Martin-Schild provided the data, participated in data interpretation, and provided critical revisions of the manuscript. Randolph Marshall participated in data interpretation and provided critical revisions of the manuscript. Ronald Lazar designed the study, interpreted the data, wrote a portion of the manuscript, and oversaw all elements of this study.
STUDY FUNDING
Dr. Boehme is supported by NINDS NIH T32 NS007153-31. Drs. Lazar and Marshall are supported in part by the Levine Family Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIH.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics: 2016 update: a report from the American Heart Association. Circulation 2016;133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 2.Elman RJ, Bernstein-Ellis E. The efficacy of group communication treatment in adults with chronic aphasia. J Speech Lang Hear Res 1999;42:411–419. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen PM, Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Aphasia in acute stroke: incidence, determinants, and recovery. Ann Neurol 1995;38:659–666. [DOI] [PubMed] [Google Scholar]
- 4.Ellis C, Simpson AN, Bonilha H, Mauldin PD, Simpson KN. The one-year attributable cost of poststroke aphasia. Stroke 2012;43:1429–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berthier ML. Poststroke aphasia: epidemiology, pathophysiology and treatment. Drugs Aging 2005;22:163–182. [DOI] [PubMed] [Google Scholar]
- 6.Galski T, Bruno RL, Zorowitz R, Walker J. Predicting length of stay, functional outcome, and aftercare in the rehabilitation of stroke patients: the dominant role of higher-order cognition. Stroke 1993;24:1794–1800. [DOI] [PubMed] [Google Scholar]
- 7.Sacco RL, Foulkes MA, Mohr JP, Wolf PA, Hier DB, Price TR. Determinants of early recurrence of cerebral infarction: The Stroke Data Bank. Stroke 1989;20:983–989. [DOI] [PubMed] [Google Scholar]
- 8.Siegler JEBA, Dorsey AM, Monlezun D, et al. A comprehensive stroke center patient registry: advantages, limitations, and lessons learned. Med Stud Res J 2013;2:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brott T, Adams HP Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–870. [DOI] [PubMed] [Google Scholar]
- 10.Lyden P, Raman R, Liu L, et al. NIHSS training and certification using a new digital video disk is reliable. Stroke 2005;36:2446–2449. [DOI] [PubMed] [Google Scholar]
- 11.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–607. [DOI] [PubMed] [Google Scholar]
- 12.DP M, JH D. Estimating mediated effects in prevention studies. Eval Rev 1993;17:144–158. [Google Scholar]
- 13.Mackinnon DP, Warsi G, Dwyer JH. A simulation study of mediated effect measures. Multivariate Behav Res 1995;30:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inatomi Y, Yonehara T, Omiya S, Hashimoto Y, Hirano T, Uchino M. Aphasia during the acute phase in ischemic stroke. Cerebrovasc Dis 2008;25:316–323. [DOI] [PubMed] [Google Scholar]
- 15.Medicare CFM. Inpatient Rehabilitation Therapy Services: Complying With Documentation Requirements [online]. Available at: https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/Inpatient_Rehab_Fact_Sheet_ICN905643.pdf. Accessed March 24, 2016. [Google Scholar]
- 16.Wessler BS, Kent DM. Controversies in cardioembolic stroke. Curr Treat Options Cardiovasc Med 2015;17:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engelter ST, Gostynski M, Papa S, et al. Epidemiology of aphasia attributable to first ischemic stroke: incidence, severity, fluency, etiology, and thrombolysis. Stroke 2006;37:1379–1384. [DOI] [PubMed] [Google Scholar]
- 18.Gottesman RF, Kleinman JT, Davis C, et al. Unilateral neglect is more severe and common in older patients with right hemispheric stroke. Neurology 2008;71:1439–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsouli S, Kyritsis AP, Tsagalis G, Virvidaki E, Vemmos KN. Significance of aphasia after first-ever acute stroke: impact on early and late outcomes. Neuroepidemiology 2009;33:96–102. [DOI] [PubMed] [Google Scholar]
- 20.Laska AC, Hellblom A, Murray V, Kahan T, Von Arbin M. Aphasia in acute stroke and relation to outcome. J Intern Med 2001;249:413–422. [DOI] [PubMed] [Google Scholar]
- 21.Messe SR, Acker MA, Kasner SE, et al. Stroke after aortic valve surgery: results from a prospective cohort. Circulation 2014;129:2253–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Fernandez M, Christian AB, Davis C, Hillis AE. Role of aphasia in discharge location after stroke. Arch Phys Med Rehabil 2013;94:851–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paolucci S, Matano A, Bragoni M, et al. Rehabilitation of left brain-damaged ischemic stroke patients: the role of comprehension language deficits: a matched comparison. Cerebrovasc Dis 2005;20:400–406. [DOI] [PubMed] [Google Scholar]
- 24.Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:2064–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russo CA, Andrews RM. Hospital stays for stroke and other cerebrovascular diseases, 2005: statistical brief #51. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Healthcare Cost and Utilization Project; 2006. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.