Abstract
Environmental pollution with heavy metals is a serious issue worldwide posing threats to humans, animals and plants and to the stability of overall ecosystem. Chromium (Cr) is one of most hazardous heavy metals with a high carcinogenic and recalcitrant nature. Aim of the present study was to select low-cost biosorbent using wheat straw and Eupatorium adenophorum through simple carbonization process, capable of removing Cr (VI) efficiently from wastewater. From studied plants a low cost adsorbent was prepared for removing Cr (VI) from aqueous solution following very simple carbonization method excluding activation process. Several factors such as pH, contact time, sorbent dosage and temperature were investigated for attaining ideal condition. For analysis of adsorption equilibrium isotherm data, Langmuir, Freundlich and Temkin models were used while pseudo-first-order, pseudo-second-order, external diffusion and intra-particle diffusion models were used for the analysis of kinetic data. The obtained results revealed that 99.9% of Cr (VI) removal was observed in the solution with a pH of 1.0. Among all the tested models Langmuir model fitted more closely according to the data obtained. Increase in adsorption capacity was observed with increasing temperature revealing endothermic nature of Cr (VI). The maximum Cr (VI) adsorption potential of E. adenophorum and wheat straw was 89.22 mg per 1 gram adsorbent at 308K. Kinetic data of absorption precisely followed pseudo-second-order model. Present study revealed highest potential of E. adenophorum and wheat straw for producing low cost adsorbent and to remove Cr (VI) from contaminated water.
Introduction
Heavy metals contamination is a growing issue affecting living organisms throughout the world [1–2]. Due to rapid industrial development, direct and indirect discharges of heavy metals to the environment through wastewater have tremendously been increased [2]. In industrial wastewater treatment chromium, copper, cadmium, lead, zinc and nickel are considered more toxic and receiving more attention of researchers [3].
Chromium (Cr) is a harmful heavy metal and exists in various oxidative forms; however, the trivalent and hexavalent states are considered more essential in term of environmental pollution point of view [4]. Cr (VI) is considered more harmful and toxic due to its high carcinogenic and resistant properties than Cr (III) [5]. There are varied Cr contamination sources such as electroplating, leather tanning, textile industries, metal finishing, nuclear power plants, and chromate preparation [6]. In many countries, concentration of Cr (VI) is severely restricted and as per Environmental Protection Agency (EPA) guidelines the maximum permitted limit for Cr (VI) in inland surface, potable and industrial wastewaters are 0.1, 0.05, and 0.25 mg L−1, respectively. Over ingestion of Cr (VI) beyond the permissible limits can cause severe gastric damage, liver, kidney and lung cancer and other health related complications [7–9]. So there is a dire need of significant management of wastewater contaminated by Cr (VI) before its release.
Different conventional methods such as chemical precipitation, filtration, chemical oxidation and reduction, reverse osmosis, evaporation techniques, electrochemical and ion exchange methods are generally recommended for Cr (VI) removal from the environment [10]. However, these methods are cost effective and generate a variety of secondary pollutants [11]. Adsorption method is known to be the most promising technique and is considered as economically and operationally very effective for removing heavy metals from contaminated water, especially with the application of low-cost and high efficiency adsorbents. Many adsorbents like rice hull [12], henna leaves, chufa corm peels, peel of banana, wheat bran, etc [13–16] been testified very effective for Cr (VI) removal. However, these adsorbents have relatively low adsorption capacity to Cr (VI), therefore, it is necessary to search for highly abundant naturally available, low cost and greater adsorption capacity adsorbent in order to alleviate Cr (VI) ions from contaminated water. Wheat (Triticum aestivum) is one of the chief cash crops around the world and approximately 0.6 billion tons of wheat straw produced annually. In addition to fuel and food importance, huge amount of wheat straw is burnt in the field, which not only wastes a potential resource, but also pollutes the environment [17]. Wheat straw is rich in cellulose, hemicellulose, linen and sugars which can be used as complexion chelation with heavy metal ions [18] E. adenophorum (Crofton weed), is a harmful invasive plant species originated from Central America and currently invaded many countries of Southeast Asia [19]. E. adenophorum invaded a varied kind of habitats such as road sides, disturbed grasslands, abandoned fields and agricultural fields posing great loss to economics and biodiversity [20]. Utilizing E. adenophorum as a raw material for adsorbent production could be a useful measure not only for controlling its invasion process but will also utilize it as an economical natural resource. Activated carbon made from E. adenophorum has already been reported in recent years [21] but the cost is very high and use of chemical drugs during its activation stage also produce new contaminations, which somewhat limit its application as a new adsorbent.
The aim of the present study is to design an adsorbent of low cost wheat straw and E. adenophorum through simple carbonization process, which can efficiently absorb and remove Cr (VI) from wastewater. The adsorbent prepared from wheat straw and E. adenophorum was studied by optimized technological parameters and effects of different parameters such as adsorbent dosage, contact time, pH and solution temperature were investigated using a batch process. Absorption capacity rate and the mechanism of Cr adsorption were measured with the help of various equilibrium isotherm and kinetic models. This study would provide baseline information to researchers about using the abundantly available low-cost adsorbent that could be helpful in reducing the problem of Cr (IV) polluted water across the world.
Materials and Methods
Ethics statement
In present investigation E. adenophorum and wheat straw were collected from Xichang city, Sichuan Province, southwestern China. No specific permission was required for the collection of these species because of its high abundance in the area, not endangered or protected species and not under any authority responsible for national park or protected areas of land or sea. Therefore, there is no restriction or need of permission for harvesting these species for research activities in Xichang city.
Preparation of the adsorbent
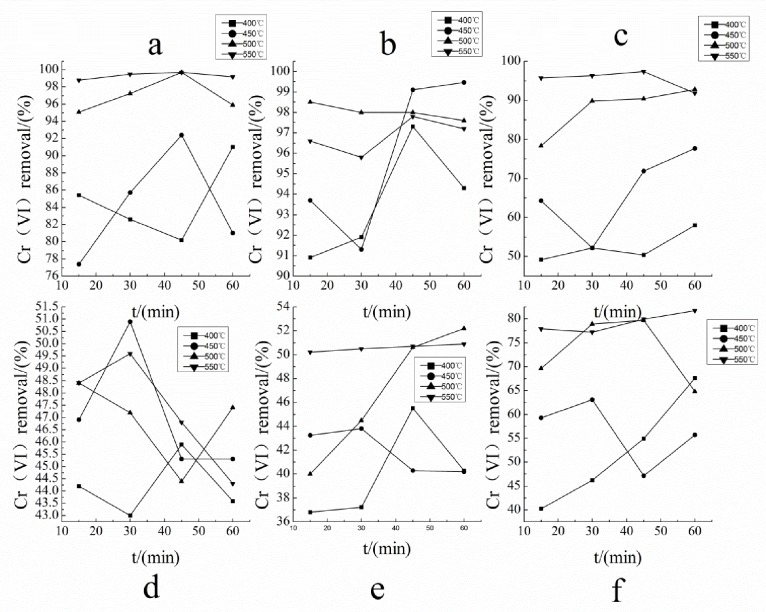
The raw materials (stems) were dried at 378 K then ground in a mill and finally passed through (-35, +60) meshes. E. adenophorum stem and wheat straw powders were mixed in different combinations as follows: (a) E. adenophorum (50%) + wheat straw (50%), (b) E. adenophorum (33%) + wheat straw (67%), (c) E. adenophorum (100%) + wheat straw (0%), (d) E. adenophorum (25%) + wheat straw (75%), (e) E. adenophorum (67%) + wheat straw (33%), and (f) E. adenophorum (0%) + wheat straw (100%). These combinations were carbonized at different temperatures (i.e., 673, 723, 773 and 823 K) for different times (i.e., 15, 30, 45 and 60 min) and these samples were then used as adsorbent.
Reagents and Equipments
For preparing Cr (VI) (45 mg/L) stock solution, 0.1415 ± 0.0001g of the analytical grade potassium dichromate (K2Cr2O7) (Chengdu, China) was dissolved in deionized water. The stock solution was further diluted for the preparation of working solutions. Digital pH (PHS-25, Shanghai, China) meter was used for measuring pH. For samples igitation electric thermostatic reciprocating shaker (SHZ-88, Jintan, China) was used. The content of chromium in standard and treated solutions was determined using a visible spectrophotometer (Model 722N, Shanghai, China). E. adenophorum and wheat were carbonized with the help of an electric furnace (Model TMF-4-13, Shanghai, China). In aqueous solution particular concentration of Cr (VI) was determined with the help of spectrophotometer using 1, 5-Diphenylcarbazide reagent (GB 7467–87, China), which specifically measures Cr (VI). Colorimetric method using diphenylcarbazide reagent (GB7466-87, China) was used for the measurement of total Cr.
Batch adsorption studies
For the investigation of solution temperature, contact time, adsorbent dosage, and pH batch studies were carried out. Designated adsorbents were added into 150 ml conical flask with a stopper, containing 100 ml of test solution and then carried out the batch adsorption at the desired contact time, pH and adsorbent dose. Solutions with varied initial concentrations of Cr (VI) were prepared using standard stock solution dilution of 45 mg/L Cr (VI). pH adjustment was done with 0.1 mol/L NaOH and 0.1 mol/L HCl. After each batch, the solution was filtered and the filtrate was analyzed for remnant Cr (VI) concentration in the sample.
Prepared biosorbent (0.3g) added to 100 mL of Cr (VI) solution (45 mg/L) in a conical flask with a stopper. The conical flask was shaken in electric thermostatic reciprocating shaker for 3 h at 298 K with the frequency of 150rpm. The removal percentage of Cr (VI) was calculated based on the measured value of Ci and Ce concentrations. The removal percentage of Cr (VI) by the adsorbent at different periods and temperatures were measured and calculated.
The removal percentage of Cr (VI) was calculated as follows:
Where %R indicates the removal percentage of metals by biosorbent, C stands for final concentration and C0 is the initial concentration of metal in the solution (mg/L).
The adsorbed amount of Cr (VI) in the equilibrium was determined with the help of following equation;
Where qe is Cr (VI) quantity adsorbed by per gram of adsorbent (mg/g); V indicates volume of the solution (L), Ci and Ce (mg/L) are the initial and equilibrium metal concentrations respectively, and m is the weight (g) of adsorbent.
Adsorption kinetic studies
The kinetic tests were also carried out with same technique as in the equilibrium experiments. In the adsorption kinetic study, pseudo-first-order, pseudo-second-order, intra-particle diffusion and external film diffusion models were followed in order to estimate the adsorption rates and possible reaction mechanisms. The solutions were shaken for 3 h at a frequency of 150 r/min. During the course of experiment, samples were periodically taken from each solution at fixed time intervals (10, 20, 30, 40, 50 and 60 mins).
The pseudo-first-order kinetic model was expressed as:
The pseudo-second-order kinetic model was described as:
Where qt and qe are Cr (VI) quantity adsorbed (mg/g) at specific adsorption time and at equilibrium, respectively. k1, k2 are pseudo-first and second-order adsorption rate constants (per minute) respectively.
External film diffusion [22] and intra-particle diffusion [23] models were followed for the investigation of rate controlling step in the process of adsorption. These two equations are defined as follows:
Where ki (mg/g min0.5) and kfd (1/min) represents intra-particle diffusion and liquid film diffusion models rate constants, respectively.
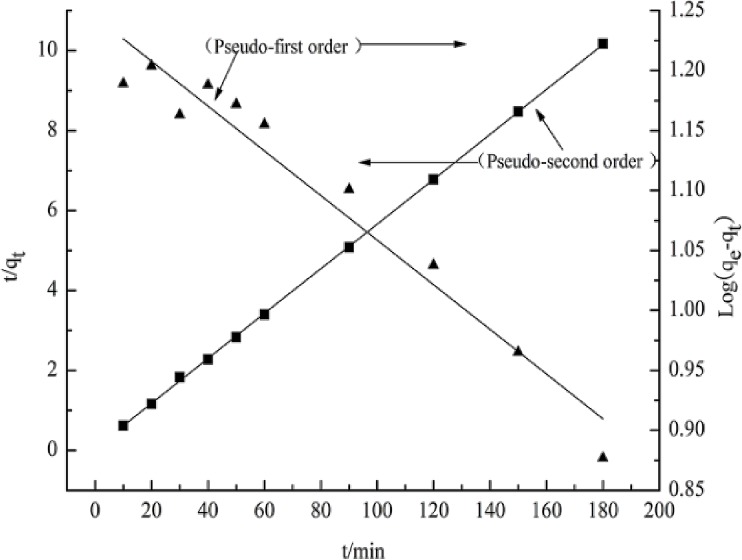
Cr (VI) adsorption kinetics was measured from the curves of time against qe (mg/g). Linearized form of pseudo-first-order model for Cr (VI) ions adsorption onto wheat straw and E. adenophorum at various adsorption times (min) at 298 K. Conformation between model predicted values and experimental results were stated by correlation coefficient (R2). Adsorption density qe and rate constant k1 or k2 values were attained from the intercepts and slopes of the plot of log(Ce−qe) against t, respectively (Table 1). If intra-particle diffusion has major role in term of controlling kinetics of adsorption process, the plot of qt versus t0.5 produces straight line passing through the origin, and the slope gives the rate constant, ki. Similarly, if external film diffusion equation is appropriate, the plot of ln(1-qt/qe) against t should give a straight line with zero intercept, from which kfd can be measured from the slope of the plot (Table 1).
Table 1. Kinetic constant parameters obtained for Cr (VI) adsorption on biosorbent.
| pseudo–first- order | pseudo–second-order | |||||
|---|---|---|---|---|---|---|
| qe,exp/ (mg/g) | qe,cal1(mg/g) | k1(10−3)(min-1) | R2 | qe,cal2(mg/g) | k2(10−3)(min-1) | R2 |
| 17.7 | 17.579 | 4.14 | 0.9529 | 17.844 | 0.06128 | 0.9998 |
Adsorption isotherms experiments
Isothermal models like Freundlich, Langmuir and Temkin were considered for describing Cr (VI) uptake carbonized plant materials. The Temkin isotherm model suggested decrease in adsorption energy linearly with surface coverage due to interaction between adsorbent and adsorbate. Equilibrium isotherm was calculated at a temperature of 298, 303, and 308 K and agitation speed of 150 rpm. In 100 ml of solution total 0.25 g adsorbent was added with different concentrations of Cr (VI), i.e.0, 50, 75,100, 150, 200, 300 and 400 mg/ L. Solution pH was adjusted to 1.0 and the model parameters were determined from the following equations:
Where qe is Cr (VI) quantity adsorbed by per gram of adsorbent (mg/g); qm stands for highest adsorption capacity (mg/g); b indicates equilibrium constants; Ce is Cr (VI) (mg/L) equilibrium concentration; Kf is Freundlich isotherm constants; n is Freundlich constant; n were determined using lnqe versus Ce intercept and the slope of a plot of lnqe versus ln Ce (Table 2). If 1/n value were found less than 1, it designated a favorable adsorption.
Table 2. Parameters of different isotherms of adsorption.
| T/K | Langmuir | Freundlich | Temkin isotherm | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Qm (mg/g) | Kc | R2 | Kf | 1/n | R2 | bT (KJ/mol) | KT (L/gm) | R2 | |
| 298 | 56.91 | 0.069 | 0.9202 | 27.98 | 0.102 | 0.8783 | 0.7266 | 6.735 | 0.7251 |
| 303 | 64.39 | 0.591 | 0.9751 | 43.479 | 0.09 | 0.6967 | 0.5049 | 6.847 | 0.5861 |
| 308 | 88.57 | 0.801 | 0.9974 | 47.197 | 0.131 | 0.893 | 0.3976 | 4.679 | 0.9497 |
Also, the linear form of the Temkin isotherm model was described as follows [24]:
Where bT is the Temkin constant related to the heat of sorption (J/mol), KT represents equilibrium constant (L/g) binding, T indicates absolute temperature (K), and R is gas constant (8.314 × 10−3 kJ/mol K-1). The Temkin constants were calculated from the intercept and slope of a linear plot of qe vs lnC0 (Table 2).
Results and Discussion
Optimizing technological parameters for the preparation of adsorbent
The optimal technological parameters were selected according to the optimum removal percentage of Cr (VI). The carbonizations parameters corresponded to the highest removal ratio of each mixed materials are shown in Fig 1. The results showed that at a carbonization temperature of 773 K, carbonization time of 45 min, with the combination of materials of E. adenophorum (50%) and wheat straw (50%), the percentage removal of Cr (VI) was 99.7% (Fig 1A). All the experiments had three replications and mean value was used.
Fig 1. Optimizing technological parameters, initial Cr (VI) concentration 45 mg/L, pH 1.0.
Effect of pH on Cr (VI) adsorption
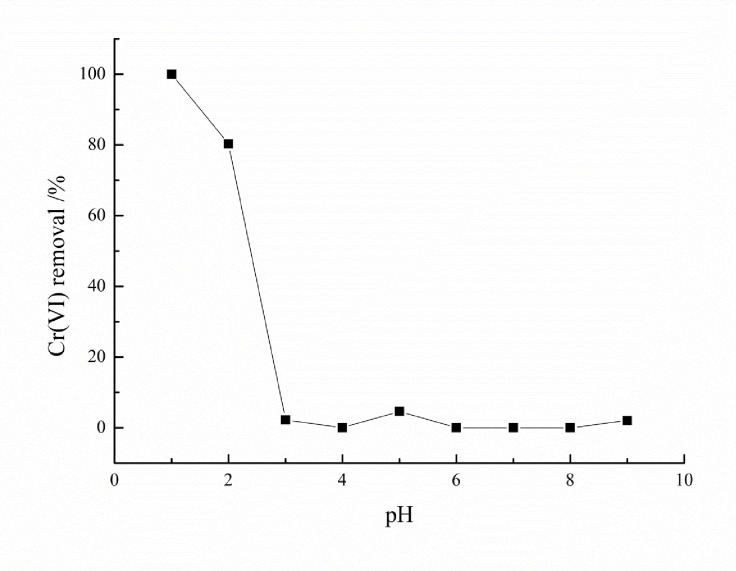
The obtained results revealed that pH strongly affected the % adsorption (Fig 2). The Cr (VI) removal percentage was found to decrease with an increase in pH. The removal efficiency maintained at 5% with little fluctuations within the pH range of 3 to 9. The Cr (VI) removal percentage reached to the maximum of 99.98% at a pH of 1. Previous studies on adsorption of heavy metals suggested pH is an essential factor affecting the efficiency of adsorption process [25], since it interferes with the solid–solution interface. It affects biomass active site charges and also influences behavior of metals in solution [26]. According to the solution pH, Cr (VI) mainly exists as: chromate (CrO42-), chromic acid (H2CrO4) hydrogen chromate (HCrO4-) or dichromate (Cr2O72-) [27–28]. Change in Cr (VI) adsorption with changing pH might be due to the fact that at low pH of solution the adsorbent can easily protonate and generate positive net charge. Thus, increase adsorption of Cr (VI) attained with decreased pH of the solution due to enhancement of electrostatic attraction [29]. A lot of positive charges (-COOH2+, -NH4+, -OH2+) were present and covered the entire adsorbent surface resulted in increase of contact area between Cr (VI) and adsorbent at pH between 1 and 2. As a result, more Cr (VI) was absorbed by the carbonized wheat straw and E. adenophorum. As pH increased, abundance of OH− ions on the adsorbent surface caused repulsive force between adsorbent and adsorbate in the solid–liquid phase, which resulted in a reduction of the adsorption [30]. Also, at pH 1, Cr mostly exists as HCrO4-, but with an increase in pH, HCrO4- shuffles to Cr2O72- and other forms such as CrO42-. Finally, as the pH is increased above 7.0, CrO42− would be the primary form [31]. These results clearly revealed that HCrO4- was preferentially adsorbed onto the biomass, thus inconsistent with previous findings [23, 32]. It can be speculated that HCrO4- preferably adsorbed on the adsorbent, however, the discrepancy observed may be due to the fact that the materials of the adsorbent in the present study were different from the earlier studies.
Fig 2. Effect of pH on Cr (VI) adsorption at initial Cr (VI) concentration of 45 mg/L, adsorbent dosage of 0.3 g/100mL, time 3 h and temperature 298 K.
Effect of adsorbent dosage on Cr (VI) adsorption
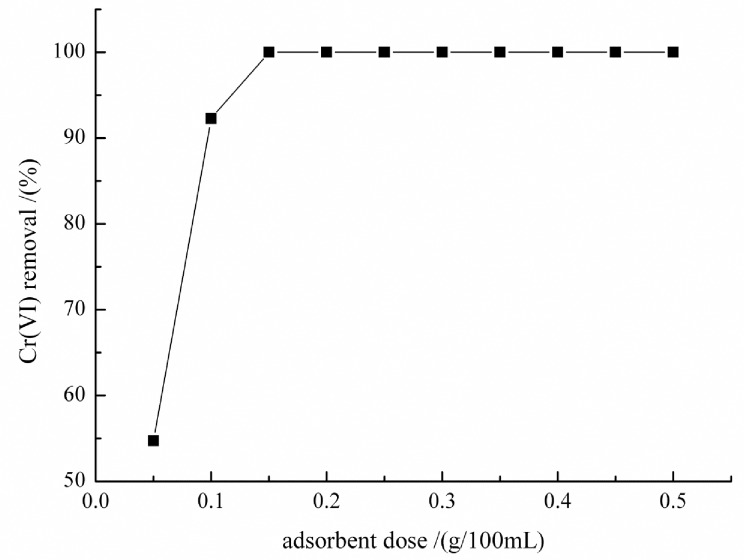
Increase in adsorbent amount from 0 to 0.15 g/100mL, Cr (VI) removal percentage increased rapidly and reached 99.9% at the dosage of 0.15 g/100mL (Fig 3). At a dosage of 0.25 g/100mL, the removal efficiency gradually reached adsorption equilibrium and there was no further increase in the adsorption of Cr with further increase in adsorbent dosage. Similar findings have also been reported in earlier studies [33–35].
Fig 3. Effect of adsorbent dosage on Cr (VI) adsorption at initial Cr (VI) concentration 45 mg/L, pH 1.0, time 3 h and temperature 298 K.
Adsorbent dosage is an important factor for the determination of adsorption potential of an adsorbent at a given initial concentration [36]. The variation in adsorption capacities at different dosage of adsorbents could be mainly attributed to the available sites of adsorption. As the adsorbent dosage increased, more active sites and surface area of the adsorbent became available for the adsorption. When the adsorption reached an equilibrium, increase in adsorbent dosage had no effect on the adsorbate uptake because it added unavailable sites [37]. Furthermore, although adsorbent dosage increased the interference of the adsorbent surface among the active groups, the equilibrium concentration of Cr (VI) was lower; because the driving force was too low for adsorb to diffuse into the adsorbent surface to be adsorbed. These findings are in accordance with previous studies [38].
Effect of the contact time and conformation changes of Cr (VI)
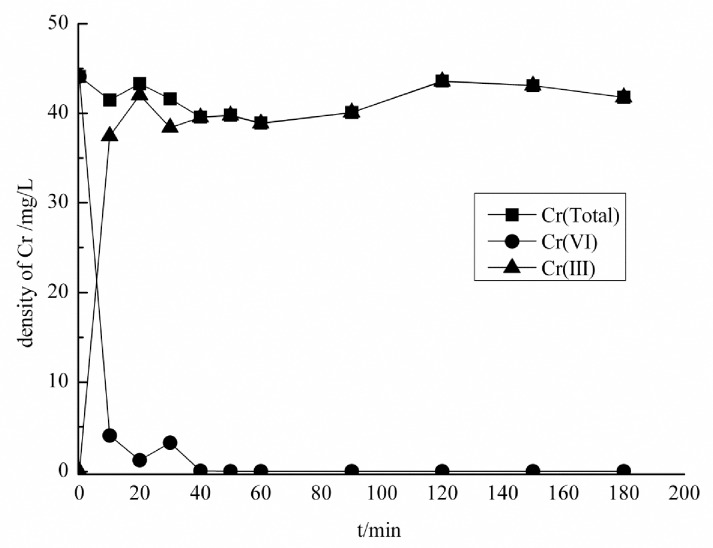
During 3 h adsorption process, the samples were withdrawn at 0, 10, 20, 30, 40, 50, 60, 90, 120, 150 180 mins intervals. The results of total chromium and Cr (VI) measured showed that Cr (VI) in the solutions decreased sharply with the extension of adsorption time, and reached adsorption equilibrium after 50 mins and was completely removed in the aqueous solution (Fig 4).
Fig 4. Effect of contact time on Cr (VI) adsorption, initial Cr (VI) concentration 45 mg/L, pH 1.0, adsorbent dosage 0.25 g/100mL and temperature 298 K.
The final concentration of Cr (III) persisted at 38.4mg/L after the complete removal of Cr (VI), suggesting that biomass adsorbed 6.6 mg/L of total Cr. As described in Fig 4 during first 20 min of adsorption process, total Cr first increased and then gradually decreased, Cr (VI) also decreased while Cr (III) generated. During 20 to 30 min, the decrease of total Cr and Cr (III) was almost equal while slight increase in Cr (VI) was observed. Moreover, from 30 to 50 min, the decrease of total Cr was less than that of Cr (VI), and Cr (III) in the solution found increase. After 50 min, the concentration of Cr (III), Cr (VI), and total Cr tended to be constant in the solution. The concentration of Cr in aqueous solution revealed that the implication of Cr (VI) removal mechanism of the adsorbent was its reduction into Cr (III). This mechanism consisted of three steps: (1) the binding of anionic Cr (VI) ions with the positively charged groups present on the biomass surface; (2) the reduction of Cr (VI) to Cr (III) by adjacent electron-donor groups; (3) the release of the Cr (III) ions into the aqueous phase due to electronic repulsion between the positively charged groups and the Cr (III) ions, or the formation of complexes of the Cr (III) with adjacent groups capable of Cr-binding. Present results are in line with previous studies conducted by Park et al. and Deng et al [39, 40]. These findings suggested that almost complete Cr (VI) was reduced spontaneously to Cr (III) as soon it was in contact with carbonized E. adenophorum and wheat straw comprising high quantity of reducing C and Cr (VI) has a high redox potential (above + 1.3 V under standard condition) [38, 41–42]. Extension in time of absorption could cost much for engineering application therefore, 50 mins is appropriate time of contact for equilibrium adsorption of Cr (VI) by wheat straw and E. adenophorum.
Adsorption kinetic studies
Table 1 shows values of k1, k2, measured adsorptive capacity, qe, cal and correlation coefficients, R2 obtained from the plots for adsorption of Cr (VI) on the wheat straw and E. adenophorum. Table 1 shows that the R2 values of pseudo-second-order model were nearly unity (0.999) for Cr (VI), indicating that the adsorption of Cr (VI) onto wheat straw and E. adenophorum fitted well with this model (Fig 5). Additionally, data obtained for equilibrium adsorption capacities (qe, cal) (17.84mg/g) from pseudo-second-order kinetic model fitted with the experimental values (qe, exp) (17.7mg/g). Therefore, this model well explained the adsorption kinetics, suggesting Cr (VI) process is a chemical behavior and in line with previous study of Chen et al. [43].
Fig 5. Pseudo–first-order and pseudo–second-order kinetics plots for the adsorption of Cr (VI) onto biosorbent, initial Cr (VI) concentration 45 mg/L, pH 1.0, adsorbent dosage 0.25 g/100mL and temperature 298 K.
The plots of ln(1-qt/qe) against t was linear and also with low intercepts (Table 1). Although, the intercepts values were smaller and not absolutely zero but it indicates that in adsorption process external film diffusion had major role [44]. Values of correlation coefficients and Intra-particle diffusion rate constant ki are given in Table 3. According to the present findings diffusion of Cr (VI) into the pores of adsorbent is not due to adsorption and kinetic studies indicates that diffusion into adsorbent pores is not a leading cause for monitoring adsorption process mechanisms.
Table 3. The intra-particle and liquid film diffusion rate constants for the Cr (VI) adsorption.
| Intra-particle diffusion | Liquid film diffusion | ||||
|---|---|---|---|---|---|
| ki(mg/(gmin0.5))×10 | c(mg/g) | R2 | kfd(1/min) ×10 | Intercept | R2 |
| 8.58 | 9.50449 | 0.4432 | 0.2125 | 2.98996 | 0.4223 |
Adsorption isotherms experiments
Temperature effect on Cr (VI) biosorption was mainly studied at 298K, 303K, and 308K at pH 1 with altered initial Cr (VI) concentration. Cr (VI) removal rate was improved with increase in temperature of the solution. Slope and intercept were used for the estimation of isotherm constants Fig 6(A) (Langmuir isotherm), Fig 6(B) (Freundlich isotherm) and Fig 6(C) (Temkin isotherm) and are presented in Table 1. Coefficient of determination (i.e. R2) value was recorded in range of 0.92 to 0.99 and comparatively higher for Langmuir isotherm than for the Temkin isotherm and Freundlich isotherm. High correlation was observed with Langmuir model within the studied temperature range. Present findings suggested that adsorption process was well representing with Langmuir equation. Therefore, development of monolayer coverage of adsorbate over surface of adsorbent was put forward. Value of maximum potential of sorbent (qm) was measured from Langmuir plots. Progressive increase from 56.91 to 88.57 mg/g was observed in maximum capacity of wheat straw and E. adenophorum for Cr (VI) with elevated temperature, suggesting endothermic adsorption process.
Fig 6.
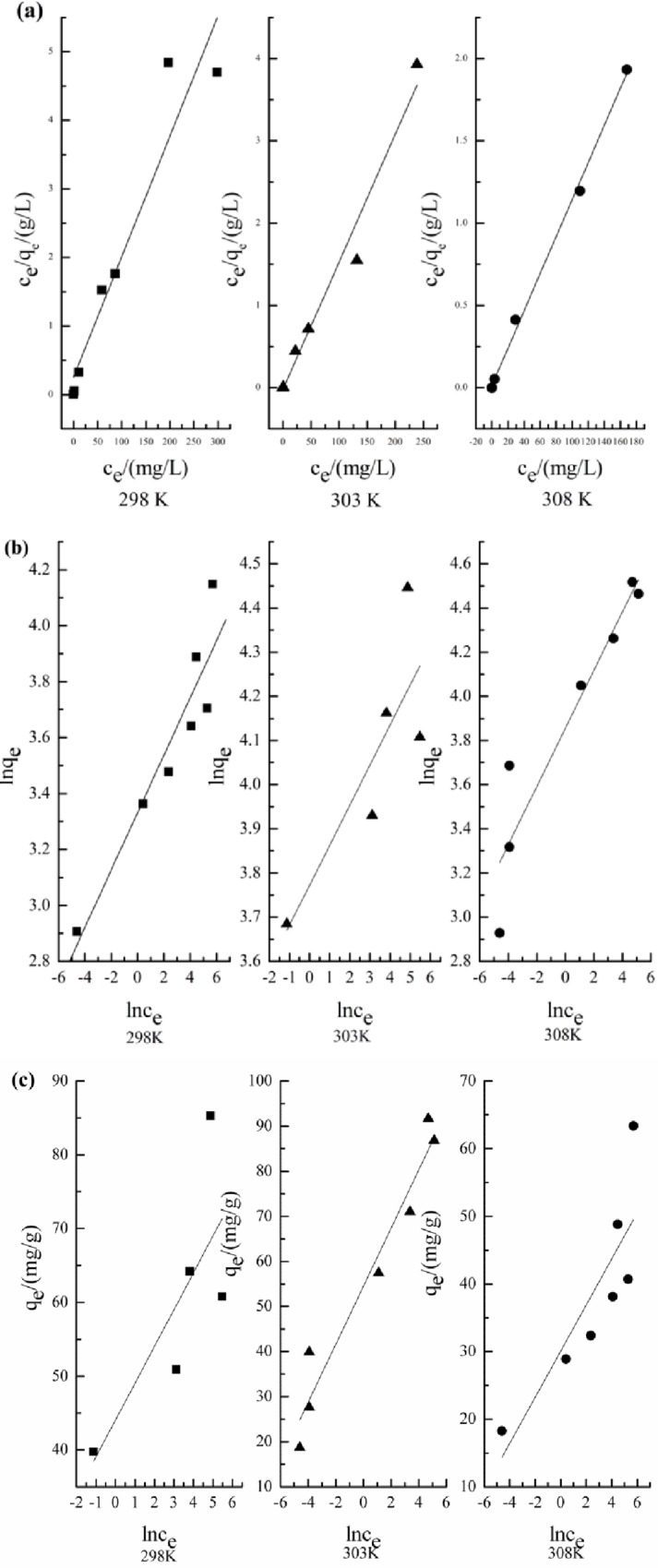
Adsorption isotherms of Cr (VI) at different temperature (Langmuir (a), Freundlich (b), and Temkin isotherm (c) models)
The adsorbents potential for removing Cr (VI) from wastewater in present study is compared with reported values from literature of different adsorbents (Table 4). Although, previous documented studies are the result of different experimental conditions, however, still it could be taken as useful criteria for comparison of adsorption capacities of varied adsorbents. Literature review showed wheat straw and E. adenophorum have highest adsorption potential for Cr (VI).
Table 4. Comparison of maximum sorption capacities of various adsorbents for Cr (VI).
| Adsorbents | Temp. (K) | qm (mg/g) | Reference |
|---|---|---|---|
| Sawdust | 303 | 41.5 | [45] |
| Wheat bran | 303 | 40.8 | [46] |
| Rice bran | 303 | 58.9 | [46] |
| Alligator weed | 303 | 82.57 | [47] |
| Parthenium weed | 293 | 24.5 | [48] |
| Silica-based adsorbent | 298 | 68 | [30] |
| Coconut shell carbon | 298 | 20 | [49] |
| E. adenophorum and buckwheat | 303 | 55.19 | [17] |
| Rice husk | 298 | 10.4 | [50] |
| Carbonized pineapple leaves | 293 | 18.77 | [51] |
| Cactus | 303 | 7.082 | [52] |
| Wheat straw and E. adenophorum | 308 | 88.57 | Present Research |
Conclusions
The maximum adsorption capacity of the studied adsorbents for Cr was observed at a pH of 1. Maximum uptake of Cr was attained at 0.25 g/100mL adsorbent dosage and it can be considered as optimum dosage level of adsorbent under the specified conditions. For the adsorption of Cr from the aqueous solution stable time was 50 min. Experimental results were more fitted to the data attained from Langmuir adsorption isotherm model at different level of temperatures. Pseudo-second-order kinetic model was best fitted for Cr (VI) adsorption and was mutually controlled by film diffusion and intra-particle diffusion for wheat straw and E. adenophorum adsorbents. Present research confirms that carbonized wheat straw and E. adenophorum were reliable low cost adsorbent. Considering the economic aspects of wastewater treatment and reducing water pollution, these materials can be utilized as promising and reliable biosorbent for effective removal of Cr (VI) from wastewater.
Acknowledgments
Authors pay special thanks to Yu Zhisheng from the Resource and Environmental Department, University of Chinese Academy of Sciences, Beijing, China for his help and support.
Data Availability
All relevant data are within the paper.
Funding Statement
Present study was financially supported by National Natural Science Foundation of China (Grant No. 31370632) and by the Ministry of Sciences and Technology of China (Grant No. 2011BAC09B04-01-03).
References
- 1.Louhab K, Sahmoune N, Bar S. Valorisation of the Fungi Waste in the Recuperation of Chromium in the Rejection of the Tannery. European Journal of Scientific Research. 2006. [Google Scholar]
- 2.Khamis M, Jumean F, Abdo N. Speciation and removal of chromium from aqueous solution by white, yellow and red UAE sand. J Hazard Mater. 2009; 169: 948–952. 10.1016/j.jhazmat.2009.04.053 [DOI] [PubMed] [Google Scholar]
- 3.Fu F, Wang Q. Removal of heavy metal ions from wastewaters: A review. J Environ Manage. 2011; 92: 407–418. 10.1016/j.jenvman.2010.11.011 [DOI] [PubMed] [Google Scholar]
- 4.Goyal N, Jain SC, Banerjee UC. Comparative studies on the microbial adsorption of heavy metals. Advances in Environmental Research. 2003; 7: 311–319. [Google Scholar]
- 5.Praveen P, Loh KC. Thermodynamic analysis of Cr(VI) extraction using TOPO impregnated membranes. J Hazard Mater. 2016; 314: 204–210. 10.1016/j.jhazmat.2016.04.054 [DOI] [PubMed] [Google Scholar]
- 6.Gholami F, Mahvi AH, Omrani GA, Nazmara S () Removal of chromium (VI) from aqueous solution by Ulmus leaves. Iran J Environ Healt. 2006;3: 97–102. [Google Scholar]
- 7.Uluozlu OD, Tuzen M, Mendil D, Kahveci B, Soylak M. 3-Ethyl-4-(p-chlorobenzylidenamino-4,5-dihydro-1H-1,2,4-triazol-5-one (EPHBAT) as precipitant for carrier element free coprecipitation and speciation of chromium(III) and chromium(VI). J Hazard Mater. 2009; 172: 395–399. 10.1016/j.jhazmat.2009.07.021 [DOI] [PubMed] [Google Scholar]
- 8.Nguyen NV, Lee JC, Jeong J, Pandey BD. Enhancing the adsorption of chromium(VI) from the acidic chloride media using solvent impregnated resin (SIR). Chem Eng J. 2013;219: 174–182. [Google Scholar]
- 9.Stout MD, Herbert RA, Kissling GE, Collins BJ, Travlos GS, Witt KL, et al. Hexavalent Chromium Is Carcinogenic to F344/N Rats and B6C3F1 Mice after Chronic Oral Exposure. Environ Health Persp. 2009; 117: 716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobya M. Removal of Cr(VI) from aqueous solutions by adsorption onto hazelnut shell activated carbon: kinetic and equilibrium studies. Bioresource Technol. 2004; 91: 317–321. [DOI] [PubMed] [Google Scholar]
- 11.Deveci H, Kar Y. Adsorption of hexavalent chromium from aqueous solutions by bio-chars obtained during biomass pyrolysis. J Ind Eng Chem. 2013;19: 190–196. [Google Scholar]
- 12.Bayrak Y, Topallar H, Karagöz B, Kılıç İ. Kinetics and Thermodynamics of Cr(VI), Cu(II), and Ni(II) Adsorption on Activated Carbon Prepared from Rice Hulls. J Disper Sci Technol. 2013; 34: 1248–1256. [Google Scholar]
- 13.Memon JR, Memon SQ, Bhanger MI, El A. Banana peel: a green and economical sorbent for the selective removal of Cr(VI) from industrial wastewater. Colloid Surface B. 2009;70: 232–237. [DOI] [PubMed] [Google Scholar]
- 14.Singh KK, Hasan SH, Talat M, Singh VK, Gangwar SK. Removal of Cr (VI) from aqueous solutions using wheat bran. Chem Eng J. 2009; 151: 113–121. [Google Scholar]
- 15.Shanthi T, Selvarajan VM. Removal of Cr(VI) and Cu(II) Ions from Aqueous Solution by Carbon Prepared from Henna Leaves. J Chem. 2012; 2013(1). [Google Scholar]
- 16.Lin Q, Wang Q, Duan Y, Wei X, Wu G, Luo Y, et al. Removal of Cu(II), Cr(III), and Cr(VI) from Aqueous Solution using a Novel Agricultural Waste Adsorbent. Sep Sci Technol. 2013; 48: 2843–2851. [Google Scholar]
- 17.Chen J, Yang P, Song D, Yang S, Zhou L, Han L, et al. Biosorption of Cr(VI) by carbonized Eupatorium adenophorum and Buckwheat straw: Thermodynamics and mechanism. Front Env Sci Eng. 2014; 8: 960–966. [Google Scholar]
- 18.Garg UK, Kaur MP, Garg VK, Sud D. Removal of hexavalent chromium from aqueous solution by agricultural waste biomass. J Hazard Mater. 2007;140: 60–68. 10.1016/j.jhazmat.2006.06.056 [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Song D.Adsorption for organic pollutants in domestic wastewater by carbonized Eupatorium adenophorum Spreng. Chinese Journal of Environmental Engineering. 2013;7: 2197–2202. [Google Scholar]
- 20.Tian YH, Feng YL, Liu C. Addition of activated charcoal to soil after clearing Ageratina adenophora stimulates growth of forbs and grasses in China. Trop Grasslands. 2007; 41: 285–291. [Google Scholar]
- 21.Weidi SKJJLJD. Preparation and Characterization of Activated Carbon from Eupatorium adenophorum. Scientia Silvae Sinicae. 2010;46: 178–182. [Google Scholar]
- 22.Bhattacharya AK, Naiya TK, Mandal SN, Das SK. Adsorption, kinetics and equilibrium studies on removal of Cr(VI) from aqueous solutions using different low-cost adsorbents. Chem Eng J. 2008; 137: 529–541. [Google Scholar]
- 23.Weber WJ, Morris JC. Kinetics of Adsorption on Carbon from Solution. Journal of the Sanitary Engineering Division. 2015; 89: 53–55. [Google Scholar]
- 24.Kiran B, Kaushik A. Chromium binding capacity of Lyngbya putealis exopolysaccharides. Biochem Eng J. 2008; 38: 47–54. [Google Scholar]
- 25.Tarley CRT, Arruda MAZ. Biosorption of heavy metals using rice milling by-products. Characterisation and application for removal of metals from aqueous effluents. Chemosphere. 2004; 54: 987–995. [DOI] [PubMed] [Google Scholar]
- 26.Gao R, Wang J. Effects of pH and temperature on isotherm parameters of chlorophenols biosorption to anaerobic granular sludge. J Hazard Mater. 2007; 145: 398–403. 10.1016/j.jhazmat.2006.11.036 [DOI] [PubMed] [Google Scholar]
- 27.Cotton FA, Wilkinson G, Sir. Advanced inorganic chemistry: a comprehensive text. Advanced Inorganic Chemistry A Comprehensive Text. 1972. [Google Scholar]
- 28.Brito F AJ, Mateo S. Equilibrium of Cr (VI) species in acid medium and adsorption studies of these species. Polyhedron. 1997;21: 3835–3846. [Google Scholar]
- 29.Li K, Zhang Y, Dang Y, Wei H, Wang Q. Removal of Cr(VI) from Aqueous Solutions Using Buckwheat (Fagopyrum esculentumMoench) Hull through Adsorption-Reduction: Affecting Factors, Isotherm, and Mechanisms. Clean—Soil Air Water. 2014; 42: 1549–1557. [Google Scholar]
- 30.Qiu J, Wang Z, Li H, Ling X, Jing P, Zhai M, et al. Adsorption of Cr(VI) using silica-based adsorbent prepared by radiation-induced grafting. J Hazard Mater. 2009; 166: 270–276. 10.1016/j.jhazmat.2008.11.053 [DOI] [PubMed] [Google Scholar]
- 31.Garbisu C, Alkorta I, Llama MJ, Serra JL. Aerobic chromate reduction by Bacillus subtilis. Biodegradation. 1998; 9: 133–141. [DOI] [PubMed] [Google Scholar]
- 32.Acar FN, Malkoc E. The removal of chromium(VI) from aqueous solutions by Fagus orientalis L. J Immunol. 2004; 94: 13–15. [DOI] [PubMed] [Google Scholar]
- 33.Gupta VK, Mittal A, Malviya A, Mittal J. Adsorption of carmoisine A from wastewater using waste materials—Bottom ash and deoiled soya. J Colloid Interf Sci. 2009;335: 24–33. [DOI] [PubMed] [Google Scholar]
- 34.Mittal A, Mittal J, Malviya A, Kaur D, Gupta VK. Adsorption of Hazardous Dye Crystal Violet from Wastewater by Waste Materials. J Colloid Interf Sci. 2010; 343: 463–473. [DOI] [PubMed] [Google Scholar]
- 35.Jain AK, Gupta VK, Shubhi J. Removal of chlorophenols using industrial wastes. Environ Sci Technol. 2004; 38: 1195–1200. [DOI] [PubMed] [Google Scholar]
- 36.De Lasa H, Xu CC. Equilibrium and Kinetic Modelling of Astrazon Yellow Adsorption by Sawdust: Effect of Important Parameters: International Journal of Chemical Reactor Engineering. Int J Chem React Eng. 2010; 8: 47–54. [Google Scholar]
- 37.Namasivayam C, Prabha D, Kumutha M. Removal of direct red and acid brilliant blue by adsorption on to banana pith. Bioresource Technol. 1998;64: 77–79. [Google Scholar]
- 38.Park D, Yun YS, Ji YK, Park JM. How to Study Cr(VI) Biosorption: Use of Fermentation Waste for Detoxifying Cr(VI) in Aqueous Solution. Chem Eng J. 2008;136: 173–179. [Google Scholar]
- 39.Park D, Lim SR, Yun YS, Park JM. Development of a new Cr(VI)-biosorbent from agricultural biowaste. Bioresource Technol. 2008;l 99: 8810–8818. [DOI] [PubMed] [Google Scholar]
- 40.Deng L, Zhang Y, Qin J, Wang X, Zhu X. Biosorption of Cr(VI) from aqueous solutions by nonliving green algae Cladophora albida. Miner Eng. 2009; 22: 372–377. [Google Scholar]
- 41.Ronghua LI, Zhang Z, Meng Z. Biosorption of Cr(VI) by corn stalk biomass: thermodynamics and mechanism. Acta Scientiae Circumstantiae. 2009. [Google Scholar]
- 42.Ahmed El N. Potential of pomegranate husk carbon for Cr(VI) removal from wastewater: Kinetic and isotherm studies. J Hazard Mater. 2009; 1: 132–141. [DOI] [PubMed] [Google Scholar]
- 43.Chen S, Yue Q, Gao B, Li Q, Xu X. Removal of Cr(VI) from aqueous solution using modified corn stalks: Characteristic, equilibrium, kinetic and thermodynamic study. Chem Eng J. 2011;168: 909–917. [Google Scholar]
- 44.Bhattacharyya KG, Gupta SS. Adsorptive accumulation of Cd(II), Co(II), Cu(II), Pb(II), and Ni(II) from water on montmorillonite: influence of acid activation. J Colloid Interf Sci. 2007;310: 411–424. [DOI] [PubMed] [Google Scholar]
- 45.Gupta S, Babu BV. Removal of Toxic Metal Cr(VI) from Aqueous Solutions Using Sawdust as Adsorbent: Equilibrium, Kinetics and Regeneration Studies. Chem Eng J. 2009; 150: 352–365. [Google Scholar]
- 46.Xue SW, Zhi ZL, Cheng S. Removal of Cr(VI) from aqueous solutions by low-cost biosorbents: Marine macroalgae and agricultural by-products. J Hazard Mater. 2008;153: 1176–1184. 10.1016/j.jhazmat.2007.09.079 [DOI] [PubMed] [Google Scholar]
- 47.Xue SW, Ye PT, Sheng RT. Kinetics, equilibrium and thermodynamic study on removal of Cr (VI) from aqueous solutions using low-cost adsorbent Alligator weed. Chem Eng J. 2009; 148: 217–225. [Google Scholar]
- 48.Venugopal V, Mohanty K. Biosorptive uptake of Cr(VI) from aqueous solutions by Parthenium hysterophorus weed: Equilibrium, kinetics and thermodynamic studies. Chem Eng J. 2011; 174: 151–158. [Google Scholar]
- 49.Alaerts GJ JV, Kelderman P. Use of coconut shell-based activated carbon for chromium (VI) removal. Water Sci Technol. 1989; 21: 1701–1704. [Google Scholar]
- 50.Bansal M, Garg U, Singh D, Garg VK. Removal of Cr(VI) from aqueous solutions using pre-consumer processing agricultural waste: A case study of rice husk. J Hazard Mater. 2009; 162: 312–320. 10.1016/j.jhazmat.2008.05.037 [DOI] [PubMed] [Google Scholar]
- 51.Ponou J, Kim J, Li PW, Dodbiba G, Fujita T. Sorption of Cr(VI) anions in aqueous solution using carbonized or dried pineapple leaves. Chem Eng J. 2011;172: 906–913. [Google Scholar]
- 52.Dakiky M, Khamis M, Manassra A, Mer’Eb M. Selective adsorption of chromium (VI) in industrial wastewater using low-cost abundantly available adsobents. Adv Environ Res. 2002; 6: 533–540 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.