Abstract
Background
Although the exact pathophysiology of preeclampsia is not fully understood, several elemental micronutrient abnormalities have been suggested to play a contributory role in preeclampsia.
Aims
To investigate the levels of calcium, magnesium, zinc and copper in women with preeclampsia.
Subjects and Methods
A case—control study was conducted in Omdurman Maternity Hospital, Sudan, during the period of September through December 2014. The cases were women with preeclampsia while healthy pregnant women were the controls. The medical and obstetrics history was gathered using questionnaires. The serum levels of calcium, magnesium, zinc and copper were measured using atomic absorption spectrophotometer.
Results
There was no significant difference between the two groups in their age, gestational age, parity and body mass index. Zinc and copper levels were not significantly different between the two groups. In comparison with the controls, women with preeclampsia had a significantly lower median (inter-quartile) serum calcium [7.6 (4.0─9.6) vs. 8.1 (10.6─14.2), mg/dl, P = 0.032] and higher levels of magnesium [1.9 (1.4─2.5) vs. 1.4 (1.0─1.9) mg/dl; P = 0.003]. In binary logistic regression, lower calcium (OR = 0.73, 95% CI = 0.56 ─ 0.95, P = 0.021) and higher magnesium (OR = 5.724, 95% CI = 1.23 ─ 26.50, P = 0.026) levels were associated with preeclampsia. There were no significant correlations between levels of hemoglobin and these trace elements.
Conclusion
The current study showed significant associations between preeclampsia and serum levels of calcium and magnesium.
Introduction
Preeclampsia is a common medical disorder affecting about 2–7% of pregnant women worldwide [1, 2] and can lead to unfavorable pregnancy outcomes such as increased maternal as well as perinatal morbidity and morbidity [3]. The etiology of preeclampsia remains ambiguous, albeit, reports that implicated placental defects and oxidative stress early during pregnancy in affected pregnancies [4, 5].
Micronutrients and trace elements play a pivotal role in metabolism and in the preservation of tissue function. Trace elements are important constituents of a number of antioxidants. Therefore they are integral part of a robust antioxidant that protect the cell from damage [6, 7].
Several elemental micronutrients abnormalities (calcium, magnesium, zinc, and copper) have been suggested to play a contributory role in preeclampsia [8–13]. However, the results of the reports describing the associations between serum concentration of zinc, copper, magnesium, calcium and preeclampsia varied greatly [2, 8, 14–17].
Therefore, research on trace elements and preeclampsia is of paramount importance for academicians, health planners as well as treating physicians. The findings of research on trace elements and preeclampsia is an evidence-base and could be implemented for prevention of preeclampsia e.g. calcium and zinc supplement [18]. In Sudan, preeclampsia/eclampsia is a major cause of maternal and perinatal morbidity and mortality [1, 19, 20]. We have previously shown that preeclampsia is associated with oxidative stress [21]. The current study was carried to investigate the levels of serum calcium, magnesium, zinc and copper in preeclampsia, to find their potential role in the etiology/pathogenesis of preeclampsia and to add to the previous research on preeclampsia in Sudan [21–23].
Materials and Methods
A case ─ control study was conducted in Omdurman Maternity Hospital, Sudan during the period of September through December 2014. Omdurman Maternity Hospital is the largest tertiary maternity hospital in the region. It serves an area of 6 million people and there are 24913 deliveries per year (around 70 deliveries per day) [24].
The cases were women presented with preeclampsia (before receiving any medications), which was defined as hypertension (occurrence of systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg after at least 20 weeks of pregnancy in a previously normotensive patient) plus at least 300 mg of protein in a 24-hour urine sample or a dipstick test result of at least 2+ [25]. Preeclampsia was further divided into mild and severe forms, according to a diastolic blood pressure of ≤110 mm Hg or > 110 mm Hg, respectively. Healthy pregnant women attended the prenatal care clinic of the same hospital during the study period were taken as the controls.
Women with diabetes mellitus, other endocrine disorder, and kidney disease were excluded from both cases and controls. After signing an informed consent a detailed history was obtained from all participants followed by physical and obstetrics examination. Weight and height were measured and body mass index was calculated and expressed as Kg/m2. An antecubital route was used to collect the blood samples from the antecubital vein of each of the patients and the controls. Sera were then centrifuged, separated and kept at 0–20°C till analysis. The serum calcium, magnesium, copper and zinc levels were measured using an atomic absorption spectrophotometer (SOLAAR, Atomic Absorption Spectrophotometer, Thermo Electron, Cambridge, UK) [26]. The quality assurance and assay accuracy were assured by using standard solutions for every ten test sample.
Statistics
Data was analyzed using SPSS for Windows (version 20.0). A total sample size of 50 participants in each group was calculated to get the difference in the mean of the proposed variables (levels of trace elements) that would provide 80% power to detect a 5% difference at α = 0.05, with an assumption that complete data might not be available for 10% of participants. T-test and X2 tests were used to compare the normally distributed data (Mann-Whitney U if the data were not normally distributed) continuous variables and proportions between the two groups. Binary regressions were performed, where preeclampsia was the dependent variable and socio-demographic parameters (age, parity, education, job, residence), trace elements levels were the independent variables. Odds ratio (OR) and 95% confidence interval (CI) were calculated. Pearson/ spearman correlation was performed. P < 0.05 was considered statistically significant.
Research Board at the Department of Obstetrics and Gynecology, Faculty of Medicine, University of Khartoum, Sudan specifically approved this study.
All procedures performed in this study were in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Results
While there was no significant difference between the two groups (50 women in each arm) of the study in their age, parity, gestational age and BMI, hemoglobin level was significantly lower in women with preeclampsia, Table 1.
Table 1. Comparing the mean (SD) of the basic characteristics between women with preeclampsia and controls.
| Variables | Preeclamptic women | Healthy controls | P |
|---|---|---|---|
| (n = 50) | (n = 50) | ||
| Age, year | 28.6 (6.4) | 28.6 (6.6) | 0.988 |
| Parity | 2.3 (2.3) | 2.7(2.8) | 0.390 |
| Gestational age, weeks | 37.1(1.0) | 36.8(1.0) | 0.190 |
| Body mass index, Kg/m2 | 29.0(5.0) | 27.0(5.1) | 0.139 |
| Hemoglobin, g/dl | 10.8(1.0) | 11.5 (1.3) | 0.018 |
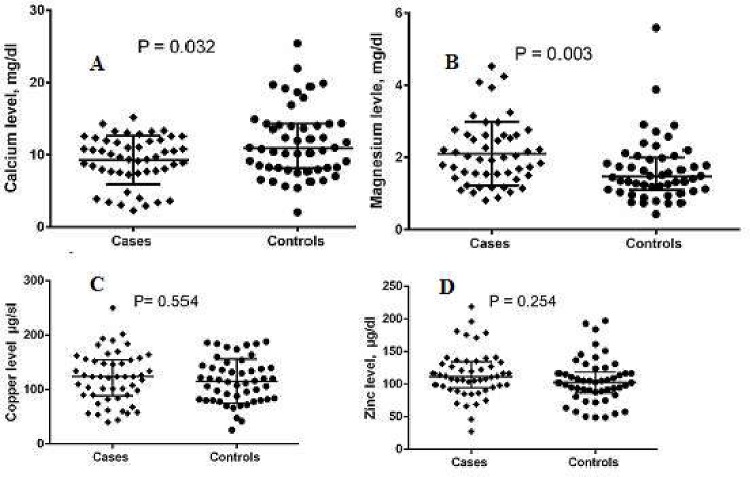
In comparison with the controls, women with preeclampsia (four and 46 were mild and severe preeclampsia, respectively) had significantly lower calcium [7.6 (4.0─9.6) vs. 8.1 (10.6─14.2), mg/dl, P = 0.032] and higher levels of magnesium [1.9 (1.4─2.5) vs. 1.4 (1.0─1.9) mg/dl; P = 0.003]. There were no significant differences in the zinc, copper levels and zinc/ copper ratio between women with preeclampsia and the controls, Table 2, Fig 1A–1D.
Table 2. Comparing the median (inter-quartile of the trace elements between women with preeclampsia and controls.
| Variables | Preeclamptic women | Healthy controls | P |
|---|---|---|---|
| (n = 50) | (n = 50) | ||
| Calcium, mg/dl | 7.6 (4.0─9.6) | 8.1 (10.6─14.2) | 0.032 |
| Magnesium, mg/dl | 1.9 (1.4─2.5) | 1.4 (1.0─1.9) | 0.003 |
| Zinc, μg/dl | 108.0 (91.6─131.7) | 102.0 (82.8─124.0) | 0.254 |
| Copper μg/dl | 111.6 (94.3─135.3) | 103.6 (86.6─126.7) | 0.554 |
Fig 1.
(A-D) Median (inter-quartile) of calcium, magnesium, zinc and copper levels women with in preeclampsia and controls.
In binary logistic regression, a low calcium (OR = 0.73, 95% CI = 0.56 ─ 0.95, P = 0.021) and a high magnesium (OR = 5.724, 95% CI = 1.23 ─ 26.50, P = 0.026) levels were associated with preeclampsia, Table 3.
Table 3. Binary logistic regression for the factors associated with preeclampsia.
| Variables | OR | 95% CI | P |
|---|---|---|---|
| Age, year | 0.98 | 0.79 ─1.21 | 0.854 |
| Parity | 1.06 | 0.69 ─ 1.64 | 0.768 |
| Gestational age, weeks | 1.61 | 0.67 ─ 3.85 | 0.283 |
| Body mass index, Kg/m2 | 1.14 | 0.95 ─ 1.36 | 0.141 |
| Hemoglobin, g/dl | 0.93 | 0.46 ─ 1.85 | 0.841 |
| Calcium, mg/dl | 0.73 | 0.56 ─ 0.95 | 0.021 |
| Magnesium, mg/dl | 5.72 | 1.23 ─ 26.50 | 0.026 |
| Zinc, μg/dl | 1.00 | 0.99 ─1.00 | 0.154 |
| Copper μg/dl | 0.98 | 0.96 ─ 1.05 | 0.056 |
There were no significant correlations between gestational age, BMI, hemoglobin and calcium, magnesium, zinc and copper. There was no significant correlation between the investigated trace elements, Table 4.
Table 4. Correlations between gestational age, body mass index, hemoglobin and trace elements.
| Variable | S. calcium | S. magnesium | S. zinc | S. copper | ||||
| r | P | r | P | r | P | r | P | |
| Gestational age, year | 0.023 | 0.820 | 0. | 119 0.237 | 0.007 | 0.941 | ─ 0.094 | 0.351 |
| Body mass index, Kg/m2 | ─ 0.187 | 0.230 | ─ 0.105 | 0.423 | ─ 0.051 | 0.698 | ─ 0.026 | 0.846 |
| Hemoglobin, g/dl | 0.031 | 0.794 | 0.272 | 0.018 | 0.014 | 0.224 | 0.102 | 0.381 |
| Calcium, mg/dl | ─ | ─ | ─ 0.077 | 0.446 | ─ 0.125 | 0.217 | 0.166 | 0.098 |
| Magnesium, mg/dl | ─ 0.077 | 0.446 | ─ | ─ | 0.086 | 0.395 | ─ 0.088 | 0.385 |
| Zinc, µg/dl | ─ 0.125 | 0.217 | 0.086 | 0.395 | ─ | ─ | ─ 0.135 | 0.182 |
Discussion
The main findings of the current study were; significantly lower calcium, higher magnesium levels in women with preeclampsia, calcium and magnesium were associated with preeclampsia. Our finding (higher magnesium level) is in concurrence with recent reports, where Katz et al found higher levels of magnesium among 43 preeclamptic women who received magnesium sulphate (the patients in the current study were enrolled before commencement of any medications) [27]. Recently, Farzin and Sajadi reported significantly lower levels of calcium, magnesium and zinc in preeclamptic women compared with the controls [16]. Earlier, Jain et al observed significantly lower levels of calcium, magnesium and zinc in women with preeclampsia (25 with mild and 25 with severe preeclampsia) than the normal pregnant controls [8]. On the other hand, Negi et al observed decreased levels of magnesium zinc, copper in the umbilical cord blood of preeclamptic and eclamptic pregnancies. Yet, Vafaei et al observed no significant difference in the serum levels of calcium, magnesium and zinc levels in the 40 normotensive pregnancies (controls), 20 mild and 20 severe preeclamptic Iranian women [15]. Furthermore, an association between preeclampsia and hypocalciuria [28], decreased urine calcium to creatinine ratio [29], and low dietary milk intake [30] has also been established.
In the current study, although there was a low calcium and a higher magnesium level in preeclamptic women, calcium and magnesium levels showed no significant correlation. Interestingly, previous reports have delineated a competition between magnesium and calcium with one another for common binding sites on plasma protein molecules [31]. The interaction at molecule level and carrier protein level should be considered when the level of calcium, magnesium and albumin is investigated [32, 33]. Moreover, studies have shown that magnesium counteracts calcium-dependent release of acetylcholine at motor endplates [31]. Thus, magnesium may be regarded as a natural ‘calcium antagonist’
Magnesium is a trace element of paramount importance; it acts as a cofactor for a number of enzyme systems [34]. Magnesium is important for tone, contractility, and reactivity of blood vessels and, therefore, plays a pivotal role in the physiological regulation of blood pressure. This enhances the understanding of the therapeutic importance of magnesium in the treatment of preeclampsia [35].
Calcium has a major role in the rise of blood pressure; however, a balance between calcium and magnesium is needed to control blood pressure. This is because blood vessels need calcium to contract and magnesium to relax and open up. Therefore, magnesium can be considered as a calcium channel blocker by antagonizing the increase in the intracellular calcium concentration leading to vasodilatation [36–38].
It is worth to be mentioned, that previous studies have reported a link between low dietary calcium intake with increased incidence of preeclampsia. Likewise, supplementation of calcium has been reported to prevent preeclampsia [39, 40].
The current study showed no significant difference in copper level between cases and controls. Previous studies, reported an association between high maternal serum copper and preeclampsia [16, 27]. However, others linked the association with caeruloplasmin activity on a background of a raised serum copper rather than just a raised serum copper, probably secondary to impaired antioxidant enzymes [41].
In the current study, there were no significant correlations between hemoglobin and the trace elements we studied. This goes with our previous observation where we observed no correlations between zinc, copper and hemoglobin levels [26, 42].
Limitations of the present study includes that, the possible interactions between trace elements and their carrying vehicle were ignored. Measurement of serum albumin, serum caeruloplasmin and pH in future studies will help in better interpretations of the present findings.
Conclusion
The current study findings indicate that the three trace elements; calcium and magnesium significantly associated with preeclampsia. On the other hand, zinc and copper were not associated with preeclampsia. Further researches that consider the complex relation between the trace elements and their carrier vehicle proteins are needed.
Supporting Information
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Adam I, Haggaz AD, Mirghani OA, Elhassan EM. Placenta Previa and Pre-Eclampsia: Analyses of 1645 Cases at Medani Maternity Hospital, Sudan. Frontiers in Physiology 2013;4:32 10.3389/fphys.2013.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mistry HD, Gill CA, Kurlak LO, Seed PT, Hesketh JE, Méplan C, et al. SCOPE Consortium. “Association between Maternal Micronutrient Status, Oxidative Stress, and Common Genetic Variants in Antioxidant Enzymes at 15 Weeks׳ Gestation in Nulliparous Women Who Subsequently Develop Preeclampsia.” Free Radical Biology & Medicine 2015; 78: 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steegers E.A, von Dadelszen P, Duvekot J.J, Pijnenborg R. Pre-eclampsia. Lancet 2010; 376:631–644. 10.1016/S0140-6736(10)60279-6 [DOI] [PubMed] [Google Scholar]
- 4.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet 2005;365:785–799. 10.1016/S0140-6736(05)17987-2 [DOI] [PubMed] [Google Scholar]
- 5.Poston L, Igosheva N, Mistry H.D, Seed P.T, Shennan A.H, Rana S, et al. Role of oxidative stress and antioxidant supplementation in pregnancy disorders. Am J Clin Nutr 2011; 94:1980S–1985S. 10.3945/ajcn.110.001156 [DOI] [PubMed] [Google Scholar]
- 6.Jaiser SR, Winston GP. Copper deficiency myelopathy: Review. J Neurol 2010; 257:869–81. 10.1007/s00415-010-5511-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins JF, Prohaska JR, Knutson MD. Metabolic crossroads of iron and copper. Nutrition reviews 2010; 68:133–147. 10.1111/j.1753-4887.2010.00271.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain S, Sharma P, Kulshreshtha S, Mohan G, Singh S. The role of calcium, magnesium, and zinc in pre-eclampsia. Biol Trace Elem Res 2010; 133:162–70. 10.1007/s12011-009-8423-9 [DOI] [PubMed] [Google Scholar]
- 9.Hovdenak N, Haram K. Influence of mineral and vitamin supplements on pregnancy outcome. Eur J Obstet Gynecol Reprod Biol 2012;164:127–32 review. 10.1016/j.ejogrb.2012.06.020 [DOI] [PubMed] [Google Scholar]
- 10.Negi R, Pande D, Karki K, Kumar A, Khanna RS, Khanna HD. Trace elements and antioxidant enzymes associated with oxidative stress in the pre-eclamptic/eclamptic mothers during fetal circulation. Clin Nutr 2012; 31:946–50. 10.1016/j.clnu.2012.04.005 [DOI] [PubMed] [Google Scholar]
- 11.Roohani N, Hurrell R, Kelishadi R, Schulin R. Zinc and its importance for human health: An integrative review. Journal of Research in Medical Sciences: The Official Journal of Isfahan University of Medical Sciences 2013; 18:144–157. [PMC free article] [PubMed] [Google Scholar]
- 12.Pathak P, Kapil U. Role of trace elements zinc, copper and magnesium during pregnancy and its outcome. Indian J Pediatr 2004; 71:1003–5. [DOI] [PubMed] [Google Scholar]
- 13.Peacock M. Calcium metabolism in health and disease. Clin J Am Soc Nephrol 2010; 5:S23–30. 10.2215/CJN.05910809 [DOI] [PubMed] [Google Scholar]
- 14.Al-Jameil N, Tabassum H, Al-Mayouf H, Aljohar HI, Alenzi ND, Hijazy SM, et al. Analysis of serum trace elements-copper, manganese and zinc in preeclamptic pregnant women by inductively coupled plasma optical emission spectrometry: a prospective case controlled study in Riyadh, Saudi Arabia. Int J Clin Exp Pathol 2014; 7:1900–10. eCollection 2014. [PMC free article] [PubMed] [Google Scholar]
- 15.Vafaei H, Dalili M, Hashemi SA. Serum concentration of calcium, magnesium and zinc in normotensive versus preeclampsia pregnant women: A descriptive study in women of Kerman province of Iran. Iran J Reprod Med 2015; 13:23–6. [PMC free article] [PubMed] [Google Scholar]
- 16.Farzin L, Sajadi F. Comparison of serum trace element levels in patients with or without pre-eclampsia. J Res Med Sci 2012; 17:938–41. [PMC free article] [PubMed] [Google Scholar]
- 17.Sarwar MS, Ahmed S, Ullah MS, Kabir H, Rahman GK, Hasnat A, et al. Comparative study of serum zinc, copper, manganese, and iron in preeclamptic pregnant women. Biol Trace Elem Res 2013; 154:14–20. 10.1007/s12011-013-9721-9 [DOI] [PubMed] [Google Scholar]
- 18.Imdad A, Jabeen A, Bhutta ZA.Role of calcium supplementation during pregnancy in reducing risk of developing gestational hypertensive disorders: a meta-analysis of studies from developing countries. BMC Public Health 2011; 11:S18 10.1186/1471-2458-11-S3-S18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali AA, Okud A, Khojali A, Adam I. High incidence of obstetric complications in Kassala Hospital, Eastern Sudan. J Obstet Gynaecol 2012; 32:148–9. 10.3109/01443615.2011.637140 [DOI] [PubMed] [Google Scholar]
- 20.Ali AA, Adam I. Lack of antenatal care, education, and high maternal mortality in Kassala hospital, eastern Sudan during 2005–2009. J Matern Fetal Neonatal Med 2011; 24:1077–8. 10.3109/14767058.2010.545908 [DOI] [PubMed] [Google Scholar]
- 21.Bueno AA, Ghebremeskel K, Bakheit KH, Elbashir MI, Adam I. Dimethyl acetals, an indirect marker of the endogenous antioxidant plasmalogen level, are reduced in blood lipids of Sudanese pre-eclamptic subjects whose background diet is high in carbohydrate. J Obstet Gynaecol 2012; 32:241–6. 10.3109/01443615.2011.641622 [DOI] [PubMed] [Google Scholar]
- 22.Bakheit KH, Ghebremeskel K, Pol K, Elbashir MI, Adam I. Erythrocyte omega-3 and omega-6 fatty acids profile in Sudanese women with pre-eclampsia. J Obstet Gynaecol 2010; 30:151–4. 10.3109/01443610903391005 [DOI] [PubMed] [Google Scholar]
- 23.Elhaj ET, Adam I, Alim A, Elhassan EM, Lutfi MF. Thyroid Function/Antibodies in Sudanese Patients with Preeclampsia. Front Endocrinol (Lausanne) 2015; 11;6:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlson VM, Omer MI, Ibrahim SA, Ahmed SE, O'Byrne KJ, Kenny LC, et al. Fifty years of Sudanese hospital-based obstetric outcomes and an international partnership. BJOG 2011; 118:1608–16. 10.1111/j.1471-0528.2011.03092.x [DOI] [PubMed] [Google Scholar]
- 25.ACOG technical bulletin. Hypertension in pregnancy. Number 219—January 1996 (replaces no. 91, February 1986). Committee on Technical Bulletins of the American College of Obstetricians and Gynecologists. Int J Gynecol Obstet 1996; 53:175–83. [PubMed] [Google Scholar]
- 26.Bushra M, Elhassan EM, Ali NI, Osman E, Bakheit KH, Adam I. Anaemia, zinc and copper deficiencies among pregnant women in central Sudan. Biol Trace Elem Res 2010; 137:255–61. 10.1007/s12011-009-8586-4 [DOI] [PubMed] [Google Scholar]
- 27.Katz O, Paz-Tal O, Lazer T, Aricha-Tamir B, Mazor M, Wiznitzer A, et al. Severe pre-eclampsia is associated with abnormal trace elements concentrations in maternal and fetal blood. J Matern Fetal Neonatal Med 2012; 25:1127–30 10.3109/14767058.2011.624221 [DOI] [PubMed] [Google Scholar]
- 28.Pal A, Roy D, Adhikary S, Roy A, Dasgupta M, Mandal AK. A Prospective Study for the Prediction of Preeclampsia with Urinary Calcium Level. Journal of Obstetrics and Gynaecology of India 2012; 62:312–316. 10.1007/s13224-012-0223-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheela CN, Beena SR, Mhaskar A. Calcium-creatinine ratio and microalbuminuria in prediction of preeclampsia. Journal of Obstetrics and Gynaecology of India 2011; 61:72–76. [Google Scholar]
- 30.Duvekot EJ, de Groot CJ, Bloemenkamp KW, Oei SG. Pregnant women with a low milk intake have an increased risk of developing preeclampsia. Eur J Obstet Gynecol Reprod Biol 2002; 105:11–4. [DOI] [PubMed] [Google Scholar]
- 31.Jahnen-Dechent W, Ketteler M. Magnesium basics. Clin Kidney J 2012; 5:i3–i14. 10.1093/ndtplus/sfr163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CARR CW. Competitive binding of calcium and magnesium with serum albumin. Proc Soc Exp Biol Med 1955; 89:546–9. [DOI] [PubMed] [Google Scholar]
- 33.Wang S, McDonnell EH, Sedor FA, Toffaletti JG. pH effects on measurements of ionized calcium and ionized magnesium in blood. Arch Pathol Lab Med 2002; 126:947–50. [DOI] [PubMed] [Google Scholar]
- 34.Sissi C, Palumbo M. Effects of magnesium and related divalent metal ions in topoisomerase structure and function. Nucleic Acids Research 2009;37:702–711. 10.1093/nar/gkp024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walsh SB, Zdebik AA, Unwin RJ. Magnesium: The Disregarded Cation. Mayo Clin Proc 2015; 90:993–5. 10.1016/j.mayocp.2015.06.011 [DOI] [PubMed] [Google Scholar]
- 36.Touyz RM. Role of magnesium in pathogenesis of hypertension. Mol Aspects Med 2003; 24:107–136. [DOI] [PubMed] [Google Scholar]
- 37.Sukonpan K, Phupong V. Serum calcium and serum magnesium in normal and preeclamptic pregnancy. Arch Gynecol Obstet 2005; 273:12–16. 10.1007/s00404-004-0672-4 [DOI] [PubMed] [Google Scholar]
- 38.Power ML, Heaney RP, Kalkwarf HJ, Pitkin RM, Repke JT, Tsang RC, et al. The role of calcium in health and disease. Am J Obstet Gynecol 1999; 181:1560–9. [DOI] [PubMed] [Google Scholar]
- 39.Belizan JM, Villar J, Repke J. The relationship between calcium intake and pregnancy-induced hypertension: up-to-date evidence. Am J Obstet Gynecol 1988; 158:898–902. [DOI] [PubMed] [Google Scholar]
- 40.Levine RJ, Hauth JC, Curet LB, Sibai BM, Catalano PM, Morris CD, et al. Trial of calcium to prevent preeclampsia. N Eng J Med 1997; 337:69–76. [DOI] [PubMed] [Google Scholar]
- 41.Mistry H, Kurlak L, Gill C, Chappell L, Morgan L, Poston L. PP027. Alterations in maternal antioxidant micronutrient concentrations in women prior to developing pre-eclampsia. Pregnancy Hypertens 2013;3:76–7. [DOI] [PubMed] [Google Scholar]
- 42.Mohamed AA, Ali AA, Ali NI, Abusalama EH, Elbashir MI, Adam I. Zinc, parity, infection, and severe anemia among pregnant women in Kassla, eastern Sudan. Biol Trace Elem Res 2011; 140:284–90. 10.1007/s12011-010-8704-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.