Abstract
Malaria is endemic in the American continent and the Amazonian rainforest is the region with the highest risk of transmission. However, the lack of suitable experimental models to infect malaria vectors from the Americas has limited the progress to understand the biology of transmission in this region. Anopheles aquasalis, a major vector in coastal areas of South America, was found to be highly refractory to infection with two strains of Plasmodium falciparum (NF54 and 7G8) and with Plasmodium berghei (mouse malaria), even when the microbiota was eliminated with antibiotics and oxidative stress was reduced with uric acid. In contrast, An. aquasalis females treated with antibiotics and uric acid are susceptible to infection with a second murine parasite, Plasmodium yoelii nigeriensis N67 (PyN67). Anopheles albimanus, one of the main malaria vectors in Central America, Southern Mexico and the Caribbean, was more susceptible to infection with PyN67 than An. aquasalis, even in the absence of any pre-treatment, but was still less susceptible than Anopheles stephensi. Disruption of the complement-like system in An. albimanus significantly enhanced PyN67 infection, indicating that the mosquito immune system is mounting effective antiplasmodial responses. PyN67 has the ability to infect a broad range of anophelines and is an excellent model to study malaria transmission by South American vectors.
Introduction
Malaria, a parasitic disease transmitted by mosquitoes, has a major impact on global public health and threatens the economy of one third of the world’s population. In the Americas, 22 countries are affected. In 2015, 660,000 cases of malaria were estimated and 120 million people in the Americas live in areas at risk of malaria [1]. The Amazonian rainforest is the region with the highest risk of transmission, however, the malaria threat extends throughout the Northern regions of South America and across Central America, the Caribbean and Mexico [2]. In Brazil, malaria affects thousands of people every year accounting for 10% of all cases reported outside Africa [1].
Anopheles mosquitoes are the vectors of several Plasmodium species that cause malaria in humans, with Plasmodium vivax (71%) and Plasmodium falciparum (29%) being the most prevalent infections in the Americas [1]. Despite the relative abundance of malaria in the New World, little is known about the biology of the mosquito vectors in this region, when compared to the vast knowledge available for vectors from Africa and Asia. Anopheline mosquitoes diverged from culicines approximately 217 million years ago (MYA;) [3] in the Pangea supercontinent. The three major subgenera of Anopheles (Anopheles, Cellia, and Nyssorhynchus) that transmit malaria to humans exhibit differing geographical ranges. Malaria vectors in Africa and Asia belong to the subgenus Cellia or Anopheles and diverged from the subgenus Nyssorhynchus about 125–115 MYA [4] as the landmasses that would become South America and Africa drifted apart. The subgenus Cellia is restricted to the Old World, while Nyssorhynchus is limited to tropical regions of the New World [5]. Several malaria vectors of the subgenus Kerteszia are also present in South America (e.g. An. cruzzi, An. bellator and An. neivai).
The laboratory colonization of American vectors of the subgenus Nyssorhynchus, such as An. aquasalis and An. albimanus, opened the possibility of studying their interactions with Plasmodium parasites. An. aquasalis is an important vector in coastal areas of South America, and its colonization was achieved in 1995 [6]. This was followed by the colonization of An. albimanus, one of the main malaria vectors in Central America, Southern Mexico and the Caribbean [7]. An. aquasalis and An. albimanus are the only two long-term colonized Central and South American malaria vectors maintained in laboratories that have been used for experimental infections mostly by feeding them on blood of patients from endemic regions infected with P. vivax, demonstrating that they can be good models to study the interaction of American vectors with Plasmodium species [8]. More recently, An. darlingi has been colonized and successfully infected with P. vivax [9].
Studies in An. gambiae infected with Plasmodium berghei, a murine malaria model, revealed that the mosquito complement-like system can greatly limit Plasmodium infection [10]. The Thioester-containing Protein TEP1 is a major effector molecule that is stabilized in the mosquito hemolymph by interacting with two leucine-rich proteins, the leucine-rich repeat immune protein 1 (LRIM1) and the Anopheles Plasmodium-responsive leucine-rich repeat 1 protein (APL1) [11–13]. LRIM1 silencing results in premature activation of TEP1 and disrupts complement-mediated mosquito antiplasmodial responses, greatly increasing An. gambiae infection with P. berghei [12, 13], and with some strains P. falciparum strains[14] [15].
The establishment of robust animal models has been key to our current understanding of the biology of malaria transmission and the mosquito responses to Plasmodium infection. Understanding the parasite/vector interactions that affect vectorial capacity is indispensable for the development of new drugs or vaccines to disrupt transmission. The most widely used laboratory models to study malaria transmission are the in vitro production of P. falciparum gametocytes, a human malaria parasite, and in vivo infection with two murine parasites, P. berghei and P. yoelii. The two mosquito species most widely studied are the African vector An. gambiae, and the Asian vector An. stephensi. Mosquitoes can differ widely in their susceptibility to infection with specific Plasmodium parasite species [16] and great differences have been documented even between parasite strains [17, 18]. An. albimanus can be infected by direct feeding on P. berghei-infected mice [19] but, in general, infections are much lower when compared to An. gambiae or An. stpehensi. An. albimanus is readily infected with a P. falciparum strain of Brazilian origin (7G8) but is highly resistant to infection with African and Asian strains [18].
The main goal of this study was to establish robust experimental models to study malaria transmission by two colonized New World vectors, An. aquasalis and An. albimanus. Their susceptibility to laboratorial infections with the human malaria parasite P. falciparum and the rodent parasites P. berghei and P. yoelii was investigated. The role of the mosquito complement-like system in their susceptibility to infections was also evaluated by silencing LRIM1. Our studies show that Plasmodium yoelii nigeriensis is a viable model system to study malaria transmission by New World vectors.
Results
Plasmodium falciparum infection in An. aquasalis
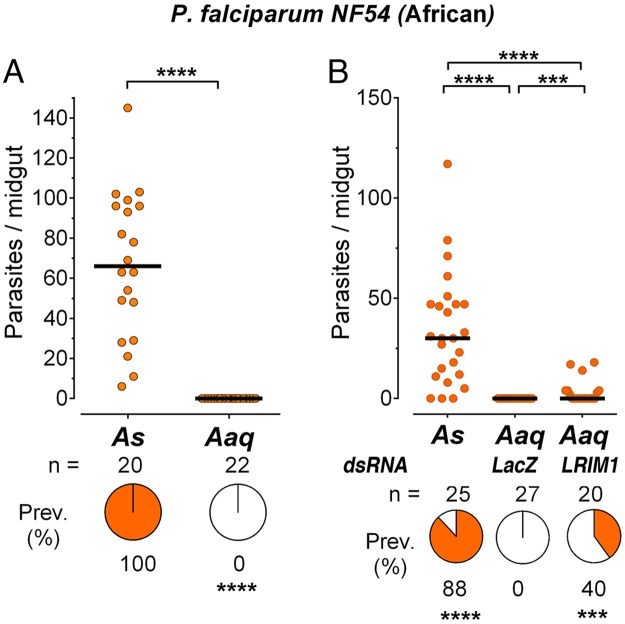
A major limitation in establishing experimental models for New World vectors is the impossibility of using mosquito vectors that are widely used elsewhere for laboratory infections (such as An. gambiae or An. stephensi) to ensure the quality of the gametocytes (positive controls) when performing experiments in laboratories located in malaria endemic areas. This problem was circumvented by transiently establishing an An. aquasalis colony at the National Institutes of Health (NIH) in the USA. The susceptibility of An. aquasalis to P. falciparum infection was evaluated using gametocyte cultures from the NF54 strain, a laboratory-adapted line of putative African origin. The quality of the gametocyte cultures was confirmed by simultaneously feeding An. stephensi (Nijmengen Sda500) mosquitoes, a laboratory strain that has been genetically selected to be highly susceptible to P. falciparum infection [20]. The An. stephensi control groups were readily infected with P. falciparum NF54, reaching infection prevalences of 88–100% and medians of 30–90 oocysts/midgut (Fig 1A and S1 Fig). In contrast, only one of the An. aquasalis mosquitoes out of 53 that fed on the same gametocyte cultures became infected with a single oocyst (p<0.0001, Fig 1A and S1 Fig).
Fig 1. Infection of A. aquasalis with P. falciparum NF54.
(A) Susceptibility of Anopheles stephensi (As) and Anopheles aquasalis (Aaq) mosquitoes to infection with Plasmodium falciparum NF54 strain. (B) Effect of disrupting the mosquito immune system by silencing LRIM1 on Aaq susceptibility to infection. Each dot represents the number of oocysts present on an individual midgut 10–12 days post-infection and the median number of oocysts is indicated by the black line. The medians were compared using the Mann-Whitney test and the infection prevalence using Chi-square (*** p<0.001, **** p<0.0001).
We investigated whether the lack of infectivity of An. aquasalis was due to activation of the mosquito immune system by silencing LRIM1 expression. We were able to clone a 1,501bp partial sequence of the An. aquasalis LRIM1 cDNA (S2 Fig) using primers designed based on the annotated An. albimanus LRIM1 transcript (AALB005865-RA). This region has 81% identity to the An. albimanus LRIM1 transcript, and the 571bp 3’-end of our partial A. aquasalis cDNA has 100% homology to a shorter non-annotated transcript in Vector Base labeled GAMD01000377.1_Aaquasalis_Anoaqua-4332_mRNA. Our partial cDNA sequence (S2 Fig) was used to design primers to generate dsRNA and to evaluate the silencing efficiency.
None of the mosquitoes injected with the dsLacZ control were infected, while 40% of the An. aquasalis mosquitoes in which LRIM1 was silenced became infected (p<0.001, Fig 1B). However, even after silencing LRIM1 the infection intensity was still low (median of 0) in An. aquasalis, when compared to An. stephensi with median of 38 oocysts (p<0.0001), and a prevalence of 88% (p<0.001); indicating that only some of the parasites are eliminated by the An. aquasalis immune system.
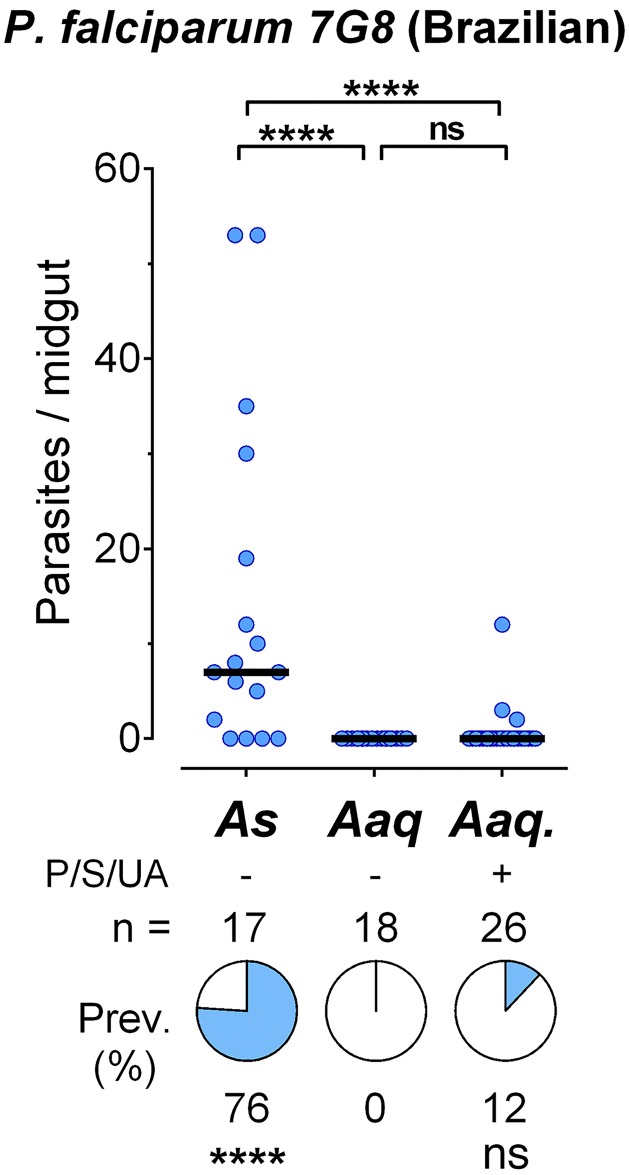
Recent studies have shown that parasite isolates from different geographic origin can exhibit dramatic differences in infectivity to the same mosquito vector. For example, a P. falciparum line of Brazilian origin (7G8) is more effective infecting An. albimanus mosquitoes than parasite lines of African origin [18]. It is also well established that oxidative stress [21] and the gut microbiota [22] can affect Plasmodium survival. Oral administration of uric acid reduces oxidative stress, decreasing loss of fecundity with age and preventing Plasmodium melanization [21, 23]. We tested the susceptibility of An. aquasalis to infection with the P. falciparum 7G8 line and the effect of reducing the gut microbiota by oral administration of antibiotics solution (Penicillin and Streptomycin). The solution was also supplemented with uric acid, to reduce oxidative stress in the mosquito. Although a few An. aquasalis subjected to this treatment became infected with P. falciparum 7G8 (Fig 2 and S3 Fig), the prevalence was low (10–12%) when compared to An. stephensi (28–76%, p<0.001). None of the An. aquasalis females that were not treated with the antibiotic + uric acid mixture became infected (p<0.0001).
Fig 2. Infection of A. aquasalis with P. falciparum 7G8.
Susceptibility of Anopheles stephensi (As) and Anopheles aquasalis (Aaq) mosquitoes to infection with Plasmodium falciparum 7G8 strain. The effect of oral administration of antibiotics (Penicillin/Streptomycin = P/S) and uric acid (UA) on Aaq infection was also tested. Each dot represents the number of oocysts present on an individual midgut 10–12 days post-infection and the median number of oocysts is indicated by the black line. The medians were compared using the Mann-Whitney test and the infection prevalence using Chi-square (**** p<0.0001).
Susceptibility of An. aquasalis to infection with murine malaria parasites
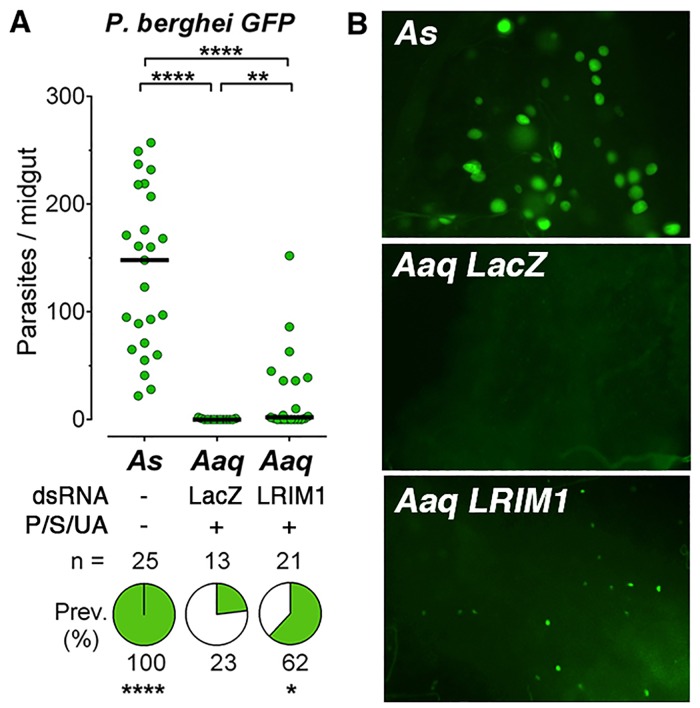
The P. berghei ANKA 2.34 strain can effectively infect An. albimanus when mosquitoes are fed ookinetes cultured in vitro, and a high prevalence (>90%) and intensity of infection (20–30 oocysts/midgut) can be obtained [19]. Multiple times we attempted to infect females from a An. aquasalis colony established in Brazil by direct feeding on P. berghei (Anka 2.34-GFP)-infected mice with no success, even when mice parasitemias and exflagellations were optimal (data not shown). We decided to confirm this lack of infectivity at the Laboratory of Malaria and Vector Research (NIH), by infecting An. stephensi and An. aquasalis using the same mouse, under optimal conditions. As expected, the An. stephensi control group was very susceptible to infection with P. berghei (Anka 2.34-GFP) with a prevalence of 100% and median of 148 oocysts 7 days post-feeding. In contrast, only three of the An. aquasalis females treated with the antibiotic + uric acid solution and injected with a dsLacZ control dsRNA became infected with a single oocyst (p<0.0001, Fig 3A). Silencing LRIM1 significantly enhanced the infection prevalence to 62% (p<0.04), but the intensity of infection remained low (median of 1) (p<0.01, Fig 3A). Furthermore, the oocysts that developed were very small at 7 days post-feeding, relative to those in A. stephensi, (Fig 3B) indicating that the ookinetes that survived in An. aquasalis did not develop normally into the oocysts stage.
Fig 3. Infection of A. aquasalis with P. berghei.
Susceptibility of Anopheles stephensi (As) and Anopheles aquasalis (Aaq) mosquitoes to infection with P. berghei. (A) Effect of disrupting the mosquito immune system by silencing LRIM1 on Aaq susceptibility to infection. (B) P. berghei oocysts in As and Aaq mosquitoes 8 days post infection. Each dot represents the number of oocysts present on an individual midgut 10–12 days post-infection and the median number of oocysts is indicated by the black line. The medians were compared using the Mann-Whitney test and the infection prevalence using Chi-square (* p<0.05, **** p<0.0001).
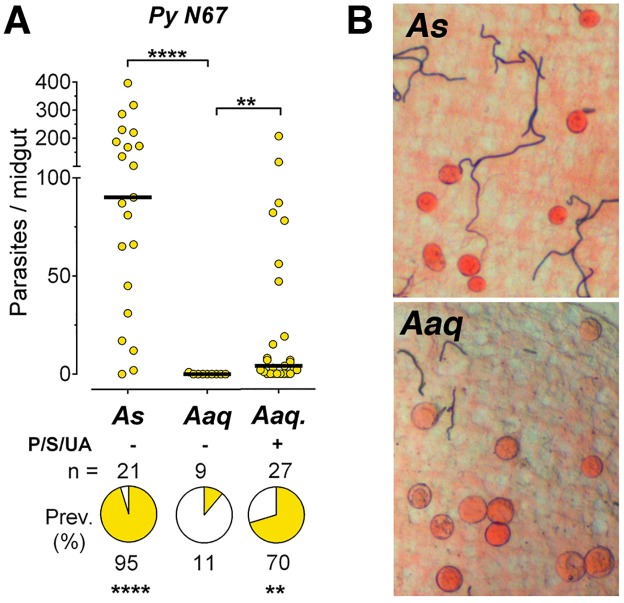
Given the extremely low infectivity of P. berghei, we decided to test a different murine malaria parasite. We found that An. aquasalis was much more susceptible to infection with P. yoelii nigeriensis N67 (PyN67) parasites, reaching a consistent high prevalence of infection (54–70%) when mosquitoes were treated with antibiotic + uric acid solution (p<0.01), (Figs 4A and 5A, and S4 Fig). In contrast to P. berghei, PyN67 oocysts developed normally in An. aquasalis and had a similar size and appearance as in An. stephensi controls (Fig 4B). We were able to recover sporozoites from the salivary glands of An. aquasalis (17,000–19,000 spz/mosquito) indicating that PyN67 can complete its life cycle in this mosquito species. The infectivity of these sporozoites to mice was not tested.
Fig 4. Infection of A. aquasalis with P. yoelii nigeriensis N67.
Susceptibility of Anopheles stephensi (As) and Anopheles aquasalis (Aaq) mosquitoes to infection with P. yoelii nigeriensis N67 (PyN67). (A) Effect of oral administration of antibiotics (Penicillin/Streptomycin = P/S) and uric acid (UA) on Aaq infection. (B) PyN67 oocysts in As and Aaq mosquitoes 8 days post infection. Image of oocysts in Aaq mosquitoes treated with P/D + UA. Each dot represents the number of oocysts present on an individual midgut 10–12 days post-infection and the median number of oocysts is indicated by the black line. The medians were compared using the Mann-Whitney test and the infection prevalence using Chi-square (** p<0.01, **** p<0.0001).
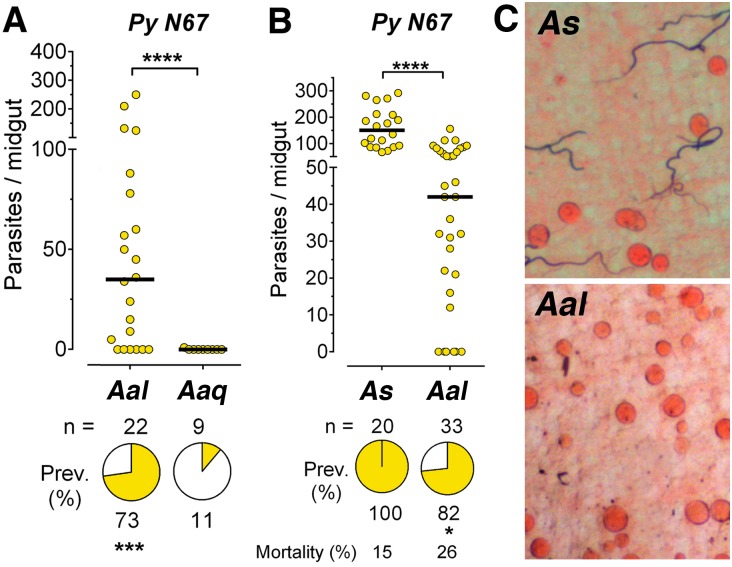
Fig 5. Infection of A. albimanus with P. yoelii nigeriensis N67.
(A and B) Susceptibility of Anopheles albimanus (Aal), Anopheles aquasalis (Aaq) and Anopheles stephensi (As) mosquitoes to infection with P. yoelii nigeriensis N67 (PyN67), without antibiotics or uric acid. (C) PyN67 oocysts in As and Aal mosquitoes 8 days post infection. Each dot represents the number of oocysts present on an individual midgut 10–12 days post-infection and the median number of oocysts is indicated by the black line. The medians were compared using the Mann-Whitney test and the infection prevalence using Chi-square (* p<0.05, *** p<0.001, **** p<0.0001).
Susceptibility of An. albimanus to infection with P. yoelii N67
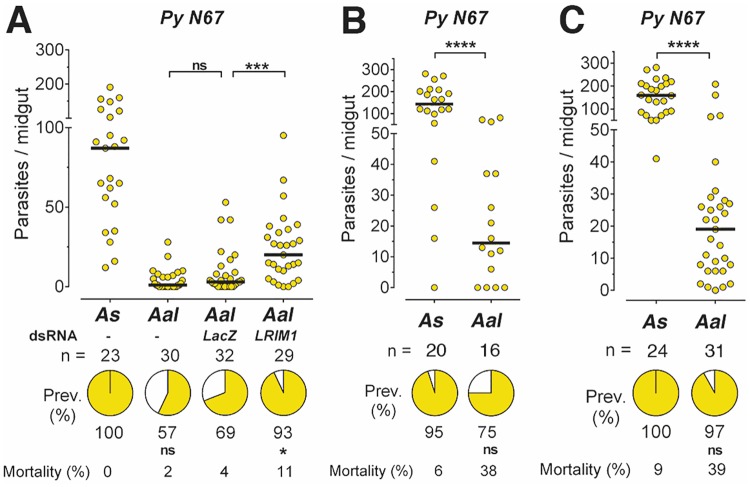
An. albimanus is an important malaria vector in Mexico, Central America and the Northern regions of South America. We explored the potential of P. yoelii N67 as a model of malaria transmission in this mosquito species. An. albimanus is more susceptible to infection with PyN67 than An. aquasalis (Fig 5A), reaching a high prevalence (73%) of infection and a median of 36 oocysts/midgut without administration of antibiotics or uric acid, while no oocysts could be detected in An. aquasalis females (p<0.0001, Fig 5A). However, the intensity and prevalence of infection was significantly lower than in An. stephensi (p<0.0001, Fig 5B and S5 Fig). PyN67 oocysts also developed normally in An. albimanus (Fig 5C). Injection of dsLacZ control dsRNA had no effect, while silencing LRIM1 significantly increased the intensity of infection (p<0.001, Fig 6A and S6 Fig), indicating that the An. albimanus complement-like system limits PyN67 infection.
Fig 6. Effect of disrupting the mosquito immune system on PyN67 infection in A. albimanus.
Susceptibility of Anopheles stephensi (As) and Anopheles albimanus (Aal) mosquitoes to infection with P. yoelii nigeriensis N67 (PyN67). (A) Susceptibiltiy to infection and effect of disrupting the mosquito immune system by silencing LRIM1. (B and C) Susceptibility of infection and mortality after PyN67 completed one developmental cycle in An. albimanus. Each dot represents the number of oocysts present on an individual midgut 10–12 days post-infection and the median number of oocysts is indicated by the black line. The medians were compared using the Mann-Whitney test and the infection prevalence using Chi-square (*p<0.05, *** p<0.001, **** p<0.0001, ns = not significant).
We were able to recover a small number of sporozoites from infected salivary glands of An. albimanus mosquitoes (200–500 spz/mosquito), and they were used to infect a recipient mouse. The infection was successful when 1000 PyN67 sporozoites extracted from A. albimanus salivary glands were injected IV into a BalbC mouse, indicating that PyN67 parasites can complete their development in An. albimanus and generate infectious sporozoites. In general, after completing one developmental cycle in An. albimanus, mosquito infections were high, often reaching medians of more than 100 oocysts in An. stephensi (Fig 6B and 6C and S7 Fig). Interestingly, these parasites seemed to be more pathogenic to An. albimanus mosquitoes, as they caused 6–9% mortality in A. stephensi, while in An. albimanus mortality was significantly higher 20–39% (p<0.01).
Discussion
An. aquasalis is susceptible to infection with P. vivax [24] and this experimental system has been used to investigate the role of the STAT pathway [25] and reactive oxygen species [26] on mosquito susceptibility to infection. Recent studies indicate that P. falciparum evasion of mosquito immunity is mediated by the Pfs47 gene and is critical for parasite survival. Different haplotypes of Pfs47 are circulating in different continents and they are major determinants of vector/parasite compatibility [18]. An. gambiae mosquitoes are very susceptible to infection with two different P. falciparum lines of African origin (NF54 and MRA1181), while An. albimanus is highly refractory to infection with these isolates. Conversely, An. albimanus is more susceptible to infection with a P. falciparum line (7G8) of Brazilian origin than An. gambiae [18]. The lack of compatibility between isolates from a different continent can be overcome by disrupting the mosquito complement-like system, indicating that the mosquito immune system is selecting for parasites that express certain haplotypes of Pfs47 and can evade immunity [18]. We found that An. aquasalis is almost completely refractory to infection with P. falciparum NF54 parasites, however, although disrupting the mosquito complement-like system by silencing LRIM1 significantly increased the prevalence and intensity of infection, the infection levels are much lower than in An. stephensi controls or in An. albimanus females in which LRIM1 expression was silenced [18]. This suggests that, besides the mosquito immune system, some other factor(s) in An. aquasalis is probably responsible for the low infectivity with P. falciparum NF54 parasites. This is in agreement with the observation that An. aquasalis is also highly refractory to infection with a P. falciparum (7G8) line expressing the most common Pfs47 haplotype in Brazil. Oral administration of antibiotics and uric acid allowed survival of a few parasites, but the prevalence and intensity of infection were still low. While it is clear that An. aquasalis is an important vector of P. vivax malaria in Brazil (reviewed by [8]) and Guyana [27], our findings indicate that this mosquito species is not a competent vector of P. falciparum malaria with the two different lines tested. In the Amazon region, An. darling, An. albitarsis and An. rondoni have been documented as vectors of P. falciparum by direct immunodetection of sporozites, with An. darling being the most prevalent infected species. This kind of direct evidence for An. aquasalis as a major vector of P. falciparum malaria is not available.
An. aquasalis is less susceptible to infection with P. berghei and P. yoelii N67 than An. albimanus. This could be due to differences in physiology or in the microbiota, as An. aquasalis larvae have adapted to brackish water. Reducing the microbiota by oral administration of antibiotics and disruption of the An. aquasalis immune system was able to rescue some low level of P. berghei infection, indicating that a few ookinetes were successful in invading the midgut and transformed into oocyts. However, the oocysts that formed were very small, indicating that they did not develop properly (Fig 3B). This could be due to physiological conditions in An. aquasalis that do not provide and adequate environment for the developing oocysts or to late phase immune responses that target the oocyst stage of the parasite. An. aquasalis females treated with antibiotics and uric acid were much more susceptible to infection with PyN67 than with P. berghei and the oocysts that developed were normal in size; indicating that murine PyN67 infection is a good animal model to study malaria transmission by An. aquasalis.
An. albimanus is more susceptible to PyN67 infection than An. aquasalis, and it is readily infected without the need for oral administration of antibiotics or uric acid. It also supports normal oocyst development. Silencing the An. albimanus immune system enhances infection, indicating that mosquitoes are mounting an active immune response to infection. Furthermore, PyN67 salivary gland sporozoites were infectious to mice, making it possible to close the transmission cycle under laboratory conditions. In all experiments, An. stephensi was more susceptible to infection than An. aquasalis and An. albimanus, probably because this line was genetically selected to be highly susceptible to P. falciparum infection and these mosquitoes take very large blood meals under laboratory conditions. We conclude that different anopheline mosquito species differ broadly in their susceptibility to infection with different Plasmodium species. P. yoelii N67 appears to have a broad ability to infect many different mosquito species, including New World vectors, making it an excellent model system to study malaria transmission.
Materials and Methods
Ethics statement
Public Health Service Animal Welfare Assurance #A4149-01 guidelines were followed according to the National Institutes of Health Animal (NIH) Office of Animal Care and Use (OACU). These studies were done according to the NIH animal study protocol (ASP) approved by the NIH Animal Care and User Committee (ACUC), with approval ID ASP-LMVR5. All the animal procedures used in this study have been approved with the NIH Animal Care and User Committee (ACUC).
Mosquito rearing
An. Stephensi, Anopheles aquasalis and Anopheles albimanus mosquitoes were reared at 27°C and 80% humidity on a 12-h light/dark cycle under insectary conditions [21]. All mosquito larvae were readed in unchlorinated water, by allowing chorinated water to rest for 48h in an open container. Tetramin Tropical Flakes® fish food was grinded into fine powder using a coffee mill and used as feeding source for larvae. An. aquasalis larvae were reared in water that contained table salt at a final concentration of 2g/L. An. aquasalis adult females were fed on cow blood for colony maintenance by membrane feeding (using hog gut sausage casings as membranes), while An. albimanus and An. stephensi females were fed on live anesthetized chickens.
Mosquitoes were fed a 10% sucrose solution in a cotton ball until two days before the infective blood meals, when some experimental groups were switched to drinking a 1% uric acid solution with Penicillin (100 units/ml) and Streptomycin (0.1 mg/ml) in water, and they obtained sugar from a solid sugar cube placed on top of the cage. They were fed this solution and the sugar cube until the end of the experimental infections. The solution was fed by placing it in an inverted glass test tube with a cotton ball plug and was changed daily.
Plasmodium infections
Female mosquitoes (4–5 days old) were infected by feeding them blood meals with mature stage IV and V P. falciparum gametocyte cultures (NF54 or 7G8 strains) through a membrane feeder at 37°C for 30 min [17]. Human blood was obtained from the Interstate Blood Bank, Memphis, Tennessee. Membrane feedings were done using hog gut sausage casings as membranes.
For P. yoelii nigeriensis (N67 strain) and P. berghei infections, parasites from frozen stocks were administered via intraperitoneal (IP) injection to 3- to 5-week-old BALB/c female mice. When the parasitemia of the donor mice reached 5–10% (in about 5–7 days), the infected blood was taken by cardiac puncture and transferred to a healthy mouse. Mouse parasitemia was determined by light microscopy inspection of Giemsa-stained thin blood smears obtained by tail snip. Experimental BALB/c mice were infected by intraperitonealy (IP) injection 20–30μl fresh blood from the donor mice. This recipient mouse was used to infect mosquitoes when it reached 3–5% parasitemia 2–3 days after inoculation. Female mosquitoes (5–7 days old) were infected by direct feeding on anesthetized infected mice. For P yoelii infections, mosquitoes were maintained at 24°C, while P. berghei-infected mosquitoes were kept at 21°C (their respective permissive temperatures for gametogenesis). Both were kept at 80% humidity. P. yoelii midgut infection was assessed by light microscopy 10–11 days after feeding with mercurochrome staining (0.1% in water) [28]; while P. berghei midgut infection was assessed by fluorescence microscopy of GFP parasites 7 days after feeding. Oocysts on individual midguts were counted to determine the prevalence and intensity of infection.
The number of PyN67 sporozoites recovered from An. aquasalis salivary glands was between 17,000–19,000 spz/mosquito. Their infectivity to mice was not tested. The number of sporozoites recovered from An. albimanus salivary glands was between 200–500 spz/mosquito. We injected 1000 P. yoelii N67 sporozoites extracted from An. albimanus salivary glands via IV into the tail vein of a Balb/c mouse in a volume of 100 μl of RPMI with 10% mouse serum, and the animal became infected.
RNAi gene silencing
Individual female A. gambiae mosquitoes were injected 1–2 d after emergence as previously described [21]. Briefly, mosquitoes were injected with 69 nL of a 3 μg/μL dsRNA solution 3–4 d before receiving a Plasmodium-infected blood meal. The control dsRNA (dsLacZ) was produced as previously described [21]. Double-stranded dsLacZ RNA was generated by introducing T7 promoters (in bold letters) thought PCR amplification of the cloned insert using the following vector primers: M13F 5' GTAAAACGACGGCCAG 3' and M13Rev- T7 5'CTCGAGTAATACGACTCACTATAGGGCAGGAAACAGCTATGAC3'. The PCR product was used as template to generate dsRNA using T7 RNA polymerase, as described below.
The An. aquasalis LRIM1 dsRNA was synthesized with primers designed based on the partial An. aquasialis LRIM1 cDNA sequence (S2 Fig). The sequence of the primers used is: LRIM1_AaqFw, 5’- TAATACGACTCACTATAGGGTTGTACGGCACGGTAAACCT-3’, and LRIM1_AaqRv, 5’-TAATACGACTCACTATAGGGCCACGGTAGCTTGTTGTGC-3’ (PCR conditions were 94°C for 3min; 40 cycles of 94°C for 30 s, 59°C for 30 s, and 72°C for 1min; final extension, 72°C, 5 min).
The primers used for An. albimanus LRIM1 were as follows: two external PCR primers were used, and the product of the first amplification was used as template for a second one using internal primers with the following sequences:
LRIM1_AalExFw, 5’-AAGGTTGAGCCGAAGAATGA-3’, andLRIM1_AalExRv,5’-GCACTTCCCATGCTGCTAAT-3’ (PCR conditions were 94°C for 3min; 35 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min; final extension, 72°C, 5 min); for internal PCR (primers containing T7 promoter): LRIM1_AalInFw, 5’-TAATACGACTCACTATAGGGCTGTACGGCACCGTTAACCT-3’, and LRIM1_AalInRv, 5’-TAATACGACTCACTATAGGGAGCTTGTTGTGCGAAAGGTC-3’ (PCR conditions were 94°C for 3min; 40 cycles of 94°C for 30 s, 59°C for 30 s, and 72°C for 1min; final extension, 72°C, 5 min; using 1 μL of a 1–20 dilution of the external primer PCR).
RNAs were synthesized simultaneously from the template, annealed and purified using the T7 RNAi Mega-script kit (Ambion) following the procedure recommended by the manufacturer. dsRNA products were eluted in water to a final concentration of 3 μg/μl. A volume of 69nl of dsRNA preparation was injected into the thorax of cold anesthetized, 2–3 day-old female mosquitoes using a nano-injector (Nanoject; Drummond Scientific, Broomall, Pennsylvania, USA) fitted with a glass capillary needle. The dsLacZ RNA was used in each experiment to control for any unspecific effect of dsRNA injection. Mosquitoes received a Plasmodium-infected blood meal 2–3 day post-dsRNA injection.
qRT-PCR gene expression
Total RNA was isolated from 15 to 20 mosquito midguts using Trizol (Invitrogen) and cDNA synthesis was performed using QuantiTect Reverse Transcription Kit (Qiagen). Gene-expression analysis was measured by SYBR green qRT-PCR (DyNAmo HS; New England Biolabs) in a CFX96 system (Biorad). Gene expression was assessed using two to three technical replicates and three biological replicates. The An. gambiae ribosomal protein S7 was used as an internal reference to normalize each sample for the amount of template present. Fold-change was calculated using the 2−ΔΔ Ct method.
An. aquasalis and An. albimanus LRIM1 gene silencing was assessed in whole sugar-fed mosquitoes by quantitative real-time PCR (qPCR) using the S7 ribosomal protein gene as internal reference. The primers used for qPCR for A. aquasalis were as follows: LRIM1_Aaq_qPFw, 5’-ACCTCAGCGGTAACAAGGTG-3’; LRIM1_Aaq_qPRv; 5’- CTGCGGGTCCTTATTGTTTG-3’; S7_Aaq_qPFw, 5’- ATCCTGGAGCTGGAGATGAA -3’; and S7_Aaq_qPRv, 5’- ACGATGGCCTTCTTGTTGTT -3’.
The primers used for qPCR for A. albimanus were as follows: LRIM1_Aal_qPFw, 5’-GACAAAAGTGTGCGCTTTGA-3’; LRIM1_Aal_qPRv; 5’-CACTCCCGGATTAGAGCTTG-3’; S7_Aal_qPFw, 5’-ACCTGGACAAGAACCAGCAG-3’; and S7_Aal_qPRv, 5’-GTTTTCTGGGAATTCGAACG-3’. The silencing efficiency in dsRNA-injected mosquitoes was 94–98% for An. aquasalis LRIM1 and 85–98% for An. albimanus LRIM1, relative to dsLacZ-injected controls.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 5 (GraphPad software). Analyses derived from at least two independent biological replicates. Gene-expression data were analyzed using Student’s t test on mean value of all independent experiments. Infection intensity of oocysts was compared with each other using Mann–Whitney tests, infection prevalence and overall mortality were compared using χ2 analysis.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank André Laughinghouse and Kevin Lee for insectary support.
Abbreviations
- APL1
Anopheles Plasmodium-responsive leucine-rich repeat 1 protein
- LRIM1
leucine-rich repeat immune protein 1
- TEP1
Thioester-containing Protein
Data Availability
All data are available in the paper and its Supporting Information files.
Funding Statement
This work was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health and the following Brazilian agencies: Conselho Nacional de Desenvolvimento Tecnologico (CNPq-PAPES), Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior (CAPES) and the Fundações de Amparo a Pesquisas do Amazonas e de Minas Gerais (FAPEAM e FAPEMIG). ASO was supported by PhD fellowships from FIOCRUZ and CNPq; and APMD by a CAPES post-doctoral fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO. World Malaria Report. Geneva: WHO, 2015.
- 2.WHO. World Malaria Report. Geneva: World Health Organization; 2014.
- 3.Reidenbach KR, Cook S, Bertone MA, Harbach RE, Wiegmann BM, Besansky NJ. Phylogenetic analysis and temporal diversification of mosquitoes (Diptera: Culicidae) based on nuclear genes and morphology. BMC Evol Biol. 2009;9:298 Epub 2009/12/24. 10.1186/1471-2148-9-298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valencio DA, Vilas JF. Age of the Separation of South America and Africa. Nature. 1969;223(5213):1353–4. [Google Scholar]
- 5.Harbach RE. the Phylogeny and Classification of Anopheles In: Manguin S, editor. Anopheles mosquitoes—New insights into malaria vectors: InTech; 2013. [Google Scholar]
- 6.Horosko S 3rd, Lima JB, Brandolini MB. Establishment of a free-mating colony of Anopheles albitarsis from Brazil. J Am Mosq Control Assoc. 1997;13(1):95–6. Epub 1997/03/01. [PubMed] [Google Scholar]
- 7.Zerpa N, Moreno J, Gonzalez J, Noya O. Colonization and laboratory maintenance of Anopheles albimanus Wiedemann in Venezuela. Revista do Instituto de Medicina Tropical de Sao Paulo. 1998;40(3):173–6. Epub 1998/11/27. [DOI] [PubMed] [Google Scholar]
- 8.Pimenta PF, Orfano AS, Bahia AC, Duarte AP, Rios-Velasquez CM, Melo FF, et al. An overview of malaria transmission from the perspective of Amazon Anopheles vectors. Mem Inst Oswaldo Cruz. 2015;110(1):23–47. 10.1590/0074-02760140266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreno M, Tong C, Guzman M, Chuquiyauri R, Llanos-Cuentas A, Rodriguez H, et al. Infection of Laboratory-Colonized Anopheles darlingi Mosquitoes by Plasmodium vivax. The American journal of tropical medicine and hygiene. 2014;90(4):612–6. 10.4269/ajtmh.13-0708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blandin S, Shiao SH, Moita LF, Janse CJ, Waters AP, Kafatos FC, et al. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell. 2004;116(5):661–70. Epub 2004/03/10. [DOI] [PubMed] [Google Scholar]
- 11.Osta MA, Christophides GK, Kafatos FC. Effects of mosquito genes on Plasmodium development. Science. 2004;303:2030–2. 10.1126/science.1091789 [DOI] [PubMed] [Google Scholar]
- 12.Fraiture M, Baxter RH, Steinert S, Chelliah Y, Frolet C, Quispe-Tintaya W, et al. Two mosquito LRR proteins function as complement control factors in the TEP1-mediated killing of Plasmodium. Cell Host Microbe. 2009;5(3):273–84. Epub 2009/03/17. 10.1016/j.chom.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 13.Povelones M, Waterhouse RM, Kafatos FC, Christophides GK. Leucine-rich repeat protein complex activates mosquito complement in defense against Plasmodium parasites. Science. 2009;324(5924):258–61. Epub 2009/03/07. 10.1126/science.1171400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garver LS, Bahia AC, Das S, Souza-Neto JA, Shiao J, Dong Y, et al. Anopheles Imd pathway factors and effectors in infection intensity-dependent anti-Plasmodium action. PLoS pathogens. 2012;8(6):e1002737 10.1371/journal.ppat.1002737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molina-Cruz A, Dejong RJ, Ortega C, Haile A, Abban E, Rodrigues J, et al. Some strains of Plasmodium falciparum, a human malaria parasite, evade the complement-like system of Anopheles gambiae mosquitoes. Proceedings of the National Academy of Sciences of the United States of America. 2012. Epub 2012/05/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaramillo-Gutierrez G, Rodrigues J, Ndikuyeze G, Povelones M, Molina-Cruz A, Barillas-Mury C. Mosquito immune responses and compatibility between Plasmodium parasites and anopheline mosquitoes. BMC Microbiol. 2009;9:154 Epub 2009/08/01. 10.1186/1471-2180-9-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molina-Cruz A, Garver LS, Alabaster A, Bangiolo L, Haile A, Winikor J, et al. The Human Malaria Parasite Pfs47 Gene Mediates Evasion of the Mosquito Immune System. Science. 2013;340(6135):984–7. 10.1126/science.1235264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molina-Cruz A, Canepa GE, Kamath N, Pavlovic NV, Mu J, Ramphul UN, et al. Plasmodium evasion of mosquito immunity and global malaria transmission: The lock-and-key theory. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(49):15178–83. Epub 2015/11/26. 10.1073/pnas.1520426112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Contreras-Garduno J, Rodriguez MC, Hernandez-Martinez S, Martinez-Barnetche J, Alvarado-Delgado A, Izquierdo J, et al. Plasmodium berghei induced priming in Anopheles albimanus independently of bacterial co-infection. Dev Comp Immunol. 2015;52(2):172–81. Epub 2015/05/26. 10.1016/j.dci.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 20.Feldmann AM, Ponnudurai T. Selection of Anopheles stephensi for refractoriness and susceptibility to Plasmodium falciparum. Medical and veterinary entomology. 1989;3(1):41–52. [DOI] [PubMed] [Google Scholar]
- 21.Molina-Cruz A, DeJong RJ, Charles B, Gupta L, Kumar S, Jaramillo-Gutierrez G, et al. Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. J Biol Chem. 2008;283(6):3217–23. 10.1074/jbc.M705873200 [DOI] [PubMed] [Google Scholar]
- 22.Gendrin M, Rodgers FH, Yerbanga RS, Ouédraogo JB, Basáñez M-G, Cohuet A, et al. Antibiotics in ingested human blood affect the mosquito microbiota and capacity to transmit malaria. Nature communications. 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeJong RJ, Miller LM, Molina-Cruz A, Gupta L, Kumar S, Barillas-Mury C. Reactive oxygen species detoxification by catalase is a major determinant of fecundity in the mosquito Anopheles gambiae. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(7):2121–6. Epub 2007/02/08. 10.1073/pnas.0608407104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.da Silva AN, Santos CC, Lacerda RN, Machado RL, Povoa MM. Susceptibility of Anopheles aquasalis and an. darlingi to Plasmodium vivax VK210 and VK247. Mem Inst Oswaldo Cruz. 2006;101(5):547–50. Epub 2006/10/31. [DOI] [PubMed] [Google Scholar]
- 25.Bahia AC, Kubota MS, Tempone AJ, Araujo HR, Guedes BA, Orfano AS, et al. The JAK-STAT pathway controls Plasmodium vivax load in early stages of Anopheles aquasalis infection. PLoS Negl Trop Dis. 2011;5(11):e1317 10.1371/journal.pntd.0001317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahia AC, Oliveira JH, Kubota MS, Araujo HR, Lima JB, Rios-Velasquez CM, et al. The role of reactive oxygen species in Anopheles aquasalis response to Plasmodium vivax infection. PLoS One. 2013;8(2):e57014 Epub 2013/02/27. 10.1371/journal.pone.0057014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laubach HE, Validum L, Bonilla JA, Agar A, Cummings R, Mitchell C, et al. Identification of Anopheles aquasalis as a possible vector of malaria in Guyana, South America. The West Indian medical journal. 2001;50(4):319–21. Epub 2002/05/08. [PubMed] [Google Scholar]
- 28.Billker O, Shaw MK, Margos G, Sinden RE. The roles of temperature, pH and mosquito factors as triggers of male and female gametogenesis of Plasmodium berghei in vitro. Parasitology. 1997;115 (Pt 1):1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All data are available in the paper and its Supporting Information files.