Abstract
Right ventricular outflow tract (RVOT) ventricular tachycardias (VT) occur in the absence of structural heart disease and are called idiopathic ventricular arrhythmias. These arrhythmias are thought to be produced by adenosine-sensitive, cyclic AMP mediated, triggered activity and are commonly observed in adolescents and young adults. In the ECG, they appear with a wide QRS complex, a left bundle branch block morphology and, usually, an inferior QRS axis. In the last few years, there has been an increasing number of reports suggesting the possibility of a curative treatment of RVOT VT by means of catheter ablation. This paper reviews the rate of cure of such arrhythmias by discussing the effects of catheter ablation on symptoms, arrhythmia detection, possibility of induction, and short- and long-term follow-up studies.
Keywords: Right Ventricular Outflow Tract, Tachycardia, Ablation, Cure
Introduction
This paper reviews the rate of cure achieved with catheter ablation in the type of arrhythmias called right ventricular outflow tract (RVOT) ventricular tachycardias (VT), the main characteristics of which have been described by Calvo et.al.[1] as follows:
RVOT VT occur in the absence of structural heart disease and are called idiopathic ventricular arrhythmias.
Ventricular arrhythmias originating in the RVOT are the most common subtype of idiopathic ventricular arrhythmias.
Idiopathic RVOT VT is thought to be produced by adenosine-sensitive, cyclic AMP mediated, triggered activity.
They are commonly observed in adolescents or young adults.
In the ECG they appear with a wide QRS complex, a left bundle branch block morphology and, usually, an inferior QRS axis.
RVOT VT is usually benign, but occasionally can induce left ventricular dysfunction, and, very rarely, ventricular fibrillation or polymorphic VT.
WordWeb® defines cure as “make healthy again” and health as “the state of being free of physical or psychological disease, illness, or malfunction”. This means that when we talk about a rate of cure, we mean the disappearance of the disease in term of symptoms and physical signs. We will thus examine the place of both (symptoms and physical signs) in order to assess the effectiveness of ablation as a curative treatment for RVOT VT.
A good example of cure on electrophysiological grounds is the successful ablation of an accessory pathway in a patient with Wolff-Parkinson-White syndrome. We can assert that the patient is cured because he/she does not present the tachycardia any longer, ECG pre-excitation disappears and bypass tract-mediated arrhythmia can no longer be induced in the electrophysiology (EP) laboratory. This example illustrates different ways of assessing the possibility of cure of RVOT VT by means of catheter ablation. We shall now discuss the symptoms, physical signs, arrhythmia detection, arrhythmia induction, acute success rate and long-term follow-up.
Symptoms
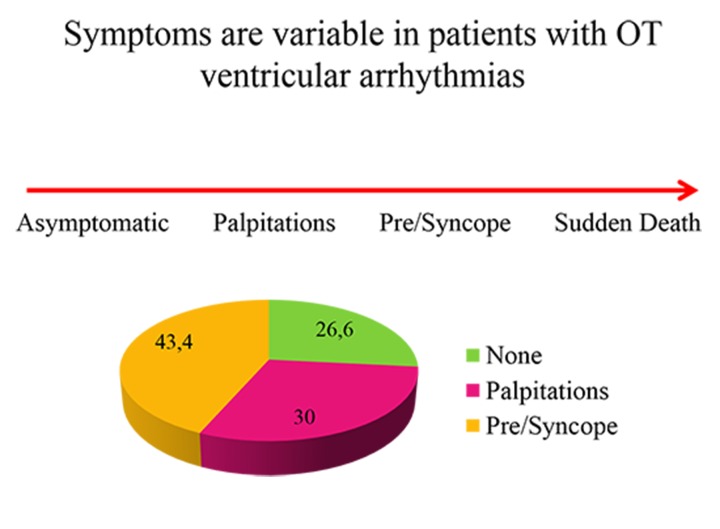
Patients who seek a consultation with a cardiologist or an electrophysiologist are usually biased. Indeed, they seek specialized medical assistance either because of their symptoms or because they underwent medical screening and were found to suffer from an arrhythmia. Patients are then generally more aware of palpitations and, as a result, more symptomatic. In 1983, Buxton et.al. published one of the first description of RVOT VT in 30 patients: a quarter of them were asymptomatic.[2] Twenty four years later, Kim described 127 patients with RVOT VT who were divided into three groups according to the type of arrhythmia that they presented: paroxysmal sustained monomorphic VT (SMVT), repetitive nonsustained VT (NSVT), and premature ventricular contractions (PVC).[3] These three clinical presentations are those we most frequently find in our daily practice. Few reports of patients with RVOT VT have described subjects with polymorphic VT, ventricular fibrillation and/or aborted sudden death.[1] Patients may present with symptoms that vary from absence of symptoms (asymptomatic) to sudden death (see Figure 1). In the two series of patients described above,[2,3] around 20% in each group (SMVT, NSVT or PVC) did not report palpitations.
Figure 1. Scale of symptoms suffered by patients with RVOT VT and previously reported percentages.
Patients who are referred to our Arrhythmia Clinic are sometimes initially asymptomatic but are later found, during a routine clinical examination, to suffer from PVC that they probably had for a long time. Once they are told they have an arrhythmia, they start feeling palpitations.But when they do not present either ventricular dysfunction or structural heart disease, they only need to be reassured about the benign course of the disease. They then become asymptomatic again without intervention (antiarrhythmic drugs or catheter ablation).
It is well known that treatments may have a placebo effect. This is why treatment effectiveness should be evaluated by studies using a placebo.In our search of the literature, we could not find any publication about catheter ablation of RVOT VT with such a design.
The aforesaid leads us to conclude that symptoms cannot be considered a reliable means of assessing either the effectiveness of a treatment or the disappearance of the arrhythmia. In other words, in terms of symptoms, no study proves that catheter ablation can cure RVOT VT.
Physical Signs
Unless they are very frequent and because of their variable nature, arrhythmias are difficult to assess either by means of a physical examination or of an ECG recorded at the physician’s office. However, in order to diagnose and follow the course of an arrhythmia, we have to either detect it or induce it.
Arrhythmia Detection
The traditional means of detection is the Holter monitoring system and, more recently, the long-term monitoring system. We could not find any study that specifically evaluates the spontaneous variability of RVOT VT or PVC. However, it is very important to bear that variability in mind when attributing a reduction in the frequency of detection of an arrhythmia to a treatment. In 1988 Bernard Lown et.al. addressed that issue in 45 patients (9 without structural heart disease). To be included in their study, the patients had to exhibit >3 consecutive PVC and more than 300 PVC per hour. The authors concluded that a decrease in PVC density > 63% was required to distinguish drug effect from spontaneous variability. The requirement for NSVT was 90%. To meet the criteria for arrhythmia aggravation, PVC had to increase by 400% and NSVT by 500%.[4]
In 1995 Wei-Xi-Zhu et.al. reported 10 patients (7 without structural heart disease) with highly symptomatic PVC who did not respond to antiarrhythmic drugs. The PVC foci were in the RVOT in 9 patients.[5] The authors report that “the ventricular ectopic activity was eliminated in all 10 patients during the procedure” and that the frequency of ectopic activity decreased from 1065 ± 631 beats/h to 0 in 7 patients.[5]
In 1999 the Bordeaux group described 12 patients who suffered from frequent and symptomatic OT PVC.[6] Nine did not present any evidence of structural heart disease, and all of them had been administered multiple antiarrhythmic drugs without success.The 12 patients were submitted to radiofrequency (RF) ablation, and the procedure “succeeded” in 11 who then became asymptomatic (2 of them with previously ineffective antiarrhythmic drugs). The PVC count on Holter decreased from 12096 ± 3326 to 1329 ± 3198.[6] These initial reports probably set the basis for a more widespread use of ablation for PVC.
A few years later, Yarlagadda et.al. and Taieb et.al. described patients with RVOT PVC who had left ventricular dysfunction and who renormalized the ejection fraction after “successful” ablation.[7,8]
Since the introduction of electroanatomic mapping systems into clinical electrophysiological practice, the number of patients with RVOT PVC who are submitted to RF ablation increased. Indeed, Baranchuk et.al. published a meta-analysis in which they reviewed 450 articles dealing with ablation in patients with PVC originating from the RVOT.[9] Fourteen articles only consistently reported the effect of ablation on PVC count and ventricular function.It is very important to note, however, that the average reduction in PVC was less than 50%. Thus, treatment effect cannot account for PVC reduction.[4,5] If we do not take spontaneous variability into consideration, the PVC reduction inferior to 50% represents and improvement but not a cure of the arrhythmia.
As previously mentioned about the spontaneous variability of arrhythmias, specifically regarding NSVT and SMVT, treatment effect can explain the reduction in arrhythmia detection by means of a Holter only if the reduction is greater than 90%. We could not find any study of patients with NSVT or SMVT submitted to ablation and evaluated by means of long-term monitoring.
Baranchuk et al. meta-analysis reported a significant improvement in left ventricular function after PVC ablation.[9] This finding would support the prescription of ablation for those patients with frequent PVC and left ventricular dysfunction. We do not know of any report about the effects of antiarrhythmic drugs on left ventricular function in patients with RVOT, frequent PVC, and left ventricular dysfunction, but it is tempting to speculate that if the antiarrhythmic drug is able to significantly reduce the PVC count, it would also be able to improve ventricular function. Indeed, ablation improves ventricular function in spite of the fact that it does not completely eliminate the PVC as has been reported elsewhere.
Arrhythmia Induction
Another means of assessing the effect of ablation as a potential cure for RVOT VT is to demonstrate that the arrhythmia cannot be induced after applying the treatment when the arrhythmia was reproducibly inducible before the ablation.As RVOT VT is produced by an adenosine-sensitive, cyclic AMP mediated, triggered activity, arrhythmia induction can be achieved by means of programmed ventricular stimulation, burst pacing, catecholamine infusion or exercise.[1]
In their group of SMVT patients, Kim et.al. found that the arrhythmia was induced at exercise stress test in 67% of the patients.[3] In the NSVT and PVC patients, the percentage of induction by means of stress test was only 10%. Considering that 33% of patients with SMVT are not inducible at stress testing, we cannot infer that the absence of arrhythmia induction during the stress test means that the arrhythmia was cured. The same reasoning applies for NSVT and PVC patients.
The other means of arrhythmia induction is programmed stimulation with premature stimuli and/or burst pacing alone or combined with the infusion of catecholamines. In Kim’s patients, 78% of the subjects with SMVT were induced at the EP study, while only 26% of the NSVT and PVC patients were inducible.[3] Neither in Kim’s study nor in other publications could we find an assessment of the reproducibility of induction. As was said before, if the induction is not reproducible before performing the ablation, we cannot state that the absence of induction is a proof of cure.
The above discussion leads us to conclude that arrhythmia induction cannot be used as a tool to evaluate the possibility of cure of RVOT VT.
Acute “Success” Rate
Successful ablation has not been uniformly defined. Many authors define it as the impossibility to induce the arrhythmia with the best method of induction found before ablation and when patient remains non-inducible for at least 30 minutes after performing the RF.[10]
It is well known that undergoing a medical or surgical procedure may have a placebo effect.[11,12] We could not find any double blind study that prospectively evaluated the “success” rate of ablation in RVOT VT patients. Besides, because of ethical considerations, this procedure is not universally accepted.[11,12] We could not find any study either with a robust scientific design comparing pharmacological treatment with ablation in a prospective follow-up. Most publications about RVOT VT ablation report an excellent acute success rate of about 90%.[3,10,13] It is worthwhile mentioning that these studies do not specify mid-term “success” rate, i.e. between 1 to 3 years.
Long-term “Success” Rate
Ventura et.al. retrospectively reviewed the long-term success rate of treatment in 133 patients with RVOT SMVT with a mean follow-up period of 135 ± 68 months.[14] Sixty two patients (47%) were administered antiarrhythmic drugs, and 32 (52%) had recurrences of the arrhythmia within 10 years on average. Seventy one patients (53%) underwent catheter ablation, and the procedure was found to be successful in 82%. From the 58 patients with a successful ablation, 30 (52%) had recurrences within 6.2 years on average.[14] It is worthwhile mentioning that from the 30 patients who received antiarrhythmic drugs, only 8 were still receiving the medication at the end of the follow-up, but all of them were free of symptoms, and the Holter recordings did not detect any arrhythmia.The authors suggest the possibility of spontaneous remission, which makes it even more difficult to assess the possibility of cure by means of catheter ablation.
The above results imply that the long-term “success” rate of initially successful RVOT SMVT ablation is 48% and is equal to that obtained with antiarrhythmic drugs. In other words, the success rate of both treatment forms is identical, and the patients who were administered antiarrhythmic drugs remained free of arrhythmia recurrence for a longer period of time.[14]
Prospective randomized trials studying large enough samples of patients with RVOT VT need to be conducted before selecting ablation over antiarrhythmic drug therapy. Current guidelines recommend catheter ablation when SMVT causes severe symptoms, when antiarrhythmic drugs are not effective, tolerated or desired, and in patients with electrical storm refractory to antiarrhythmic therapy.[15]
Conclusions
In conclusion, until we have a solid and scientifically validated evidence, we cannot assert that catheter ablation cures RVOT VT. We, as electrophysiologists,should temper our enthusiasm before performing catheter ablation for a condition that has a significant chance of recurrence and for which antiarrhythmic drugs may offer a similar “success” rate.
The clinical course of patients with RVOT has almost uniformly been described as benign. This makes it hard to advocate for the application of any treatment to improve the clinical course of the disease. A particular subgroup could be one consisting of patients with frequent RVOT and left ventricular dysfunction. Indeed, we do not know what the long-term course of patients with frequent RVOT and left ventricular dysfunction is. In other forms of cardiac disease, it is well known that the presence of left ventricular dysfunction constitutes a powerful risk factor for predicting morbidity and mortality. As a consequence, it would be unethical not to treat patients with frequent RVOT and left ventricular dysfunction.This is another area where it would be advisable to conduct randomized studies comparing antiarrhythmic drugs with ablation.
Disclosures
None.
References
- 1.Calvo Naiara, Jongbloed Monique, Zeppenfeld Katja. Radiofrequency catheter ablation of idiopathic right ventricular outflow tract arrhythmias. Indian Pacing Electrophysiol J. 2013 Jan;13 (1):14–33. doi: 10.1016/s0972-6292(16)30585-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buxton A E, Waxman H L, Marchlinski F E, Simson M B, Cassidy D, Josephson M E. Right ventricular tachycardia: clinical and electrophysiologic characteristics. Circulation. 1983 Nov;68 (5):917–27. doi: 10.1161/01.cir.68.5.917. [DOI] [PubMed] [Google Scholar]
- 3.Kim Robert J, Iwai Sei, Markowitz Steven M, Shah Bindi K, Stein Kenneth M, Lerman Bruce B. Clinical and electrophysiological spectrum of idiopathic ventricular outflow tract arrhythmias. J. Am. Coll. Cardiol. 2007 May 22;49 (20):2035–43. doi: 10.1016/j.jacc.2007.01.085. [DOI] [PubMed] [Google Scholar]
- 4.Raeder E A, Hohnloser S H, Graboys T B, Podrid P J, Lampert S, Lown B. Spontaneous variability and circadian distribution of ectopic activity in patients with malignant ventricular arrhythmia. J. Am. Coll. Cardiol. 1988 Sep;12 (3):656–61. doi: 10.1016/s0735-1097(88)80052-4. [DOI] [PubMed] [Google Scholar]
- 5.Zhu D W, Maloney J D, Simmons T W, Nitta J, Fitzgerald D M, Trohman R G, Khoury D S, Saliba W, Belco K M, Rizo-Patron C. Radiofrequency catheter ablation for management of symptomatic ventricular ectopic activity. J. Am. Coll. Cardiol. 1995 Oct;26 (4):843–9. doi: 10.1016/0735-1097(95)00287-7. [DOI] [PubMed] [Google Scholar]
- 6.Lauribe P, Shah D, Jaïs P, Takahashi A, Haïssaguerre M, Clémenty J. Radiofrequency catheter ablation of drug refractory symptomatic ventricular ectopy: short- and long-term results. Pacing Clin Electrophysiol. 1999 May;22 (5):783–9. doi: 10.1111/j.1540-8159.1999.tb00544.x. [DOI] [PubMed] [Google Scholar]
- 7.Yarlagadda Ravi K, Iwai Sei, Stein Kenneth M, Markowitz Steven M, Shah Bindi K, Cheung Jim W, Tan Vivian, Lerman Bruce B, Mittal Suneet. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation. 2005 Aug 23;112 (8):1092–7. doi: 10.1161/CIRCULATIONAHA.105.546432. [DOI] [PubMed] [Google Scholar]
- 8.Taieb Jerome M, Maury Philippe, Shah Dipen, Duparc Alexandre, Galinier Michel, Delay Marc, Morice Ronan, Alfares Ali, Barnay Claude. Reversal of dilated cardiomyopathy by the elimination of frequent left or right premature ventricular contractions. J Interv Card Electrophysiol. 2007 Nov;20 (1-2):9–13. doi: 10.1007/s10840-007-9157-2. [DOI] [PubMed] [Google Scholar]
- 9.Lamba Jasmine, Redfearn Damian P, Michael Kevin A, Simpson Christopher S, Abdollah Hoshiar, Baranchuk Adrian. Radiofrequency catheter ablation for the treatment of idiopathic premature ventricular contractions originating from the right ventricular outflow tract: a systematic review and meta-analysis. Pacing Clin Electrophysiol. 2014 Jan;37 (1):73–8. doi: 10.1111/pace.12243. [DOI] [PubMed] [Google Scholar]
- 10.Issa ZF, Miller JM, DP Zipes. Adenosine-sensitive (outflow tract) ventricular tachycardia. In Clinical Arrhythmology and Electrophysiology . A companion to Braunwald’s Heart Disease. 2009;0:562–586. [Google Scholar]
- 11.Freeman T B, Vawter D E, Leaverton P E, Godbold J H, Hauser R A, Goetz C G, Olanow C W. Use of placebo surgery in controlled trials of a cellular-based therapy for Parkinson's disease. N. Engl. J. Med. 1999 Sep 23;341 (13):988–92. doi: 10.1056/NEJM199909233411311. [DOI] [PubMed] [Google Scholar]
- 12.Macklin R. The ethical problems with sham surgery in clinical research. N. Engl. J. Med. 1999 Sep 23;341 (13):992–6. doi: 10.1056/NEJM199909233411312. [DOI] [PubMed] [Google Scholar]
- 13.Prystowsky Eric N, Padanilam Benzy J, Joshi Sandeep, Fogel Richard I. Ventricular arrhythmias in the absence of structural heart disease. J. Am. Coll. Cardiol. 2012 May 15;59 (20):1733–44. doi: 10.1016/j.jacc.2012.01.036. [DOI] [PubMed] [Google Scholar]
- 14.Ventura Rodolfo, Steven Daniel, Klemm Hanno U, Lutomsky Boris, Müllerleile Kai, Rostock Thomas, Servatius Helge, Risius Tim, Meinertz Thomas, Kuck Karl-Heinz, Willems Stephan. Decennial follow-up in patients with recurrent tachycardia originating from the right ventricular outflow tract: electrophysiologic characteristics and response to treatment. Eur. Heart J. 2007 Oct;28 (19):2338–45. doi: 10.1093/eurheartj/ehm293. [DOI] [PubMed] [Google Scholar]
- 15.Aliot Etienne M, Stevenson William G, Almendral-Garrote Jesus Ma, Bogun Frank, Calkins C Hugh, Delacretaz Etienne, Della Bella Paolo, Hindricks Gerhard, Jaïs Pierre, Josephson Mark E, Kautzner Josef, Kay G Neal, Kuck Karl-Heinz, Lerman Bruce B, Marchlinski Francis, Reddy Vivek, Schalij Martin-Jan, Schilling Richard, Soejima Kyoko, Wilber David. EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA). Heart Rhythm. 2009 Jun;6 (6):886–933. doi: 10.1016/j.hrthm.2009.04.030. [DOI] [PubMed] [Google Scholar]