Abstract
Aim:
Tissue Doppler Imaging (TDI) detects early signs of left ventricular dysfunction. The prognostic potential of TDI in patients with atrial fibrillation (AF) has, however, not yet been clarified. This study evaluates the prognostic value of TDI in patients with atrial fibrillation.
Methods and Results:
In total, echocardiograms from 313 patients with AF during examination were analyzed offline. Longitudinal systolic velocity (s’), early diastolic velocity (e’) and longitudinal displacement (LD) were measured by color TDI. During a median follow-up of 891 days, 64 patients (20%) died.
TDI was significantly associated with all-cause mortality, and the risk of dying increased significantly per 1 cm/s decrease in s’ (HR of 1.31, 95% CI 1.05-1.63; p=0.018) and e’ (HR of 1.17, 95% CI 1.01-1.35; p=0.038) respectively, even after adjustment for age, gender, heart rate, aortic stenosis, DM and LVEF quartiles.
LD also proved to be a significant predictor of outcome after multivariate adjustment (HR 1.23; 95% CI 1.05-1.44; p=0.012).
The population was stratified according to high or low s’ and e’. Patients with low s’ and e’ had more than three times the risk of mortality compared to the patients with high s’ and e’ (HR 3.64; 95% CI 1.83-7.26; p<0.001) and remained in significantly higher risk after adjustment for various risk factors.
Conclusions:
Both systolic and diastolic performance, as assessed by TDI, are strong predictors of mortality in patients with atrial fibrillation, and especially the combination of systolic and diastolic dysfunction is a significant prognostic marker.
Keywords: Echocardiography, Tissue Doppler Imaging, Atrial Fibrillation, Outcome, Mortality
Introduction
Atrial fibrillation (AF) is the most common of all cardiac arrhythmias and affects about 1-2% of the general population. It occurs in less than 4% of those under the age of 60 but in more than 9% of those over 80 years of age.[1] The high prevalence makes atrial fibrillation a frequent cause of illness and hospitalisation in the western world and the prevalence is expected to double over the next 50 years.[2] AF is responsible for impaired quality of life as well as more severe conditions such as heart failure, every fifth stroke, and in the worst cases, death.[3]
As the incidence of AF and its complications are expected to increase in the population, an increasing amount of resources in health care will be needed in the form of frequent hospitalisations, increased consumption of drugs and invasive interventions, care and other support measures. Consequently, efforts to improve risk-stratification, clarify pathophysiological mechanisms and identify targets for therapeutic intervention in AF are of the utmost importance.
Tissue Doppler Imaging (TDI) has proven to be a useful and reproducible tool for assessment of systolic and diastolic function.[4,5] The prognostic role of tissue Doppler velocities has been explored and proven to have strong predictive power in various diseases[6] such as hypertension,[7] diabetes,[8] ischemic heart disease[9,10] and heart failure.[11,12] Relatively small populations have limited current studies of TDI and AF and evaluation of longitudinal systolic and diastolic function of the left ventricle in AF determined by TDI remains to be investigated. The aim of this study was to evaluate the prognostic value of longitudinal systolic and diastolic function by TDI in patients with AF.
Methods
Data Source
The Department of Cardiology, Gentofte Hospital Copenhagen performs routine echocardiograms according to a standardised protocol. These echocardiograms have been stored in a database on a local hard disk since 2005.
Study Population
The study is a retrospective echocardiographic cohort study. Relevant patients (n=1,016) were identified in the database by searching for echocardiograms from patients with AF. Of the 1,016 AF patients identified, 418 patients fulfilled the inclusion criteria: 1) presence of AF during examination and 2) the echocardiographic examination included color TDI-recordings. 105 patients were excluded due to various reasons: invalid social security number (n=10), poor echocardiographic quality (n=68), and duplicates (n=27). Thus, the study sample consisted of 313 patients.
Information about co-morbidity at baseline was collected in the form of ICD-10 codes (A- and B-diagnoses) noted in the same hospitalisation as the examination was conducted. Also, information about condition and co-morbidity was noted from the report attached to the echocardiographic examination included in the study.
Echocardiography
All echocardiograms were stored digitally using Image Vault (GE Healthcare, Horten Norway) and analysed offline with commercially available software (EchoPac, BT11) by a single investigator who was blinded to all other patient data. If the examination contained more than one heart cycle, the first cycle was used. All measurements were performed in the 4-chamber, 2-chamber and apical long axis view, meaning that all parameters were calculated based on an average of three cardiac cycles to account for beat-to-beat variations.
Conventional Echocardiography
From the parasternal long-axis view, the left ventricle (LV) diameter and wall thicknesses (posterior and interventricular wall) were measured at the level of the mitral valve tips, thereby ensuring a measurement perpendicular to the long axis of the ventricle. From the apical four and two chamber positions, LV ejection fraction (LVEF) was obtained using modified biplane Simpson’s method. Left atrial (LA) volume was estimated by the biplane area-length method. Pulsed wave Doppler at the apical position was used to obtain mitral inflow velocities between the tips of the mitral leaflets. E/e’ was calculated as early mitral inflow velocity (E) divided by the average of septal and lateral mitral annular peak early diastolic velocity (e’) obtained by pulsed wave TDI.
Color Tissue Doppler Imaging
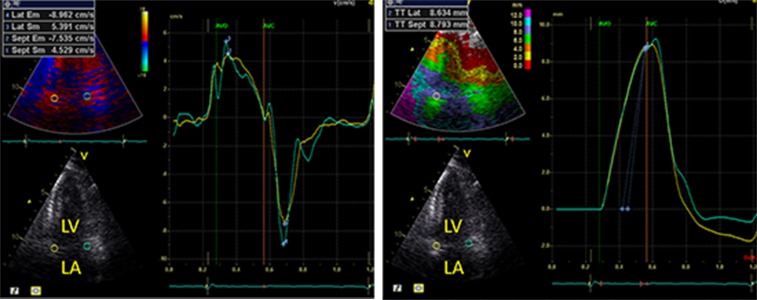
Color TDI loops were obtained in the apical 4-chamber, 2-chamber and apical long-axis view. Peak longitudinal systolic (s’) and early diastolic (e’) velocities were measured at six mitral annular sites (Fig. 1a) obtained by dividing the LV into six segments of interest: the septal, lateral, anterior, inferior, posterior and anteroseptal myocardial wall. By averaging the myocardial velocities from the six mitral annular sites, an assessment of global longitudinal performance of the left ventricle is obtained. Inter- and intra-observer variability of mitral anular veloities estimated by color TDI have been reported to be low.[13,14,15] Also, longitudinal displacement was measured by tissue tracking in all six segments in the three apical projections and averaged to assess global longitudinal displacement (Fig. 1b).
Figure 1. Color Tissue Doppler Imaging Curves Examples of mitral annular longitudinal A) myocardial velocity curves and B) displacement curves.
Strain and Strain Rate Imaging
If frame rate was sufficient (i.e. ≥40 frames per second), strain and strain rate (SR) was measured by speckle tracking (n = 305, 61 events). Peak global longitudinal systolic strain and SR was measured and averaged to provide global strain and SR.
Follow-Up and Endpoint
The endpoint was defined as all-cause mortality. Data was obtained using a unique personal social security number issued by the Central Office of Civilian Registration. Follow-up was 100%.
Statistics
In Table 1, continuous Gaussian distributed variables were compared using student’s t-test and χ2-test was used for proportions.
Table 1. Baseline clinical and echocardiographic characteristics in patients with atrial fibrillation stratified according to death.
| Baseline characteristics | Alive at follow-up (n = 249) | Dead at follow-up (n = 64) | P-value | |||
|---|---|---|---|---|---|---|
| Age (years) | 71±10 | 79±8 | <0.001 | |||
| Male gender | 70% | 58% | 0.14 | |||
| Heart rate (beats/min) | 88±26 | 95±35 | 0.08 | |||
| Diabetes Mellitus (%) | 5% | 17% | 0.002 | |||
| Hypertension (%) | 9% | 17% | 0.07 | |||
| Thyroid toxicity/metabolic disease (%) | 0% | 3% | 0.11 | |||
| Ischaemic heart disease (%) | 16% | 16% | 1.00 | |||
| STEMI (%) | 2% | 5% | 0.40 | |||
| NSTEMI (%) | 4% | 8% | 0.20 | |||
| Valvular disease | 35% | 45% | 0.15 | |||
| Aortic stenosis (%) | 12% | 23% | 0.027 | |||
| Mitral stenosis (%) | 2% | 2% | 1.00 | |||
| Aortic insufficiency (%) | 10% | 9% | 1.00 | |||
| Mitral insufficiency (%) | 19% | 22% | 0.60 | |||
| Mitral valve prosthesis (%) | 2% | 2% | 1.00 | |||
| Aortic valve prosthesis (%) | 5% | 8% | 0.36 | |||
| Heart failure (%) | 27% | 34% | 0.28 | |||
| Conventional echocardiographic measurements | ||||||
| LVEF (%) | 47±8 | 41±10 | <0.001 | |||
| IVSd (cm) | 1.1±0.00 | 1.1±0.00 | 0.12 | |||
| LVIDd (cm) | 4.9±0.01 | 4.8±0.01 | 0.32 | |||
| LVPWd (cm) | 1.1±0.00 | 1.1±0.00 | 0.12 | |||
| Color Tissue Doppler Imaging | ||||||
| s’ (cm/s) | 4.4 ±1.5 | 3.7±1.3 | <0.001 | |||
| e’ (cm/s) | 7.5±2.2 | 5.9±1.9 | <0.001 | |||
| LD (mm) | 6.0±2.2 | 4.7±1.9 | <0.001 | |||
| Strain | ||||||
| Global strain (%) | -11.7±3.99 | -10.9±4.20 | 0.13 | |||
| Global strain rate s’ (s-1) | -0.78±0.24 | -0.75±0.27 | 0.14 | |||
| 1.02±0.40 | 0.94±0.39 | 0.48 |
Cumulative survival curves were established by the Kaplan–Meier method. Hazards ratios (HR) were calculated by uni- and multivariable Cox proportional hazards regression analyses. The assumption of proportional hazards was tested. Harrell’s c-statistics were calculated for the conventional echocardiographic parameters (LVEF and E/e’) and for the conventional echocardiographic parameters in combination with s’+e’. First order interaction was tested among valve diseases and TDI parameters and Bonferroni correction was used to exclude multiple significances.
Bland Altman analysis was used to assess intra and interobserver variability of tissue Doppler measures of systolic and diastolic heart function in 20 randomly selected patients and was expressed as mean difference ± 1.96 standard deviation (SD) and as coefficient of variation (CV).
P-values ≤ 0.05 in 2-sided tests were considered statistically significant. The statistical software used was SPSS for Mac version 20.0.
Results
Baseline characteristics are displayed in Table 1. During a median follow-up of 891 days (range: 5-2493 days) 64 patients (20%) died.
Myocardial Longitudinal Function
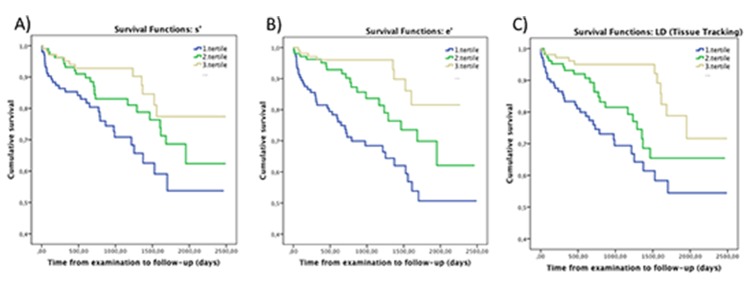
s’ and e’ were significantly lower in the group of patients who died compared to the patients still alive at follow-up (Table 1). The risk of mortality increased with decreasing systolic and diastolic performance by TDI (Fig. 2a-b), even after multivariable adjustments (Table 2). No significant interaction was found between systolic and diastolic performance and valvular disease.
Figure 2. Cumulative survival for patients stratified into tertiles of s’, e’ and LD A) Kaplan-Meier curves depicting cumulative probability of staying event free for patients stratified into tertiles of global s’, upper cutoff point being (4.8 cm/s) and lower cutoff point being (3.6 cm/s). B) Kaplan-Meier curves depicting cumulative probability of staying event free for patients stratified into tertiles of global e’, upper cutoff point being (8.0 cm/s) and lower cutoff point being (6.1 cm/s). C) Kaplan-Meier curves depicting cumulative probability of staying event free for patients stratified into tertiles according to values of global LD, upper cutoff point being (6.6 mm) and lower cutoff point being (4.6 mm).
Table 2. Unadjusted and adjusted Cox proportional hazards regression models of global tissue Doppler velocities as predictors of outcome.
DM = diabetes mellitus, LVEF = Left Ventricular Ejection Fraction, s’ = global peak systolic longitudinal mitral annular velocity determined by color Tissue Doppler Imaging, e’ = global peak early diastolic longitudinal mitral annular velocity determined by color Tissue Doppler Imaging, LD = global longitudinal displacement determined by color Tissue Doppler Imaging, E/E’ = transmitral to mitral anular early diastolic velocity ratio
| Endpoint (64 events) | Hazard Ratio (95% CI) | P-value |
|---|---|---|
| Unadjusted model | ||
| s’ per 1 cm/s decrease | 1.42 (1.18-1.72) | <0.001 |
| e’ per 1 cm/s decrease | 1.34 (1.18-1.52) | <0.001 |
| LD pr 1 mm decrease | 1.32 (1.17-1.50) | <0.001 |
| Sum of s’ and e’ per 1 cm/s decrease | 1.22 (1.13-1.33) | <0.001 |
| E/e' | 1.04 (1.02-1.07) | <0.001 |
| Multivariable model adjusted for age and gender | ||
| s’ per 1 cm/s decrease | 1.39 (1.14-1.70) | <0.001 |
| e’ per 1 cm/s decrease | 1.25 (1.09-1.42) | <0.001 |
| LD per 1 mm decrease | 1.28 (1.12-1.46) | <0.001 |
| Sum of s’ and e’ per 1 cm/s decrease | 1.19 (1.09-1.30) | <0.001 |
| E/e' | 1.03 (1.01-1.06) | 0.014 |
| Multivariable model adjusted for age, gender, heart rate, aortic stenosis and DM | ||
| s’ per 1 cm/s decrease | 1.41 (1.16-1.72) | <0.001 |
| e’ per 1 cm/s decrease | 1.23 (1.08-1.41) | 0.002 |
| LD per 1 mm decrease | 1.29 (1.12-1.49) | <0.001 |
| Sum of s’ and e’ per 1 cm/s decrease | 1.18 (1.08-1.30) | <0.001 |
| E/e' | 1.03 (1.00-1.05) | 0.06 |
| Multivariable model adjusted for age, gender, heart rate, aortic stenosis, DM and LVEF quartiles | ||
| s’ per 1 cm/s decrease | 1.33 (1.05-1.67) | 0.016 |
| e’ per 1 cm/s decrease | 1.19 (1.02-1.38) | 0.029 |
| LD per 1 mm decrease | 1.23 (1.05-1.43) | 0.010 |
| Sum of s’ and e’ per 1 cm/s decrease | 1.15 (1.03-1.27) | 0.009 |
| E/e' | 1.02 (1.00-1.05) | 0.13 |
Longitudinal function was assessed by global strain and strain rate and although statistically insignificant (Table 1), there was a trend towards lower strain and strain rate in the patients who died at follow-up.
Combining Tissue Doppler Velocities
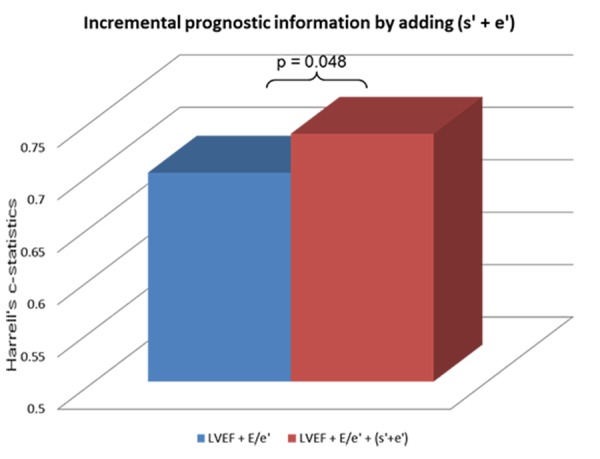
Combining the information on systolic and diastolic function by combining global s’ and global e’ (s’+e’) provided a significant risk marker of death (Table 2). Furthermore, using the mean value of global s’ and e’ as cutoff, the population was stratified according to high or low s’ and e’ and patients were divided into four groups: patients with high s’ and e’, high s’ and low e’, low s’ and high e’, and low s’ and e’. Isolated impaired systolic or diastolic function determined by a low value of either s’ or e’ did not seem to provide prognostic information (Table 3). Compared to patients with high global s’ and e’, patients with both low global s’ and e’ had more than three times the risk of death (Table 3). Even after adjustment for age, gender, heart rate, aortic stenosis, diabetes mellitus (DM) and LVEF quartiles, these patients remained in significantly higher risk of death (HR 2.21, 95% CI 1.00-4.86, p=0.049). Furthermore, the combined systolic and diastolic global tissue Doppler velocities (s’+e’) provided incremental prognostic information to the conventional echocardiographic parameters of systolic and diastolic function (LVEF and E/e’), determined by a significant increase in the Harrell’s c-statistics (LVEF+E/e’: 0.70 (0.62-0.66) vs. LVEF+E/e’+(s’+e’): 0.74 (0.66-0.81), p=0.048) (Fig. 3).
Table 3. Cox proportional hazardsregression model depicting the risk of reaching the combined endpoint for patients stratified according to high and low global s’ and e’.
High and low global s’ and e’ refer to above or below the mean (cut-off points 4.27 cm/s for s’ and 7.15 cm/s for e’). global peak systolic longitudinal mitral annular velocity determined by color Tissue Doppler Imaging, e’ = global peak early diastolic longitudinal mitral annular velocity determined by color Tissue Doppler Imaging. * = Remained in significantly higher risk of death after adjustment for age, gender, heart rate, aortic stenosis, DM and LVEF quartiles
| Hazard Ratio | 95% CI | P-value | |
|---|---|---|---|
| High s’, high e’ (n = 99) | |||
| High s’, low e’ (n = 47) | 0.70 | 0.19 - 2.53 | 0.58 |
| Low s’, high e’ (n = 41) | 2.32 | 0.94 - 5.72 | 0.07 |
| Low s’, low e’ (n = 126) | 3.74* | 1.88 - 7.46 | <0.001 |
Figure 3. Incremental prognostic information by adding an assessment of s’+e’ to the conventional echocardiographic parameters The Harrell’s c-statistic values obtained from the multivariate Cox proportional hazards regression models. Model 1 includes LVEF and E/e’. Model 2 includes LVEF, E/e’ and (s’+e’).
The cutoff values determined in the current study were 4.27 cm/s for s’ and 7.15 cm/s for e’, respectively and were found to be very similar to the prespecified cutoff-values of 5 cm/s for s’ and 7 cm/s for e’ proposed by Shin and colleagues in heart failure with preserved ejection fraction and AF.
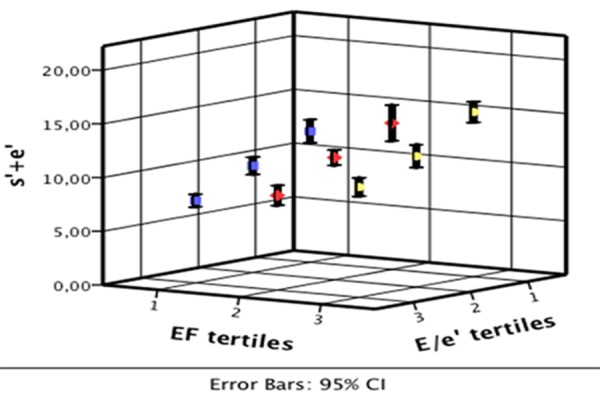
The association between (s’+e’) and conventional echocardiographic parameters of systolic and diastolic function (LVEF and E/e’) are depicted in Fig. 4. s’+e’ increased independently with decreasing E/e’ (p<0.001) and increasing LVEF (p<0.001).
Figure 4. The association between s’+e’, LVEF and E/e’ The association between the combined systolic and early diastolic tissue Doppler velocities and two conventional echocardiographic measurements reflecting systolic (LVEF) and diastolic function (E/e’), showing that s’+e’ correlates with both.
Reproducibility
Intraobserver and interobserver variability was low for all obtained tissue Doppler measures, as is shown by the mean difference being close to zero, a narrow standard deviation, and a low CV for each measurement (Table 4). The smallest variability was seen in intraobserver measures.
Table 4. Intra and interobserver variabilty of tissue Doppler derived measures of peak systolic and diastolic velocity and maximal systolic displacement.
Displaying the mean difference and the coefficient of variation. SD = standard deviation, CV = coefficient of variation, s’ = global peak systolic longitudinal mitral annular velocity determined by color Tissue Doppler Imaging, e’ = global peak early diastolic longitudinal mitral annular velocity determined by color Tissue Doppler Imaging, LD = global longitudinal displacement determined by color Tissue Doppler Imaging
| Intraobserver variability (n=20) | Interobserver variability (n=20) | |||
|---|---|---|---|---|
| Mean±1.96SD | CV (%) | Mean±1.96SD | CV (%) | |
| High s’, high e’ (n = 99) | 0.03±0.15 | 2 | -0.06±0.47 | 5 |
| Low s’, high e’ (n = 41) | -0.09±0.30 | 2 | 0.15±0.55 | 4 |
| Low s’, low e’ (n = 126) | 0.04±0.30 | 2 | -0.10±0.54 | 4 |
Discussion
So far, evaluation of systolic and diastolic function in patients with AF has been a clinical challenge, especially due to variable cycle lengths and the lack of the late diastolic velocity wave, the A and the a’ wave[16] (Fig. 1a). Also, AF patients are often excluded in echocardiographic studies. This study is the first to illustrate that the prognostic information obtained by tissue Doppler imaging is applicable to patients with AF.
In the literature, many suggestions have been put forward with regards to risk stratification of patients with AF. Among these, mitral valve deceleration time (DT), which provides information on left ventricular diastolic function, has been suggested. Matsukida and colleagues proposed a cutoff value of DT ≤ 100 ms as a predictor of pulmonary capillary wedge pressure and thus an evaluation of diastolic function in patients with AF. In the present study, however, we found no prognostic value of DT (Table 1).
E/e’ has been shown to be a predictor of clinical outcome in patients with non-valvular AF.[17] Similarly we found that E/e’ predicted risk of mortality independent of age and gender. E/e’ did not, however, seem to provide prognostic information after multivariable adjustments for age, gender, heart rate, aortic stenosis, DM and LVEF (Table 2). This might be due to the varied population, as the current study included both valvular and non-valvular AF patients. However, we did not find any interaction between valve disease and echocardiographic parameters for death.
Strain and Strain Rate Imaging
Both strain and SR have proven to be associated with various diseases such as hypertension, diabetes, ischemic heart disease, mitral regurgitation and cardiomyopathies.[18] Additionally, due to the regional myocardial changes that accompany myocardial ischemia, strain and SR have been found to have great prognostic value, especially in patients with myocardial infarction.[19] Strain and SR might be advantageous in the assessment of diastolic function in patients with AF.[16]
The majority of research within this area has mainly investigated strain and SR measured in the left atrium in patients with AF. SR, measured in the left atrium, has been shown to provide a valid quantification of LA function in patients with LA dilatation due to AF and aging.[20] Furthermore, in patients with paroxysmal AF, decreased left atrial SR has been shown to be independently associated with paroxysmal AF.[21] Also, left atrial SR was significantly lower in patients with AF than in age-matched controls.[20]
Tops and collegues[22] demonstrated that global longitudinal strain and SR measured in the LV improved significantly in patients who maintained sinus rhythm following catheter ablation as opposed to patients with recurrent AF. The patients with a recurrence of AF had a significant decrease in LV longitudinal strain and SR. This might be due to the longitudinal fibers in the subendocardium that are be more prone to pathological changes in response to the fibrillating atria, and global longitudinal strain and SR imaging might better detect these pathological changes during diastolic filling. Nevertheless, global strain and SR did not seem to be significant prognosticators of death in the present study (Table 1). It has previously been stated that strain and SR measurements are difficult to apply in patients with tachycardia as the optimal frame rate for speckle tracking is 50-70 frames per second (FPS).[18,23] Compared to TDI which has an optimal frame rate of >180 FPS, the low frame rate of strain and SR imaging might result in undersampling in patients with tachycardia or other patients with a high heart rate, which is often the case with AF patients. This might to some extent explain our findings with regards to strain and SR being insignificantly associated with death in patients with AF.
Color Tissue Doppler Imaging
The prognostic value of TDI velocities has been validated in several studies.[6,24]
In the current study, global systolic and diastolic peak velocities and longitudinal displacement was shown to provide independent prognostic information in an otherwise difficult patient population. These echocardiographic markers were shown to be highly significant in predicting outcome in patients with AF, even after adjusting for other conventional risk factors and LVEF quartiles.
In a study by Shin and colleagues,[25] the relationship between systolic and diastolic parameters and the prognosis of patients with heart failure with preserved ejection fraction and AF was investigated. In concordance with our findings, Shin et al. found that s’ and e’ velocities had prognostic potential to predict adverse clinical outcomes. Furthermore they suggested that the combination of the two velocities would be a good marker of prognosis, and that the combined information of systolic and diastolic dysfunction may be used for risk stratification. Cutoff points at s’< 5 cm/s and e’< 7 cm/s were suggested as clinical binary cutoff-points and, according to the study, patients with values below these cutoffs had a 12-fold higher risk of cardiac events. As systole and diastole are coherent and interdependent, it may be valuable to consider assessment of both s’ and e’ in the same risk stratification strategy in patients with AF. In accordance with this, the combined information on systolic and diastolic function in the present study seems to provide valuable information about prognosis in patients with AF. Our group has previously demontstrated the same pattern in patients with ST-segment elevation myocardial infarction.[26]
Furthermore, we found an association between the combined systolic and diastolic global tissue Doppler velocities (s’+e’) and conventional echocardiographic measurements of systolic (LVEF) and diastolic function (E/e’) (Fig. 4). Interestingly, s’+e’ seems to provide additional prognostic information as it is a significant independent predictor, even after adjustment for E/e’ and LVEF (and age, gender, heart rate, aortic stenosis and DM).
Although all velocity measurements proved to have prognostic potential in the evaluation of the numeric values (Table 2), isolated impaired systolic or diastolic dysfunction, determined by a low value of one velocity alone (Table 3), did not seem to provide statistically significant information on prognosis when the other velocity was high (Table 3). The combination of an impaired systolic and diastolic function, however, provides great prognostic value as this patient group had more than 3 times the risk of death compared to the group with high values of both s’ and e’ (p<0.001). Furthermore, (s’+e’) provided prognostic information above and beyond conventional echocardiographic parameters of systolic and diastolic function (Fig. 3).
Limitations
This retrospective study included only clinical echocardiographic examinations from the period 2005-2012 and echocardiograms were analyzed in the light of previous clinical reports, i.e. conventional echocardiographic data already noted in the downloaded examination was included in the study. This may have resulted in interobserver errors when assessing the echocardiographic examinations. Many previous studies states, that TDI is an independent predictor in sinus rhythm. We have not assessed the TDI velocities in SR as the purpose of the study was to evaluate the predictive value of TDI in AF.
Conclusions
The systolic and diastolic performances, as assessed by TDI, are strong predictors of mortality in patients with AF. The combination of systolic and diastolic dysfunction is especially a significant marker, which provides prognostic information incremental to conventional echocardiographic parameters of systolic and diastolic function.
Disclosures
None.
References
- 1.Go A S, Hylek E M, Phillips K A, Chang Y, Henault L E, Selby J V, Singer D E. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001 May 9;285 (18):2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Camm A John, Kirchhof Paulus, Lip Gregory Y H, Schotten Ulrich, Savelieva Irene, Ernst Sabine, Van Gelder Isabelle C, Al-Attar Nawwar, Hindricks Gerhard, Prendergast Bernard, Heidbuchel Hein, Alfieri Ottavio, Angelini Annalisa, Atar Dan, Colonna Paolo, De Caterina Raffaele, De Sutter Johan, Goette Andreas, Gorenek Bulent, Heldal Magnus, Hohloser Stefan H, Kolh Philippe, Le Heuzey Jean-Yves, Ponikowski Piotr, Rutten Frans H. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace. 2010 Oct;12 (10):1360–420. doi: 10.1093/europace/euq350. [DOI] [PubMed] [Google Scholar]
- 3.Wolf P A, Abbott R D, Kannel W B. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991 Aug;22 (8):983–8. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 4.Yu Cheuk-Man, Sanderson John E, Marwick Thomas H, Oh Jae K. Tissue Doppler imaging a new prognosticator for cardiovascular diseases. J. Am. Coll. Cardiol. 2007 May 15;49 (19):1903–14. doi: 10.1016/j.jacc.2007.01.078. [DOI] [PubMed] [Google Scholar]
- 5.Mogelvang Rasmus, Sogaard Peter, Pedersen Sune A, Olsen Niels T, Schnohr Peter, Jensen Jan S. Tissue Doppler echocardiography in persons with hypertension, diabetes, or ischaemic heart disease: the Copenhagen City Heart Study. Eur. Heart J. 2009 Mar;30 (6):731–9. doi: 10.1093/eurheartj/ehn596. [DOI] [PubMed] [Google Scholar]
- 6.Wang Mei, Yip Gabriel W K, Wang Angela Y M, Zhang Yan, Ho Pik Yuk, Tse Mui Kiu, Lam Peggo K W, Sanderson John E. Peak early diastolic mitral annulus velocity by tissue Doppler imaging adds independent and incremental prognostic value. J. Am. Coll. Cardiol. 2003 Mar 5;41 (5):820–6. doi: 10.1016/s0735-1097(02)02921-2. [DOI] [PubMed] [Google Scholar]
- 7.Wang Mei, Yip Gabriel Wk, Wang Angela Ym, Zhang Yan, Ho Pik Yuk, Tse Mui Kiu, Yu Cheuk-Man, Sanderson John E. Tissue Doppler imaging provides incremental prognostic value in patients with systemic hypertension and left ventricular hypertrophy. J. Hypertens. 2005 Jan;23 (1):183–91. doi: 10.1097/00004872-200501000-00029. [DOI] [PubMed] [Google Scholar]
- 8.Andersson Charlotte, Gislason Gunnar H, Møgelvang Rasmus, Hoffmann Søren, Mérie Charlotte, Køber Lars, Torp-Pedersen Christian, Søgaard Peter. Importance and inter-relationship of tissue Doppler variables for predicting adverse outcomes in high-risk patients: an analysis of 388 diabetic patients referred for coronary angiography. Eur Heart J Cardiovasc Imaging. 2012 Aug;13 (8):643–9. doi: 10.1093/ejechocard/jer297. [DOI] [PubMed] [Google Scholar]
- 9.Westholm Carl, Johnson Jonas, Sahlen Anders, Winter Reidar, Jernberg Tomas. Peak systolic velocity using color-coded tissue Doppler imaging, a strong and independent predictor of outcome in acute coronary syndrome patients. Cardiovasc Ultrasound. 2013;11 () doi: 10.1186/1476-7120-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann Søren, Mogelvang Rasmus, Sogaard Peter, Iversen Allan Zeeberg, Hvelplund Anders, Schaadt Bente Krogsgaard, Fritz-Hansen Thomas, Galatius Soren, Risum Niels, Biering-Sørensen Tor, Jensen Jan Skov. Tissue Doppler echocardiography reveals impaired cardiac function in patients with reversible ischaemia. Eur J Echocardiogr. 2011 Aug;12 (8):628–34. doi: 10.1093/ejechocard/jer094. [DOI] [PubMed] [Google Scholar]
- 11.Lee Cheng-Hung, Hung Kuo-Chun, Chang Shang-Hung, Lin Fun-Chung, Hsieh Ming-Jer, Chen Chun-Chi, Chu Chi-Ming, Hsieh I-Chang, Wen Ming-Shien, Wu Delon. Reversible left ventricular diastolic dysfunction on Doppler tissue imaging predicts a more favorable prognosis in chronic heart failure. Circ. J. 2012;76 (5):1145–50. doi: 10.1253/circj.cj-11-0929. [DOI] [PubMed] [Google Scholar]
- 12.Wang Mei, Yip Gabriel, Yu Cheuk-Man, Zhang Qing, Zhang Yan, Tse Deko, Kong Shun-Ling, Sanderson John E. Independent and incremental prognostic value of early mitral annulus velocity in patients with impaired left ventricular systolic function. J. Am. Coll. Cardiol. 2005 Jan 18;45 (2):272–7. doi: 10.1016/j.jacc.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 13.Olsen Niels Thue, Jons Christian, Fritz-Hansen Thomas, Mogelvang Rasmus, Sogaard Peter. Pulsed-wave tissue Doppler and color tissue Doppler echocardiography: calibration with M-mode, agreement, and reproducibility in a clinical setting. Echocardiography. 2009 Jul;26 (6):638–44. doi: 10.1111/j.1540-8175.2008.00872.x. [DOI] [PubMed] [Google Scholar]
- 14.Manouras Aristomenis, Shahgaldi Kambiz, Winter Reidar, Nowak Jacek, Brodin Lars-Ake. Comparison between colour-coded and spectral tissue Doppler measurements of systolic and diastolic myocardial velocities: effect of temporal filtering and offline gain setting. Eur J Echocardiogr. 2009 May;10 (3):406–13. doi: 10.1093/ejechocard/jen298. [DOI] [PubMed] [Google Scholar]
- 15.de Knegt Martina Chantal, Biering-Sorensen Tor, Sogaard Peter, Sivertsen Jacob, Jensen Jan Skov, Mogelvang Rasmus. Concordance and reproducibility between M-mode, tissue Doppler imaging, and two-dimensional strain imaging in the assessment of mitral annular displacement and velocity in patients with various heart conditions. Eur Heart J Cardiovasc Imaging. 2014 Jan;15 (1):62–9. doi: 10.1093/ehjci/jet119. [DOI] [PubMed] [Google Scholar]
- 16.Kim Tae-Seok, Youn Ho-Joong. Role of echocardiography in atrial fibrillation. J Cardiovasc Ultrasound. 2011 Jun;19 (2):51–61. doi: 10.4250/jcu.2011.19.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okura H, Takada Y, Kubo T, Iwata K, Mizoguchi S, Taguchi H, Toda I, Yoshikawa J, Yoshida K. Tissue Doppler-derived index of left ventricular filling pressure, E/E', predicts survival of patients with non-valvular atrial fibrillation. Heart. 2006 Sep;92 (9):1248–52. doi: 10.1136/hrt.2005.082594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dandel Michael, Hetzer Roland. Echocardiographic strain and strain rate imaging--clinical applications. Int. J. Cardiol. 2009 Feb 6;132 (1):11–24. doi: 10.1016/j.ijcard.2008.06.091. [DOI] [PubMed] [Google Scholar]
- 19.Gjesdal Ola, Hopp Einar, Vartdal Trond, Lunde Ketil, Helle-Valle Thomas, Aakhus Svend, Smith Hans-Jørgen, Ihlen Halfdan, Edvardsen Thor. Global longitudinal strain measured by two-dimensional speckle tracking echocardiography is closely related to myocardial infarct size in chronic ischaemic heart disease. Clin. Sci. 2007 Sep;113 (6):287–96. doi: 10.1042/CS20070066. [DOI] [PubMed] [Google Scholar]
- 20.Inaba Yoshie, Yuda Satoshi, Kobayashi Naoko, Hashimoto Akiyoshi, Uno Kikuya, Nakata Tomoaki, Tsuchihashi Kazufumi, Miura Tetsuji, Ura Nobuyuki, Shimamoto Kazuaki. Strain rate imaging for noninvasive functional quantification of the left atrium: comparative studies in controls and patients with atrial fibrillation. J Am Soc Echocardiogr. 2005 Jul;18 (7):729–36. doi: 10.1016/j.echo.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Tsai Wei-Chuan, Lee Cheng-Han, Lin Chih-Chan, Liu Yen-Wen, Huang Yao-Yi, Li Wei-Ting, Chen Ju-Yi, Lin Li-Jen. Association of left atrial strain and strain rate assessed by speckle tracking echocardiography with paroxysmal atrial fibrillation. Echocardiography. 2009 Nov;26 (10):1188–94. doi: 10.1111/j.1540-8175.2009.00954.x. [DOI] [PubMed] [Google Scholar]
- 22.Tops Laurens F, Den Uijl Dennis W, Delgado Victoria, Marsan Nina Ajmone, Zeppenfeld Katja, Holman Eduard, van der Wall Ernst E, Schalij Martin J, Bax Jeroen J. Long-term improvement in left ventricular strain after successful catheter ablation for atrial fibrillation in patients with preserved left ventricular systolic function. Circ Arrhythm Electrophysiol. 2009 Jun;2 (3):249–57. doi: 10.1161/CIRCEP.108.838748. [DOI] [PubMed] [Google Scholar]
- 23.Biswas Monodeep, Sudhakar Selvin, Nanda Navin C, Buckberg Gerald, Pradhan Manish, Roomi Asad Ullah, Gorissen Willem, Houle Helene. Two- and three-dimensional speckle tracking echocardiography: clinical applications and future directions. Echocardiography. 2013 Jan;30 (1):88–105. doi: 10.1111/echo.12079. [DOI] [PubMed] [Google Scholar]
- 24.Mogelvang Rasmus, Sogaard Peter, Pedersen Sune A, Olsen Niels T, Marott Jacob L, Schnohr Peter, Goetze Jens P, Jensen Jan S. Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population. Circulation. 2009 May 26;119 (20):2679–85. doi: 10.1161/CIRCULATIONAHA.108.793471. [DOI] [PubMed] [Google Scholar]
- 25.Shin Hong-Won, Kim Hyungseop, Son Jihyun, Yoon Hyuck-Jun, Park Hyoung-Seob, Cho Yun-Kyeong, Han Chun-Duk, Nam Chang-Wook, Hur Seung-Ho, Kim Yoon-Nyun, Kim Kwon-Bae. Tissue Doppler imaging as a prognostic marker for cardiovascular events in heart failure with preserved ejection fraction and atrial fibrillation. J Am Soc Echocardiogr. 2010 Jul;23 (7):755–61. doi: 10.1016/j.echo.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Biering-Sørensen Tor, Jensen Jan Skov, Pedersen Sune, Galatius Søren, Hoffmann Soren, Jensen Magnus Thorsten, Mogelvang Rasmus. Doppler tissue imaging is an independent predictor of outcome in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Soc Echocardiogr. 2014 Mar;27 (3):258–67. doi: 10.1016/j.echo.2013.11.005. [DOI] [PubMed] [Google Scholar]